Abstract
The effect of gamma radiation on the ultrastructure of the cell membranes of red blood cells has been probed using a powerful tool, namely, atomic force microscopy. We used mice erythrocytes as a model. Blood samples withdrawn from mice were gamma-irradiated using a 60Co source unit with doses of 10,15,20,25 and 30 Gy. Structural changes appeared in the form of nanoscale potholes, depressions and alterations of the cell membrane roughness. The roughness of the cell membrane increased dramatically with increasing doses, although at 10 Gy , the cell membrane roughness was less than that of normal red blood cells (controls). Therefore, such modifications at the nano-scale level may affect the biophysical properties of membranes, resulting in impairment of their function.
Keywords: Erythrocytes, Gamma radiation, Nanostructure, Atomic force microscopy
Introduction
Irradiation of blood components prior to transfusion using gamma radiation has become a routine practice in many clinical institutions, and the main purpose for such a practice is to eliminates the risk of transfusion-associated graft versus host disease (TA-GVHD) [1–4]. The reason for irradiating blood instead of treating the patients with medications, such as immunoglobulins and corticosteroids, is the lack of medication efficacy. Although the use of gamma radiation is routine worldwide, there are no unified standards for the gamma radiation dose [5], so the recommended dose varies between 25 and 50 Gy [6]. Such high doses may have deleterious effects on the quality of the blood components [7]. For example, the rate of efflux of intracellular potassium, sodium and hemoglobin increases following the irradiation of RBC, which may lead to a reduction in the RBC shelf-life [8–10]. An increase in plasma potassium and sodium concentrations has been attributed to the damage of potassium and sodium pumps induced by irradiation [9, 11]. However, Brugnara et al. [11] showed that the increase in plasma potassium and sodium concentrations may be not due to damage to the pumps, but may be due to hemolysis of the RBCs’ membranes that results in increasing the membrane permeability. However, Jacobs [12] showed that irradiation of RBCs at very high doses, e.g., 200 Gy, has no an evident impact on the cell morphology.
Oxygenation of body cells is the main function of RBCs. The mammalian anucleated RBC has a biconcave disk shape of approximately 8 µm in diameter and is mainly composed of a concentrated solution of hemoglobin encapsulated by a thin film called the membrane. The key role of hemoglobin solution is to carry oxygen. Thus, the erythrocyte’s membrane is strongly connected to its function. The membrane consists of two layers; the outer layer is composed primarily of a phospholipid bilayer studded with membrane proteins, and the inner layer is a 2D filamentous network called a cytoskeleton, which is composed mainly of spectrin and actin. The role of the cytoskeleton is to maintain the structural integrity of the red blood cells. The cytoskeleton is anchored to the phospholipid bilayer by transmembrane proteins [13].
Irradiation of cells using ionizing radiation, such as gamma radiation, increases the level of reactive oxygen species (ROS), causing damage to RBC membranes by peroxidation of membrane lipids and oxidation of membrane-associated proteins [14]. Such damage to the cellular membrane results in impairment of the cell membrane functions or deterioration of the cell viability [15–21]. Although the ultrastructure of the RBC membrane plays a pivotal role in cellular regulation and function, little information is available concerning the impact of gamma irradiation on the RBC membrane ultrastructure. RBCs, and the RBC membranes can be used as a model to study the effects of ionizing radiation (essentially γ-radiation and X-rays) on whole blood and the susceptibility of RBCs to oxidative stress damage resulting from irradiation.
In this work, we evaluate the effects of gamma irradiation on the ultrastructure (nanostructure) of the RBC membrane using atomic force microscopy (AFM). We call the hemolysis of the RBC membrane at nanostructural level as “nanohemolysis”, which may not affect the whole morphology of cell but has a profound impact on the cell membrane at the molecular level and thus its function.
Materials and methods
Sample preparation
Blood samples were drawn from Wister albino rats (~200 g), and anticoagulated with heparin. The blood sample was split into six aliquots of equal volume. One aliquot was left untreated as a control, and the other five aliquots were individually irradiated with gamma radiation (60Co source) at doses of 10, 15, 20, 25 and 30 Gy (at KACST, Riyadh, KSA). Immediately after exposure, the irradiated and control samples were processed. The samples were diluted in PBS (pH 7.4) and centrifuged for 10 min at 3000 rpm at room temperature. The yellowish supernatants were discarded, and the erythrocytes were resuspended in PBS. A droplet of each erythrocyte solution was manually spread onto around glass coverslip with a diameter of 12 mm [22] and allowed to air dry for few minutes prior to imaging by AFM (Fig. 1). The coverslips coated with erythrocytes were glued to 12 mm-magnetic steel discs using double-sided adhesive tape and then mounted on the AFM scanning stage.
Fig. 1.
An optical image of RBCs distributed on a glass coverslip
AFM measurements
All AFM topographic images were acquired using a commercial AFM (Multimode V, Bruker, USA) operating in intermittent contact mode (tapping mode) in air. Tapping-mode etched silicon probes (TESP, Bruker probes) with a nominal spring constant of k = 42 N/m, a nominal resonance frequency of f = 320 kHz and a tip radius of approximately 8 nm were used for imaging. All AFM measurements were performed in air at room temperature with a scan rate of approximately 0.7 Hz. A top-view optical microscope was used to direct the AFM tip to the desired cells. For each sample, 10 cells at different positions were scanned. The AFM images were processed and analyzed using Nanoscope Analysis Software (Bruker Corp). The experiment was repeated several times, each time with new samples, in order to eliminate any chance of artifacts.
Results
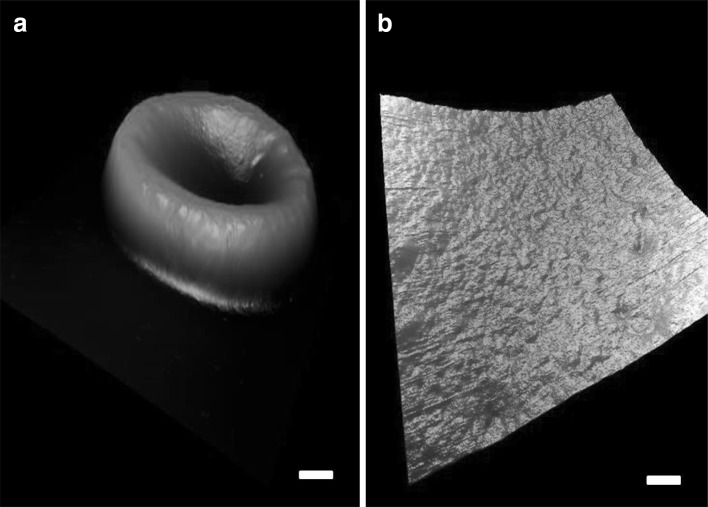
The morphological properties of non-irradiated RBCs (controls) have been investigated by means of AFM. Figure 2a displays a typical three-dimensional topographical AFM image of non-irradiated erythrocyte. At a small scan size (1.2 µm square scanning), the ultrastructure of the membrane surface of the RBC appeared smooth and uniform (Fig. 2b). The overall morphologies of irradiated RBCs are similar to those of non-irradiated ones, and no considerable changes in their morphology can be reported. However, by zooming in on the surface using a smaller scan size (e.g., ~1 µm square scanning), clear differences between cells can be revealed; nano-scale potholes and pores appeared on the membrane surface, and alterations in the roughness of the surface, emphasizing the role of irradiation in alternating the structure of the cell membrane, were seen.
Fig. 2.
a A three-dimensional AFM image of a single non-irradiated RBC; scale bar 1.5 µm. b Ultrastructure of the RBC membrane, scale bar 200 nm
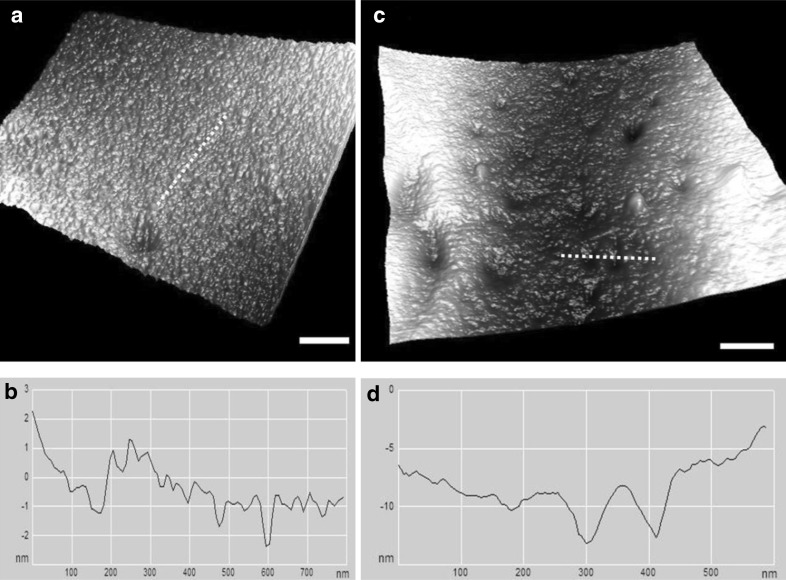
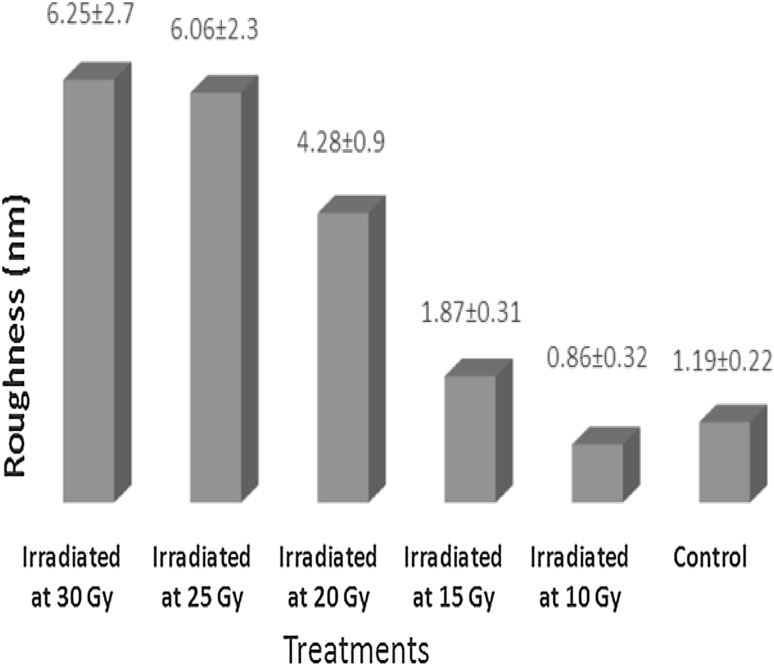
In the samples irradiated at 10 Gy, the ultrastructure of the membrane surface of the RBCs did not show major differences compared with non-irradiated RBCs (Fig. 3). However, variations were observed in the cell membrane roughness. The samples irradiated at 10 Gy showed a significant decrease in the surface roughness by 30 % compared to the controls (Fig. 4). The roughness decreased from ~1.2 to 0.86 nm. For the samples irradiated at doses higher than 10 Gy, a remarkable increase in the surface roughness was observed. For example, the surface roughness of RBCs irradiated at 25 and 30 Gy is approximately five times higher than that of the controls (~1.2 nm).
Fig. 3.
a Ultrastructure of an RBC membrane irradiated at 10 Gy. b The line profile of the image along the dashed line in (a). c Ultrastructure of RBC membrane irradiated at 15 Gy; few potholes and depressions on the cell membrane can be observed. d The line profile of the image along the dashed line in (c). Scale bar 200 nm
Fig. 4.
The cell membrane roughness of RBCs after irradiation. RBCs were irradiated at different gamma doses. The mean of the roughness (nN) ± standard division (n = 100) of RBC membrane for irradiated and non-irradiated cells
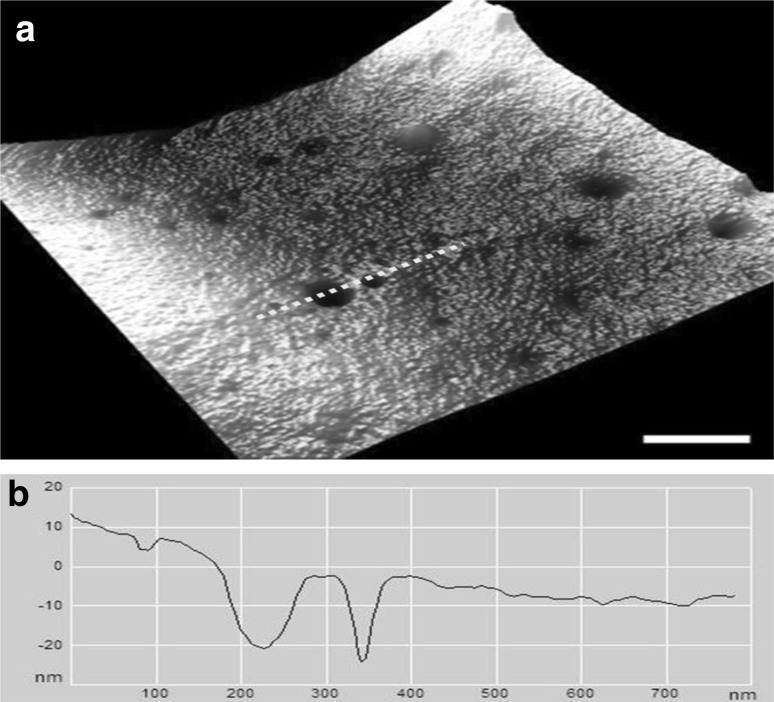
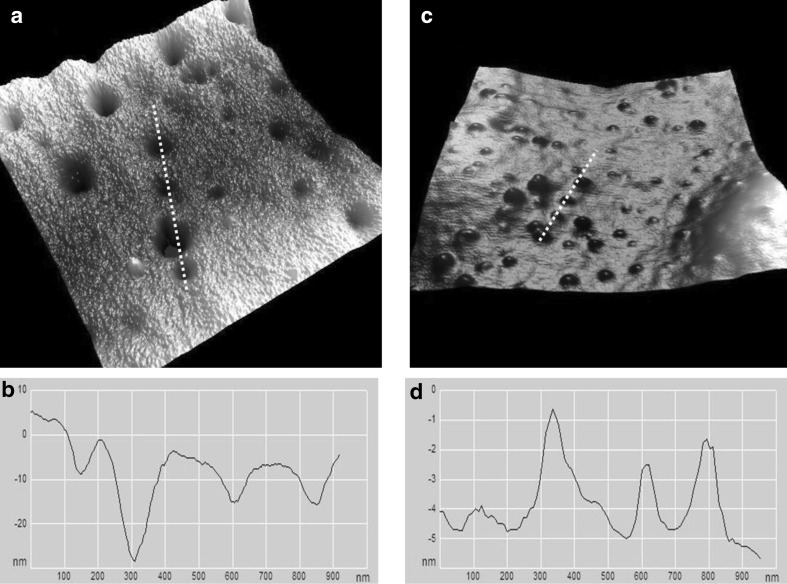
In Fig. 5, the RBCs exposed to 15 Gy showed nontrivial ultrastructural alterations; few small nano-pores appeared on the membrane surface. The depth of the pores varied between 3 and 8 nm. The number of potholes, their depth and the surface roughness increased at higher doses (20, 25 and 30 Gy) (Figs. 5, 6). An increase of the gamma radiation dose beyond 15 Gy dramatically changed the ultrastructure of the RBC surface membrane. The size of the potholes and the roughness of the cell membrane surface became more prominent. As seen in Fig. 6c, another noteworthy alteration is the appearance of many protuberances on the membrane surface of the irradiated erythrocytes at doses of 25 and 30 Gy. There are large protrusions or blebs with an average height on the order of 3–6 nm and an approximate diameter of 100–200 nm.
Fig. 5.
a Large, deep potholes are observed on the membrane of RBCs irradiated at 20 Gy. b The line profile of the cell membrane in (a) along the dashed line. Scale bar 200 nm
Fig. 6.
Ultrastructure of RBC membranes irradiated at 25 and 30 Gy. The effect of irradiation is manifested as large potholes (a) or as blebs (c) on the membrane. b The height profile along the dashed line in (a). d The height profile along the dashed line in (c)
Discussion
The structural properties of the membranes of RBCs are essential for membrane function. The presence of reactive oxygen species (ROS) has the most substantial effects on the properties of the lipid bilayer, membrane-associated proteins and, therefore, the structure and function of the RBCs [23].
Irradiation of RBCs at gamma radiation doses of 10–30 Gy had no effect on their overall morphology. However, this finding is inconsistent with the previous work by Xu et al. [5]. They used scanning electron microscopy to evaluate the changes in the cell membrane structures due to irradiation during storage. It has been found that the shape of the irradiated RBCs changed markedly, and the proportions of echinocytes and sphero-echinocytes increased. Such controversial findings can be explained as follows. The deformations of RBCs are not due to exposure to radiation but are due to storage. Storage time can play a key role in the development of such deformations. In our study, the time elapsed between the blood sample collection and the sample processing was a few hours.
In this study, nano-hemolysis and nano-structural changes were observed on the cell membranes of irradiated cells as a function of radiation dose. Such hemolysis was due to the effect of increased levels of ROS, which are the most prominent example of oxidizing substances and are highly reactive due to the presence of unpaired electrons that cause immense alterations in the cell membrane structure. Therefore, the nano-hemolysis (nano-pores, crater-like structures) observed on the cell membrane can be attributed to the oxidation-induced damage, which may be triggered via one of two mechanisms. First, the presence of a high level of polyunsaturated fatty acids in the RBC membrane compared to other cells is negatively correlated with oxidative stress [24, 25]. The formation of ROS leads to the degradation of polyunsaturated lipids and an increase of the oxidative stress level, which can compromise the cell membrane integrity and could lead to cell death [14, 26]. Therefore, RBCs may be more susceptible to oxidative damage than other cells. Second, denaturation of hemoglobin (Hb) can cause the damage. Accumulating evidence has shown that released hemin, one of the end product of Hb denaturation, is a potent catalyst of lipid peroxidation, which in turn causes hemolysis [27–29]. Many microscopy studies have shown that hemin can affect the conformation of the cell cytoskeleton, which in turn affects the cell structure [27, 29, 30]. An increase in the rate of lipid peroxidation of RBCs membranes as a function of radiation dose was characterized by an increase in the density and size of nano-pores on the cell membrane (or nano-hemolysis). The higher the radiation dose is, the higher is the concentration of ROS.
Although the typical overall morphology of RBCs was preserved, the formation of nano-hemolysis within the cell membrane can causes a reduction in the structural strength of the cell membrane, making the cells more fragile and thus reducing their life span. It should be noted that the storage of irradiated RBCs could enhance the cell membrane lesions [31].
We also observed an increase in the cell membrane roughness as a function of irradiation dose. This finding provides evidence that ROS are capable of triggering structural deficits in the lipid bilayer and cytoskeleton of RBC membranes. However, RBCs have the ability to resist such deformation and recover. Benderitter et al [32]. demonstrated that the level of lipid peroxidation increased in the hours after radiation exposure but was not observed after 3 days post-irradiation. However, the recovery system may repair damage caused by ROS faster than was previously thought. At a dose of 10 Gy, no morphological alterations or geometrical changes have been observed compared to the controls, but the value of membrane roughness was smaller than that of the control. The reason for the decrease in the surface roughness is not yet clear, but it may be an attempt to reorganize the cell membrane components to maintain the integrity of the surface. We hypothesize that at 10 Gy, relatively small and unstable nano-pores were initially formed that were then obliterated by a recovery mechanism within the cells, which occurred a few hours after irradiation. However, the level of damage induced by doses higher than 10 Gy could be beyond the repair ability of the cell, at least at the nano-scale level.
It is worth noting that the irregularities observed on the cell membrane of irradiated RBC are unlikely to be artifacts due to imaging or drying. During air-drying, the RBCs are subjected to stress and strain effects that may cause some structural abnormalities on their cell membranes. However, such abnormalities have not been detected on the membrane surface of non-irradiated RBCs, and are indicative of an oxidation effect.
Moreover, some anticoagulants may induce morphological changes in erythrocytes [33, 34], such as Hemolysis anisocytosis and swelling. Walencik and Witeska [35] compared the effects of three anticoagulants on the erythrocytes of Cyprinus carpio L. They found that sodium citrate and EDTA induced hemolysis in the erythrocytes of C. carpio L, while heparin caused no significant alterations. Similar results have been observed by Maqbool et al. [36] who investigated the impacts of EDTA and heparin on the RBC morphology of Oncorhynchus mykiss. These observations may be further supported by our results that no significant structural changes were observed in heparin treated samples (controls).
Conclusions
Irradiation of RBCs by gamma radiation could cause various degrees of damage to RBCs membranes. The damage to the cell membrane is dose-dependent. The differences between the fine structure of the cell membranes of irradiated and non-irradiated cells are clear; however, these dissimilarities were indistinguishable when using a large scan size. Nano-pores and crater-like structures were distributed over the entire cell membrane. Additionally, irradiation of the cells induced a remarkable change in their roughness. Such alterations could result in impairment of the cells’ function.
Acknowledgments
This work was supported by King Saud University, Deanship of Scientific Research, College of Science Research Center.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed.
References
- 1.Reiser H, Stadecker MJ. Costimulatory B7 molecules in the pathogenesis of infectious and autoimmune diseases. N Engl J Med. 1996;335(18):1369–1377. doi: 10.1056/NEJM199610313351807. [DOI] [PubMed] [Google Scholar]
- 2.Button LN, DeWolf WC, Newburger PE, Jacobson MS, Kevy SV. The effects of irradiation on blood components. Transfusion. 1981;21(4):419–426. doi: 10.1046/j.1537-2995.1981.21481275998.x. [DOI] [PubMed] [Google Scholar]
- 3.Williamson LM, Warwick RM. Transfusion-associated graft-versus-host disease and its prevention. Blood Rev. 1995;9(4):251–261. doi: 10.1016/S0268-960X(95)90016-0. [DOI] [PubMed] [Google Scholar]
- 4.Hart S, Cserti-Gazdewich C, McCluskey S. Red cell transfusion and the immune system. Anaesthesia. 2015;70(s1):38-e16. doi: 10.1111/anae.12892. [DOI] [PubMed] [Google Scholar]
- 5.Xu D, Peng M, Zhang Z, Dong G, Zhang Y, Yu H. Study of damage to red blood cells exposed to different doses of gamma-ray irradiation. Blood Transfus. 2012;10:321–330. doi: 10.2450/2012.0076-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maia G, de Oliveira Renó C, Medina J, da Silveira A, Mignaco J, Atella G, et al. The effect of gamma radiation on the lipid profile of irradiated red blood cells. Ann Hematol. 2014;93(5):753–760. doi: 10.1007/s00277-013-1944-5. [DOI] [PubMed] [Google Scholar]
- 7.Olivo RA, da Silva MV, Garcia FB, Soares S, Rodrigues Junior V, Moraes-Souza H. Evaluation of the effectiveness of packed red blood cell irradiation by a linear accelerator. Revista Brasileira de Hematologia e Hemoterapia. 2015;37(3):153–159. doi: 10.1016/j.bjhh.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirayama J, Abe H, Azuma H, Ikeda H. Leakage of potassium from red blood cells following gamma ray irradiation in the presence of dipyridamole, trolox, human plasma or mannitol. Biol Pharm Bull. 2005;28(7):1318. doi: 10.1248/bpb.28.1318. [DOI] [PubMed] [Google Scholar]
- 9.Dinning G, Doughty R, Reid M, Lloyd H. Potassium concentrations in irradiated blood. BMJ. 1991;303(6810):1110. doi: 10.1136/bmj.303.6810.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barjas-Castro M, Brandao M, Fontes A, Costa F, Cesar C, Saad S. Elastic properties of irradiated RBCs measured by optical tweezers. Transfusion. 2002;42(9):1196–1199. doi: 10.1046/j.1537-2995.2002.00201.x. [DOI] [PubMed] [Google Scholar]
- 11.Brugnara C, Churchill W. Effect of irradiation on red cell cation content and transport. Transfusion. 1992;32(3):246–252. doi: 10.1046/j.1537-2995.1992.32392213809.x. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs GP. A review on the effects of ionizing radiation on blood and blood components. Radiat Phys Chem. 1998;53(5):511–523. doi: 10.1016/S0969-806X(98)00185-6. [DOI] [Google Scholar]
- 13.Fournier J-B, Lacoste D, Raphaël E. Fluctuation spectrum of fluid membranes coupled to an elastic meshwork: jump of the effective surface tension at the mesh size. Phys Rev Lett. 2004;92(1):018102. doi: 10.1103/PhysRevLett.92.018102. [DOI] [PubMed] [Google Scholar]
- 14.Anand A, Dzik W, Imam A, Sadrzadeh S. Radiation-induced red cell damage: role of reactive oxygen species. Transfusion. 1997;37(2):160–165. doi: 10.1046/j.1537-2995.1997.37297203518.x. [DOI] [PubMed] [Google Scholar]
- 15.Adams F, Bellairs G, Bird AR, Oguntibeju OO. Biochemical storage lesions occurring in nonirradiated and irradiated red blood cells: a brief review. BioMed Res Int. 2015;2015:8. doi: 10.1155/2015/968302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi Y, Li R-y Tu, Z-c Ma D, Wang H, X-q Huang, et al. Effect of γ-irradiation on the physicochemical properties and structure of fish myofibrillar proteins. Radiat Phys Chem. 2015;109:70–72. doi: 10.1016/j.radphyschem.2014.12.016. [DOI] [Google Scholar]
- 17.Winter K, Johnson L, Kwok M, Reid S, Alarimi Z, Wong J, et al. Understanding the effects of gamma-irradiation on potassium levels in red cell concentrates stored in SAG-M for neonatal red cell transfusion. Vox Sang. 2014;108:141–150. doi: 10.1111/vox.12194. [DOI] [PubMed] [Google Scholar]
- 18.Chadwick K, Leenhouts H. Radiation risk is linear with dose at low doses. Br J Radiol. 2014;78:8–10. doi: 10.1259/bjr/51173413. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y-K, Kwon E-H, Kim D-H, Won D-I, Shin S, Suh J-S. Susceptibility of oxidative stress on red blood cells exposed to gamma rays: hemorheological evaluation. Clin Hemorheol Microcirc. 2008;40(4):315–324. [PubMed] [Google Scholar]
- 20.Anand AJ, Dzik WH, Imam A, Sadrzadeh SMH. Radiation-induced red cell damage: role of reactive oxygen species. Transfusion. 1997;37(2):160–165. doi: 10.1046/j.1537-2995.1997.37297203518.x. [DOI] [PubMed] [Google Scholar]
- 21.Relevy H, Koshkaryev A, Manny N, Yedgar S, Barshtein G. Blood banking-induced alteration of red blood cell flow properties. Transfusion. 2008;48(1):136–146. doi: 10.1111/j.1537-2995.2007.01491.x. [DOI] [PubMed] [Google Scholar]
- 22.Zachée P, Snauwaert J, Vandenberghe P, Hellemans L, Boogaerts M. Imaging red blood cells with the atomic force microscope. Br J Haematol. 1996;95(3):472–481. doi: 10.1111/j.1365-2141.1996.tb08991.x. [DOI] [PubMed] [Google Scholar]
- 23.Maia GAS, de Oliveira Renó C, Medina JM, da Silveira AB, Mignaco JA, Atella GC, et al. The effect of gamma radiation on the lipid profile of irradiated red blood cells. Ann Hematol. 2014;93(5):753–760. doi: 10.1007/s00277-013-1944-5. [DOI] [PubMed] [Google Scholar]
- 24.Asgary S, Naderi G, Ghannady A. Effects of cigarette smoke, nicotine and cotinine on red blood cell hemolysis and their-SH capacity. Exp Clin Cardiol. 2005;10(2):116. [PMC free article] [PubMed] [Google Scholar]
- 25.Edwards R, Peet M, Shay J, Horrobin D. Omega-3 polyunsaturated fatty acid levels in the diet and in red blood cell membranes of depressed patients. J Affect Disord. 1998;48(2–3):149–155. doi: 10.1016/S0165-0327(97)00166-3. [DOI] [PubMed] [Google Scholar]
- 26.Wu D, Cederbaum AI. Alcohol, oxidative stress, and free radical damage. Alcohol Res Health. 2003;27:277–284. [PMC free article] [PubMed] [Google Scholar]
- 27.Chiu DT, Huang T-M, Hung I-J, Wei J-S, Liu T, Stern A. Hemin-induced membrane sulfhydryl oxidation: possible involvement of thiyl radicils. Free Radical Res. 1997;27(1):55–62. doi: 10.3109/10715769709097838. [DOI] [PubMed] [Google Scholar]
- 28.Chiu DT-Y, Van Den Berg J, Kuypers FA, Hung I-J, Wei J-S, Liu T-Z. Correlation of membrane lipid peroxidation with oxidation of hemoglobin variants: possibly related to the rates of hemin release. Free Radic Biol Med. 1996;21(1):89–95. doi: 10.1016/0891-5849(96)00035-4. [DOI] [PubMed] [Google Scholar]
- 29.Kozlova E, Chernysh A, Moroz V, Gudkova O, Sergunova V, Kuzovlev A. Transformation of membrane nanosurface of red blood cells under hemin action. Sci Rep. 2014;4:6033. doi: 10.1038/srep06033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaklai N, Avissar N, Rabizadeh E, Shaklai M. Disintegration of red cell membrane cytoskeleton by hemin. Biochem Int. 1986;13(3):467–477. [PubMed] [Google Scholar]
- 31.Agarwal P, Ray V, Choudhury N, Chaudhary R. Effect of pre-storage gamma irradiation on red blood cells. Indian J Med Res. 2005;122(5):385. [PubMed] [Google Scholar]
- 32.Benderitter M, Vincent-Genod L, Pouget J, Voisin P. The cell membrane as a biosensor of oxidative stress induced by radiation exposure: a multiparameter investigation. Radiat Res. 2009;159:471–483. doi: 10.1667/0033-7587(2003)159[0471:TCMAAB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 33.Hattingh J, Smith EM. Anticoagulants for avian and reptilian blood: heparin and EDTA. Pflug Arch. 1976;363(3):267–269. doi: 10.1007/BF00594613. [DOI] [PubMed] [Google Scholar]
- 34.Muro J, Cuenca R, Pastor J, Vinas L, Lavin S. Effects of lithium heparin and tripotassium EDTA on hematologic values of Hermann’s tortoises (Testudo hermanni) J Zoo Wildl Med. 1998;29:40–44. [PubMed] [Google Scholar]
- 35.Walencik J, Witeska M. The effects of anticoagulants on hematological indices and blood cell morphology of common carp (Cyprinus carpio L.) Comp Biochem Physiol C: Toxicol Pharmacol. 2007;146(3):331–335. doi: 10.1016/j.cbpc.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Maqbool A, Ahmed I, Sheikh ZA. Effects of two commonly used anticoagulants on haematology and erythrocyte morphology of rainbow trout (Oncorhynchus mykiss) Int J Fish Aquat Stud. 2013;2:239–243. [Google Scholar]