Abstract
Recent innovations in treatment of multiple myeloma include autologous stem cell transplantation (ASCT) along with high dose chemotherapy (HDC). We undertook this study to estimate incremental cost per quality adjusted life year gained (QALY) with use of ASCT along with HDC as compared to conventional chemotherapy (CC) alone in treatment of multiple myeloma. A combination of decision tree and markov model was used to undertake the analysis. Incremental costs and effects of ASCT were compared against the baseline scenario of CC (based on Melphalan and Prednisolone regimen) in the patients of multiple myeloma. A lifetime study horizon was used and future costs and consequences were discounted at 5%. Consequences were valued in terms of QALYs. Incremental cost per QALY gained using ASCT as against CC for treatment of multiple myeloma was estimated using both a health system and societal perspective. The cost of providing ASCT (with HDC) for multiple myeloma patients was INR 500,631, while the cost of CC alone was INR 159,775. In the long run, cost per patient per year for ASCT and CC arms was estimated to be INR 119,740 and INR 111,565 respectively. The number of QALYs lived per patient in case of ASCT and HDC alone were found to be 4.1 and 3.5 years respectively. From a societal perspective, ASCT was found to incur an incremental cost of INR 334,433 per QALY gained. If the ASCT is initiated early to patients, the incremental cost for ASCT was found to be INR 180,434 per QALY gained. With current mix of patients, stem cell treatment for multiple myeloma is not cost effective at a threshold of GDP per capita. It becomes marginally cost-effective at 3-times the GDP per capita threshold. However, accounting for the model uncertainties, the probability of ASCT to be cost effective is 59%. Cost effectiveness of ASCT can be improved with early detection and initiation of treatment.
Keywords: Cost-effectiveness analysis, Autologous stem cell transplant, Multiple myeloma, Quality adjusted life year, Health technology assessment
Introduction
Multiple myeloma is a malignancy that is part of spectrum of diseases ranging from Monoclonal Gammopathy of Unknown Significance (MGUS) to Plasma Cell Leukaemia. It occurs at an annual incidence of 1% of all malignancies, and 13% of all hematological malignancies [1]. In India the reported incidence varies from 0.3 to 1.9 per 100,000 for men and 0.4 to 1.3 per 100,000 for women [2]. In turn this amounts to nearly 6000 new multiple myeloma cases each year in India [3]. It is more common in men than women with male to female ratio of 1.4:1. The median age at diagnosis is 62 years for men and 61 years for women [1]. This severe disease may range from asymptomatic to being severely symptomatic with especially complications that require emergent treatment [4].
Over the years since 1960s, treatment of disease has evolved from Melphalan and Prednisolone to introduction of high dose drugs with autologous stem cell transplantation (ASCT) and during 1990s, a new era of regimen initiated by Thalidomide, its analogue Lenalidomide and Bortezomib. The median survival reported after conventional treatment is 3–4 years, while with high dose drugs along with ASCT, the median survival has been reported to be 5–7 years [1]. Some studies have shown that it may lead to improvements in terms of progression free survival and complete responses seen up to 40–50% in treated patients [5–7].
However, despite better health gains with treatment of multiple myeloma using ASCT, it comes at a higher cost. This cost has to be borne by either the households in the form of out-of-pocket expenditures (OOP) or by the Government. In case of OOP expenditures, it imposes significant financial barriers to treatment or results in financial catastrophe for the family. Nearly 1/3rd of all illness episodes for which care is not sought in rural India, financial barriers are cited as the reason [8]. Further, among those who get treated, high OOP expenditures lead to catastrophic outcomes and impoverishment. As against an episode of communicable disease, the risk of households facing catastrophic health expenditures increases by 170% in case of treatment for cancer [9].
In order to provide protection from high costs, Government of India as well as several State Governments have initiated programs for provision of free treatment. Under the National Program for Prevention and Control of Cancer, Diabetes, Cardiovascular Diseases and Stroke (2010), it is envisaged to support establishment of 20 State Cancer Institutes in 20 states and 50 Tertiary Cancer Care Centres in different parts of India [10]. This implies significant investments in cancer care. Further, several State Governments such as Andhra Pradesh, Tamil Nadu, Karnataka and Himachal Pradesh have initiated publicly financed cashless insurance schemes for free tertiary care treatment, including cancer, in public and private hospitals [11]. A scheme specifically designed for free treatment of cancer alone has been implemented in Punjab state. As a result, it is imperative to explore for cost-effectiveness of newer treatment options so as to justify the additional investments to be made.
Currently, there is no evidence from an economic viewpoint, to evaluate ASCT for treatment of multiple myeloma [12]. We aim to bridge this gap by assessing the cost effectiveness of ASCT as compared to conventional chemotherapy (based on Melphalan and Prednisolone regimen) for multiple myeloma. Specifically, we estimate the incremental cost per QALY gained with High Dose Treatment (HDT) i.e., High Dose Chemotherapy (HDC) and Autologous Stem Cell Transplantation (ASCT) as compared to Conventional Chemotherapy (CC) alone in patients of multiple myeloma in Indian settings.
Methodology
General Model Overview
A mathematical markov model along with decision tree was parameterised on an MS Excel spreadsheet to estimate the incremental cost effectiveness of High Dose Chemotherapy with Autologous Stem Cell Transplant as compared to Conventional Chemotherapy. Incremental costs and effects of Autologous Stem Cell Treatment were compared against the baseline scenario of Conventional Chemotherapy (Melphalan and Prednisolone regimen) in the patients of multiple myeloma. Future costs and consequences were discounted at 5% for time preferences of cost and utility [13]. Consequences were valued in terms of life years and quality adjusted life years (QALY) in both intervention and comparator scenarios. Clinical, cost and effectiveness parameters were used to model the lifetime costs and consequences for a hypothetical cohort of 1000 multiple myeloma patients, who could be treated by either of treatment regimens, using both the health system and societal perspective. Cost effectiveness was assessed by estimating incremental cost per QALY gained with treatment using ASCT as against CC.
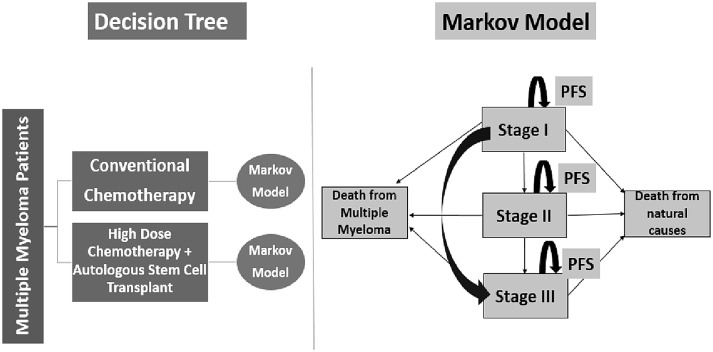
The markov model comprised of a finite number of health states to represent pre and post disease progression and death. Health states were modelled according to ISS staging for multiple myeloma, i.e., Stage-I, Stage-II and Stage-III [14]. Apart from it, two absorbing health states were also considered, i.e., death from multiple myeloma and death from natural causes. A conceptual framework of decision tree and markov model used in economic modelling is depicted in Fig. 1.
Fig. 1.
Conceptual framework of decision tree and Markov model for economic modelling. PFS refers to progression free survival in this figure
The markov model, which has been used in our analysis to undertake cost effectiveness, classically comprises of different health states which are used to denote the biologically plausible life-course of an individual who develops a given disease [15]. For modelling the multiple myeloma (MM) disease, we undertook an extensive review of literature to determine the markov states. There are two systems for staging MM. The Durie–Salmon (DS) Staging system, which has been in use since 1975, is one of the systems but this is gradually being replaced by an updated system, the International Staging System (ISS). This new system is based on measurement of two serum proteins, β2-microglobulin and albumin. A patient with stage I disease will not necessarily proceed linearly through disease stages. Stage III disease can be reached without a requirement to pass through stage II first [16, 17].
Clinical and Epidemiological Parameters
A review of literature supplemented with expert opinion was undertaken to determine the clinical parameters such as overall survival and progression free survival with the two treatment options (Table 1). Markov state transition probabilities of moving from one health state to another were computed using date on progression free survival, while the probability to die due to multiple myeloma was computed using the overall survival [18, 19].
Table 1.
Epidemiological parameters used in Markov Model for valuing consequences
| Parameters | High dose chemotherapy + autologous stem cell transplant | Conventional chemotherapya | ||
|---|---|---|---|---|
| Base valueb | Source | Base value | Source | |
| Overall survival (median in months) | ||||
| ISS Stage I | 67 | Kumar et al. [18] | 58.8 | Ludwig et al. [19] |
| ISS Stage II | 79 | 42 | ||
| ISS Stage III | 20 | 26.4 | ||
| Progression free survival (median in months) | ||||
| ISS Stage I | 32 | Kumar et al. [18] | 24 | Primary analysis of PGI Data |
| ISS Stage II | 75 | 18 | ||
| ISS Stage III | 16 | 12 | ||
aConventional chemotherapy refers to Melphalan and Prednisone regimen
bBase value represent epidemiological parameters based on extensive review of literature, used in the model to form part of our base case analysis
Patients in the initial year are placed in different stages, based on the existing evidence on stage-wise distribution of multiple myeloma patients in India [20]. Subsequently, patients were modelled to move on to other states if their condition worsens or if they have any adverse event. Each of the health state is assigned a utility score based on review of literature. For stage I, II and III, the quality of life scores was assumed as 0.865, 0.660 and 0.501 respectively [21, 22].
Cost of Treatment: ASCT and Conventional Chemotherapy
Cost of treatment of multiple myeloma patients was estimated for both the arms—ASCT and conventional chemotherapy in Post Graduate Institute of Medical Education and Research—a tertiary care hospital. Both health system cost and OOP expenditures were estimated. In order to assess the health system cost, a bottom-up approach was followed to determine all resources used to provide treatment to patients with multiple myeloma [23, 24]. Adaptation of standard methods of economic costing [25] and which have been used elsewhere for costing studies in India were used for the present study [26–30]. Specific services provided to multiple myeloma patients i.e., ASCT in bone marrow centre, outpatient consultation, inpatient hospitalization in a general ward as well as intensive care unit (or high dependency unit), dialysis, conventional chemotherapy etc. were identified and its cost assessed. Unit cost for each of these services was estimated.
Data was collected on human resources and their time allocation for delivery of care to multiple myeloma patients, building and space, equipment, drugs and consumables, other non-consumables and overheads. Besides the quantity of resources consumed during a 1 year period from March 2015 to April 2016, data on their prices was obtained from the procurement department of the institute. Life of the capital items was assessed by interviewing the staff members. Annual number of patients who sought treatment for each service during study period was estimated from respective departmental records. Annualized cost of capital items was estimated based on its useful life and discounting the future cost at 5% [13]. Joint costs were apportioned for specific service based on appropriate apportioning statistics.
Out-of-pocket expenditures were assessed for specific services by interviewing the patients who received a service—outpatient consultation, hospitalization, dialysis, ASCT and chemotherapy; during the period of data collection. In case of ASCT, we also interviewed all patients who had received the procedure during the last 1 year. A total of 26 and 108 patients who were administered CC and dialysis respectively were interviewed to assess the OOP costs. Five of the total fourteen patients who had undergone ASCT in the last 1 year were interviewed to assess OOP expenditure for ASCT. Structured interview schedules were used to elicit data on OOP expenditure for drugs, diagnostics, user charges (including consultation, hospitalization or procedure), travel, boarding and lodging, food etc. [31–34]. Data from the 71st round of National Sample Survey on Morbidity and Cost of Care was analysed in order to assess the OOP expenditure for an outpatient consultation and hospitalization [8].
All costs are reported in Indian National Rupee (INR) and US Dollars (USD) using the average conversion of 1 USD = 65 INR in 2015 [35].
Valuation of Consequences and Cost Effectiveness Analysis
Estimation of lifetime costs and health consequences resulting from both treatment modalities was done using a combination of decision tree and markov model. The efficacy of two treatment options was assessed in terms of overall and progression free survival rates based on a review of literature. Together, the survival rates were used to determine the transition probabilities. Health related quality of life utility values were assigned to each of health states from literature review [21, 22]. The model estimated lifetime incremental cost and benefits of treatment from both health system and societal perspective. Future costs and benefits were discounted at a rate of 5% for time preferences of cost and utility. Cost effectiveness was assessed in terms of incremental cost per QALY gained using ASCT versus CC for multiple myeloma treatment. Various parameters and assumptions used for analysis are mentioned below in Table 2.
Table 2.
Demographic, disease progression and treatment parameters used in the model
| Base value | Lower limit | Upper limit | Source of estimate (see reference list) | |
|---|---|---|---|---|
| Discount rate | 0.05 | 0.03 | 0.08 | [13] |
| Median age of onset | 50 | 34 | 65 | Estimates from hospital records |
| Quality of life parameters | [22] | |||
| Stage I | 0.865 | 0.78 | 0.88 | |
| Stage II | 0.66 | 0.617 | 0.747 | |
| Stage III | 0.501 | 0.404 | 0.548 | |
| Disease progression parameters | ||||
| Incidence of multiple myeloma in India | 0.7 | 0.3 | 1.2 | [36] |
| Proportion of multiple myeloma patients reporting at study hospital in stage I | 0.148 | 0.1184 | 0.1776 | Author estimates from hospital data |
| Proportion of multiple myeloma patients reporting at study hospital in stage II | 0.426 | 0.3408 | 0.5112 | |
| Proportion of multiple myeloma patients reporting at study hospital in stage III | 0.426 | 0.3408 | 0.5112 | |
| Probability of Stage-I patient to progress to Stage-II in conventional chemotherapy | 0.125 | 0.1 | 0.15 | Author estimates from hospital data |
| Probability of Stage-I patient to progress to Stage-III in conventional chemotherapy | 0.125 | 0.1 | 0.15 | |
| Probability of Stage-II patient to progress to Stage-III in conventional chemotherapy | 0.33 | 0.27 | 0.4 | |
| Probability of Stage-I patient to progress to Stage-II in ASCT | 0.09375 | 0.075 | 0.1125 | Author estimates based on [16 , 18] |
| Probability of Stage-I patient to progress to Stage-III in ASCT | 0.09375 | 0.075 | 0.1125 | |
| Probability of Stage-II patient to progress to Stage- III in ASCT | 0.08 | 0.064 | 0.096 | |
| Death | ||||
| Probability of Stage-1 patient to die from multiple myeloma in conventional chemotherapy | 0.10204 | 0.05102 | 0.15306 | Author estimates based on [19] |
| Probability of Stage-2 patient to die from multiple myeloma in conventional chemotherapy | 0.14285 | 0.07142 | 0.21428 | |
| Probability of Stage-3 patient to die from multiple myeloma in conventional chemotherapy | 0.22727 | 0.11363 | 0.34090 | |
| Probability of Stage-1 patient to die from multiple myeloma in ASCT | 0.089552 | 0.04477 | 0.13432 | Calculations based on [18] |
| Probability of Stage-2 patient to die from multiple myeloma in ASCT | 0.075949 | 0.03797 | 0.11392 | |
| Probability of Stage-3 patient to die from multiple myeloma in ASCT | 0.3 | 0.15 | 0.45 | |
| Progression free survival | ||||
| Probability of PFS in ISS Stage I in chemotherapy patients | 0.25 | 0.125 | 0.375 | Author estimates based on hospital records |
| Probability of PFS in ISS Stage II in chemotherapy patients | 0.33333 | 0.16666 | 0.5 | |
| Probability of PFS in ISS Stage I in ASCT | 0.1875 | 0.16666 | 0.07832 | Calculations based on [18] |
| Probability of PFS in ISS Stage II in ASCT | 0.08 | 0.16666 | 0.07832 | |
| Treatment pattern parameters | ||||
| Proportion of multiple myeloma patients reporting with renal failure | 0.75 | 0.52 | 0.88 | [37] |
| Proportion of multiple myeloma patients requiring Dialysis before treatment | 0.4125 | 0.33 | 0.495 | Author estimates based on expert opinion and hospital records |
| Proportion of multiple myeloma patients requiring Plasmapheresis before treatment | 0.4125 | 0.33 | 0.495 | |
| Average number of cycles of chemotherapy in a year in CC and ASCT group | 6 | 3 | 9 | |
| For CC group | ||||
| Proportion of patients requiring dialysis in one year | 0.1 | 0.05 | 0.15 | |
| Average number of dialysis cycles required in one year in CC group | 17 | 8.5 | 25.5 | |
| Proportion of patients requiring plasmapheresis in one year | 0.5 | 0.25 | 0.75 | |
| Average number of Plasmapheresis cycles required in patient in one year | 5 | 2.5 | 7.5 | |
| Proportion of patients treated in OPD | 0.6 | 0.3 | 0.9 | |
| Proportion of patients treated in IPD | 0.3 | 0.15 | 0.45 | |
| Proportion of patients treated in HDU ICU | 0.1 | 0.05 | 0.15 | |
| Proportion of patients developing relapse in one year | 0.254 | 0.127 | 0.381 | |
| For ASCT group | ||||
| Proportion of patients requiring dialysis in one year | 0.05 | 0.025 | 0.075 | |
| Average number of dialysis cycles required in patient in one year | 17 | 8.5 | 25.5 | |
| Proportion of patients treated in OPD | 0.8 | 0.4 | 1.2 | |
| Proportion of patients treated in IPD | 0.1 | 0.05 | 0.15 | |
| Proportion of patients treated in HDU | 0.1 | 0.05 | 0.15 | |
| Proportion of patients developing relapse in one year | 0.08466 | 0.042333 | 0.127 | |
| Cost parameters | ||||
| Per patient total (health system + OOP) expenditure on chemotherapy | 62,785 | 31,392.5 | 94,177.5 | Author estimates based on primary costing survey and analysis of NSSO 71st Round [8] |
| Per patient total expenditure on ASCT | 395,527 | 197,763.5 | 593,290.5 | |
| Per Patient total cost of OPD visit | 6342 | 3171 | 9513 | |
| Per patient total cost for treatment in IPD | 47,350 | 23,675 | 71,025 | |
| Per patient total cost for treatment in HDU ICU | 99,808 | 49,904 | 149,712 | |
| Per patient health system cost of dialysis | 3974 | 1987 | 5961 | |
| Per patient OOP expenditure on dialysis in chemotherapy | 2838 | 2203 | 3546 | |
| Per patient total expenditure on dialysis | 6812 | 4190 | 9507 | |
| Per patient health system cost of plasmapheresis in chemotherapy | 2402 | 1201 | 3603 | [38] |
| Per patient OOP expenditure on plasmapheresis | 16,396 | 8198 | 24,594 | |
| Per patient total expenditure on plasmapheresis | 18,798 | 9399 | 28,197 | |
| Per patient health system cost of treating bone complications | 19,648.8 | 9824.4 | 29,473.2 | [39] |
Sensitivity Analysis
Uncertainties in parameters and model structure were assessed in a sensitivity analysis. A scenario analysis was undertaken to determine the cost effectiveness of ASCT, if all patients are diagnosed early and initiated therapy, as against the base scenario of current mix of stage-wise distribution at the time of detection. Secondly, while the base case used Indian evidence on effectiveness, in an alternate scenario analysis we used the international evidence on overall survival rates with ASCT and CC. The effect of uncertainty in parameter values on overall incremental cost effectiveness ratio (ICER) was assessed using a univariate sensitivity analysis. Discount rate was varied from 3 to 8%.
Effect of joint parameter sensitivity was analysed by applying a probabilistic sensitivity analysis. Probability of ASCT program to remain cost effective at a willingness to pay threshold equal to per capita gross domestic product (GDP) and 3-times the GDP per capita was estimated, using a health system and societal perspective. For undertaking PSA analysis, we used log-normal distribution for cost parameters; and beta distribution for parameters related to overall and progression free survival. For rest of the parameters we used uniform distribution to simulate random values. Upper and lower bound were computed assuming a variation of 20% on either side of base estimate for disease progression and other clinical parameters, and 50% variation for risk of mortality, treatment patterns, cost parameters [40]. Monte Carlo method was used for simulating the results over 999 times. Median was computed along with 2.5th and 97.5th percentile to estimate 95% confidence interval.
Ethical Approval
The study was approved by the Institute Ethics Committee of the Post Graduate Institute of Medical Education and Research, Chandigarh.
Results
Cost of ASCT and Conventional Chemotherapy
Unit costs of providing specific services to multiple myeloma patients are presented in Table 3. The average cost of hospitalization in a bone marrow transplant centre was INR 395,527 (USD 6085), while it was INR 99,808 (USD 1535) in an ICU setting. While nearly 60% of the total cost of admission in BMT centre is borne out-of-pocket by patient, the share of cost borne by patient is only 37% in case of an ICU admission. The overall unit cost of an outpatient consultation, inpatient hospitalization and a dialysis procedure were estimated as INR 2114 (USD 32.5), INR 47,350 (USD 728.5) and INR 6812 (USD 104.8) respectively.
Table 3.
Unit cost for treatment provided to multiple myeloma patients
| Cost centre | Cost per patient, INR (USD) | ||
|---|---|---|---|
| Health system | Out of Pocket (OOP) | Total | |
| Bone marrow transplant centre | 1,60,027 (USD 2462) | 235,500 (USD 3623) | 395,527 (USD 6085) |
| High dependency unit (intensive care setting) | 62,565 (USD 963) | 37,243 (USD 573)a | 99,808 (USD 1535.5) |
| IPD hospitalization | 10,107 (USD 155) | 37,243 (USD 573) | 47,350 (USD 728.5) |
| Outpatient visit | 510 (USD 7.84) | 1604 (USD 24.7)b | 2114 (USD 32.5) |
| Dialysis | 3974 (USD 61.1) | 2838 (USD 43.6) | 6812 (USD 104.8) |
aAverage total OOP expenditure on hospitalization at public hospital in Chandigarh in NSSO 71st Round
bAverage medical OOP on per outpatient visit for cancers in last 15 days in NSSO 71st Round
Based on these unit costs, we estimated that the overall per patient cost of treatment using ASCT and conventional chemotherapy is INR 500,631 (USD 7702) and INR 159,775 (USD 2458) respectively (Table 3). However, considering the overall life of the patient, and after accounting for cost of treating failure or complication, cost per quality adjusted life year for a patient who undergoes treatment using ASCT and conventional chemotherapy is INR 160,922 (USD 2476) and INR 157,438 (USD 2422) respectively.
Cost Effectiveness
Following the pattern of actual distribution of patients at time of diagnosis of disease, a total of 819 out of 1000 cohort patients survived at the end of first year, 658 patients at second year and 319 patients at end of 5 years in CC group based on our model analysis. While for ASCT group, 818 patients out of 1000 cohort survived at the end of first year, 671 at second year and 374 patients at end of 5 years in the model analysis.
Quality adjusted life year lived by a multiple myeloma patient using ASCT and conventional chemotherapy patient was estimated to be 4.1 and 3.5 years respectively. Estimates of incremental cost per life year gained and per quality adjusted life year gained are presented in Table 4. From a societal perspective, ASCT incurs an incremental cost of INR 334,433 (USD 5245) per QALY gained as against treatment with conventional chemotherapy. Similarly, using a health system perspective incremental cost per QALY gained with ASCT is INR 263,440 (USD 4053).
Table 4.
Costs, effects and cost-effectiveness of ASCT versus conventional chemotherapy for treatment of multiple myeloma
| Outcome parameters | Scenarios | |
|---|---|---|
| HDC + ASCT | Conventional chemotherapy | |
| Costs, INR (USD) | ||
| Cost per patient per life year | 119,740 (USD 1842) | 111,565 (USD 1716.4) |
| Cost per patient per QALY | 160,922 (USD 2476) | 157,438 (USD 2422) |
| Costs per patient for initial treatment | 500,631 (USD 7702) | 159,775 (USD 2458) |
| Consequences | ||
| Life year per patient | 5.5 | 4.9 |
| QALY per patient | 4.1 | 3.5 |
| Incremental cost [INR (USD)] per QALY gained | ||
| Base scenario (current patient mix) | 3,34,433 (USD 5245) | |
| Early therapy | 1,80,434 (USD 4053) | |
Sensitivity Analysis
If the patients are detected early in stage I and initiated for therapy, the health system will spend an extra INR 180,434 per QALY gained for treatment with ASCT, which is nearly 1.5 times the GDP per capita. The incremental cost per QALY gained from a societal perspective was estimated to be INR 193,270 (1.6 times GDP per capita) for early therapy.
In the base scenario, we assumed gains in survival from ASCT based on Indian evidence which was lower than what has been reported in international literature. In case the latter is assumed as gains in survival rates, ASCT was found to be significantly more cost effective—incremental cost per QALY of INR 212,414 (1.76 times GDP per capita).
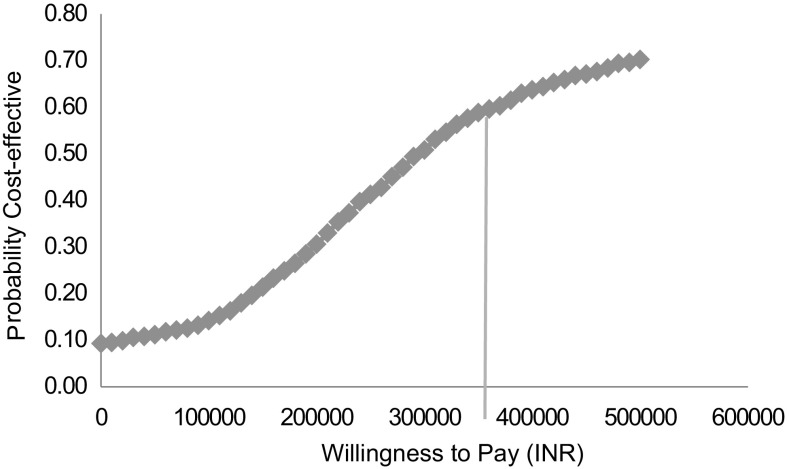
The incremental cost effectiveness ratio varied from INR 266,934 (USD 4106) to INR 447,162 (USD 6879) when the discount rate was varied from 3 to 8% respectively. Probability of ASCT to be cost effective at a threshold of GDP per capita and 3-times the GDP per capita was found to be 16 and 59% respectively (Fig. 2).
Fig. 2.
Cost effectiveness acceptability curve at different willingness to pay
Discussion
We undertook this study to estimate the cost effectiveness of treating multiple myeloma in a public sector setting in India, using autologous stem cell transplant (along with high dose chemotherapy) as compared to conventional chemotherapy. Lifetime costs associated with treatment of multiple myeloma in the two alternate therapies were estimated and compared with gains in terms of overall and progression free survivals. Overall, we found that the ASCT incurs an incremental cost of INR 334,433 (USD 5245) per QALY gained, as compared to conventional chemotherapy. The approach suggested by the Commission for Macroeconomics on Health (2001) and the World Health Organization (2005) is that interventions with an incremental cost less than the per capita GDP in low middle income countries (LMICs) are “very cost effective”, and those costing less than triple the per capita GDP are “cost-effective”. India had a GDP per capita of USD 1805 (INR 117,325) in 2015. At per capita GDP threshold, the incremental cost of ASCT suggests that it is not cost effective in Indian context. If one considers the threshold to be 3-times the GDP per capita, ASCT is marginally cost effective. However, given the uncertainties in current evidence, the probability for ASCT to be cost effective even at a 3-times the GDP per capita threshold is 59%.
We undertook several sensitivity and scenario analyses. In the base scenario, we had assumed the distribution of patients with multiple myeloma in ISS different stages based on the current patterns of detection of disease. In the first scenario analysis, we estimated the cost effectiveness of ASCT if all patients are detected and treated in Stage I. In such as case, ASCT becomes much more cost effective, with an incremental cost of INR 180,434 per QALY gained. Secondly, we had used Indian evidence on survival gains with ASCT which does not show too much difference as compared to conventional chemotherapy. Based on this evidence, our model estimates that the life years per patient treated with ASCT and conventional chemotherapy were 5.5 and 4.9 years respectively. In case international evidence on overall survival with ASCT is assumed, it is found to incur an incremental cost per QALY of INR 212,414.
As per our knowledge, this is the first study to report on the cost-effectiveness of multiple myeloma treatment using ASCT. We used a decision model which is plausible based on the current understanding of the disease progression and its outcomes. As far as possible, Indian evidence on epidemiology, clinical effectiveness in terms of overall and progression free survival and cost of care was taken. For costing, a primary study was undertaken in a tertiary care hospital to assess the unit health system cost as well as OOP expenditures for different services. We also performed a detailed sensitivity analysis in order to account for the effect of uncertainties in parameters and other assumptions.
A comparative analysis of different modelling methodologies for assessing cost effectiveness of treating multiple myeloma had reported two classical type of models. These include the SHTAC model and the Janssen-Cilag model. While the former used 3 health states, the Janssen-Cilag model used a model with 4 health states defined by disease progression and occurrence of adverse effects. Both the models use a cohort of newly diagnosed myeloma patients treated with alternate options. The models used a survival analysis approach to model the transition probabilities using data on overall survival (OS) and progression-free survival (PFS) for each of the interventions for a patient with newly diagnosed MM. We have also followed the similar methodology for modelling [17].
However, there are several data limitations based on which more work needs to be undertaken in this area of research to determine robust estimates. Firstly, we did not obtain data on quality of life from Indian studies. This would be a critical area where more work needs to be done. Secondly, the data on survival rates are based on analysis of data from a single centre in India. Moreover, this is also based on experience of treating for about 7–8 years. Further, research with head-to-head comparison between ASCT and conventional chemotherapy in terms of overall and progression free survival is recommended. Thirdly, our estimates on cost are based on a single public sector hospital. We do acknowledge that there can be significant variations in the costing in different centres. Moreover, the costs in private sector are entirely different and likely to be higher than what it costs in public sector. In view of this, ASCT is likely to be even less cost effective in such as setting.
Our findings hold significant importance for the purchasing of care under the publicly financed health insurance schemes. The findings suggest that there is little economic argument in treating all multiple myeloma cases with ASCT. Instead, there is a greater value for money if these patients are treated using conventional chemotherapy. Role of ASCT should be limited to only those cases which are detected early in Stage I and therapy started immediately. Secondly, there is a need to undertake greater clinical research in this field to estimate with more robustness, parameters pertaining to epidemiology and clinical effectiveness of treatment.
Compliance with Ethical Standards
Conflict of interest
None.
References
- 1.Raab M, Podar K, Breitkreutz I, Richardson P, Anderson K. Multiple myeloma. Lancet. 2009;374:324–339. doi: 10.1016/S0140-6736(09)60221-X. [DOI] [PubMed] [Google Scholar]
- 2.Kumar L, Vikram P, Kochupillai V. Recent advances in the management of multiple myeloma. Natl Med J India. 2006;19:80. [PubMed] [Google Scholar]
- 3.Ghalaut P, Chaudhuri S, Singh R (2013) Recent advances in diagnosis and management of multiple myeloma: an update. The Association of Physicians of India, Medicine Update, pp 360–365
- 4.Agarwal M (2016) Multiple myeloma: treatment is getting individualized. Indian Soc Haematol Transfus Med 32(1) [DOI] [PMC free article] [PubMed]
- 5.Attal M, Harousseau J, Stoppa A, Sotto E, Fuzibet J, Rossi J, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. N Engl J Med. 1996;335(2):91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 6.Child J, Morgan G, Davies F, Owen R, Bell S, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348(19):1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 7.Koreth J, Cutler C, Djulbegovic B, Behl R, Schlossman R, Munshi N, et al. High-dose therapy with single autologous transplantation versus chemotherapy for newly diagnosed multiple myeloma: a systematic review and meta-analysis of randomized controlled trials. Biol Blood Marrow Transplant. 2007;13:183–196. doi: 10.1016/j.bbmt.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 8.NSSO . Key indicators of social consumption in India Health NSS 71st Round (January–June 2014) New Delhi: National Sample Survey Organization, Ministry of Statistics and Programme Implementation, Government of India; 2015. [Google Scholar]
- 9.Mahal A, Karan A, Engelau M. The economic implications of non-communicable disease for India. Washington: World Bank; 2010. [Google Scholar]
- 10.Twelfth Five Year Plan (2012–2017) Social sectors, volume III. SAGE Publications India Pvt Ltd. http://planningcommission.gov.in/plans/planrel/12thplan/pdf/12fyp_vol3.pdf
- 11.PHFI . A critical assessment of the existing health insurance models in India. New Delhi: Public Health Foundation of India; 2011. [Google Scholar]
- 12.Prinja S, Chauhan AS, Angell B, Gupta I, Jan S. A systematic review of the state of economic evaluation for health care in India. Appl Health Econ Health Policy. 2015;13(6):595–613. doi: 10.1007/s40258-015-0201-6. [DOI] [PubMed] [Google Scholar]
- 13.ISPOR (2016) Pharmacoeconomics & outcomes research guidelines for India PEOR guidelines [Internet]. https://www.ispor.org/consortiums/asia/PEGuidelines_India_March2016.pdf
- 14.Greipp P, Miguel J, Durie B, Crowley J, Barlogie B, Bladé J, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 15.Fesenfeld M, Hutubessy R, Jit M. Cost-effectiveness of human papilloma virus vaccination in low and middle income countries: a systematic review. Vaccine. 2013;31:3786–3804. doi: 10.1016/j.vaccine.2013.06.060. [DOI] [PubMed] [Google Scholar]
- 16.Picot J, Cooper K, Bryant J, Clegg AJ (2011) The clinical effectiveness and cost-effectiveness of bortezomib and thalidomide in combination regimens with an alkylating agent and a corticosteroid for the first-line treatment of multiple myeloma: a systematic review and economic evaluation. Health Technol Assess 15(41):23–47 [DOI] [PMC free article] [PubMed]
- 17.Cooper K, Picot J, Bryant J, Clegg A. Comparative cost-effectiveness models for the treatment of multiple myeloma. Int J Technol Assess Health Care. 2014;30(1):90–97. doi: 10.1017/S0266462313000615. [DOI] [PubMed] [Google Scholar]
- 18.Kumar L, Ghosh J, Ganessan P, Gupta A, Hariprasad R, Kochupillai V. High-dose chemotherapy with autologous stem cell transplantation for multiple myeloma: what predicts the outcome? Experience from a developing country. Bone Marrow Transplant. 2009;43(6):481–489. doi: 10.1038/bmt.2008.343. [DOI] [PubMed] [Google Scholar]
- 19.Ludwig H, Durie B, Bolejack V, Turesson I, Kyle R, Blade J, et al. Myeloma in patients younger than age 50 years presents with more favorable features and shows better survival: an analysis of 10 549 patients from the International Myeloma Working Group. Blood. 2008;111(8):4039–4047. doi: 10.1182/blood-2007-03-081018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta M, Pal R, Tikoo D. Multiple myeloma: the disease and its treatment. Int J Basic Clin Pharmacol. 2013;2(2):103–121. doi: 10.5455/2319-2003.ijbcp20130302. [DOI] [Google Scholar]
- 21.Van Agthoven M, Segeren C, Buijt I, Uyl-de Groot C, van der Holt B, Lokhorst H, et al. A cost-utility analysis comparing intensive chemotherapy alone to intensive chemotherapy followed by myeloablative chemotherapy with autologous stem-cell rescue in newly diagnosed patients with stage II/III multiple myeloma. Eur J Cancer. 2004;40(8):1159–1169. doi: 10.1016/j.ejca.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 22.Proskorovsky I, Lewis P, Williams C, Jordan K, Kyriakou C, Ishak J, et al. EORTC QLQ-C30 and QLQ-MY20 to EQ-5D in patients with multiple myeloma. Health and Quality of Life Outcomes. 2014;12(1):35. doi: 10.1186/1477-7525-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beck EJ, Harling G, Gerbase S, DeLay P. The cost of treatment and care for people living with HIV infection: implications of published studies, 1999–2008. Curr Opin HIV AIDS. 2010;5(3):215–224. doi: 10.1097/COH.0b013e32833860e9. [DOI] [PubMed] [Google Scholar]
- 24.Hendriks ME, Kundu P, Boers AC, Bolarinwa OA, Te Pas MJ, Akande TM, et al. Step-by-step guideline for disease-specific costing studies in low- and middle-income countries: a mixed methodology. Glob Health Action. 2014;7:23573. doi: 10.3402/gha.v7.23573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adam T, Bishai D, Khan MM, Evans DB. Methods for the costing component of the multi-country evaluation of IMNCI. Geneva: Department of Child and Adolescent Health and Development, World Health Organization; 2004. [Google Scholar]
- 26.Prinja S, Gupta A, Verma R, Bahuguna P, Kumar D, Kaur M, Kumar R. Cost of delivering health care services in public sector primary and community health centres in North India. PLoS ONE. 2016;11(8):e0160986. doi: 10.1371/journal.pone.0160986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prinja S, Balasubramanian D, Jeet G, Verma R, Kumar D, Bahuguna P, Kaur M, Kumar R (2016) Cost of delivering secondary level health care services through public sector district hospitals in India. Indian J Med Res (forthcoming) [DOI] [PMC free article] [PubMed]
- 28.Prinja S, Jeet G, Verma R, Kumar D, Bahuguna P, Kaur M, Kumar R. Economic analysis of delivering primary health care services through community health workers in 3 North Indian States. PLoS ONE. 2014;9(3):e91781. doi: 10.1371/journal.pone.0091781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prinja S, Mazumder S, Taneja S, Bahuguna P, Bhandari P, Mohan P, Hombergh H, Kumar R. Cost of delivering child health care through community level health workers: how much does extra does IMNCI cost? J Trop Pediatr. 2013;59(6):489–495. doi: 10.1093/tropej/fmt057. [DOI] [PubMed] [Google Scholar]
- 30.Prinja S, Manchanda N, Mohan P, Gupta G, Sethy G, Sen A, Hombergh HVD, Kumar R. Cost of neonatal intensive care delivered through district level public hospitals in India. Indian Pediatr. 2013;50:765–772. doi: 10.1007/s13312-013-0234-6. [DOI] [PubMed] [Google Scholar]
- 31.Gupta I, Chowdhury S, Prinja S, Trivedi M. Out-of-pocket spending on out-patient care in india: assessment and options based on results from a district level survey. PLoS ONE. 2016;11(11):e0166775. doi: 10.1371/journal.pone.0166775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prinja S, Bahuguna P, Gupta R, Sharma A, Rana SK, Kumar R. Coverage and financial risk protection for institutional delivery: how universal Is provision of maternal health care in India? PLoS ONE. 2015;10(9):e0137315. doi: 10.1371/journal.pone.0137315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prinja S, Gupta R, Bahuguna P, Sharma A, Aggarwal AK, Phogat A, Kumar R (2016) A composite indicator to measure universal health care coverage in india: way forward for post-2015 health system performance monitoring framework. Health Policy Plann. pii:czw097 (Epub ahead of print) [DOI] [PubMed]
- 34.Prinja S, Jagnoor J, Chauhan AS, Aggarwal S, Nguyen H, Ivers R. Economic burden of hospitalization due to injuries in North India: A cohort study. Int J Environ Res Public Health. 2016;13:673–26. doi: 10.3390/ijerph13070673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.(2015) The economic times: forex rates. http://economictimes.indiatimes.com/markets/forex
- 36.GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide 2012: international agency for research on cancer. http://globocan.iarc.fr/Pages/factsheetspopulation.aspx
- 37.Prakash J, Niwas SS, Parekh A. Multiple myeloma–presenting as acute kidney injury. J Assoc Physicians India. 2009;57:23–6. [PubMed] [Google Scholar]
- 38.Maheshwari A (2014) Cost effectiveness of treatment of guillain barre syndrome with iv immunoglobulins versus plasmapharesis [MD thesis]. PGIMER, Chandigarh
- 39.Sangwan A (2015) Cost of trauma care in secondary and tertiary care public sector hospitals in north india [MPH thesis]. PGIMER, Chandigarh [DOI] [PubMed]
- 40.Kouroukis CT, O’brien BJ, Benger A, Marcellus D, Foley R, Garner R, Ingram J, Haines C, Henderson-O’Connor R, Meyer P. Cost-effectiveness of a transplantation strategy compared to melphalan and prednisone in younger patients with multiple myeloma. Leuk Lymphoma. 2003;44(1):29–37. doi: 10.3109/10428190309178811. [DOI] [PubMed] [Google Scholar]