Abstract
We combined cryopreservation of intact Drosophila larvae and electron tomography with comprehensive segmentation of key features to reconstruct the complete ultrastructure of a model glutamatergic synapse in vivo. Presynaptically, we detail a complex network of filaments that connects and organizes synaptic vesicles. We link the complexity of this synaptic vesicle network to proximity to the active zone cytomatrix, consistent with the model that these protein structures function together to regulate synaptic vesicle pools. We identify a net-shaped network of electron-dense filaments spanning the synaptic cleft that suggests conserved organization of transsynaptic adhesion complexes at excitatory synapses. Postsynaptically, we characterize a regular pattern of macromolecules that yields structural insights into the scaffolding of neurotransmitter receptors. Together, these analyses extend our understanding of the ultrastructural correlates of the molecular machines that regulate synaptic communication and reveal an unexpected level of conservation in the nanoscale organization of diverse glutamatergic synapses.
Keywords: neuromuscular junction, electron tomography, high-pressure freezing, active zone, presynaptic cytomatrix, synaptic vesicles, synaptic cleft, postsynaptic density
Introduction
Synapses are the fundamental units of signal transmission between cells in neural circuits. In presynaptic neurons, neurotransmitter release occurs at a specialized molecular machine termed the active zone. Neurotransmitter-containing synaptic vesicles are trafficked to the active zone membrane where Ca2+ influx triggers molecularly primed synaptic vesicles to fuse with the membrane and release their contents. Neurotransmitter released from the active zone diffuses across the narrow synaptic cleft to signal neurotransmitter receptors clustered on the postsynaptic cell. Thus, synaptic function depends critically on the coordinated organization of the presynaptic active zone, synaptic cleft, and postsynaptic specializations.
Ultrastructural studies have the potential to drive conceptual understanding of the formation, function, and plasticity of synapses. To date, these studies have primarily focused on presynaptic terminals where the morphological correlates of active zone function are most readily discerned at ultrastructural level. For example, synaptic vesicles were first observed in the 1950s and immediately informed models of quantal release (De Robertis and Bennett, 1955; Palay and Palade, 1955). Beyond the presynaptic terminal, morphological investigation has been hampered by the difficulty of discerning patterned structures from complex, protein-dense environments. Illustrating this point, many synapses are most easily identified by their postsynaptic specialization, commonly referred to as the postsynaptic density for its dense appearance in electron micrographs.
Much of our understanding of synaptic ultrastructure comes from thin-section electron microscopy of chemically fixed preparations (Bruckner et al., 2015; Torrealba and Carrasco, 2004; Sheng and Hoogenraad, 2007). More recently, electron tomography has been employed at multiple model synapses to resolve synaptic structure in three dimensions (Fernández-Busnadiego et al., 2010; Jiao et al., 2010; Helmprobst et al., 2015; Stigloher et al., 2011; Chen et al., 2008b; Rostaing et al., 2006; Szule et al., 2015; Perez de Arce et al., 2015; High et al., 2015; Linsalata et al., 2014; Burette et al., 2012; Chen et al., 2008a; 2011; Lučić et al., 2005). Further, rapid cryo-preservation of samples for electron microscopy has enabled the preservation and study of cellular features in as near-to-native state as possible (Korogod et al., 2015; Fernández-Busnadiego et al., 2011). However, cryo-preservation and electron tomography have been difficult to apply to endogenous synapses. The Drosophila larval neuromuscular junction (NMJ) is a genetically and experimentally accessible glutamatergic model synapse at which the molecular regulation of synapse function has been studied extensively. The recent application of high-pressure freeze/freeze substitution (HPF/FS) electron tomography to the Drosophila NMJ is already advancing our understanding of the structural underpinnings of presynaptic function (Jiao et al., 2010; Liu et al., 2011; Matkovic et al., 2013). Here, we have combined HPF/FS preparation of intact Drosophila larvae and electron tomography with comprehensive segmentation of motor synapses to reconstruct the complete ultrastructure of this model synapse in vivo. We present a detailed investigation of the presynaptic terminal, synaptic cleft and postsynaptic density, and discuss the organizational themes that emerge from their ultrastructural characterization.
Results and Discussion
The active zone cytomatrix clusters interconnected synaptic vesicles
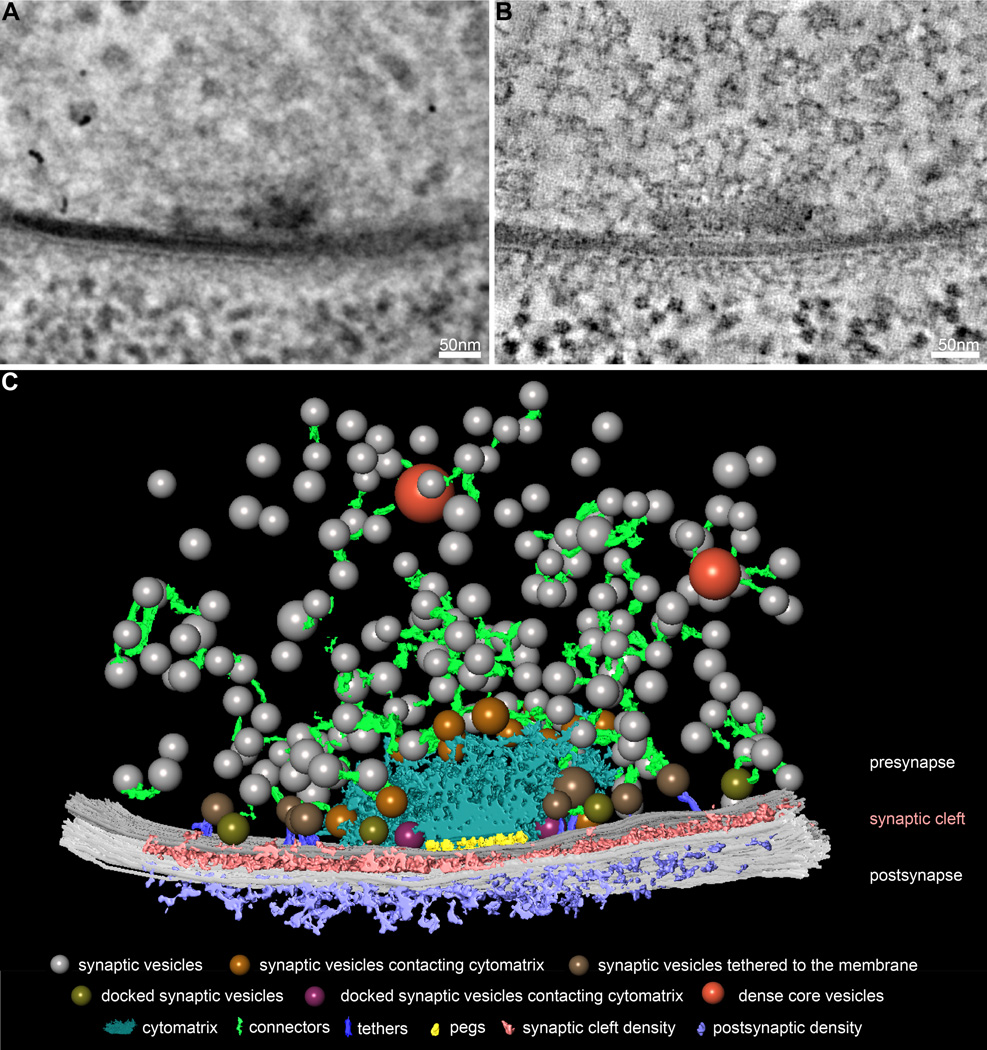
We collected double-tilt series of 250 nm sections from intact second-instar Drosophila larvae, and computationally reconstructed tomograms of motor synapses to obtain a 3D view with nanometer resolution (Figure 1A, B). Using semi-automatic segmentation of synaptic features in tomogram virtual slices, we identified key features and structural patterns of the presynaptic terminal, synaptic cleft, and postsynaptic density (Figure 1C). The most prominent features of Drosophila NMJ presynaptic terminals are the active zone cytomatrix and synaptic vesicles. As has been previously observed, the electron-dense active zone cytomatrix is a filamentous structure that extends for approximately 150 nm in each direction from the active zone center (Figure 1C, blue structure; Fouquet et al., 2009; Jiao et al., 2010). The many neurotransmitter-containing synaptic vesicles found at presynaptic terminals can be separated into distinct morphological classes, including vesicles contacting the active zone cytomatrix (Figure 1C, gold spheres), vesicles linked to the presynaptic membrane by long tethers (Figure 1C, brown spheres), vesicles directly contacting the presynaptic membrane (Figure 1C, olive spheres), and vesicles contacting both the cytomatrix and the membrane (Figure 1C, magenta spheres). We also observe neuropeptide-containing dense core vesicles, distinguished by their electron-dense content and large diameter, at a subset of synapses (Figure 1C, orange spheres). In close agreement with previous studies, the average diameter of synaptic vesicles in our tomograms of cryopreserved synapses is 34.9 ± 0.1 nm (Figure 2A; Karunanithi et al., 2002; Daniels et al., 2006).
Figure 1.
Comprehensive three-dimensional reconstruction of synaptic ultrastructure at the Drosophila NMJ. (A) A representative electron micrograph of a Drosophila NMJ synapse from a central position during a 120° tilt series of a 250-nm section. (B) A representative virtual slice of the tomogram generated from the tilt series in A. (C) 3D model of segmented features of the Drosophila NMJ synapse in A and B, including synaptic vesicles (colored spheres), dense core vesicles (orange spheres), the active zone cytomatrix (blue), connectors (green), presynaptic membrane (dark gray), synaptic cleft density (pink), postsynaptic membrane (light grey), and postsynaptic density (purple). Among synaptic vesicles, we observe subsets contacting the active zone cytomatrix (gold spheres), linked to the presynaptic membrane by long tethers (brown spheres, purple filaments), directly contacting the presynaptic membrane (olive spheres), and contacting both the cytomatrix and presynaptic membrane (magenta spheres).
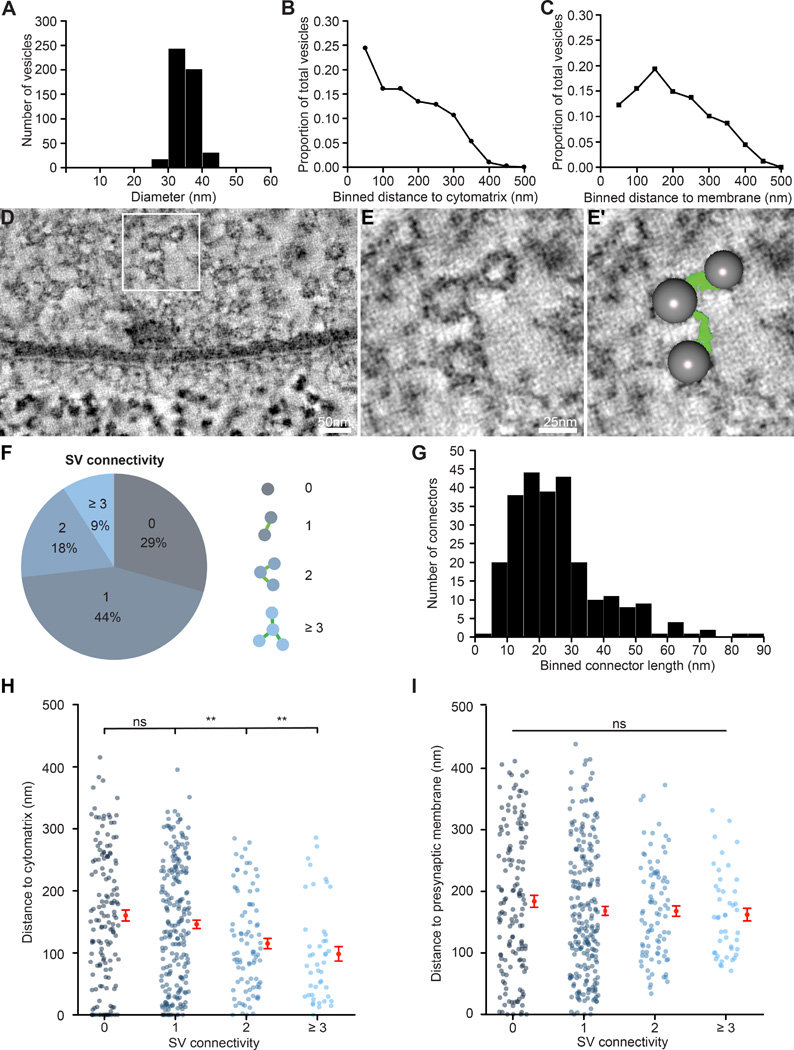
Figure 2.
Synaptic vesicles are linked by filamentous connectors and clustered at the active zone cytomatrix. (A) Histogram of synaptic vesicle diameter (mean 34.9 ± 0.1 nm, n = 497 vesicles from 2 synapses). (B,C) The proportion of total synaptic vesicles plotted in 50 nm bins relative to their distance to the active zone cytomatrix (B) or presynaptic membrane (C) (n = 497 vesicles from 2 synapses). (D–E′) Representative tomogram virtual slices (D,E) illustrating filamentous connectors linking synaptic vesicles clustered at the active zone, and the same virtual slice as in E with segmented features superimposed (E′). Panels E and E′ are magnifications of the boxed region in D. (F) Distribution of the number of connections linking each synaptic vesicle to its neighbors (n = 497 vesicles from 2 synapses). (G) Histogram of connector length (25.6 ± 0.9 nm, n = 253 connectors from 2 synapses). (H) Proximity to the active zone cytomatrix is significantly different between vesicles with higher-order vs. lower-order interconnectivity (1 vs. 0 connections, n.s., n = 219 and 145, respectively; 2 vs. 0 connections, p = 0.009, n = 88 and 145, respectively; 3 or more vs. 0 connections, p = 0.002, n = 45 and 145, respectively). (I) Proximity to the presynaptic membrane is not significantly different between vesicles with different levels of connectivity (p = 0.62). n.s. = not significant, ** p < 0.01. Kruskal-Wallis test followed by Dunn’s multiple comparisons tests. Error bars represent s.e.m.
The close association between the active zone cytomatrix and clustered synaptic vesicles has long suggested a key role for the cytomatrix in organizing synaptic vesicles at presynaptic terminals. To evaluate this model, we measured the distance from every visible synaptic vesicle to the active zone cytomatrix in fully segmented tomograms of two synapses. We observe a strong negative correlation between the density of synaptic vesicles and distance from the active zone cytomatrix (Figure 2B; Pearson’s correlation test: p < 0.0001, correlation coefficient = −0.97). We observe a similar correlation between the density of synaptic vesicles and distance from the active zone membrane with a peak in vesicle density between 100–150 nm from the membrane (Figure 2C; Pearson’s correlation test: p = 0.006, correlation coefficient = −0.83). Notably, this distance corresponds well with the extent of the active zone cytomatrix in these tomograms, consistent with a role for active zone cytomatrix in clustering synaptic vesicles near the active zone center.
A number of functional synaptic vesicle pools have been described (Alabi and Tsien, 2012). The readily releasable vesicle pool comprises those vesicles molecularly primed for fusion upon Ca2+ influx, while the recycling pool replenishes the readily releasable pool for rapid release. In contrast, vesicles in the reserve pool are only released upon sustained, high-frequency stimulation. The morphological correlates of these pools remain unknown, and there is evidence that the recycling and reserve pools are not spatially segregated in presynaptic terminals (Denker and Rizzoli, 2010; Rizzoli and Betz, 2005). The greatest progress has been made in correlating the functionally defined readily releasable pool with membrane-associated vesicles observed in ultrastructural studies. A consensus is converging on the model that vesicles either in direct contact with the active zone membrane or attached by multiple short filaments, thought to correspond to the molecular release apparatus, constitute the readily releasable pool (Bruckner et al., in press; Fernández-Busnadiego et al., 2010; 2013; Siksou et al., 2011; 2009; Imig et al., 2014). As this pool has been well characterized elsewhere, here we focus our analysis on the remaining synaptic vesicles.
In the bouton interior a complex network of filaments, termed connectors, links synaptic vesicles to each other and to the cytomatrix (Figure 2D–E, green filaments). This network of filaments was first observed in freeze-etch electron microscopy of mouse cerebellar synapses, and has now been observed in electron tomograms of rat hippocampal slices and cerebrocortical synaptosomes and C. elegans and zebrafish neuromuscular junctions (Rostaing et al., 2006; Landis et al., 1988; Fernández-Busnadiego et al., 2010; Helmprobst et al., 2015; Stigloher et al., 2011; Siksou et al., 2007). We find that over 70% of synaptic vesicles at Drosophila NMJ active zones are connected to at least one of their neighbors (Figure 2F). While the majority of vesicles are linked to one other vesicle, a subset exhibit higher order connectivity, with 9% of synaptic vesicles connected to three or more neighbors. This is in close agreement with the level of vesicle interconnectivity quantified at worm NMJs and rodent cerebrocortical synaptosomes, suggesting a conserved functional significance at both glutamatergic and cholinergic excitatory synapses (Stigloher et al., 2011; Fernández-Busnadiego et al., 2010).
We find that connectors average 25.6 ± 0.9 nm in length, and range from less than 2 to nearly 90 nm (Figure 2G). These measurements are very similar to observations in other model synapses, supporting conservation of their molecular composition (Siksou et al., 2007; Stigloher et al., 2011). The molecular identity of connectors remains unclear. Members of the Synapsin family of phosphoproteins were initially implicated; however, the persistence of connectors in Synapsin triple-knockout mice suggests that other molecules are involved (Hirokawa et al., 1989; Siksou et al., 2007). Diverse molecules have been shown to regulate the distribution of synaptic vesicles, including cytomatrix proteins and the vesicular SNARE protein Synaptobrevin, and are therefore compelling candidates (Fernández-Busnadiego et al., 2010; 2013; Ackermann et al., 2015).
Models have been proposed suggesting that connectors either limit or promote trafficking of synaptic vesicles to the presynaptic membrane for Ca2+-triggered fusion (Landis et al., 1988; Fernández-Busnadiego et al., 2010). Recent functional studies in rat synaptosomes demonstrate that the number of connectors is reduced by synaptic activity, and led to the model that connectors play a dual role in limiting dispersion of vesicles from presynaptic terminals and facilitating their mobilization upon stimulation – a function long hypothesized for the active zone cytomatrix as well (Fernández-Busnadiego et al., 2010; Shtrahman et al., 2005; Rizzoli and Betz, 2005; Ackermann et al., 2015). Interestingly, we find that synaptic vesicles with higher order connectivity reside significantly closer to the active zone cytomatrix than unconnected vesicles or vesicles linked only to a single neighbor (Figure 2H). In contrast, synaptic vesicle connectivity is not related to vesicle distance from the presynaptic membrane (Figure 2I). The specific correlation between the accumulation of connectors and proximity to the active zone cytomatrix supports the model that these structures act together to coordinate synaptic vesicles at the functional core of the active zone, but cannot distinguish roles in clustering and mobilization. Future genetic studies will be important for determining the molecular composition and function of connectors.
Arrayed organization of putative Ca2+ channel complexes beneath the active zone cytomatrix
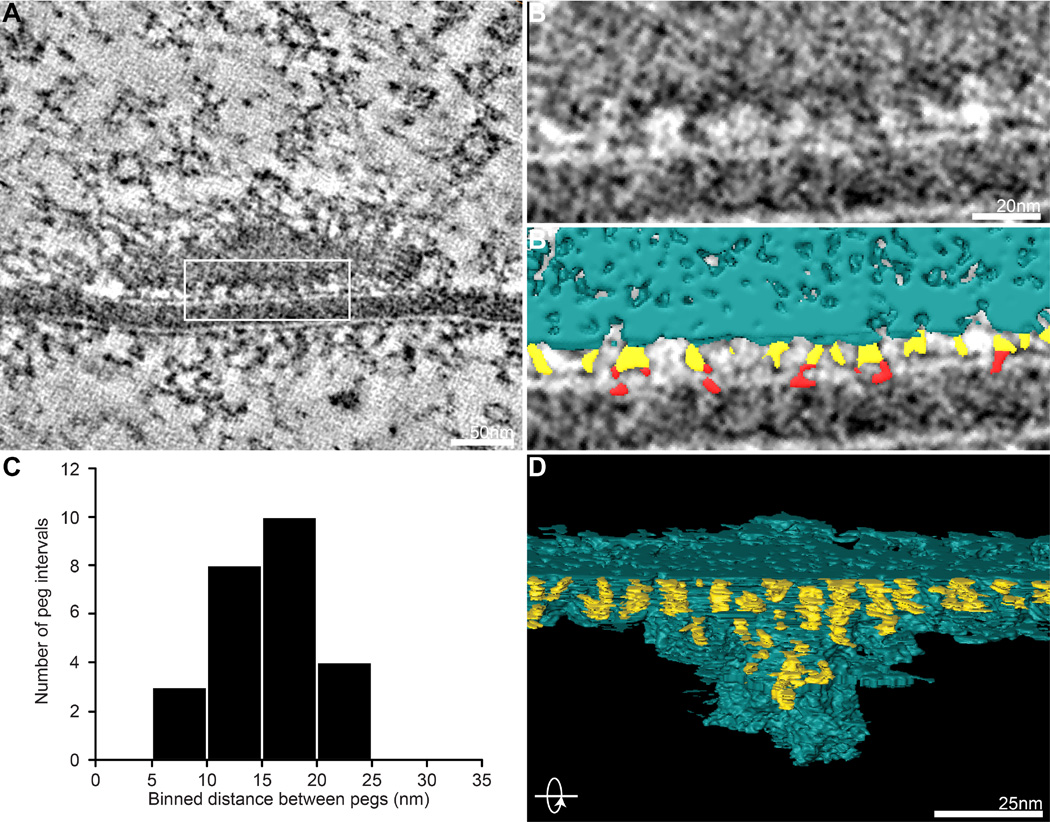
Voltage-gated Ca2+ channels open upon arrival of an action potential, resulting in local increases in Ca2+ concentration that trigger vesicle fusion. Thus, a key determinant of vesicle release probability is proximity to Ca2+ channels (Eggermann et al., 2012). At the cholinergic frog NMJ, detailed ultrastructural analysis indicates that a portion of the cytomatrix is attached to the presynaptic membrane by short ‘pegs’ proposed to be protein complexes that include Ca2+ channels (Harlow et al., 2001). Similar pegs have also been identified in tomographic reconstructions of mouse and Drosophila NMJs, and are consistent with light-level localization of Ca2+ channels (Nagwaney et al., 2009; Fouquet et al., 2009; Jiao et al., 2010). We observe similar structures separated by ~15 nm in virtual slices and 3D models of our tomograms, and can detect pegs penetrating the presynaptic membrane, consistent with the hypothesis that these complexes include Ca2+ channels (Figure 3A–C). Pegs become difficult to distinguish from the active zone cytomatrix at its core, but by focusing on less dense regions we were able to confidently segment pegs extending between the cytomatrix and presynaptic membrane. In 3D models rotated 90 degrees, it becomes apparent that pegs are organized in an array of regularly spaced rows (Figure 3D). It is interesting to hypothesize that an arrayed distribution of Ca2+ channels might yield an optimal range of coupling distances to vesicles docked around the perimeter of the active zone cytomatrix and beyond. Immuno-electron microscopy experiments to confirm the localization of Ca2+ channels in pegs, together with computational modeling of the release properties supported by such an arrangement will be important tools for taking advantage of this unique opportunity to elucidate the geometry of neurotransmitter release.
Figure 3.
An arrayed pattern of putative Ca2+ channel complexes. (A–B′) In representative tomogram virtual slices (A,B) and the same virtual slice with segmented features superimposed (B′), short ‘pegs’ (yellow) between the active zone cytomatrix (blue) and presynaptic membrane are visible and sometimes observed crossing the presynaptic membrane (red). (C) Histogram of the size of the interval between pegs, binned in 5 nm increments (n = 25 peg intervals from 4 synapses). (D) Rotating the 3D model 90° reveals that pegs are organized in a regularly spaced array beneath the active zone cytomatrix.
The synaptic cleft: a net-like organization of transsynaptic particles
The close proximity of pre- and postsynaptic cells is critical for rapid communication in neural networks. Consistently, we find that the width of the synaptic cleft is tightly controlled at the Drosophila NMJ, varying only slightly between 15.2 and 16.5 nm in 14 tomograms (average = 15.9 ± 0.09 nm). This distance agrees with previous observations at the Drosophila NMJ and is similar to the cleft width at glutamatergic synapses of the mammalian CNS (Zuber et al., 2005; Burette et al., 2012; Perez de Arce et al., 2015; Lučić et al., 2005; High et al., 2015; Koper et al., 2012). In contrast, cholinergic synapses of the mammalian NMJ exhibit very different synaptic clefts, measuring 60–70 nm in width with a prominent basement membrane that is not present at Drosophila NMJs (Koper et al., 2012).
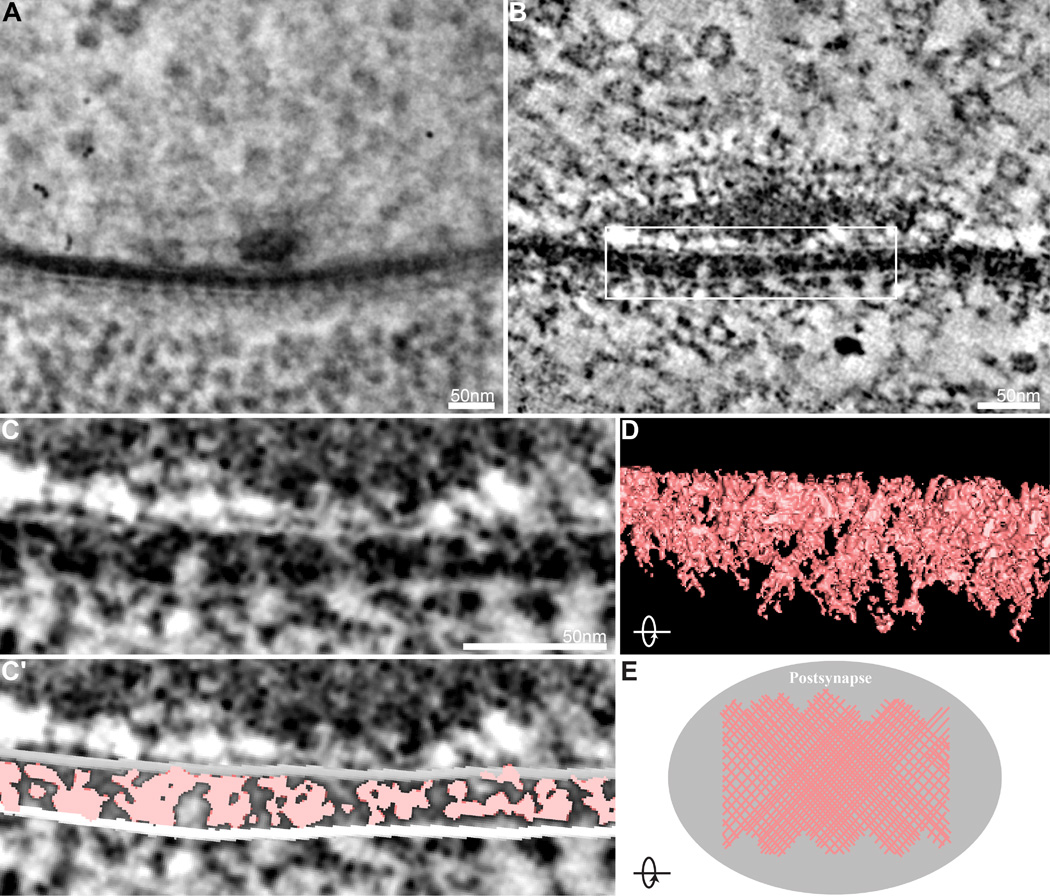
Although significant effort has been applied to the molecular and functional understanding of the transsynaptic adhesion molecules that connect pre- and postsynaptic cells, few studies have explored synaptic cleft ultrastructure due to the significant barrier posed by its size and complexity. Nonetheless, work in multiple models indicates that the synaptic cleft is a dense proteinaceous structure and hints at an underlying order (Lučić et al., 2005; Zuber et al., 2005; Burette et al., 2012; Perez de Arce et al., 2015; Ichimura and Hashimoto, 1988; High et al., 2015). A particularly compelling demonstration of an ordered ultrastructural arrangement of transsynaptic complexes comes from early studies of transverse sections of Drosophila embryonic and larval NMJs prepared and imaged using conventional EM techniques (Prokop, 1999). Here, regularly spaced clusters of dense material extended asymmetrically from the postsynaptic membrane part way across the cleft. In our tilt series, we observe similar densities with regular spacing of ~25 nm that fully span the cleft (Figure 4A–C). This difference likely reflects the different sample preparation and imaging approaches employed in the two studies. To assess the 3D organization of the synaptic cleft, we conducted semiautomatic segmentation of cleft electron densities in virtual slices. We find that the synaptic cleft is filled with electron-dense material arranged as a net-like scaffold of macromolecular complexes connecting the pre-and postsynaptic membranes that increases with proximity to the synapse center (Figure 4D, E). This organization is in remarkable agreement with the model proposed by Lučić and colleagues based on cryo-electron tomography of synaptosomes isolated from rat cerebral cortex and observations from tomograms prepared from chemically fixed hippocampal slices (Lučić et al., 2005; Burette et al., 2012). This suggests that a net-like network of transsynaptic molecules is a conserved organizational strategy at glutamatergic synaptic clefts, and points to the functional importance of this arrangement. A potential role for this regular arrangement is suggested by the recent demonstration of transsynaptic ‘nanocolumns’ that align presynaptic neurotransmitter release sites and postsynaptic neurotransmitter receptors (Tang et al., 2016).
Figure 4.
Net-like organization of synaptic cleft densities. (A) Representative electron micrograph of a Drosophila motor synapse from a central position during a 120° tilt series of a 250-nm section shows periodic organization of the synaptic cleft. (B) A representative virtual slice of the tomogram generated from the tilt series in A, where fine details of the transsynaptic ultrastructure are readily identified. (C,C′) Higher magnification of the boxed region of the tomogram virtual slice in B (C) and the same virtual slice with segmentation of synaptic cleft ultrastructure superimposed (C′). (D) Rotating the 3D model of the segmented synaptic cleft 90° reveals a net-like scaffold of interwoven molecules. (E) Top-view model of the organization of ultrastructural elements in the synaptic cleft, where the density of the net-like scaffold increases at the synapse core.
Lattice pattern of postsynaptic densities
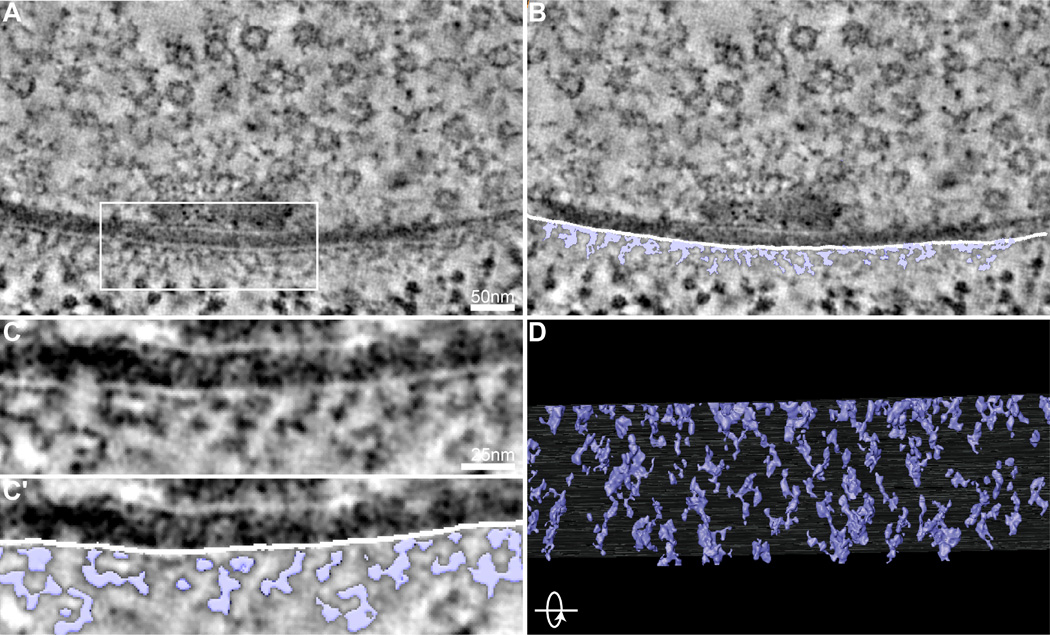
The tightly regulated, yet highly plastic, localization of neurotransmitter receptors is determined in large part by scaffolding molecules at postsynaptic densities (Feng and Zhang, 2009). Though numerous studies have addressed the localization of postsynaptic scaffolding proteins at the light level, combining this work with an ultrastructural spatial model of postsynaptic organization will yield insights into the mechanisms by which postsynaptic complexes promote synaptic function (Gold, 2012). However, the complexity of the mammalian postsynaptic density makes this a challenging task, and only a few studies have undertaken detailed characterization of postsynaptic organization. Postsynaptic densities of the Drosophila neuromuscular junction have less prominent postsynaptic densities than central glutamatergic synapses, facilitating the identification of discrete structures (Figure 5A–C).
Figure 5.
Lattice pattern of postsynaptic densities. (A,A′) Representative tomogram virtual slice (A) and the same virtual slice with segmentation of postsynaptic ultrastructural features superimposed (A′; purple). (B,B′) Higher magnification of the boxed region in A and A′. (C) Rotating the 3D model of segmented postsynaptic ultrastructure (purple) 90° reveals that the postsynaptic density is arranged as an ordered lattice-like scaffold beneath the postsynaptic membrane.
In virtual slices of our tomograms of Drosophila NMJ synapses, we observe a clear repeating network of electron-dense filaments linked to each other and the postsynaptic membrane (Figure 5A–C). A rotated view of the 3D model obtained by semi-automatic segmentation of these features reveals macromolecular complexes linked together in a loose lattice-like scaffold with a strikingly regular pattern (Figure 5D). This scaffold extends less than 50 nm from the postsynaptic membrane into the postsynaptic cytoplasm and excludes ribosomes observed more distally (Figure 5B–C). A subset of cytoplasmic electron densities appears to either insert into the postsynaptic membrane or extend laterally across it, suggesting this postsynaptic lattice may comprise both neurotransmitter receptors and the scaffolding molecules that regulate their localization (Figure 5B–C). Intriguingly, this structure is reminiscent of postsynaptic densities recently characterized in rat hippocampal slices (Burette et al., 2012). This suggests that the postsynaptic specializations at Drosophila NMJs are organized similarly to central glutamatergic synapses and is consistent with molecular studies demonstrating conservation of key scaffolding proteins (Budnik et al., 1996; Chen et al., 2011; Kim and Sheng, 2004; Sheng and Hoogenraad, 2007). The repeating lattice pattern hints at a physical model for how scaffolding proteins might regulate the lateral diffusion of neurotransmitter receptors within the postsynaptic membrane (Choquet and Triller, 2013). Further studies to delineate the molecular components of this postsynaptic lattice will be of great interest (Chen et al., 2008a; 2011).
Conclusions
By combining cryopreservation of intact Drosophila larvae and electron tomography with comprehensive segmentation of synaptic features, we have obtained a detailed view of glutamatergic synapse architecture in vivo. Presynaptically, we detail a complex network of filaments that connects and organizes synaptic vesicles. We link the complexity of this network of vesicles to proximity to the active zone cytomatrix, reinforcing the idea that these molecules function together to control synaptic vesicle clustering and/or mobilization. Within the synaptic cleft, we characterize a net of electron-dense filaments that yields insights into the conserved organization of transsynaptic adhesion complexes. Postsynaptically, we characterize the organization of scaffolding macromolecules, revealing the surprising similarity of these structures at diverse glutamatergic synapses. Together, these analyses extend our understanding of the ultrastructural correlates of the molecular machines that regulate neurotransmission at a genetically accessible endogenous synapse.
Materials and Methods
Fly stocks
All experiments were performed on w1118 (wild type) reared at 25°C prior to high-pressure freeze preservation for electron microscopy.
High-pressure freeze and freeze substitution
High-pressure freeze (HPF) and freeze substitution (FS) preservation techniques were adapted from previous work in C. elegans and Drosophila (Stigloher et al., 2011; Fouquet et al., 2009; McDonald et al., 2012; Gracheva et al., 2010; Rostaing et al., 2004). Individual late second-instar larvae were mounted in the cavity of aluminum specimen carriers with a depth of 200µm (Aluminum carrier type A 100/200, carrier type B 0/300, Technotrade International), filled with a mixture of 10% BSA and OP50 E. coli in PBS, and sealed with 1-Hexadecene (Sigma). Larvae were frozen in a Bal-Tec HPM 010 at a speed of >20,000 K/s and pressure >2000 bar. After freezing, samples were rapidly immersed in liquid nitrogen and carefully transferred to a Leica AFS1, where they were incubated in 0.5% glutaraldehyde, 0.1% tannic acid, and 1% H2O in anhydrous acetone for 98 hours at −90°C, and rinsed with 1% H2O in acetone for 2 hours at −90°C followed by incubation in 1% OsO4 and 1% H2O in acetone for 7 hours at −90°C. Samples were warmed to −20°C over 14 hours (5°C/hour), then incubated at −20°C for 16 hours. Temperature was increased to 4°C over 2.4 hours (10°/h). Finally, samples were rinsed with 1% H2O in acetone for 2 hours, transferred to room temperature, and embedded in Epon resin using standard procedures.
Electron tomography and tomogram reconstruction
250-nm sections were cut from three HPF-FS-prepared larvae, collected onto pioloform-coated copper slot grids, and stained with 2% aqueous uranyl acetate and Reynold’s lead. For fiducial-aided image alignment, grids were treated with 10-nm Aurion Gold solution (Electron Microscopy Sciences). Tilt acquisition of 14 synapses was conducted at 31,000× magnification using SerialEM software (Mastronarde, 2005) on a Tecnai TF-30 (FEI) transmission electron microscope with Gatan 2k × 2k ultrascan camera at 300 KeV in 1° increments from −60° to 60°. For the second tilt series each sample was rotated 90° and the series repeated. Virtual tomogram series were generated using eTOMO from the IMOD software package (Kremer et al., 1996).
Segmentation
Key protein structures of two tomograms were exhaustively segmented using the magic wand tool with the “labelfield” function in Amira 6.0.1 (FEI) to semi-automatically detect electron-dense structures. Synaptic vesicles were segmented using the Amira “landmark” function, where the size of the landmark was adjusted to the maximum diameter of the synaptic vesicle. Pre- and postsynaptic membranes were manually segmented using the brush tool and Amira “labelfield” function, with the width of the brush trajectory adjusted to fill the lipid double-layer of the membrane.
Quantification and statistics
Measurements of synaptic features were performed in Amira. Normality was tested using the Shapiro-Wilk test. Multiple comparisons were conducted via a Kruskal-Wallis test followed by Dunn’s multiple comparisons tests. Correlations were tested using Pearson’s product-moment correlation test.
Acknowledgments
We thank Ben August, Alex Kvit, the UW Medical School Electron Microscope Facility and the UW-Madison Materials Science Center for excellent technical support. We are grateful to Desiree Benefield, Janice Pennington, Marisa Otegui, Paul Ahlquist and Bill Hickey for generously sharing equipment and expertise. Janet Richmond, Szi-Chieh Yu, and Shigeki Watanabe provided helpful advice on optimizing use of HPF/FS techniques in vivo. Finally, we thank members of the O’Connor-Giles lab for helpful discussions and comments on the manuscript. This work was supported by a grant to Kate O’Connor-Giles from the National Institutes of Health (NS078179) and a National Science Foundation Graduate Research Fellowship to Joseph Bruckner.
References
- Ackermann F, Waites CL, Garner CC. Presynaptic active zones in invertebrates and vertebrates. EMBO Rep. 2015 doi: 10.15252/embr.201540434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alabi AA, Tsien RW. Synaptic vesicle pools and dynamics. Cold Spring Harb Perspect Biol. 2012;4:a013680–a013680. doi: 10.1101/cshperspect.a013680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckner JJ, Zhan H, O’Connor-Giles KM. Advances in imaging ultrastructure yield new insights into presynaptic biology. Front Cell Neurosci. 2015;9:1–16. doi: 10.3389/fncel.2015.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckner JJ, Zhan H, Gratz SJ, Rao X, Zilberg G, O’Connor-Giles KM. Fife organizes synaptic vesicles and calcium channels for high-probability neurotransmitter release. Journal of Cell Biology. doi: 10.1083/jcb.201601098. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles KM. Fife organizes synaptic vesicles and calcium. doi: 10.1083/jcb.201601098. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnik V, Koh YH, Guan B, Hartmann B, Hough C, Woods D, Gorczyca M. Regulation of synapse structure and function by the Drosophila tumor suppressor gene dlg. Neuron. 1996;17:627–640. doi: 10.1016/s0896-6273(00)80196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burette AC, Lesperance T, Crum J, Martone M, Volkmann N, Ellisman MH, Weinberg RJ. Electron tomographic analysis of synaptic ultrastructure. J. Comp. Neurol. 2012;520:2697–2711. doi: 10.1002/cne.23067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Winters C, Azzam R, Li X, Galbraith JA, Leapman RD, Reese TS. Organization of the core structure of the postsynaptic density. Proceedings of the National Academy of Sciences. 2008a;105:4453–4458. doi: 10.1073/pnas.0800897105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Winters CA, Reese TS. Life inside a thin section: tomography. J Neurosci. 2008b;28:9321–9327. doi: 10.1523/JNEUROSCI.2992-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Nelson CD, Li X, Winters CA, Azzam R, Sousa AA, Leapman RD, Gainer H, Sheng M, Reese TS. PSD-95 is required to sustain the molecular organization of the postsynaptic density. J Neurosci. 2011;31:6329–6338. doi: 10.1523/JNEUROSCI.5968-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choquet D, Triller A. The Dynamic Synapse. Neuron. 2013;80:691–703. doi: 10.1016/j.neuron.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Daniels RW, Collins CA, Chen K, Gelfand MV, Featherstone DE, DiAntonio A. A Single Vesicular Glutamate Transporter Is Sufficient to Fill a Synaptic Vesicle. Neuron. 2006;49:11–16. doi: 10.1016/j.neuron.2005.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis ED, Bennett HS. Some features of the submicroscopic morphology of synapses in frog and earthworm. J Biophys Biochem Cytol. 1955;1:47–58. doi: 10.1083/jcb.1.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denker A, Rizzoli SO. Synaptic vesicle pools: an update. Front Synaptic Neurosci. 2010 doi: 10.3389/fnsyn.2010.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermann E, Bucurenciu I, Goswami SP, Jonas P. Nanodomain coupling between Ca2 channels and sensors of exocytosis at fast mammalian synapses. Nat Rev. Neurosci. 2012;13:7–21. doi: 10.1038/nrn3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Zhang M. Organization and dynamics of PDZ-domain-related supramodules in the postsynaptic density. Nat Rev Neurosci. 2009;10:87–99. doi: 10.1038/nrn2540. [DOI] [PubMed] [Google Scholar]
- Fernández-Busnadiego R, Zuber B, Maurer UE, Cyrklaff M, Baumeister W, Lučie V. Quantitative analysis of the native presynaptic cytomatrix by cryoelectron tomography. J Cell Biol. 2010;188:145–156. doi: 10.1083/jcb.200908082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Busnadiego R, Schrod N, Kochovski Z, Asano S, Vanhecke D, Baumeister W, Lučić V. Insights into the molecular organization of the neuron by cryo-electron tomography. Journal of Electron Microscopy. 2011;60(Suppl 1):S137–S148. doi: 10.1093/jmicro/dfr018. [DOI] [PubMed] [Google Scholar]
- Fernández-Busnadiego R, Asano S, Oprisoreanu A-M, Sakata E, Doengi M, Kochovski Z, Zürner M, Stein V, Schoch S, Baumeister W, Lučić V. Cryo-electron tomography reveals a critical role of RIM1a in synaptic vesicle tethering. J Cell Biol. 2013;201:725–740. doi: 10.1083/jcb.201206063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouquet W, Owald D, Wichmann C, Mertel S, Depner H, Dyba M, Hallermann S, Kittel RJ, Eimer S, Sigrist SJ. Maturation of active zone assembly by Drosophila Bruchpilot. J Cell Biol. 2009;186:129–145. doi: 10.1083/jcb.200812150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MG. A frontier in the understanding of synaptic plasticity: solving the structure of the postsynaptic density. Bioessays. 2012;34:599–608. doi: 10.1002/bies.201200009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow ML, Ress D, Stoschek A, Marshall RM, McMahan UJ. The architecture of active zone material at the frog’s neuromuscular junction. Nature. 2001;409:479–484. doi: 10.1038/35054000. [DOI] [PubMed] [Google Scholar]
- Helmprobst F, Frank M, Stigloher C. The presynaptic architecture of the larval zebrafish neuromuscular junction. J. Comp. Neurol. 2015 doi: 10.1002/cne.23775. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- High B, Cole AA, Chen X, Reese TS. Electron microscopic tomography reveals discrete transcleft elements at excitatory and inhibitory synapses. Front Synaptic Neurosci. 2015;7:9. doi: 10.3389/fnsyn.2015.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Sobue K, Kanda K, Harada A, Yorifuji H. The cytoskeletal architecture of the presynaptic terminal and molecular structure of synapsin 1. J Cell Biol. 1989;108:111–126. doi: 10.1083/jcb.108.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura T, Hashimoto PH. Structural components in the synaptic cleft captured by freeze-substitution and deep etching of directly frozen cerebellar cortex. J. Neurocytol. 1988;17:3–12. doi: 10.1007/BF01735373. [DOI] [PubMed] [Google Scholar]
- Imig C, Min S-W, Krinner S, Arancillo M, Rosenmund C, Südhof TC, Rhee J, Brose N, Cooper BH. The morphological and molecular nature of synaptic vesicle priming at presynaptic active zones. Neuron. 2014;84:416–431. doi: 10.1016/j.neuron.2014.10.009. [DOI] [PubMed] [Google Scholar]
- Jiao W, Masich S, Franzén O, Shupliakov O. Two pools of vesicles associated with the presynaptic cytosolic projection in Drosophila neuromuscular junctions. J. Struct. Biol. 2010;172:389–394. doi: 10.1016/j.jsb.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Karunanithi S, Marin L, Wong K, Atwood HL. Quantal size and variation determined by vesicle size in normal and mutant Drosophila glutamatergic synapses. J Neurosci. 2002;22:10267–10276. doi: 10.1523/JNEUROSCI.22-23-10267.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- Koper A, Schenck A, Prokop A. Analysis of Adhesion Molecules and Basement Membrane Contributions to Synaptic Adhesion at the Drosophila Embryonic NMJ. PLoS ONE. 2012;7:e36339. doi: 10.1371/journal.pone.0036339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korogod N, Petersen CCH, Knott GW. Ultrastructural analysis of adult mouse neocortex comparing aldehyde perfusion with cryo fixation. Elife. 2015;4:241. doi: 10.7554/eLife.05793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer JR, Mastronarde DN, MCINTOSH JR. Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 1996;116:71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- Landis DM, Hall AK, Weinstein LA, Reese TS. The organization of cytoplasm at the presynaptic active zone of a central nervous system synapse. Neuron. 1988;1:201–209. doi: 10.1016/0896-6273(88)90140-7. [DOI] [PubMed] [Google Scholar]
- Linsalata AE, Chen X, Winters CA, Reese TS. Electron tomography on γ-aminobutyric acid-ergic synapses reveals a discontinuous postsynaptic network of filaments. Journal of Comparative Neurology. 2014;522:921–936. doi: 10.1002/cne.23453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KSY, Siebert M, Mertel S, Knoche E, Wegener S, Wichmann C, Matkovic T, Muhammad K, Depner H, Mettke C, Buckers J, Hell SW, Muller M, Davis GW, Schmitz D, Sigrist SJ. RIM-Binding Protein, a Central Part of the Active Zone, Is Essential for Neurotransmitter Release. Science. 2011;334:1565–1569. doi: 10.1126/science.1212991. [DOI] [PubMed] [Google Scholar]
- Lučie V, Yang T, Schweikert G, Förster F, Baumeister W. Morphological characterization of molecular complexes present in the synaptic cleft. Structure. 2005;13:423–434. doi: 10.1016/j.str.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Matkovic T, Siebert M, Knoche E, Depner H, Mertel S, Owald D, Schmidt M, Thomas U, Sickmann A, Kamin D, Hell SW, Bürger J, Hollmann C, Mielke T, Wichmann C, Sigrist SJ. The Bruchpilot cytomatrix determines the size of the readily releasable pool of synaptic vesicles. J Cell Biol. 2013;202:667–683. doi: 10.1083/jcb.201301072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagwaney S, Harlow ML, Jung JH, Szule JA, Ress D, Xu J, Marshall RM, McMahan UJ. Macromolecular connections of active zone material to docked synaptic vesicles and presynaptic membrane at neuromuscular junctions of mouse. Journal of Comparative Neurology. 2009;513:457–468. doi: 10.1002/cne.21975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay SL, Palade GE. The fine structure of neurons. J Biophys Biochem Cytol. 1955;1:69–88. doi: 10.1083/jcb.1.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez de Arce K, Schrod N, Metzbower SWR, Allgeyer E, Kong GKW, Tang A-H, Krupp AJ, Stein V, Liu X, Bewersdorf J, Blanpied TA, Lučić V, Biederer T. Topographic Mapping of the Synaptic Cleft into Adhesive Nanodomains. Neuron. 2015;88:1165–1172. doi: 10.1016/j.neuron.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop A. Integrating bits and pieces: synapse structure and formation in Drosophila embryos. Cell Tissue Res. 1999;297:169–186. doi: 10.1007/s004410051345. [DOI] [PubMed] [Google Scholar]
- Rizzoli SO, Betz WJ. Synaptic vesicle pools. Nat Rev Neurosci. 2005 doi: 10.1038/nrn1583. [DOI] [PubMed] [Google Scholar]
- Rostaing P, Real E, Siksou L, Lechaire J-P, Boudier T, Boeckers TM, Gertler F, Gundelfinger ED, Triller A, Marty S. Analysis of synaptic ultrastructure without fixative using high-pressure freezing and tomography. Eur. J. Neurosci. 2006;24:3463–3474. doi: 10.1111/j.1460-9568.2006.05234.x. [DOI] [PubMed] [Google Scholar]
- Sheng M, Hoogenraad CC. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu. Rev. Biochem. 2007;76:823–847. doi: 10.1146/annurev.biochem.76.060805.160029. [DOI] [PubMed] [Google Scholar]
- Shtrahman M, Yeung C, Nauen DW, Bi G-Q, Wu X-L. Probing vesicle dynamics in single hippocampal synapses. Biophysical Journal. 2005;89:3615–3627. doi: 10.1529/biophysj.105.059295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siksou L, Triller A, Marty S. Ultrastructural organization of presynaptic terminals. Curr Opin Neurobiol. 2011;21:261–268. doi: 10.1016/j.conb.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Siksou L, Varoqueaux F, Pascual O, Triller A, Brose N, Marty S. A common molecular basis for membrane docking and functional priming of synaptic vesicles. Eur. J. Neurosci. 2009;30:49–56. doi: 10.1111/j.1460-9568.2009.06811.x. [DOI] [PubMed] [Google Scholar]
- Siksou L, Rostaing P, Lechaire J-P, Boudier T, Ohtsuka T, Fejtová A, Kao H-T, Greengard P, Gundelfinger ED, Triller A, Marty S. Three-dimensional architecture of presynaptic terminal cytomatrix. J Neurosci. 2007;27:6868–6877. doi: 10.1523/JNEUROSCI.1773-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stigloher C, Zhan H, Zhen M, Richmond J, Bessereau J-L. The presynaptic dense projection of the Caenorhabditis elegans cholinergic neuromuscular junction localizes synaptic vesicles at the active zone through SYD-2/liprin and UNC-10/RIM-dependent interactions. J Neurosci. 2011;31:4388–4396. doi: 10.1523/JNEUROSCI.6164-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szule JA, Jung JH, McMahan UJ. The structure and function of “active zone material” at synapses. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2015:370. doi: 10.1098/rstb.2014.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang A-H, Chen H, Li TP, Metzbower SR, MacGillavry HD, Blanpied TA. A trans-synaptic nanocolumn aligns neurotransmitter release to receptors. Nature. 2016 doi: 10.1038/nature19058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrealba F, Carrasco MA. A review on electron microscopy and neurotransmitter systems. Brain Res. Brain Res. Rev. 2004;47:5–17. doi: 10.1016/j.brainresrev.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Zuber B, Nikonenko I, Klauser P, Muller D, Dubochet J. The mammalian central nervous synaptic cleft contains a high density of periodically organized complexes. Proc Natl Acad Sci USA. 2005;102:19192–19197. doi: 10.1073/pnas.0509527102. [DOI] [PMC free article] [PubMed] [Google Scholar]