Abstract
Sokal index was developed in the pre-imatinib era to predict and prognosticate the outcome of Chronic myeloid leukemia (CML) patients. In the Imatinib era, a new scoring system called EUTOS scoring system has been validated as a predictive marker in CML. The scores have shown variable correlation with complete cytogenetic response (CCyR) and major molecular response (MMR). To assess the performance of Sokal score and EUTOS score as a predictive marker for CCyR and MMR for newly diagnosed CML-CP patients treated with TKIs. 273 patients with newly diagnosed CML were included in the study. They were treated with upfront imatinib. They were followed up for a median period of 3 years. Cytogenetic and Molecular response to the treatment were monitored regularly. Out of 273 patients, 174 patients (63 %) were having low EUTOS score and 99 (37 %) were having high EUTOS score. Patients with low, intermediate and high sokal scores were 237 (86.8 %), 28 (10.3 %) and 8 (2.9 %) respectively. 122 patients with low EUTOS score achieved CCyR within 18 months compared to 42 patients with high EUTOS score (p = 0.000).113 patients with low EUTOS score achieved MMR in 18 months compared to 33 patients with high EUTOS score (p = 0.000). 148, 14, 2 patients with low, intermediate and high Sokal score respectively have achieved CCyR in 18 months (p = 0.054). 133, 11, 2 patients with low intermediate and high sokal score respectively have achieved MMR in 18 months.(p = 0.06). EUTOS is better than Sokal score in predicting the outcome of patients of CML treated with imatinib.
Keywords: Chronic myeloid leukemia, Sokal, EUTOS, Imatinib, Cytogenetic, Molecular response
Introduction
Chronic myeloid leukemia (CML) is a myeloproliferative disease characterized by clonal expansion of bone marrow stem cells with a unique cytogenetic abnormality. The cytogenetic hallmark is the t(9;22) (q34;q11) translocation leading to 9q+ and 22q− known as Philadelphia chromosome [1]. It results in a fusion BCR-ABL gene leading to a protein with constitutive tyrosine kinase activity. The expression of this fusion protein is responsible for the transformed phenotype of CML cells [2].
Various prognostic systems have been used in patients with CML. Sokal score has been used to risk stratify the patients [3]. EURO scoring system was developed in the era of Interferon alpha [4]. European Leukemia.Net has developed a new scoring system called the EUTOS score [5].
Advances in targeted therapy led to the discovery of imatinib mesylate, a selective competitive antagonist of the BCR-ABL protein resulting in hematological, cytogenetic and molecular remission in a significant proportion of CML patients. Recent advances have led to the development of new tyrosine kinase inhibitors (TKIs) like dasatinib, nilotinib which give much better response than imatinib [6].
The aim of the study was to study the performance of Sokal and EUTOS score as predictive markers for achieving complete cytogenetic response (CCyR) and major molecular response (MMR) in newly diagnosed patients treated with imatinib.
Materials and Methods
Newly diagnosed cases of CML in chronic phase were enrolled prospectively in this study after obtaining written informed consent. Spleen size, total count, basophil and eosinophil counts, platelet count, cytogenetic abnormalities and RT-PCR for BCR-ABL were documented at baseline. Sokal and EUTOS scores were calculated and were used to stratify patients in different risk groups. All patients were started on imatinib 400 mg/day and were monitored for tolerability and adverse effects during treatment. Women of child bearing age were advised to use barrier contraceptive measures and to report if there was an undue delay of menstrual cycle. Patients were followed up at regular intervals for response assesment. Routine hemogram was done every month to look for hematological response. Cytogenetic response was assessed by doing bone marrow karyotyping study every 6 months until patients achieved a CCyR and thereafter annually. CCyR was defined when no Ph+ chromosomes were identified after analysis of 20 metaphases [7]. Molecular response was observed by doing RT-PCR study for BCR-ABL fusion protein every 6 months till achievement of MMR and then yearly. MMR was identified when there is 3 log fold reduction from baseline BCR-ABL/ABL ratio, that is represented by when the BCR-ABL/ABL ratio is 0.1 % or less [8]. Drug was withheld if the patients developed neutropenia (<1000/cumm) or thrombocytopenia (<50,000/cumm).
Events were defined either as any loss of previously achieved hematological response, cytogenetic response or molecular response or an increase in the dosage of imatinib when the patient didn’t achieve hematological response at 3 months, CCyR and MMR at 18 months. Cut off for achieving CCyR was kept at 18 months in both the groups of our study as per the criteria used for defining EUTOS score. Statistical analysis was done by Chi-Square test using SPSS 21. Kaplan-Meir Graph along with log rank test was used to determine the event free survival.
Results
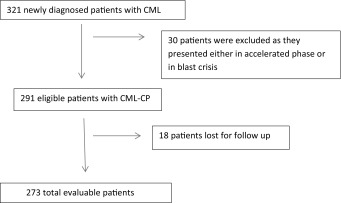
Study profile has been shown in Table 1. Characteristics of the patients are described in Table 2. Distribution of patients according to EUTOS and Sokal score is shown in Tables 3 and 4 respectively. Response was assessed in all the patients. 60.07 % patients achieved CCyR within 18 months and 53.47 % patients achieved MMR within 18 months.
Table 1.
Study profile
Table 2.
Baseline charcteristics of the patients
| Parameters | Mean | Range |
|---|---|---|
| Age (years) | 37.93 | 8–70 |
| Spleen size (cm) | 10.2 | 4–16 |
| Basophil (%) | 5.26 | 3–8 |
| Blast (%) | 4.62 | 2–6 |
| Time to achieve hematological remission (months) | 2.8 | 1–4 |
Table 3.
Risk stratification according to EUTOS score
| EUTOS score | Low risk(≤87) | High Risk (>87) |
|---|---|---|
| Patients | 174 | 99 |
Table 4.
Risk stratification according to Sokal score
| Sokal score | Low (<0.8) | Intermediate (0.8–1.2) | High (>1.2) |
|---|---|---|---|
| Patients | 237 | 28 | 8 |
In EUTOS group, 64.9 % patients achieved MMR within 18 months in the low risk group compared to 33.3 % in the high risk group (p < 0.01) as in Table 5. CCyR was achieved within 18 months by 70.11 % and 42.42 % in the low and high risk group respectively. (p < 0.01) as in Table 6.
Table 5.
EUTOS score and major molecular response
| EUTOS score | MMR achieved in 18 months | MMR not achieved in 18 months |
|---|---|---|
| Low | 113 | 61 |
| High | 33 | 66 |
Table 6.
EUTOS score and complete cytogenetic response
| EUTOS score | CCyR in 18 months | CCyR not in 18 months |
|---|---|---|
| Low | 122 | 52 |
| High | 42 | 57 |
In Sokal group, 56.11 %, 39.2 %, and 25 % achieved MMR among low, intermediate and high risk group respectively (p = 0.62) and CCyR was achieved by 62.44 %, 50 %, and 25 % respectively (p = 0.54) as in Tables 7 and 8 respectively.
Table 7.
Sokal score and major molecular response
| Sokal score | MMR in 18 months | MMR not in 18 months |
|---|---|---|
| Low | 133 | 104 |
| Intermediate | 11 | 17 |
| High | 2 | 6 |
Table 8.
Sokal score and complete cytogenetic response
| Sokal score | CCyR in 18 months | CCyR not in 18 months |
|---|---|---|
| Low | 148 | 89 |
| Intermediate | 14 | 14 |
| High | 2 | 6 |
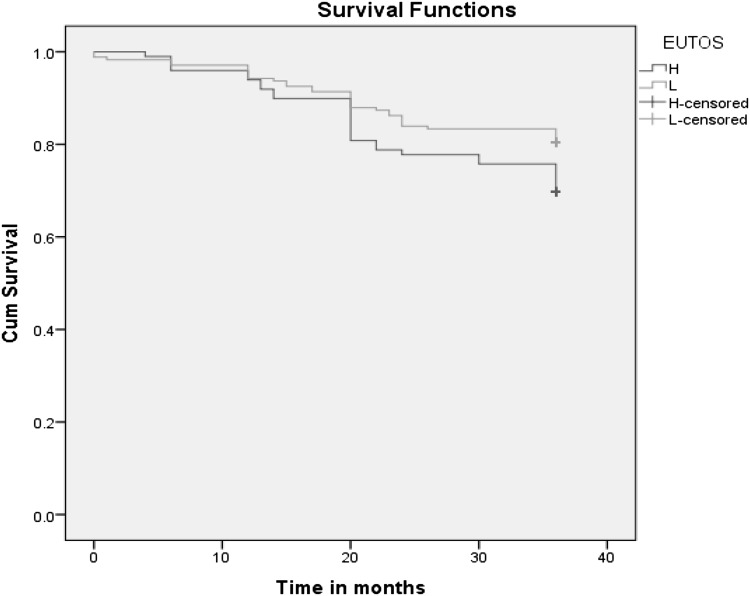
3 year event free survival among the low and high risk EUTOS group was 80.5 % and 69.7 % (p = 0.05) as shown in Fig. 1. 3 year EFS for the different Sokal groups was not significant. (p = 0.58).
Fig. 1.
Kaplan Meir graph showing event free survival among patients in EUTOS group
Discussion
In the modern era, biology of the disease has replaced burden of the disease as a prognostic factor. However in CML the disease burden is still considered as a prognostic factor. Sokal, EURO and EUTOS score assess the disease burden. Sokal score and EURO score were considered to be prognostic in the pre imatinib era. Sokal score still stands tall in predicting outcome for patients treated with imatinib and even with second generation TKIs [9]. EUTOS score was developed in 2011 as it was thought that using a scoring system developed during preimatinib era was improper [10]. Moreover with the usage of imatinib in all age groups of patients, age which was an important risk factor in previous scoring system was deleted in EUTOS score. The scoring system has been made simple by considering only percentage of basophils and spleen size. The role of spleen size and basophil percentage in predicting the prognosis of CML is still not clear. However, there are studies which indicate that the percentage of basophilia correlates with disease stage and splenic Ph+ cells behave differently from bone marrow Ph+ cells. [11–13]
Average age of the population was 37.93 years. Mean spleen size was 10.2 cm which was grossly larger than what has been observed by Hoffman et al. [14] This can be attributed to the lack of awareness in India about the disease amongst the patients and the basic healthcare personnel. Hence patients present usually in the late chronic phase. The proportion of patients in high risk EUTOS was less than 30 % in majority of the studies. In our study, 36.27 % patients belonged to high risk EUTOS group. Majority of our patients belong to low risk Sokal group. Hasford et al. [10] had 39 % in low risk group where as Marin et al. [15] had majority of patients in intermediate risk Sokal group. This disparity can be explained by the mean age of our study population which was less compared to other studies.
Among EUTOS risk groups, 70.11 % low risk patients achieved CCyR within 18 months. Pagnano et al. [16] have demonstrated a high CCyR of 83 % in similar subset of patients. But the difference among high and low risk group in achieving CCyR was statistically significant like that of Hoffman et al. [14]. However, among Sokal risk group, the difference was not statistically significant. Marin et al. [15] found a significant difference among CCyR incidence among the various risk groups. However, a point to be noted is that they have considered 8 year cumulative incidence of achieving CCyR.
In terms of achieving MMR at 18 months among low risk EUTOS, the percentages were 61 %, 56 %, 51 % [17–19] in various studies while in our study it was 64.9 %. The difference among high and low risk subset reached statistical significant value like what has been observed by Yahng et al. The difference among the subsets of Sokal risk group was not significant [19].
The Sokal group didn’t reach statistical significance in terms of predicting CCyR and MMR. This was due to less number of patients in high risk group. This can be explained by the low median age of the study population as age is an important factor in calculating the Sokal score.
In our study, the estimated 3 year EFS was 80.5 % among low risk EUTOS group in standard imatinib dosage group. Most of the studies have observed a higher progression free survival period except the one done by Jabbur et al. [20] which observed a PFS (80.5 %). This difference can be attributed to either the proportion of patients in high risk EUTOS group in our study which was higher than other studies or using a standard dose of imatinib in all the patients where as other studies have used a combination of standard, high dose of imatinib or even second generation TKIs.
There are some limitations in our study. First is the disproportionate distribution of cases among the Sokal risk groups with majority being in the low category. Thus the 3 year survival graph has shown an improved survival in the Sokal high risk group because of too few numbers of patients in that group. Second the compliance of the patients was determined by their verbal response and there were no objective assessment for the same.
However, it is still unclear about the exact value of EUTOS scores in present clinical practice. There are many questions still unanswered. Recent European Leukemia Net guidelines have fixed 12 month as cut off period for defining treatment failure. [21] So what is the value of EUTOS score in present practice which has decided 18 month period for the same. Another question lingers on whether EUTOS score will influence decision making in selecting the generation of TKIs in upfront settings. Further studies are required to answer these queries.
Acknowledgments
We are grateful to the staff members of Department of Medical Oncology, Kidwai Memorial Institute of Oncology for helping us in making this manuscript.
Compliance with Ethical Standards
Conflict of interest
All the authors declare that they have no conflict of interest
Informed Consent
Informed consent was obtained from all the individual participants
Ethical Approval
For this type of study formal consent is not required.
References
- 1.Rowley JD. A new consistent abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243:290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- 2.Heisterkamp N, Stam K, Groffen J, de Klein A, Grosveld G. Structural organization of the bcr gene and its role in the Ph’ translocation. Nature. 1985;315:758–761. doi: 10.1038/315758a0. [DOI] [PubMed] [Google Scholar]
- 3.Sokal JE, Cox EB, Baccarani M, et al. Prognostic discrimination in “good-risk” chronic granulocytic leukemia. Blood. 1984;63:789–799. [PubMed] [Google Scholar]
- 4.Hasford J, Pfirrmann M, Hehlmann R, et al. A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa. J Natl Cancer Inst. 1998;90(11):850–858. doi: 10.1093/jnci/90.11.850. [DOI] [PubMed] [Google Scholar]
- 5.Baccarani M, Cortes J, Pane F, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27(35):6041–6051. doi: 10.1200/JCO.2009.25.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pavey T, Hoyle M, Ciani O, Crathorne L, Jones-Hughes T, Cooper C, et al. Dasatinib, nilotinib and standard-dose imatinib for the first-line treatment of chronic myeloid leukaemia: systematic reviews and economic analyses. Health Technol Assess. 2012;16(42):i–vii. doi: 10.3310/hta16420. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 8.Hughes T, Deininger M, Hochhaus A, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28–37. doi: 10.1182/blood-2006-01-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Druker JB, Guilhot F, O’Brien S, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 10.Hasford J, Baccarani M, Hoffmann V, Guilhot J, Saussele S, Rosti G, et al. Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: the EUTOS score. Blood. 2011;118:686–692. doi: 10.1182/blood-2010-12-319038. [DOI] [PubMed] [Google Scholar]
- 11.Sokal JE, Baccarani M, Russo D. Staging and prognosis in chronic myelogenous leukemia. Semin Hematol. 1988;25:49–61. [PubMed] [Google Scholar]
- 12.Muller-Berat CN, Wantzin GL, Philip P, Baccarani M, Killmann S-A. Agar culture studies of bone marrow, blood, spleen, and liver in chronic myeloid leukemia. Leuk Res. 1977;1:123–131. doi: 10.1016/0145-2126(77)90012-1. [DOI] [Google Scholar]
- 13.Schemionek M, Spieker T, Kerstiens L, Elling C, Essers M, Trumpp A, et al. Leukemic spleen cells are more potent than bone marrow-derived cells in a transgenic mouse model of CML. Leukemia. 2011;26:1030–1037. doi: 10.1038/leu.2011.366. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann VS, Baccarani M, Lindoerfer D, et al. The EUTOS prognostic score: review and validation in 1288 patients with CML treated frontline with imatinib. Leukemia. 2013;27:2016–2022. doi: 10.1038/leu.2013.171. [DOI] [PubMed] [Google Scholar]
- 15.Marin D, Ibrahim AR, Goldman JM. European treatment and outcome study (EUTOS) score for chronic myeloid leukemia still requires more confirmation. J Clin Oncol. 2011;29:3944–3945. doi: 10.1200/JCO.2011.37.6962. [DOI] [PubMed] [Google Scholar]
- 16.Pagnano KB, Lorand-Metze I, Miranda ECM, et al (2012) EUTOS score is predictive of event-free survival, but not for progression-freeand overall survival in patients with early chronic phase chronic myeloidleukemia treated with imatinib: a single Institution experience. Blood, 120 (ASH abstract no. 1681)
- 17.Tiribelli M, Bonifacio M, Calistri E, et al (2012) EUTOS score identifies cases with poor outcome in patients with early phase chronic myeloid leukemia though not predictive for optimal response to imatinib. Blood, 120 (ASH abstract 3778)
- 18.Than H, Kuan L, Seow CH, Li et al (2012) The EUTOS score is highly predictive for clinical outcome and survival in Asian patients with early chronic phase chronic myeloid leukemia treated with imatinib. Blood, 120 (ASH abstract 3758)
- 19.Yahng S-A, Jang E-J, Choi SY, et al (2012) Comparison of Sokal, Hasford and EUTOS scores in term of long-term treatment outcome according to the risks in each prognostic model: a single center data analysed in 255 early chronic phase chronic myeloid leukemia patients treated with frontline imatinib mesylate. Blood, 120: (ASH abstract no. 2794)
- 20.Jabbour E, Cortes J, Nazha A, O’Brien S, et al. EUTOS score is not predictive for survival and outcome in patients with early chronic phase chronic myeloid leukemia treated with tyrosine kinase inhibitors:a single institution experience. Blood. 2012;119:4524–4526. doi: 10.1182/blood-2011-10-388967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872–884. doi: 10.1182/blood-2013-05-501569. [DOI] [PMC free article] [PubMed] [Google Scholar]