Abstract
Renal denervation (RDN) is a new therapy used to treat drug-resistant hypertension in the clinical setting. Published human trials show substantial inter-individual variability in the blood pressure (BP) response to RDN, even when technical aspects of the treatment are standardized as much as possible between patients. Widespread acceptance of RDN for treating hypertension will require accurate identification of patients likely to respond to RDN with a fall in BP that is clinically significant in magnitude, well-maintained over time and does not cause adverse consequences. In this paper we review and evaluate clinical studies that address possible predictors of the BP response to RDN. We conclude that only one generally reliable predictor has been identified to date, namely pre-RDN BP level, although there is some evidence for a few other factors. Experimental interventions in laboratory animals provide the opportunity to explore potential predictors that are difficult to investigate in human patients. Therefore we also describe results (from our lab and others) with RDN in spontaneously hypertensive rats. Since virtually all patients receiving RDN are taking three or more antihypertensive drugs, a particular focus of our work was on how ongoing antihypertensive drug treatment might alter the BP response to RDN. We conclude that patient age (or duration of hypertension) and concomitant treatment with certain drugs can affect the blood pressure response to RDN and that this information could help predict a favorable clinical response.
Keywords: Renal nerves, hypertension, anti-hypertensive drugs, spontaneously hypertensive rat
Introduction
Variability in the magnitude of BP response to catheter-based RDN reported by research centers around the world would not be surprising if there are important technical differences in the way the procedure is performed in different centers. In addition, however, responses are reported to be quite variable from patient-to-patient even within a given study population, ranging from very large falls to small increases in BP [1-6]. This suggests that specific patient characteristics also can be a key determinant of variability in response magnitude. As with other antihypertensive treatment strategies, it is important to understand the source of this variability, since a very large fall in BP could cause serious adverse effects due to hypotension, whereas a very small or transient reduction in BP would not be expected to produce the long-term reduction in adverse cardiovascular events that is the main purpose of anti-hypertensive therapy. These concerns are magnified by the fact that the BP response to RDN appears to persist in at least some patients for many years [7]. The clinical desirability of being able to accurately predict the quantitative response of BP to RDN in an individual patient has been noted repeatedly [8, 9]. Factors likely to be involved in the variability of responses include: 1) technical issues with the procedure itself or with the measurement of BP (e.g. operator experience, type of catheter, number and/or location of ablations, placebo effect, regression to the mean, altered drug adherence, etc.), 2) baseline patient characteristics (e.g. sex, age, race, body weight, overall health status, etc.), and 3) concomitant anti-hypertensive therapies (since virtually all studies have been performed in patients already taking three or more antihypertensive medications). This review will not focus on technical issues because they have been well covered elsewhere [10, 11]. Instead we will mainly discuss how patient characteristics, and in particular concomitant anti-hypertensive drug therapy, might affect the BP response to RDN. There are important limitations in the ability to study potential drug effects in the clinical setting, so we also will present experimental animal data on this issue that we (and others) have recently obtained using the spontaneously hypertensive rat (SHR) as a model system.
How have response predictors been identified in clinical studies?
Attempts to identify patient characteristics that predict the BP response to RDN have mostly relied on post-hoc analyses of data from studies evaluating the impact of RDN in a broad range of drug resistant hypertensive patients. This has been done either by comparing the magnitude of BP response in different groups of subjects (e.g. men versus women) or by classifying patients as “responders” or “non-responders” to RDN and then comparing the baseline characteristics of the patients in the two groups. However, a few studies have been designed, at least in part, to test prospectively the effect of RDN on BP in specific patient groups, e.g. elderly subjects [12].
What patient characteristics have been shown to affect BP response?
A very common finding across RDN studies is that higher baseline SBP predicts a blood pressure lowering response [6, 11, 13-18]. A study focusing more specifically on this question provides support: RDN did not significantly lower BP in patients with mild hypertension [19]. On the whole then there is good agreement that BP can be expected to fall more after RDN in patients with higher pre-intervention BPs, although this finding may well be confounded by technical issues involved in BP measurement [4, 20, 21].
One report indicates that renal artery anatomy can predict the response to RDN, i.e. a simple renal artery anatomy is favorable for a larger BP response [22]. Cardiac baroreflex activity also was reported to predict the BP response to RDN [23]. Although interesting if confirmed, neither of these would likely be a practical approach to screening patients for RDN treatment. Several other variables, including sex, BMI, eGFR, and number of attempted ablations have demonstrated a positive predictive role in at least one study [6, 11, 14, 16, 18, 24]; however the importance of these factors remains disputed as other studies have not supported similar predictive power [13, 15, 17, 25]. Other baseline patient characteristics, such as age, comorbidities, isolated systolic HTN, baseline DBP, and race, have been consistently shown not to predict the magnitude of BP response to RDN [6, 11, 13, 15, 16, 24, 25]. The impact of antihypertensive drug treatment will be considered in a later section of the review.
Limitations of efforts to identify response predictors in clinical studies
The methodology of the studies examining the effect of RDN on BP has been criticized in several reviews. Key limitations include: open-label design, lack of statistical power, lack of a confirmatory test to ensure denervation, small effect size, and confounding due to inappropriate adjustment of anti-hypertensive regimen during the observation period after RDN [4, 10, 20, 21, 26, 27]. As noted above, critics have pointed to the regression to mean phenomenon observed in similarly designed, open label drug trials, where significant discrepancies between office BP and ambulatory BP reductions are reported, as evidence that RDN data from office BP measurements should be interpreted cautiously [4]. Since the post-hoc analyses attempting to identify predictors of the BP response to RDN also were based on these data sets, the likelihood of some spurious associations is high. Thus, at this point in time there does not appear to be any reliable way to predict a clinically desirable BP response to RDN based on any specific characteristics of individual patients, with the exception of higher pre-RDN BP.
Effects of antihypertensive drug treatment on BP response to RDN
Virtually all clinical trials investigating RDN have been performed in patients with treatment resistant hypertension, defined by a hypertensive BP despite treatment with at least 3 anti-hypertensive medications. In many trials, patients were taking 5 or more medications [3, 28-34], and modification of drug therapy after RDN was generally allowed if deemed clinically necessary or desirable. In many studies, the number of antihypertensive drugs taken was modestly reduced after RDN. Therefore, it is unavoidable that anti-hypertensive drug therapy may have been an important factor influencing the BP response to RDN. This issue was first explored in the 24 month follow-up analysis of Symplicity HTN 1 patients. Somewhat surprisingly, since reduced sympathetic activity to the whole cardiovascular system (not just the kidney) has been proposed to be a major cause of the BP fall after RDN [35], the BP reduction following RDN was actually greater in patients that presumably already had low sympathetic activity due to treatment with central sympatholytics [6, 24]. Later analysis of Symplicity HTN 3 data revealed that patients receiving aldosterone antagonists responded to RDN with a greater BP reduction [11]. These findings are counterintuitive as both classes of medications are known sympatholytics; if the mechanism responsible for the BP reduction to RDN is sympatholysis (either renal or systemic), one might expect RDN to be less effective in the patients taking sympatholytic medication. To our knowledge, there are no other reports of treatment with a specific antihypertensive drug class being a predictor of the magnitude of BP response to RDN, and other studies have failed to find an association with use of central sympatholytics or aldosterone antagonists [11, 13-18, 24, 36].
There are significant problems, however, when assessing the role of drugs as response modifiers in clinical RDN studies: 1) patient adherence to prescribed drug therapy is variable and difficult to evaluate accurately [37]; 2) there is substantial inter-individual heterogeneity in BP responsiveness to antihypertensive drugs [38]; 3) by definition, patients in RDN studies were receiving “ineffective” (for them) antihypertensive doses of the various drugs they were prescribed; and 4) as discussed by Vink et al. [35] there may be meaningful inter-individual differences in the way antihypertensive drug combinations affect sympathetic activity to the kidneys and other cardiovascular targets. The latter is important since, as noted earlier, diminished sympathetic activity is one presumed mechanism by which RDN lowers BP. Therefore we need more clinical studies that specifically address how the BP response to RDN is influenced by drug therapy, and this is a major goal of a new clinical trial just getting underway [39].
Rationale for using animal models to predict BP response to RDN
Difficulties in monitoring patient adherence to prescribed drug treatment, the impact of the placebo effect, patient heterogeneity, and patient safety concerns all limit the extent to which the impact of drug therapy on the response to RDN can be fully addressed in human studies. Therefore, it is reasonable to explore laboratory animal models as an alternative approach and use the resulting findings as guidance for further clinical investigation. Selecting an appropriate animal model is critical for obtaining data with the greatest relevance to the clinical situation. For translational purposes, an ideal animal model would exhibit: 1) stable “primary” hypertension with BP levels similar to those reported in clinical trials of drug resistant hypertension, 2) objectively successful loss of efferent and/or afferent nerves to the kidneys after RDN, and 3) chronic and sustained reductions in BP reductions of a similar magnitude to those observed in clinical trials of RDN.
Renal denervation has been performed in many animal models of hypertension and the results are extensively documented and analyzed in reviews by DiBona and Kopp [40-42]. The majority of these studies have demonstrated that RDN effectively prevents or delays the development of experimental hypertension when RDN is performed prior to the onset of hypertension. Nevertheless, these data cannot be applied in a straightforward way to the current clinical use of RDN, since RDN is only undertaken in patients after they have experienced many years of established hypertension. Moreover, when RDN is performed in experimental animals after hypertension is well established, RDN does not reliably produce a sustained fall in BP in most models [40]. One rat model of hypertension, however, has shown promise in this regard: the SHR. SHR develop high blood pressure spontaneously with age as is observed in humans. Additionally, the relative elevation in BP in the SHR, and the resulting end-organ damage, is similar to that reported in patients selected for clinical RDN [43]. Previous studies showed that RDN performed in young SHR delays or attenuates development of hypertension [40, 42, 44-46]. More importantly, several authors have reported that RDN performed during the established phase of hypertension results in a significant and sustained reduction in BP [47-53], although this has been disputed by others [54]. One pattern that seemed to emerge from our review of the published studies on RDN in SHR is that older animals appeared more likely than younger animals to respond with a significant and sustained fall in BP after RDN [49-53].
Those reports prompted our lab to explore using the SHR, especially older SHR, as a pre-clinical model to identify the factors that might affect, and therefore predict, the magnitude of the fall in blood pressure occurring after RDN. As described in the remainder of this paper, we first confirmed that RDN causes a fall in BP in SHR, and then showed that the magnitude of that fall is affected by both the age of the rats and the completeness of denervation; and that concomitant treatment with some antihypertensive drugs materially altered the blood pressure response to RDN. With these findings, we propose the SHR as a suitable pre-clinical model for exploring how drug pretreatment and other factors can alter the blood pressure response to RDN. We suggest that those results may be useful to guide further thinking on the proper clinical application of RDN as therapy for hypertension.
Materials and Methods
All of our studies in SHR were approved by the Institutional Animal Use and Care Committee at Michigan State University and were in accordance with the with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996).
Rats
Male SHR were purchased at various ages ranging from 13 weeks to retired breeder status. All animals were purchased from Charles River Laboratories, Portage, IN. The vendor estimated the retired breeder rats to be 24-52 weeks of age (median age: 36 weeks). Rats were singly housed with ad libitum access to food and distilled water. Normal laboratory chow consisted of Harlan Teklad Diet 8640 (0.4% NaCl). Select studies utilized a low sodium intake containing 0.1% NaCl (Harlan Teklad diet 7034). Euthanasia was performed by exsanguination in deeply anesthetized rats (5% isoflurane) concomitantly injected with a lethal dose of sodium pentobarbital (100mg/kg).
General surgical procedures
Prior to all surgical procedures carprofen (5 mg/kg; SC), enrofloxacin (2.5 mg/kg; IM), and piperacillin (120mg/kg; SC) were administered for analgesia and bacterial prophylaxis. Surgical anesthesia was induced with 4% isoflurane in 100% oxygen and maintained throughout the procedures at 2-3%. Post-operative recovery occurred under a heat lamp until animals were conscious and stable. Health and welfare of the rats were continuously monitored throughout the studies.
Radiotelemeter implantation
Radiotelemeters (TA11PA-C40, Data Science International) were used to record BP in conscious rats. The catheter of the telemeter was advanced from the femoral artery to the abdominal aorta in anesthetized rats. The telemeter body was housed subcutaneously. Rats were allowed 5-7 days of recovery from telemeter implantation before hemodynamic variables were recorded. These included systolic (SBP), diastolic (DBP), and mean (MAP) arterial pressures, and heart rate (HR). Measurements were collected for ten seconds every 10 minutes (24 hours/day) throughout the experimental period. Recorded variables were stored on a computer and analyzed using Dataquest ART 4.1 software.
Bilateral renal denervation
The renal vasculature was accessed through a midline incision in anesthetized rats. Abdominal contents were gently displaced and kept hydrated using 0.9% saline. The renal arteries were blunt dissected away from the renal vein, and nerve fibers within the connective tissue were mechanically stripped away. A phenol solution (20% phenol in 100% ethanol) was applied to the renal vasculature to destroy any remaining nerve fibers. The abdominal contents were returned, and the incision was closed with 5-0 silk sutures. Sham operation (SO) consisted of exposing the renal vessels without further blunt dissection or application of phenol. We refer to this specific surgical RDN procedure in the rat as RDX to differentiate it from the other approaches to renal denervation (mainly catheter-based) discussed here.
Drug administration
All drugs were given via the drinking water except clonidine; it was administered via mini-osmotic pumps (Alzet, Model 2006). Drug dosages were selected based on literature reports on their use in SHR, and were confirmed to lower BP in our rats. The drugs tested and their dosages (or concentrations) are: clonidine hydrochloride (125 μg/kg/day via mini-osmotic pump); prazosin hydrochloride (87.5 mg/L drinking water); atenolol (1 mg/ml drinking water); losartan potassium (100 mg/L drinking water); chlorthalidone (100 mg/L drinking water); and amlodipine besylate (60 mg/L drinking water). Some rats were used to test more than one drug after an appropriate washout period.
Verification of renal denervation
At the conclusion of each study, the kidneys and spleen were collected from the euthanized rats. The lateral aspect of the kidneys was saved to evaluate efferent sympathetic denervation. The spleen was collected to serve as a negative control for the efferent sympathetic denervation procedure. The tissues were flash frozen in liquid nitrogen and stored at −80°C until analyzed for catecholamine content using HPLC with electrochemical detection.
Statistical analyses
All statistical analyses were performed using GraphPad Prism 6.0 software. All studies utilized a mixed-model repeated measures design. Between group differences were analyzed using a mixed-model two-way ANOVA, and post-hoc analyses was performed on steady-state BP using a Bonferroni's multiple comparison test. Steady-state BP was determined by averaging the values reported during last three days of a treatment interval once BP had stabilized. Regression analyses were performed using Deming regression. A p-value < 0.05 was considered significant. BP values are reported as 24 hour averages. All results are reported at mean ± SEM.
Results
Blood pressure response to renal denervation – effects of age
In 13 week old SHR (SO: n=7 vs RDX: n=8) with similar baseline blood pressures (SO: 143.9±3.4 vs RDX: 139.3±4.1 mmHg), RDX significantly lowered BP within 48hrs compared to SO rats (SO: 147.6±2.6 vs RDX: 132.3±2.4mmHg); however, this BP effect did not persist beyond 48hrs. In fact, the BP lowering effect was abolished within 3 days of reaching nadir, and MAP returned to baseline levels in both groups (SO: 144.8±0.3 vs RDX: 140.6±0.4 mmHg). Continued monitoring of BP over several weeks did not reveal any delayed fall in BP in the RDX rats.
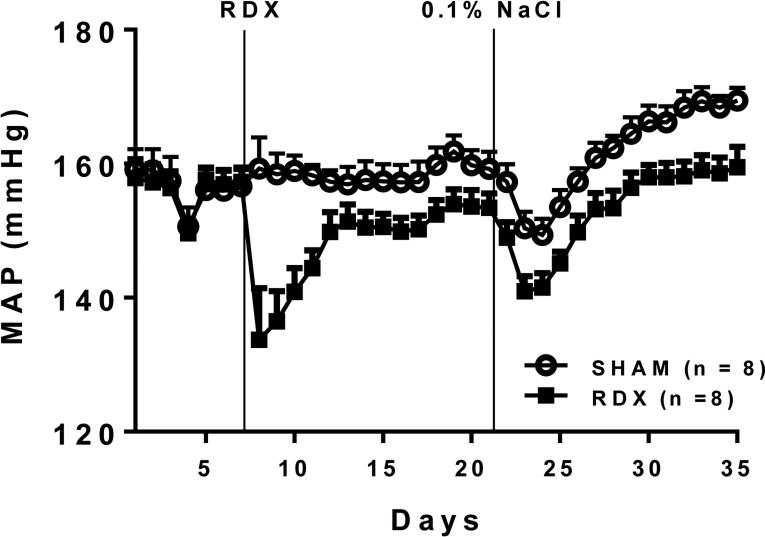
In 36 week old SHR a different pattern of response was observed (Figure 1). Baseline MAP was higher prior to RDX as expected in older SHR, but nearly identical in the two groups (SO: 156.6±3.0 vs RDX: 157.3±2.0 mmHg). Denervation of both kidneys caused a dramatic fall in MAP immediately after surgery, whereas SO had no effect. Over the next several days, however, MAP in the RDX group rose to reach steady-state values but remained significantly lower than in the SO group at two weeks after surgery (SO: 160.5±0.7 vs RDX: 153.3±0.4 mmHg). One mechanism proposed to explain the antihypertensive effect of RDN is promotion of sodium and water loss by the kidney. We reasoned therefore that low salt intake perhaps would lower BP more in RDX versus SO rats. But as shown in Figure 1, provision of a low salt diet only modestly augmented the antihypertensive effects of the RDX procedure.
Figure 1.
Mean arterial pressure (MAP) in 13 week old SHR subjected to bilateral renal denervation (RDX) or sham-operation (SHAM). Two weeks after surgery, rats were placed on a low salt diet (0.1% NaCl) for two weeks. MAP was significantly lower in the RDX rats from the time of RDX to the end of the study.
The rats shown in Figure 1 were placed back on a normal salt diet and their BP was followed for an additional seven weeks to assess the durability of the response to RDX. At 11 weeks after intervention, the reduction in MAP from baseline was significantly greater in RDX compared to SO controls (SO: −1.6±1.8 vs RDN: −9.4±1.4 mmHg). Significant differences in the two groups also were observed at this time for falls in systolic blood pressure (SO: −2.2±1.8 vs RDX: −10.2±1.8 mmHg) and diastolic blood pressure (SO: −0.9±1.1 vs RDX: −7.0±1.3 mmHg) compared to baseline values. These changes in BP observed 11 weeks after RDX in SHR are very similar to those reported using ambulatory BP monitoring in human patients obtained weeks to months after RDN [55].
Collectively then these data confirm previous reports that older SHR show a reliable antihypertensive response to RDX. More importantly they demonstrate that the reduction in BP seen in older SHR is similar in both magnitude and durability to the BP effect reported in patients treated with RDN. They also confirm the value of continuous ambulatory measurement of BP, in patients and in animals, for assessing the effects of RDN, since the expected changes in BP after RDN are small.
Predictors of BP response to RDX in 36 week old SHR
We performed RDX and chronic BP recording in 37 SHR in our studies. At the end of each separate study, we also measured the content of norepinephrine (NE) in both kidneys as a measure of the “completeness” of RDX. Regression analysis indicated that the magnitude of the chronic fall in BP caused by RDX (from baseline to the end of a study) was significantly related (p = 0.0015) to the effectiveness of denervation: Δ [steady-state MAP] = 0.18 [NE content] - 28.9. We also tested whether pre-RDX baseline BP predicted the magnitude of the fall in BP to RDX; this relationship was not statistically significant (p = 0.15): Δ [steady-state MAP] = −1.57 [baseline BP] + 236.7.
Effects of drug treatment on blood pressure response to RDX
Two examples of experiments to determine the influence of concomitant antihypertensive drug treatment on the BP response to RDX in 36 week old SHR are shown below. Figure 2 illustrates the effects of the centrally acting sympatholytic drug clonidine. Administration of clonidine for one week lowered BP as expected. When half the treated animals were subjected to RDX and the other half to sham operation, BP fell in the RDX group but didn't change in the sham operated group. However, by 4-5 days after surgery BP was not significantly different in the RDX and sham operated groups. When clonidine treatment was terminated, BP rose in both groups, but at steady-state levels was significantly higher in the sham-operated than in the RDX rats. Thus, clonidine largely abrogated the antihypertensive effect of RDX.
Figure 2.
Mean arterial pressure (MAP) in rats subjected to surgical renal denervation (RDX) or sham operation (SHAM) during treatment with the centrally acting sympatholytic drug clonidine. MAP in the two groups was not significantly different on the last three days of clonidine treatment, but were significantly different on the last three days of the study.
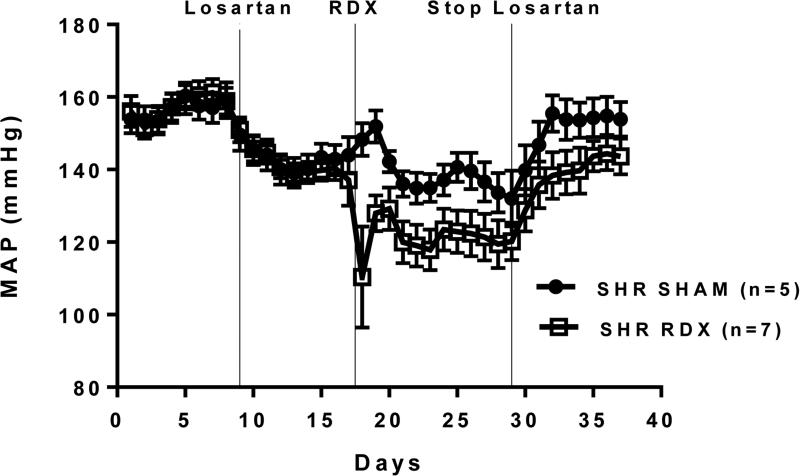
Figure 3 illustrates the effects of the angiotensin type I receptor blocking drug losartan. Administration of losartan for ten days lowered BP as expected. When half the treated animals were subjected to RDX and the other half to sham operation, BP fell markedly in the RDX group, but much less so in the sham operated group. On days 9-11 after surgery BP was significantly lower in the RDX versus sham operated rats. When losartan treatment was terminated, BP rose in both groups, but at steady-state levels remained significantly higher in the sham-operated than in the RDX rats. Thus, losartan treatment did not materially affect the antihypertensive effect of RDX.
Figure 3.
Mean arterial pressure (MAP) in rats subjected to surgical renal denervation (RDX) or sham operation (SHAM) during treatment with the angiotensin type I receptor antagonist losartan. MAP in the two groups was significantly different during the last three days of losartan treatment, and during the last three days of the study.
In additional studies using a similar approach, we found no significant effect of the diuretic chlorthalidone, the alpha-1 adrenergic receptor blocker prazosin or the calcium channel blocker amlodipine on the BP response to RDX (data not shown). In preliminary studies, however, we found RDX failed to produce a sustained fall in BP in animals treated with the beta-blocker atenolol. The data are shown in Figure 4.
Figure 4.
Mean arterial pressure (MAP) in rats subjected to surgical renal denervation (RDX) during treatment with the beta-1 selective adrenergic receptor antagonist atenolol. MAP after RDX was significantly lower than during atenolol treatment alone only on the first two days after surgery.
Discussion
Our studies in SHR reported here confirm the findings of others that older SHR respond to RDX with a significant fall in BP that is sustained for many weeks. Young SHR show a fall in BP after RDX that is not reliably maintained beyond a few days. Factors contributing to this difference in response could include: the impact of aging itself on cardiovascular regulation; the fact that older rats have higher BP at the time of RDX; or the fact that the duration of hypertension is much longer in older versus younger SHR (leading to more extensive cardiovascular remodeling). We did not find a significant relationship between basal BP and the response to RDX in our cohort of 36 week old rats, but this could be explained by the fact that the range of those BP values was fairly narrow. In general though, the results in SHR agree with the clinical findings that a higher basal BP predicts a larger and more sustained decrease in BP after RDN. Clinical studies typically have not assessed the duration of pre-existing hypertension, so it is not clear what influence this would have. And we could not evaluate this in our system, since the timing of the onset of hypertension in SHR is quite uniform. Our overall conclusion, based on human and rat data, is that advanced age may be a modest predictor of a good BP response to RDN, although the mechanisms involved are not clear.
It was not our goal to evaluate technical aspects of RDN, but in light of the questions raised about the efficacy of catheter based RDN in published trials [10] we took the opportunity to examine, in our cohort of older SHR, the impact of completeness of denervation on the fall in BP to RDX (not easily testable in routine clinical trials). We found a statistically significant relationship between denervation effectiveness and the fall in BP after RDX. In the one set of clinical patients in which RDN effectiveness was measured using NE spillover methodology [31], a mean reduction of 47% was reported. When that degree of denervation effectiveness is inserted into the regression equation derived from our data in SHR, an average fall in MAP of ~ 11 mmHg is predicted. That value aligns reasonably well with the average falls in MAP reported from ambulatory measurements in published clinical trials, supporting the utility of the SHR as an animal model for studies on RDN.
Our findings with antihypertensive drug treatment in SHR suggests that a few drugs, particularly centrally acting sympatholytic agents (and possibly beta-blockers), could under some circumstances modify the BP response to RDN; whereas most commonly employed classes (diuretics, angiotensin receptor blockers and calcium channel blockers) seem not likely to do so (consistent with most published clinical trial data). Although our studies were not designed to explore the mechanism(s) by which RDN lowers BP, we interpret our results as being consistent with the idea that RDN does not affect BP by inhibiting the renin-angiotensin system or promoting sodium and water loss, but instead mainly by directly reducing renal vascular resistance. Selective renal vasodilation is a proven method for reducing BP in SHR [56]. Of course there are important caveats to extending our findings in SHR to the clinical setting. First and foremost, the SHR rats we studied were not necessarily drug resistant, since all showed a substantial fall in BP during drug treatment. Second, unlike drug resistant human patients receiving RDN, our rats were given only one antihypertensive drug at a time prior to RDX. Third, the rats were given a substantially higher dose of all drugs (on a per body weight basis) than is typically used in the clinic, even in drug resistant patients. With these limitations in mind, the results of our rat studies still suggest that concomitant antihypertensive drug treatment (particularly with drugs affecting sympathetic nervous system control of BP) could be a factor in predicting which patients are more likely to respond to RDN with a therapeutically useful fall in BP.
Conclusions
Data neither from clinical trials nor experimental animals have yet identified a clinically useful way, aside from higher initial BP, of predicting the magnitude of the BP fall that will occur after RDN. Experiments in SHR rats point to factors related to aging and some drug actions that may deserve further investigation in clinical trials.
Highlights.
Renal denervation lowers blood pressure in drug resistant hypertension
Variability of the blood pressure response to renal denervation is large
No patient characteristic predicts response except high initial blood pressure
Studies in rats suggest that some antihypertensive drugs may reduce the response
Acknowledgements
The animal studies reported in this paper were supported by NIH grant R01 HL076312 and an American Heart Association pre-doctoral fellowship to J.T. Phelps. The authors gratefully acknowledge the technical assistance of Robert Burnett and Hannah Garver.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Azizi M, et al. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet. 2015;385(9981):1957–65. doi: 10.1016/S0140-6736(14)61942-5. [DOI] [PubMed] [Google Scholar]
- 2.Brinkmann J, et al. Catheter-based renal nerve ablation and centrally generated sympathetic activity in difficult-to-control hypertensive patients: prospective case series. Hypertension. 2012;60(6):1485–90. doi: 10.1161/HYPERTENSIONAHA.112.201186. [DOI] [PubMed] [Google Scholar]
- 3.Esler MD, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 376(9756):1903–9. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 4.Howard JP, Nowbar AN, Francis DP. Size of blood pressure reduction from renal denervation: insights from meta-analysis of antihypertensive drug trials of 4,121 patients with focus on trial design: the CONVERGE report. Heart. 2013;99(21):1579–87. doi: 10.1136/heartjnl-2013-304238. [DOI] [PubMed] [Google Scholar]
- 5.Bhatt DL, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370(15):1393–401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 6.Symplicity HTNI. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension. 2011;57(5):911–7. doi: 10.1161/HYPERTENSIONAHA.110.163014. [DOI] [PubMed] [Google Scholar]
- 7.Esler MD, et al. Catheter-based renal denervation for treatment of patients with treatment-resistant hypertension: 36 month results from the SYMPLICITY HTN-2 randomized clinical trial. Eur Heart J. 2014;35(26):1752–9. doi: 10.1093/eurheartj/ehu209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y. It is urgent to investigate predictors of the response of blood pressure to renal denervation. Can J Cardiol. 2014;30(4):465 e7. doi: 10.1016/j.cjca.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 9.Donazzan L, Ewen S, Bohm M. Until questions on intraprocedural efficacy control, renal nerve distribution and predictors of BP response are answered, the interpretation of clinical trials on renal denervation will remain uncertain. Evid Based Med. 2014;19(6):227–8. doi: 10.1136/ebmed-2014-110028. [DOI] [PubMed] [Google Scholar]
- 10.Esler M. Illusions of truths in the Symplicity HTN-3 trial: generic design strengths but neuroscience failings. J Am Soc Hypertens. 2014;8(8):593–8. doi: 10.1016/j.jash.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Kandzari DE, et al. Predictors of blood pressure response in the SYMPLICITY HTN-3 trial. Eur Heart J. 2015;36(4):219–27. doi: 10.1093/eurheartj/ehu441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ziegler AK, et al. Efficacy and safety of renal denervation in elderly patients with resistant hypertension. Catheter Cardiovasc Interv. 2015;86(2):299–303. doi: 10.1002/ccd.25166. [DOI] [PubMed] [Google Scholar]
- 13.Ewen S, et al. Reduced effect of percutaneous renal denervation on blood pressure in patients with isolated systolic hypertension. Hypertension. 2015;65(1):193–9. doi: 10.1161/HYPERTENSIONAHA.114.04336. [DOI] [PubMed] [Google Scholar]
- 14.Id D, et al. Predictors of Blood Pressure Response: Obesity Is Associated With a Less Pronounced Treatment Response After Renal Denervation. Catheter Cardiovasc Interv. 2015 doi: 10.1002/ccd.26068. [DOI] [PubMed] [Google Scholar]
- 15.Prochnau D, et al. Resistant hypertension: multivariate predictors of blood pressure response to renal denervation. Int J Cardiol. 2013;168(3):3130–2. doi: 10.1016/j.ijcard.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 16.Rohla M, et al. Predictors of response to renal denervation for resistant arterial hypertension: a single center experience. J Hypertens. 2016;34(1):123–9. doi: 10.1097/HJH.0000000000000764. [DOI] [PubMed] [Google Scholar]
- 17.Vogel B, et al. Renal sympathetic denervation therapy in the real world: results from the Heidelberg registry. Clin Res Cardiol. 2014;103(2):117–24. doi: 10.1007/s00392-013-0627-5. [DOI] [PubMed] [Google Scholar]
- 18.Persu A, et al. Hyperresponders vs. nonresponder patients after renal denervation: do they differ? J Hypertens. 2014;32(12):2422–7. doi: 10.1097/HJH.0000000000000347. discussion 2427. [DOI] [PubMed] [Google Scholar]
- 19.Desch S, et al. Randomized sham-controlled trial of renal sympathetic denervation in mild resistant hypertension. Hypertension. 2015;65(6):1202–8. doi: 10.1161/HYPERTENSIONAHA.115.05283. [DOI] [PubMed] [Google Scholar]
- 20.Fadl Elmula FE, et al. Renal sympathetic denervation after Symplicity HTN-3 and therapeutic drug monitoring in severe hypertension. Front Physiol. 2015;6:9. doi: 10.3389/fphys.2015.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howard JP, et al. Quantifying the 3 Biases That Lead to Unintentional Overestimation of the Blood Pressure-Lowering Effect of Renal Denervation. Circ Cardiovasc Qual Outcomes. 2016 doi: 10.1161/CIRCOUTCOMES.115.002533. [DOI] [PubMed] [Google Scholar]
- 22.Hering D, et al. Renal artery anatomy affects the blood pressure response to renal denervation in patients with resistant hypertension. Int J Cardiol. 2016;202:388–93. doi: 10.1016/j.ijcard.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Zuern CS, et al. Impaired cardiac baroreflex sensitivity predicts response to renal sympathetic denervation in patients with resistant hypertension. J Am Coll Cardiol. 2013;62(22):2124–30. doi: 10.1016/j.jacc.2013.07.046. [DOI] [PubMed] [Google Scholar]
- 24.Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension. 2011;57(5):911–7. doi: 10.1161/HYPERTENSIONAHA.110.163014. [DOI] [PubMed] [Google Scholar]
- 25.Flack JM, et al. An analysis of the blood pressure and safety outcomes to renal denervation in African Americans and Non-African Americans in the SYMPLICITY HTN-3 trial. J Am Soc Hypertens. 2015;9(10):769–79. doi: 10.1016/j.jash.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Epstein M, de Marchena E. Is the failure of SYMPLICITY HTN-3 trial to meet its efficacy endpoint the “end of the road” for renal denervation? J Am Soc Hypertens. 2015;9(2):140–9. doi: 10.1016/j.jash.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Bohm M, Mahfoud F. SYMPLICITY HTN-3 trial: what is it and what does it mean? Eur Heart J. 2014;35(26):1697–8. [PubMed] [Google Scholar]
- 28.Brandt MC, et al. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol. 2012;59(10):901–9. doi: 10.1016/j.jacc.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 29.Ezzahti M, et al. Blood pressure and neurohormonal responses to renal nerve ablation in treatment-resistant hypertension. J Hypertens. 2013 doi: 10.1097/HJH.0b013e3283658ef7. [DOI] [PubMed] [Google Scholar]
- 30.Goliasch G, et al. [Percutaneous renal denervation in patients with resistant hypertension--first experiences in Austria]. Wien Klin Wochenschr. 2010;122(23-24):723–6. doi: 10.1007/s00508-010-1486-y. [DOI] [PubMed] [Google Scholar]
- 31.Krum H, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373(9671):1275–81. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 32.Persu A, et al. Blood pressure changes after renal denervation at 10 European expert centers. J Hum Hypertens. 2013 doi: 10.1038/jhh.2013.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosa J, et al. Randomized comparison of renal denervation versus intensified pharmacotherapy including spironolactone in true-resistant hypertension: six-month results from the Prague-15 study. Hypertension. 2015;65(2):407–13. doi: 10.1161/HYPERTENSIONAHA.114.04019. [DOI] [PubMed] [Google Scholar]
- 34.Voskuil M, et al. Percutaneous renal denervation for the treatment of resistant essential hypertension; the first Dutch experience. Neth Heart J. 2011;19(7-8):319–23. doi: 10.1007/s12471-011-0143-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vink EE, Blankestijn PJ. Catheter-Based Renal Nerve Ablation and Centrally Generated Sympathetic Activity in Difficult-to-Control Hypertensive Patients. Hypertension. 2013;61(2):e8. doi: 10.1161/HYPERTENSIONAHA.111.00477. [DOI] [PubMed] [Google Scholar]
- 36.Kario K, et al. SYMPLICITY HTN-Japan - First Randomized Controlled Trial of Catheter-Based Renal Denervation in Asian Patients. Circ J. 2015;79(6):1222–9. doi: 10.1253/circj.CJ-15-0150. [DOI] [PubMed] [Google Scholar]
- 37.Ceral J, et al. Difficult-to-control arterial hypertension or uncooperative patients? The assessment of serum antihypertensive drug levels to differentiate non- responsiveness from non-adherence to recommended therapy. Hypertens Res. 2011;34(1):87–90. doi: 10.1038/hr.2010.183. [DOI] [PubMed] [Google Scholar]
- 38.Fontana V, Luizon MR, Sandrim VC. An update on the pharmacogenetics of treating hypertension. J Hum Hypertens. 2015;29(5):283–91. doi: 10.1038/jhh.2014.76. [DOI] [PubMed] [Google Scholar]
- 39.Kandzari DE, et al. The SPYRAL HTN Global Clinical Trial Program: Rationale and design for studies of renal denervation in the absence (SPYRAL HTN OFF-MED) and presence (SPYRAL HTN ON-MED) of antihypertensive medications. Am Heart J. 2016;171(1):82–91. doi: 10.1016/j.ahj.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 40.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77(1):75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- 41.Kopp UC. Role of renal sensory nerves in physiological and pathophysiological conditions. Am J Physiol Regul Integr Comp Physiol. 2015;308(2):R79–95. doi: 10.1152/ajpregu.00351.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johns EJ, Kopp UC, DiBona GF. Neural control of renal function. Compr Physiol. 2011;1(2):731–67. doi: 10.1002/cphy.c100043. [DOI] [PubMed] [Google Scholar]
- 43.Sun ZJ, Zhang ZE. Historic perspectives and recent advances in major animal models of hypertension. Acta Pharmacol Sin. 2005;26(3):295–301. doi: 10.1111/j.1745-7254.2005.00054.x. [DOI] [PubMed] [Google Scholar]
- 44.Kline RL, Kelton PM, Mercer PF. Effect of renal denervation on the development of hypertension in spontaneously hypertensive rats. Can J Physiol Pharmacol. 1978;56(5):818–22. doi: 10.1139/y78-128. [DOI] [PubMed] [Google Scholar]
- 45.Liard JF. Renal denervation delays blood pressure increase in the spontaneously hypertensive rat. Experientia. 1977;33(3):339–40. doi: 10.1007/BF02002815. [DOI] [PubMed] [Google Scholar]
- 46.Norman RA, Jr., Dzielak DJ. Role of renal nerves in onset and maintenance of spontaneous hypertension. Am J Physiol. 1982;243(2):H284–8. doi: 10.1152/ajpheart.1982.243.2.H284. [DOI] [PubMed] [Google Scholar]
- 47.Gattone VH, 2nd, et al. Effect of denervation on the afferent arteriole in the SHR. Jpn Heart J. 1984;25(5):745–53. doi: 10.1536/ihj.25.745. [DOI] [PubMed] [Google Scholar]
- 48.Hart EC, et al. Translational Examination of Changes in Baroreflex Function After Renal Denervation in Hypertensive Rats and Humans. Hypertension. 2013 doi: 10.1161/HYPERTENSIONAHA.113.01261. [DOI] [PubMed] [Google Scholar]
- 49.Krueger AD, et al. Selective vasodilation produced by renal denervation in adult spontaneously hypertensive rats. Hypertension. 1986;8(5):372–8. doi: 10.1161/01.hyp.8.5.372. [DOI] [PubMed] [Google Scholar]
- 50.Iliescu R, et al. Role of the renal nerves in blood pressure in male and female SHR. Am J Physiol Regul Integr Comp Physiol. 2006;290(2):R341–4. doi: 10.1152/ajpregu.00035.2005. [DOI] [PubMed] [Google Scholar]
- 51.Maranon RO, et al. Postmenopausal hypertension: role of the sympathetic nervous system in an animal model. Am J Physiol Regul Integr Comp Physiol. 2014;306(4):R248–56. doi: 10.1152/ajpregu.00490.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maranon RO, Reckelhoff JF. Mechanisms responsible for postmenopausal hypertension in a rat model: Roles of the renal sympathetic nervous system and the renin-angiotensin system. Physiol Rep. 2016;4(2) doi: 10.14814/phy2.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee JY, Walsh GM. Systemic and regional haemodynamic effects of renal denervation in spontaneously hypertensive rats. J Hypertens. 1983;1(4):381–6. doi: 10.1097/00004872-198312000-00010. [DOI] [PubMed] [Google Scholar]
- 54.Winternitz SR, Katholi RE, Oparil S. Role of the renal sympathetic nerves in the development and maintenance of hypertension in the spontaneously hypertensive rat. J Clin Invest. 1980;66(5):971–8. doi: 10.1172/JCI109966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bakris GL, et al. Impact of renal denervation on 24-hour ambulatory blood pressure: results from SYMPLICITY HTN-3. J Am Coll Cardiol. 2014;64(11):1071–8. doi: 10.1016/j.jacc.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 56.Struyker-Boudier HA, Nievelstein HM, Smits JF. Antihypertensive and haemodynamic properties of the renal vasodilator CGP 22 979A in conscious spontaneously hypertensive rats. J Hypertens Suppl. 1985;3(3):S183–6. [PubMed] [Google Scholar]