Abstract
Background
Age at menarche impacts patterns of pubertal growth and skeletal development. These effects may carry over into variation in biomechanical profiles involved in sports-related traumatic and overuse knee injuries. The present study investigated whether age at menarche is a potential indicator of knee injury risk through its influence on knee biomechanics during normal walking.
Objective
To test the hypothesis that earlier menarche is related to post-pubertal biomechanical risk factors for knee injuries, including a wider, more immature gait base of support, and greater valgus knee angles and moments.
Design
Cross-sectional observational study.
Setting
University research facility.
Participants
Healthy, post-menarcheal, adolescent females.
Methods
Age at menarche was obtained by recall questionnaire. Pubertal growth and anthropometric data were collected using standard methods. Biomechanical data were taken from tests of walking gait at self-selected speed. Reflective marker position data were collected using a three-dimensional quantitative motion analysis system, and three force plates recorded kinetic data.
Main Outcome Measures
Age at menarche; growth and anthropometric measurements; base of support; static knee frontal plane angle; dynamic knee frontal plane angles and moments during stance.
Results
Earlier menarche was significantly correlated with abbreviated pubertal growth and post-pubertal retention of immature traits, including a wider base of support. Earlier menarche and wider base of support were both correlated with more valgus static knee angles, more valgus knee abduction angles and moments at foot-strike, and a more valgus peak knee abduction angle during stance. Peak knee abduction moment during stance was not correlated with age at menarche or base of support.
Conclusions
Earlier menarche and its effects on growth are associated with retention of a relatively immature gait base of support and a tendency for static and dynamic valgus knee alignment. This biomechanical profile may put girls with earlier menarche at higher risk for sports-related knee injuries.
Introduction
Compared to male peers playing the same sports, adolescent and young adult females have a higher incidence of knee-related overuse and traumatic injuries, including iliotibial band syndrome (ITBS), patellofemoral pain syndrome (PFPS), and non-contact anterior cruciate ligament (ACL) tears [1-5]. Each injury is associated with significant pain, reduced physical activity, interrupted sports participation, and, in some cases, increased risk for musculoskeletal diseases such as osteoarthritis [4,6-11]. There is, accordingly, substantial clinical interest in early identification of high-risk individuals for the purposes of prevention [9,12-15].
The injury disparity between females and males may arise in part from sex differences in frontal plane knee biomechanics. Females tend to exhibit greater knee abduction angles and moments in the stance, or plant limb, during a variety of movements compared to males [16-20]. The female tendency toward greater knee abduction places elevated tensile strain on the iliotibial band [21], increases patellofemoral joint contact forces [5,12,17,22-24], and elevates shear stress on the ACL [25-29]. This specific biomechanical pattern is therefore likely to be broadly involved in the etiology of knee injuries among young females.
Fortunately, not all young females will sustain overuse or traumatic knee injuries, and, likewise, not all females exhibit exaggerated knee abduction angles or moments during movement. It is therefore important to assess variability within females in order to identify key factors that affect knee biomechanics, and, by proxy, elevate individual injury risk. One promising avenue of research is to examine relationships between variation in pubertal developmental timing and patterns of movement. This approach is derived from the observations that sex differences in both knee injury risk and frontal plane knee biomechanics first emerge during puberty [13,30-34].
Given that puberty has a strong effect on biomechanical differentiation between females and males, it stands to reason that variation in pubertal timing and progression within females also affects intra-sex variability in movement patterns and therefore injury risk. In females, the age at menarche (first onset of menses) provides a clear indicator of pubertal timing [35,36], and varies considerably between individuals [37]. Menarche is associated with the slowing and eventual cessation of skeletal growth [38-40], and may therefore have important implications for lower limb alignment and biomechanics. Specifically, it is possible that earlier menarche stops growth processes prematurely, resulting in the post-pubertal retention of immature traits.
The following study explores relationships between age at menarche, knee biomechanics, and knee injury risk factors through the framework of gait base of support, which we quantify as the ratio of pelvic breadth to step width during normal walking [41]. This framework has three advantages. First, base of support includes aspects of ankle and hip positioning, and is a determinant of overall lower limb alignment which has implications for knee alignment and biomechanics [42]. Variation in base of support can thus provide an indicator of the effects of puberty on dynamic lower limb frontal plane alignment [42] reflecting variability in pelvic and lower limb growth, and in the development of neuromuscular control of the knee and ankle [43,44].
Second, our recent research [45] shows that base of support matures during puberty, developing from the more immature condition of wider steps to the more mature pattern of narrower steps relative to pelvic breadth. Females undergo a substantial degree of pubertal change in base of support, and the age at which base of support reaches static adult levels is variable (mean age is 14.3 ± 1.2 years). Some of the variation in post-pubescent base of support, as well as in the age at which it is reached, may be related to variability in age at menarche. If earlier menarche results in an early halt to musculoskeletal and neuromuscular developmental processes, this may affect variation in base of support and its relationship to knee alignment and biomechanics.
Third and finally, walking gait base of support can be easily measured in clinical settings. Pelvic breadth can be measured with a set of calipers, and step width can be assessed inexpensively and quickly using a short metric walkway and ink pads [46]. If base of support is correlated with lower limb alignment and biomechanical injury risk factors, then this simple measure could be used to pre-screen adolescent girls prior to sports participation, and encourage those at greater risk to engage in preventive training programs [9]. Age at menarche itself may also provide an easily assessed indicator of risk, although if obtained by recall it is ideally recorded within 6-12 months of its occurrence [47].
The present study analyzed a sample of healthy adolescent females from the Fels Longitudinal Study (Fels). This long-running longitudinal study of human growth and development has had over 1200 serial participants [48] since 1929, many of whom had walking gait data collected between 2003 and 2009. Fels participants are mainly of European ancestry, live chiefly in southwest Ohio, and theoretically represent normal population variation since they have not been selected for any specific disease or trait. The Fels study has traditionally focused on patterns of childhood and adolescent growth in biomedical and public health contexts, and the present study represents an extension of that tradition into the realm of sports medicine, rehabilitation, and musculoskeletal injury prevention.
We tested two hypotheses in this sample. Hypothesis 1: earlier age at menarche is correlated with post-pubescent retention of a wider, more immature base of support. Hypothesis 2: earlier age at menarche and wider base of support are related to more valgus static knee alignment, as well as greater knee abduction angles and external moments during the stance phase of normal walking. If these hypotheses are supported, it would indicate that earlier menarche and wider base of support are related to lower limb biomechanical profiles that increase risk for knee injury in adolescent and young adult females.
Methods
Participants
All procedures, including the present data analysis, were approved by the local Institutional Review Board. All participants provided informed parental consent and individual assent before testing. The present analysis used a cross-sectional sample, incorporating data from only the most recent available test visit with gait data. This approach was meant to include only the oldest available age at measurement visit, and thus the greatest available level of overall developmental maturity, for each participant.
Any post-menarcheal female Fels participant with at least one gait test between the ages of 11-18 years was eligible for inclusion in the study. Participants were excluded for abnormally early or late menarche (< 11.0 years; > 15.0 years [49,50]), obesity (body mass index ≥ 95th percentile for age [51]), toe-walking, prescription shoe inserts, or chronic musculoskeletal conditions. Given these criteria, a total of 52 individuals were initially eligible. Participants were then removed from the sample if they reported any recent (≤ 6 months before gait test) lower limb, pelvic, or vertebral musculoskeletal injury, or any history of ITBS, PFPS, or ACL tear (6 individuals), resulting in a total sample of 46 uninjured post-menarcheal females with normal gait.
Data collection and processing
Age at menarche was assessed by self-report, typically within six months of its occurrence, therefore minimizing potential recall bias [47]. Standard methods were used for anthropometric measurements [52], which included stature, sitting height, and bicristal breadth (all to the nearest mm), and weight to the nearest 0.1 kg. Body mass index (BMI; kg · m-2) was calculated as (weight / stature2). Subischial leg length was calculated as (stature – sitting height). Skeletal age, an indicator of the degree of maturation of the skeleton, was assessed from hand-wrist radiographs using the FELS method [53]. Relative skeletal age was calculated as (skeletal age – chronological age) to express the degree of acceleration or delay in skeletal maturation compared to population average expectations for chronological age. Age at onset of the pubertal growth spurt was derived from longitudinal stature measurements in a subset of individuals with sufficient data (N = 29). Both age at onset itself, as well as the amount of time between age at onset and age at menarche, were used to analyze the timing and duration of pubertal growth relative to menarche and growth cessation [54].
Three-dimensional quantitative gait analysis was performed in our Motion Analysis Laboratory. The lab is equipped with six high-speed Hawk cameras (Motion Analysis Corp., Santa Rosa, CA) synchronized with three force plates which are embedded in a 15m walkway (two AMTI OR6-7-1000, Advanced Medical Technology, Inc., Watertown, MA; one Kistler Type 9281B11, Kistler Instruments, Winterthur, Switzerland). External passive reflective markers were placed on joints and body segments using the Helen Hayes system [55], and motion capture was performed using EvArt software (Motion Analysis Corp., Santa Rosa, CA). Five-second static trials were recorded with the participant standing, feet shoulder-width apart and arms spread laterally. Walking trials at self-selected “normal” speed were then recorded for each participant. Three trials with clean force plate strikes and high-fidelity marker recognition were analyzed and averaged for subsequent analyses.
Data were processed using OrthoTrak software (Motion Analysis Corp., Santa Rosa, CA) with hip joint centers calculated as offsets from anterior superior iliac spine (ASIS) and sacral markers [56]. To simplify analysis, data were restricted to the dominant limb, defined as the most frequent leadoff limb during the walking trials (participants were instructed to begin with whichever leg felt most comfortable) [57]. If neither limb was dominant, values for both limbs were averaged. Static trials were used to calculate inter-ASIS breadth (a measure of pelvic development), lower limb segmental lengths (distances between joint centers: hip-knee = thigh segment length; knee-ankle = shank segment length), and static knee frontal plane angle (see [42]). Step width from walking trials was used to calculate base of support as (bicristal breadth / step width). To further quantify maturity of base of support, we also calculated age-specific residuals from our previously published equations [45]. Dynamic knee frontal plane angle and moment at foot-strike, as well as peak abduction angle and moment during stance phase, were derived from walking trials. The knee frontal plane moments described here are external moments, calculated using inverse dynamics and normalized for body weight.
Statistical analysis
Statistical analysis was performed in SAS 9.3 (SAS Inc., Cary, NC) with α = 0.05. Descriptive statistics and Shapiro-Wilk tests of normality were computed for each variable. Correlations between variables were analyzed using Pearson's product moment correlation (or Spearman's rank order correlation where appropriate) and ordinary least squares regression analysis.
Results
To interpret the results, the following data conventions should be noted:
Base of support is calculated as bicristal breadth / step width. Smaller values therefore indicate a wider base of support relative to the breadth of the pelvis, which is the more immature condition.
Negative values of residual base of support indicate a relatively immature base of support for one's age.
For kinematic and kinetic variables negative values indicate valgus/abduction and positive values indicate varus/adduction.
Table 1 provides descriptive statistics for the anthropometric and developmental variables in this sample, as well as statistics for their degree of correlation with age at menarche. Mean age at menarche was 12.5 ± 0.8 years (median = 12.5 years, 25th percentile = 11.9 years, 75th percentile = 13.0 years), which is similar to the overall U.S. population [49]. Mean age at test visit was 16.2 ± 1.6 years, and was not correlated with age at menarche (p=.59). In other words, data from participants with earlier menarche were not collected at systematically younger chronological ages, thereby reducing or eliminating any age bias in the analyses. Sensitivity analyses controlling for age in partial correlations confirmed the lack of age bias in the study (data not shown).
Table 1. Sample descriptive statistics and correlations with age at menarche.
| N = 46 | Mean ± SD | Range | Pearson's r |
|---|---|---|---|
| Age at menarche (y) | 12.5 ± 0.8 | (11.0 - 14.1) | --- |
| Age at visit (y) | 16.2 ± 1.6 | (11.4 - 18.5) | 0.06 |
| Skeletal age at visit (y) | 16.8 ± 1.6 | (12.8 - 18.0) | -0.11 |
| Relative skeletal age at visit (y) | 0.7 ± 0.7 | (-0.6 - 2.9) | -0.46 ** |
| Age at onset of the pubertal growth spurt (y)*** | 8.4 ± 0.8 | (7.3 - 10.0) | 0.80 ** |
| Time between age at onset and age at menarche (y)*** | 4.1 ± 0.5 | (3.2 - 5.3) | 0.54 ** |
| Weight (kg) | 61.0 ± 9.7 | (44.7 - 85.3) | -0.17 |
| Height (cm) | 164.4 ± 7.4 | (152.6 - 179.9) | 0.29 * |
| Sitting height (cm) | 87.3 ± 3.7 | (80.6 - 96.4) | 0.17 |
| Subischial leg length (cm) | 77.3 ± 4.6 | (70.0 - 89.9) | 0.31 * |
| Thigh segment length (cm) | 42.2 ± 3.3 | (35.7 - 50.1) | 0.42 ** |
| Shank segment length (cm) | 38.1 ± 2.5 | (33.6 - 43.3) | 0.13 |
| Inter-ASIS breadth (cm) | 24.9 ± 2.1 | (21.0 - 29.2) | -0.33 * |
| Bicristal breadth (cm) | 27.6 ± 1.8 | (23.1 - 32.6) | 0.02 |
| Static knee frontal plane angle (°) | -2.0 ± 3.1 | (-9.3 - 5.4) | 0.28 * |
Significantly correlated with age at menarche: p<.05.
Significantly correlated with age at menarche: p<.01.
Data only available for a subset of 29 individuals.
Several growth-and-development variables were significantly correlated with age at menarche (see Table 1 for r-values). Earlier age at menarche was associated with an earlier age at the onset of the pubertal growth spurt and a shorter period of time between the onset of accelerated pubertal growth and menarche (for each, p≤.01). Earlier age at menarche was also correlated with greater relative skeletal age (p≤.01). Stature, subischial leg length, and thigh segment length were all significantly shorter with earlier menarche (for each, p<.05), whereas inter-ASIS breadth was significantly wider with earlier menarche (for each, p=.02). Earlier menarche was also related to greater BMI (p=.02). Bicristal breadth was not significantly correlated with age at menarche (p=.89).
Hypothesis 1
Age at menarche was significantly correlated with base of support (r = 0.44; p=.002) and residual base of support (r = 0.43; p=.003; see Fig. 1). The results for base of support and residual base of support were essentially identical, so we report only results for base of support below. The majority of variation in base of support was associated with variability in step width (r = -0.95; p<.001; see Fig. 1), and base of support was not related to variation in bicristal breadth (r = 0.01; p=.94). Base of support was not significantly correlated with pubertal duration (r = 0.32; p=.09), but each of its two subcomponents were (step width: r = -0.44; p=.02; bicristal breadth: r = -0.39; p=.04).
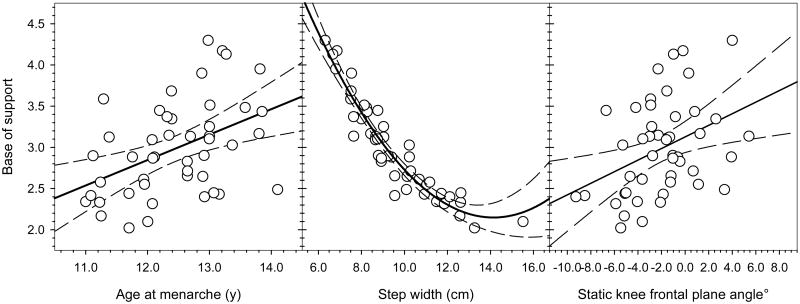
Fig. 1.
Relationships of base of support to age at menarche, step width, and static knee frontal plane angle. Base of support was significantly correlated with age at menarche (r = 0.44; p=.002), such that earlier menarche was associated with a wider base of support. Step width explained the majority of variation in base of support with a quadratic best fit line (r2 = 0.91; p<.001; the correlation coefficient r is not reported as it is not appropriate for a quadratic relationship). Static knee frontal plane angle was also significantly correlated with base of support (r = 0.38; p=.008), such that wider base of support was related to more valgus knee alignment.
Hypothesis 2
Descriptive statistics for gait and biomechanical variables are presented in Table 2. Static knee frontal plane alignment was slightly valgus in the sample as a whole, and was significantly correlated with both age at menarche (r = 0.28; p=.05; see Table 1) and base of support (r = 0.38; p=.008; see Fig. 1). Static knee frontal plane angle was also significantly correlated with the length of the pubertal growth spurt, such that a shorter period of growth was associated with more valgus alignment (r = 0.48; p=.009). Knee kinematic and kinetic values were overwhelmingly significantly correlated with age at menarche and base of support. Knee frontal plane angle and moment at foot-strike were each significantly related to age at menarche (respectively: r = 0.42; p=.004; r = 0.33; p=.02; see Fig. 2). The same was true for peak knee abduction angle during support (r = 0.45; p=.002), but not peak knee abduction moment (r = -0.16; p=.30; see Fig. 3). Knee frontal plane angle and moment at foot-strike were also each significantly related to base of support (respectively: r = 0.41; p=.005; r = 0.33; p=.03; see Fig. 2), as was peak knee abduction angle during stance (r = 0.38; p=.01), but not peak abduction moment (r = -0.17; p=.25; see Fig. 3).
Table 2. Gait variable descriptive statistics.
| Mean ± SD | Range | |
|---|---|---|
| Step width (cm) | 9.56 ± 1.98 | (6.32 - 15.51) |
| Base of support* | 2.99 ± 0.57 | (2.02 - 4.30) |
| Residual base of support* | 0.09 ± 0.57 | (-0.89 - 1.39) |
| Knee frontal plane angle at foot-strike (°) | -2.73 ± 2.43 | (-8.35 - 2.40) |
| Knee frontal plane moment at foot-strike (Nm · kg-1) | 0.04 ± 0.05 | (-0.09 - 0.14) |
| Peak knee abduction angle during support (°) | -4.26 ± 3.01 | (-10.77 - 1.76) |
| Peak knee abduction moment during support (Nm · kg-1) | -0.50 ± 0.34 | (-1.41 - -0.02) |
Because of the way it is calculated, smaller values of base of support indicate wider step widths relative to pelvic breadth. This is important to note because is not necessarily intuitive. A wider base of support (i.e. a smaller value) is the more immature condition.
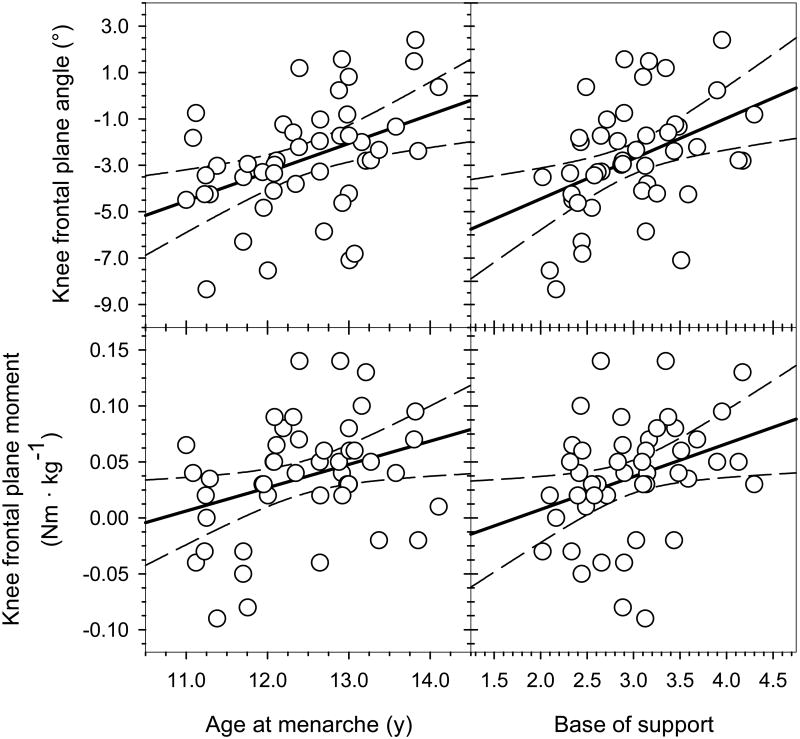
Fig. 2.
Knee frontal plane angle at foot-strike was significantly correlated with both age at menarche (r = 0.42; p=.004) and base of support (r = 0.41; p=.005). The knee frontal plane moment at foot-strike was also significantly correlated with both age at menarche (r = 0.33; p=.02) and base of support (r = 0.33; p=.03). Overall, earlier menarche and wider base of support were associated with more valgus knee angle and a tendency toward an external abduction moment at foot-strike.
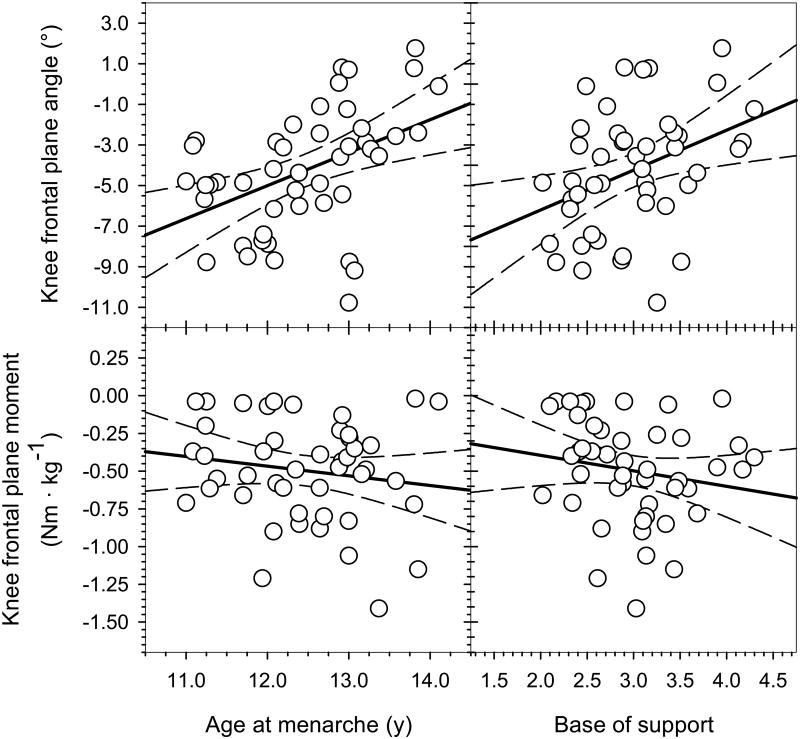
Fig. 3.
The peak knee abduction angle during stance phase was significantly correlated with both age at menarche (r = 0.45; p=.002) and base of support (r = 0.38; p=.01). Peak knee abduction moment during stance, however, was not significantly correlated with either age at menarche (r = -0.16; p=.30) or base of support (r = -0.17; p=.25). Earlier menarche and wider base of support were therefore associated with a more valgus peak knee frontal plane angle during stance.
Discussion
This study tested two hypotheses on relationships between age at menarche, musculoskeletal development, and frontal plane knee biomechanics in walking gait. These issue are of clinical relevance in the context of overuse and traumatic knee injuries including ITBS, PFPS, and ACL tears. In our sample of healthy, uninjured girls, earlier age at menarche was correlated with post-pubescent retention of a relatively immature, wider base of support. Earlier age at menarche and wider base of support were also both related to increased static and dynamic knee abduction angles and external moments during the stance phase of normal walking. These results indicate that variation in age at menarche is related to variation in biomechanical parameters that are risk factors for knee overuse and non-contact traumatic injuries.
Age at menarche has important effects on pubertal growth, and these effects explain associations between menarche and walking gait biomechanics. Females with earlier menarche tended to retain immature-looking traits after puberty relative to their peers with later menarche, including a wider base of support, which, in this sample, was related to a more valgus knee under static and dynamic conditions. A key observation is that earlier menarche was associated with both an earlier start to puberty and a shorter overall period of pubertal growth (see Table 1). Comparing age at menarche quartiles shows that girls in the earliest quartile started pubertal growth on average 1.6 years before their peers in the latest quartile (7.7 ± 0.4 vs. 9.3 ± 0.4 years), and had an accelerated period of growth from onset to menarche that was 0.5 years shorter (3.8 ± 0.5 vs. 4.3 ± 0.5 years, respectively). This points to an early start, but also an early stop, to pubertal growth in girls with earlier menarche. Their musculoskeletal systems are therefore likely to be more immature when they begin puberty, and have less time to mature during puberty, resulting in overall retention of immature traits once they cease developing at the accelerated pubertal rate.
Given that menarche is linked to the onset of epiphyseal closure [40], a shorter period between the onset of rapid growth and menarche theoretically provides less time for long bones and other bony structures to continue longitudinal extension. Consistent with this explanation is the suite of relatively immature post-pubescent skeletal traits related to earlier menarche in this sample. These include shorter stature, shorter thigh segment (i.e., femur) lengths, and shorter overall subischial leg lengths. Wider inter-ASIS breadth with earlier menarche may also indicate retention of relative pelvic immaturity, given patterns of iliac apophyseal closure that allow for medially-directed extension of the ASIS with prolonged growth [58]. Furthermore, earlier menarche in this sample was associated with higher values of relative skeletal age, indicating a more advanced stage of skeletal maturation for their age relative to their peers with later menarche. Again, this observation is consistent with the idea that earlier menarche is concomitant with an earlier start to epiphyseal closure, resulting in a younger age at which longitudinal bone growth ceases relative to peers who have a later onset of the growth spurt, later age at menarche, and an extended period of pubertal growth.
In terms of gait kinematics, earlier menarche is correlated with postpubescent retention of a wider, more immature base of support, which may also be a product of premature curtailment of developmental processes. Again, comparing this sample's earliest and latest age at menarche quartiles helps illustrate this point. Girls in the earliest quartile had an average age at measurement of 16.2 years, but compared to our previous study's results [45], their average base of support was 2.62, typical of females aged 11.2 years in the Fels sample. In contrast, the latest quartile was measured at an average age of 16.4 years, and had mean base of support of 3.28, narrower than expected for the typical postpubescent female. Although it may be coincidental, it is interesting to note that the average age at menarche in the earliest quartile was 11.4 years, very similar to the expected age for individuals with their type of postpubescent base of support.
A relatively immature pelvic and lower limb skeleton and a wider base of support both contribute to greater knee abduction angles and moments in girls with earlier menarche. Greater knee abduction angles during walking associated with earlier menarche and wider base of support are related to wider foot placement under both static and dynamic conditions. The tendency toward valgus alignment with earlier menarche is likely related to both anatomical constraints and aspects of neuromuscular control. For example, the angles of each femoral epiphysis relative to the diaphysis develop during puberty, reaching a plateau at an average age of 13 years [59]. If earlier menarche leads to a premature end to this process, it may result in femoral morphology that contributes to more valgus anatomical knee alignment. With regard to neuromuscular development, there is evidence that ankle control matures from child-like to adult-like between the ages of 8-18 [43], such that earlier menarche may result in retention of more immature ankle strategies related to wider base of support.
The wider base of support and more valgus knees in females with earlier menarche are also coupled with more laterally placed ASIS. This combination of factors may explain the relationship between age at menarche, base of support, and greater external knee abduction moments at foot-strike, which may in turn be ultimately related to injury risk. Greater valgus knee alignment and a more laterally-placed ASIS are indicative of a greater Q-angle, which is consistent with a larger lateral force vector exerted by the quadriceps at the knee [60,61]. In particular, larger Q-angles indicate a more valgus moment arm for the vastus lateralis, the largest sub-component of the quadriceps with the lateral-most force vector at the knee [62]. Zhang and Wang [63] demonstrated that the overall valgus moment arm of vastus lateralis tends to resist external knee adduction loads. The converse is thus that vastus lateralis contraction exacerbates external valgus loading of the knee, whereas the medial musculature, including vastus medialis, resists valgus loading [64].
Females in general tend to exhibit greater contraction in vastus lateralis relative to vastus medialis during jumping and landing tasks, a pattern associated with higher peak knee abduction moments and angles [62,65]. Females also tend to be quadriceps dominant, with relatively weak hamstrings that further limit the ability resist external abduction loads on the knee [3,65,66]. These findings suggest that female-typical quadriceps co-contraction patterns are in and of themselves risky with regard to knee injuries, relative to males. Within females, this general pattern may be exaggerated in individuals with earlier menarche due to their lower limb skeletal alignment. Because a greater Q-angle is associated with an increased lateral component of the quadriceps force vector, the alignment patterns associated with earlier menarche should tend to increase the lateral, or valgus, moment of the vastus lateralis, while diminishing the magnitude of the varus moment of the vastus medialis. Together, these factors likely contribute to a lower overall ability of the quadriceps to resist external valgus loads, which is consistent with the observed kinetics at foot-strike in this sample and indicative of greater injury risk.
A seeming paradox is that a wide base of support is the pre-pubescent norm in both males and females, and yet knee injuries are much rarer prior to puberty than afterward. If a wider base of support is associated with riskier biomechanical profiles, then why are prepubescent females and males not at high risk of injury? As previous researchers have noted, the increase in injury risk after puberty is at least in part attributable to an increase in the length of the moment arms of the lower limb musculature, the addition of mass, and changes in mass distribution [67]. All of these alterations to the body increase the loads experienced by the lower limb, including at the knee. Additionally, the tendency for quadriceps dominance in females develops during puberty [67,68], and extensive research has demonstrated a significant reduction in neuromuscular control of the knee in post-pubescent vs. pre-pubescent females [13,32-34,44,68,69]. Therefore, it seems that having a wider base of support in a child's body may not be especially risky, but keeping a wide base of support in an adolescent or young adult female's body may increase knee injury risk.
A limitation of this study is its retrospective, observational nature, with a focus on healthy participants. We cannot present any data on direct relationships between age at menarche, base of support, knee biomechanics, and actual injury incidence. We do, however, have interesting anecdotal data from six individuals secondarily excluded from the study sample for overuse or traumatic knee injuries. Of these six individuals, five reported previous or current ITBS, PFPS, or ACL tears. Their ages at menarche were 11.0, 11.2, 11.3, 12.0, and 13.7 years, meaning that 80% of them fell below the median age at menarche for the overall sample, and 60% were in the earliest age at menarche quartile. This pattern is provocative in its consistency with the results of the study, and points toward a potentially higher incidence of knee-related injuries among females with earlier menarche, at least within the Fels sample. Two of these individuals also have post-pubescent, pre-injury walking gait data in the Fels database, and our future research plans include exploring their knee biomechanics as a case series.
This study is also limited in that it is focused on walking rather than the higher-impact conditions under which most overuse and traumatic knee injuries develop or occur. The stress of walking is generally well below the threshold for injury to most musculoskeletal tissues (e.g. [70]), and so actual injury risk may be difficult to gauge from walking biomechanical profiles. Still, the general biomechanical profile associated with early menarche mirrors high-risk kinematic and kinetic profiles during other activities [28,62] and thus merits further investigation. If walking gait patterns anticipate biomechanical responses to higher loads under which knee injuries occur (and a recent study suggests they do: [71]), then we would expect that gait profiles typical of earlier menarche would signify greater injury risk. We are in the process of testing this hypothesis in a sample of young adult female athletes by analyzing the degree of correspondence between walking gait biomechanics and landing strategies in a drop-vertical jump test.
Finally, it is worth noting that in addition to skeletal and gross biomechanical traits, age at menarche is related to variation in body composition, with possible downstream hormonal effects that impact tissue biomechanics. Girls with earlier menarche in this sample tended to have higher BMI, consistent with previous findings [72]. This is noteworthy because adult premenopausal women with earlier menarche and greater BMI have been shown to exhibit elevated levels of circulating estradiol across the menstrual cycle, compared to peers with later menarche and lower BMI [73]. Higher estradiol levels are associated with increased ligamentous laxity [74,75] (but see [76]) as well as changes in multiple parameters related to neuromuscular control [77-81]. It is therefore plausible that in addition to its effects on musculoskeletal development, estradiol may have an acute impact on injury risk which may be exacerbated in cases of higher childhood BMI and earlier menarche. Although not addressed directly in the present study, these relationships have especially important implications for girls who are obese as children and then go on to play sports in adolescence. Higher cyclical exposure to estradiol may place these individuals at greater injury risk via effects on tissue biomechanics and on neuromuscular control.
Conclusion
In conclusion, this study demonstrates a relationship between earlier age at menarche, a wider, more immature base of support, and walking gait knee biomechanical profiles consistent with risk for overuse and non-contact traumatic knee injuries under higher-intensity conditions. These findings provide preliminary support for new screening strategies to identify females at increased injury risk prior to or during puberty. Tracking of age at menarche and walking gait base of support during puberty are clinically quite feasible, and may enhance the ability of clinicians to identify individuals at high biomechanical risk. Early identification of such individuals allows for the timely initiation of interventions to modify base of support and other movement strategies [15,82,83]. Ultimate, such strategies can help maximize the benefits of female sports participation by preventing injuries and avoiding their long-term health consequences [14,84].
Acknowledgments
Funding Source: This work was supported by grants from Wright State University Boonshoft School of Medicine (gait analysis) and the National Institutes of Health (R01HD012252: growth, development, and anthropometric data). Funding sources had no role in study design, data collection, analysis or interpretation, the writing of this manuscript, or the decision to submit it for publication.
Footnotes
This material has not been presented at an AAPM&R Annual Assembly
Device Status: No medical devices were used in this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Andrew W. Froehle, Lifespan Health Research Center, Department of Community Health and Department of Orthopaedic Surgery, Sports Medicine and Rehabilitation, Wright State University Boonshoft School of Medicine, 3171 Research Blvd., Kettering, OH 45420, Phone: 937.775.1413, Fax: 937.775.1456.
Kimberly A. Grannis, Department of Orthopaedic Surgery, University of California, San Francisco, Fresno Medical Education and Research, 2823 Fresno St, 7th Floor, Fresno, CA 93721, Phone: 559.499.6400.
Richard J. Sherwood, Department of Pathology and Anatomical Sciences, School of Medicine, University of Missouri, M263 Medical Science Building, One Hospital Drive, Columbia, MO 65212, Phone: 573.882.6174.
Dana L Duren, Department of Orthopaedic Surgery, School of Medicine, University of Missouri, 1100 Virginia Ave., Columbia, MO 65201, Phone: 573.884.7023.
References
- 1.Knowles SB. Is there an injury epidemic in girls' sports? British Journal of Sports Medicine. 2010;44:38–44. doi: 10.1136/bjsm.2009.065763. [DOI] [PubMed] [Google Scholar]
- 2.Murphy DF, Connolly DAJ, Beynnon BD. Risk factors for lower extremity injury: a review of the literature. British Journal of Sports Medicine. 2003;37:13–29. doi: 10.1136/bjsm.37.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dugan SA. Sports-related knee injuries in female athletes - What gives? American Journal of Physical Medicine & Rehabilitation. 2005;84:122–130. doi: 10.1097/01.phm.0000154183.40640.93. [DOI] [PubMed] [Google Scholar]
- 4.Taunton JE, Ryan MB, Clement DB, et al. A retrospective case-control analysis of 2002 running injuries. Br J Sports Med. 2002;36:95–101. doi: 10.1136/bjsm.36.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myer GD, Ford KR, Barber Foss KD, et al. The incidence and potential pathomechanics of patellofemoral pain in female athletes. Clin Biomech (Bristol, Avon) 2010;25:700–707. doi: 10.1016/j.clinbiomech.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lohmander LS, Ostenberg A, Englund M, et al. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis and Rheumatism. 2004;50:3145–3152. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 7.Schmale GA, Kweon C, Larson RV, et al. High Satisfaction Yet Decreased Activity 4 Years After Transphyseal ACL Reconstruction. Clin Orthop Relat Res. 2014;472:2168–2174. doi: 10.1007/s11999-014-3561-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Natri A, Kannus P, Jarvinen M. Which factors predict the long-term outcome in chronic patellofemoral pain syndrome? A 7-yr prospective follow-up study. Med Sci Sports Exerc. 1998;30:1572–1577. doi: 10.1097/00005768-199811000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Campbell CJ, Carson JD, Diaconescu ED, et al. Canadian Academy of Sport and Exercise Medicine position statement: Neuromuscular training programs can decrease anterior cruciate ligament injuries in youth soccer players. Clin J Sport Med. 2014;24:263–267. doi: 10.1097/JSM.0000000000000068. [DOI] [PubMed] [Google Scholar]
- 10.Lohmander LS, Englund PM, Dahl LL, et al. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35:1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 11.Noehren B, Wilson H, Miller C, et al. Long-term gait deviations in anterior cruciate ligament-reconstructed females. Med Sci Sports Exerc. 2013;45:1340–1347. doi: 10.1249/MSS.0b013e318285c6b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powers CM. The influence of abnormal hip mechanics on knee injury: a biomechanical perspective. J Orthop Sports Phys Ther. 2010;40:42–51. doi: 10.2519/jospt.2010.3337. [DOI] [PubMed] [Google Scholar]
- 13.Hewett TE, Myer GD, Kiefer AW, et al. Longitudinal Increases in Knee Abduction Moments in Females during Adolescent Growth. Med Sci Sports Exerc. 2015;47:2579–2585. doi: 10.1249/MSS.0000000000000700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hewett TE, Myer GD, Ford KR, et al. The 2012 ABJS Nicolas Andry Award: The sequence of prevention: a systematic approach to prevent anterior cruciate ligament injury. Clin Orthop Relat Res. 2012;470:2930–2940. doi: 10.1007/s11999-012-2440-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Myer GD, Sugimoto D, Thomas S, et al. The Influence of Age on the Effectiveness of Neuromuscular Training to Reduce Anterior Cruciate Ligament Injury in Female Athletes. American Journal of Sports Medicine. 2013;41:203–215. doi: 10.1177/0363546512460637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakagawa TH, Moriya ET, Maciel CD, et al. Frontal plane biomechanics in males and females with and without patellofemoral pain. Med Sci Sports Exerc. 2012;44:1747–1755. doi: 10.1249/MSS.0b013e318256903a. [DOI] [PubMed] [Google Scholar]
- 17.Ferber R, Davis IM, Williams DS., 3rd Gender differences in lower extremity mechanics during running. Clin Biomech (Bristol, Avon) 2003;18:350–357. doi: 10.1016/s0268-0033(03)00025-1. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs CA, Uhl TL, Mattacola CG, et al. Hip abductor function and lower extremity landing kinematics: sex differences. J Athl Train. 2007;42:76–83. [PMC free article] [PubMed] [Google Scholar]
- 19.McLean SG, Lipfert SW, van den Bogert AJ. Effect of gender and defensive opponent on the biomechanics of sidestep cutting. Med Sci Sports Exerc. 2004;36:1008–1016. doi: 10.1249/01.mss.0000128180.51443.83. [DOI] [PubMed] [Google Scholar]
- 20.Ford KR, Myer GD, Toms HE, et al. Gender differences in the kinematics of unanticipated cutting in young athletes. Med Sci Sports Exerc. 2005;37:124–129. [PubMed] [Google Scholar]
- 21.Ferber R, Noehren B, Hamill J, et al. Competitive female runners with a history of iliotibial band syndrome demonstrate atypical hip and knee kinematics. J Orthop Sports Phys Ther. 2010;40:52–58. doi: 10.2519/jospt.2010.3028. [DOI] [PubMed] [Google Scholar]
- 22.Stefanyshyn DJ, Stergiou P, Lun VM, et al. Knee angular impulse as a predictor of patellofemoral pain in runners. Am J Sports Med. 2006;34:1844–1851. doi: 10.1177/0363546506288753. [DOI] [PubMed] [Google Scholar]
- 23.Mizuno Y, Kumagai M, Mattessich SM, et al. Q-angle influences tibiofemoral and patellofemoral kinematics. J Orthop Res. 2001;19:834–840. doi: 10.1016/S0736-0266(01)00008-0. [DOI] [PubMed] [Google Scholar]
- 24.Powers CM. The influence of altered lower-extremity kinematics on patellofemoral joint dysfunction: a theoretical perspective. J Orthop Sports Phys Ther. 2003;33:639–646. doi: 10.2519/jospt.2003.33.11.639. [DOI] [PubMed] [Google Scholar]
- 25.Kanamori A, Woo SL, Ma CB, et al. The forces in the anterior cruciate ligament and knee kinematics during a simulated pivot shift test: A human cadaveric study using robotic technology. Arthroscopy. 2000;16:633–639. doi: 10.1053/jars.2000.7682. [DOI] [PubMed] [Google Scholar]
- 26.Markolf KL, Burchfield DM, Shapiro MM, et al. Combined knee loading states that generate high anterior cruciate ligament forces. J Orthop Res. 1995;13:930–935. doi: 10.1002/jor.1100130618. [DOI] [PubMed] [Google Scholar]
- 27.Lloyd DG, Buchanan TS. Strategies of muscular support of varus and valgus isometric loads at the human knee. J Biomech. 2001;34:1257–1267. doi: 10.1016/s0021-9290(01)00095-1. [DOI] [PubMed] [Google Scholar]
- 28.Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33:492–501. doi: 10.1177/0363546504269591. [DOI] [PubMed] [Google Scholar]
- 29.Fukuda Y, Woo SL, Loh JC, et al. A quantitative analysis of valgus torque on the ACL: a human cadaveric study. J Orthop Res. 2003;21:1107–1112. doi: 10.1016/S0736-0266(03)00084-6. [DOI] [PubMed] [Google Scholar]
- 30.Gallagher SS, Finison K, Guyer B, et al. The incidence of injuries among 87,000 Massachusetts children and adolescents: results of the 1980-81 Statewide Childhood Injury Prevention Program Surveillance System. Am J Public Health. 1984;74:1340–1347. doi: 10.2105/ajph.74.12.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andrish JT. Anterior cruciate ligament injuries in the skeletally immature patient. Am J Orthop (Belle Mead NJ) 2001;30:103–110. [PubMed] [Google Scholar]
- 32.Hewett TE, Myer GD, Ford KR. Decrease in neuromuscular control about the knee with maturation in female athletes. J Bone Joint Surg Am. 2004;86-A:1601–1608. doi: 10.2106/00004623-200408000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Ford KR, Shapiro R, Myer GD, et al. Longitudinal sex differences during landing in knee abduction in young athletes. Med Sci Sports Exerc. 2010;42:1923–1931. doi: 10.1249/MSS.0b013e3181dc99b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quatman CE, Ford KR, Myer GD, et al. Maturation leads to gender differences in landing force and vertical jump performance: a longitudinal study. Am J Sports Med. 2006;34:806–813. doi: 10.1177/0363546505281916. [DOI] [PubMed] [Google Scholar]
- 35.Hagg U, Taranger J. Maturation indicators and the pubertal growth spurt. Am J Orthod. 1982;82:299–309. doi: 10.1016/0002-9416(82)90464-x. [DOI] [PubMed] [Google Scholar]
- 36.Mendle J, Harden KP, Brooks-Gunn J, et al. Development's tortoise and hare: pubertal timing, pubertal tempo, and depressive symptoms in boys and girls. Dev Psychol. 2010;46:1341–1353. doi: 10.1037/a0020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demerath EW, Towne B, Chumlea WC, et al. Recent decline in age at menarche: The fels longitudinal study. American Journal of Human Biology. 2004;16:453–457. doi: 10.1002/ajhb.20039. [DOI] [PubMed] [Google Scholar]
- 38.Seselj M, Nahhas RW, Sherwood RJ, et al. The influence of age at menarche on cross-sectional geometry of bone in young adulthood. Bone. 2012;51:38–45. doi: 10.1016/j.bone.2012.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinyerd B, Zipf WB. Puberty-timing is everything! J Pediatr Nurs. 2005;20:75–82. doi: 10.1016/j.pedn.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 40.Weise M, De-Levi S, Barnes KM, et al. Effects of estrogen on growth plate senescence and epiphyseal fusion. Proc Natl Acad Sci U S A. 2001;98:6871–6876. doi: 10.1073/pnas.121180498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sutherland D, Olshen RA, Biden EN, et al. The Development of Mature Walking. London: Mac Keith Press; 1988. [Google Scholar]
- 42.Vanwanseele B, Parker D, Coolican M. Frontal knee alignment: three-dimensional marker positions and clinical assessment. Clin Orthop Relat Res. 2009;467:504–509. doi: 10.1007/s11999-008-0545-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cherng RJ, Lee HY, Su FC. Frequency spectral characteristics of standing balance in children and young adults. Med Eng Phys. 2003;25:509–515. doi: 10.1016/s1350-4533(03)00049-3. [DOI] [PubMed] [Google Scholar]
- 44.Quatman-Yates CC, Quatman CE, Meszaros AJ, et al. A systematic review of sensorimotor function during adolescence: a developmental stage of increased motor awkwardness? Br J Sports Med. 2012;46:649–655. doi: 10.1136/bjsm.2010.079616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Froehle AW, Nahhas RW, Sherwood RJ, et al. Age-related changes in spatiotemporal characteristics of gait accompany ongoing lower limb linear growth in late childhood and early adolescence. Gait Posture. 2013;38:14–19. doi: 10.1016/j.gaitpost.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wall JC, Devlin J, Khirchof R, et al. Measurement of step widths and step lengths: a comparison of measurements made directly from a grid with those made from a video recording. J Orthop Sports Phys Ther. 2000;30:410–417. doi: 10.2519/jospt.2000.30.7.410. [DOI] [PubMed] [Google Scholar]
- 47.Towne B, Czerwinski SA, Dernerath EW, et al. Heritability of age at menarche in girls from the Fels Longitudinal Study. American Journal of Physical Anthropology. 2005;128:210–219. doi: 10.1002/ajpa.20106. [DOI] [PubMed] [Google Scholar]
- 48.Sherwood RJ, Duren DL. Growth of a Species, an Association, a Science: 80 Years of Growth and Development Research. American Journal of Physical Anthropology. 2013;150:1–4. doi: 10.1002/ajpa.22178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chumlea WC, Schubert CM, Roche AF, et al. Age at menarche and racial comparisons in US girls. Pediatrics. 2003;111:110–113. doi: 10.1542/peds.111.1.110. [DOI] [PubMed] [Google Scholar]
- 50.Medicine PCotASfR. Current evaluation of amenorrhea. Fertil Steril. 2004;82(1):S33–S39. doi: 10.1016/j.fertnstert.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 51.Freedman DS, Wang J, Maynard LM, et al. Relation of BMI to fat and fat-free mass among children and adolescents. International Journal of Obesity. 2005;29:1–8. doi: 10.1038/sj.ijo.0802735. [DOI] [PubMed] [Google Scholar]
- 52.Lohman TG, Roche AF, Martorell R, et al. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics Publishers; 1988. [Google Scholar]
- 53.Nahhas RW, Sherwood RJ, Chumlea WC, et al. An update of the statistical methods underlying the FELS method of skeletal maturity assessment. Ann Hum Biol. 2013;40:505–514. doi: 10.3109/03014460.2013.806591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bock RD, Du Toit SHC, Thomas CDL. AUXAL: Auxological analysis of longitudinal measurements of human stature, Version 3. Chicago: Scientific Software International; 2003. [Google Scholar]
- 55.Kadaba MP, Ramakrishnan HK, Wootten ME. Measurement of lower extremity kinematics during level walking. J Orthop Res. 1990;8:383–392. doi: 10.1002/jor.1100080310. [DOI] [PubMed] [Google Scholar]
- 56.Harrington ME, Zavatsky AB, Lawson SE, et al. Prediction of the hip joint centre in adults, children, and patients with cerebral palsy based on magnetic resonance imaging. J Biomech. 2007;40:595–602. doi: 10.1016/j.jbiomech.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 57.Sadeghi H, Allard P, Prince F, et al. Symmetry and limb dominance in able-bodied gait: a review. Gait Posture. 2000;12:34–45. doi: 10.1016/s0966-6362(00)00070-9. [DOI] [PubMed] [Google Scholar]
- 58.Scheuer L, Black S. Developmental Juvenile Osteology. San Diego: Academic Press; 2000. [Google Scholar]
- 59.Pujol A, Rissech C, Ventura J, et al. Ontogeny of the female femur: geometric morphometric analysis applied on current living individuals of a Spanish population. J Anat. 2014;225:346–357. doi: 10.1111/joa.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith TO, Hunt NJ, Donell ST. The reliability and validity of the Q-angle: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2008;16:1068–1079. doi: 10.1007/s00167-008-0643-6. [DOI] [PubMed] [Google Scholar]
- 61.Hewett TE, Myer GD, Ford KR. Anterior cruciate ligament injuries in female athletes: Part 1, mechanisms and risk factors. Am J Sports Med. 2006;34:299–311. doi: 10.1177/0363546505284183. [DOI] [PubMed] [Google Scholar]
- 62.Palmieri-Smith RM, Mclean SG, Ashton-Miller JA, et al. Association of quadriceps and hamstrings cocontraction patterns with knee joint loading. J Athl Train. 2009;44:256–263. doi: 10.4085/1062-6050-44.3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang LQ, Wang G. Dynamic and static control of the human knee joint in abduction-adduction. J Biomech. 2001;34:1107–1115. doi: 10.1016/s0021-9290(01)00080-x. [DOI] [PubMed] [Google Scholar]
- 64.Buchanan TS, Kim AW, Lloyd DG. Selective muscle activation following rapid varus/valgus perturbations at the knee. Med Sci Sports Exerc. 1996;28:870–876. doi: 10.1097/00005768-199607000-00014. [DOI] [PubMed] [Google Scholar]
- 65.Palmieri-Smith RM, Wojtys EM, Ashton-Miller JA. Association between preparatory muscle activation and peak valgus knee angle. J Electromyogr Kinesiol. 2008;18:973–979. doi: 10.1016/j.jelekin.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 66.Besier TF, Lloyd DG, Ackland TR. Muscle activation strategies at the knee during running and cutting maneuvers. Med Sci Sports Exerc. 2003;35:119–127. doi: 10.1097/00005768-200301000-00019. [DOI] [PubMed] [Google Scholar]
- 67.Wild CY, Steele JR, Munro BJ. Musculoskeletal and estrogen changes during the adolescent growth spurt in girls. Med Sci Sports Exerc. 2013;45:138–145. doi: 10.1249/MSS.0b013e31826a507e. [DOI] [PubMed] [Google Scholar]
- 68.Kim KW, Lim BO. Effects of menarcheal age on the anterior cruciate ligament injury risk factors during single-legged drop landing in female artistic elite gymnasts. Archives of orthopaedic and trauma surgery. 2014;134:1565–1571. doi: 10.1007/s00402-014-2055-z. [DOI] [PubMed] [Google Scholar]
- 69.Hass CJ, Schick EA, Tillman MD, et al. Knee biomechanics during landings: comparison of pre- and postpubescent females. Med Sci Sports Exerc. 2005;37:100–107. doi: 10.1249/01.mss.0000150085.07169.73. [DOI] [PubMed] [Google Scholar]
- 70.Pandy MG, Andriacchi TP. Muscle and joint function in human locomotion. Annu Rev Biomed Eng. 2010;12:401–433. doi: 10.1146/annurev-bioeng-070909-105259. [DOI] [PubMed] [Google Scholar]
- 71.Barrios JA, Heitkamp CA, Smith BP, et al. Three-dimensional hip and knee kinematics during walking, running, and single-limb drop landing in females with and without genu valgum. Clin Biomech (Bristol, Avon) 2016;31:7–11. doi: 10.1016/j.clinbiomech.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 72.Currie C, Ahluwalia N, Godeau E, et al. Is obesity at individual and national level associated with lower age at menarche? Evidence from 34 countries in the Health Behaviour in School-aged Children Study. J Adolesc Health. 2012;50:621–626. doi: 10.1016/j.jadohealth.2011.10.254. [DOI] [PubMed] [Google Scholar]
- 73.Emaus A, Espetvedt S, Veierod MB, et al. 17-beta-estradiol in relation to age at menarche and adult obesity in premenopausal women. Hum Reprod. 2008;23:919–927. doi: 10.1093/humrep/dem432. [DOI] [PubMed] [Google Scholar]
- 74.Heitz NA, Eisenman PA, Beck CL, et al. Hormonal changes throughout the menstrual cycle and increased anterior cruciate ligament laxity in females. J Athl Train. 1999;34:144–149. [PMC free article] [PubMed] [Google Scholar]
- 75.Park SK, Stefanyshyn DJ, Loitz-Ramage B, et al. Changing Hormone Levels During the Menstrual Cycle Affect Knee Laxity and Stiffness in Healthy Female Subjects. American Journal of Sports Medicine. 2009;37:588–598. doi: 10.1177/0363546508326713. [DOI] [PubMed] [Google Scholar]
- 76.Van Lunen BL, Roberts J, Branch JD, et al. Association of Menstrual-Cycle Hormone Changes with Anterior Cruciate Ligament Laxity Measurements. J Athl Train. 2003;38:298–303. [PMC free article] [PubMed] [Google Scholar]
- 77.Bell DR, Myrick MP, Blackburn JT, et al. The effect of menstrual-cycle phase on hamstring extensibility and muscle stiffness. J Sport Rehabil. 2009;18:553–563. doi: 10.1123/jsr.18.4.553. [DOI] [PubMed] [Google Scholar]
- 78.Park SK, Stefanyshyn DJ, Ramage B, et al. Alterations in knee joint laxity during the menstrual cycle in healthy women leads to increases in joint loads during selected athletic movements. Am J Sports Med. 2009;37:1169–1177. doi: 10.1177/0363546508330146. [DOI] [PubMed] [Google Scholar]
- 79.Cesar GM, Pereira VS, Santiago PR, et al. Variations in dynamic knee valgus and gluteus medius onset timing in non-athletic females related to hormonal changes during the menstrual cycle. Knee. 2011;18:224–230. doi: 10.1016/j.knee.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 80.Bell DR, Blackburn JT, Hackney AC, et al. Jump-landing biomechanics and knee-laxity change across the menstrual cycle in women with anterior cruciate ligament reconstruction. J Athl Train. 2014;49:154–162. doi: 10.4085/1062-6050-49.2.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dedrick GS, Sizer PS, Merkle JN, et al. Effect of sex hormones on neuromuscular control patterns during landing. J Electromyogr Kinesiol. 2008;18:68–78. doi: 10.1016/j.jelekin.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 82.Aruin AS, Hanke TA, Sharma A. Base of support feedback in gait rehabilitation. Int J Rehabil Res. 2003;26:309–312. doi: 10.1097/00004356-200312000-00009. [DOI] [PubMed] [Google Scholar]
- 83.Nyman E, Jr, Armstrong CW. Real-time feedback during drop landing training improves subsequent frontal and sagittal plane knee kinematics. Clin Biomech (Bristol, Avon) 2015;30:988–994. doi: 10.1016/j.clinbiomech.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 84.Strong WB, Malina RM, Blimkie CJ, et al. Evidence based physical activity for school-age youth. J Pediatr. 2005;146:732–737. doi: 10.1016/j.jpeds.2005.01.055. [DOI] [PubMed] [Google Scholar]