Abstract
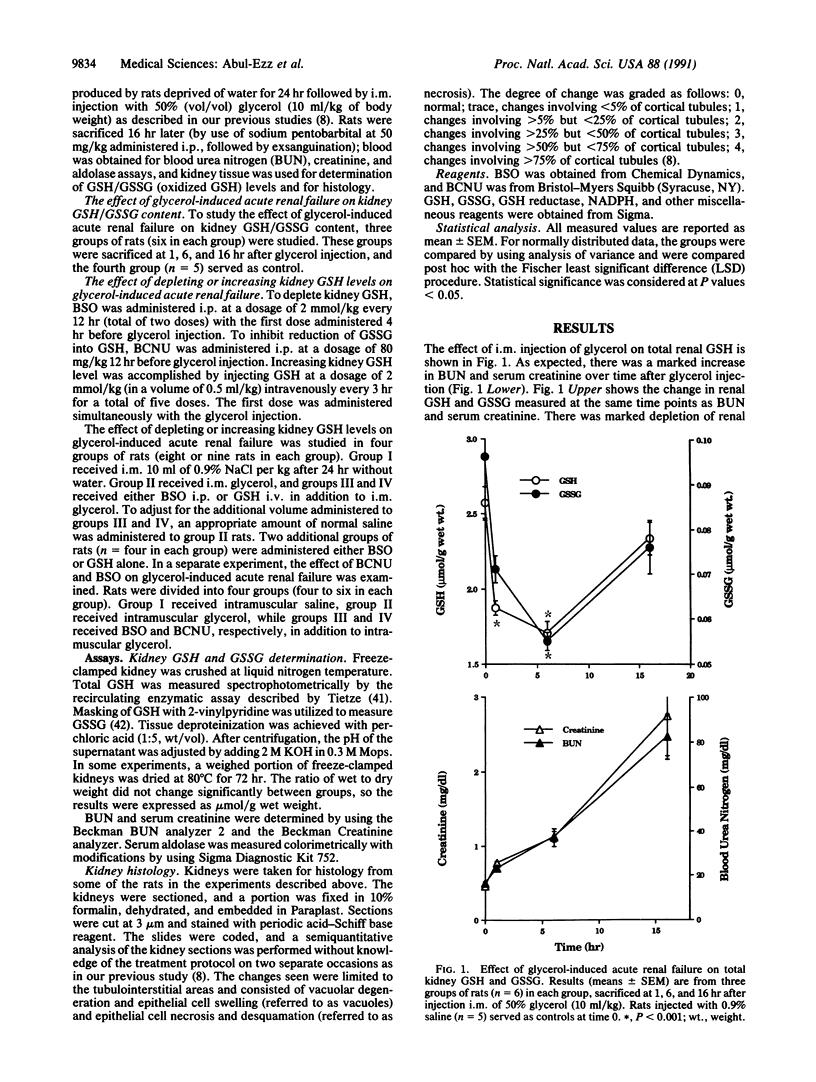
In a previous study we have shown a role for reactive oxygen metabolites in glycerol-induced acute renal failure, a well-established model for myoglobinuric acute renal failure. In the present study we examined the role of glutathione in this model of acute renal failure. Administration of 50% (vol/vol) glycerol at a dose of 10 ml/kg of body weight to rats intramuscularly resulted in significant renal failure associated with depletion of total kidney glutathione (GSH) from 2.6 +/- 0.1 mumol/g (mean +/- SEM control level) to 1.7 +/- 0.1 mumol/g after 6 hr (P less than 0.001). If GSH were important in glycerol-induced acute renal failure, one would anticipate that exogenously administered GSH should afford protection, while injury should be potentiated if endogenous GSH is depleted. We examined the effect of i.p. administration of L-buthionine-(S,R)-sulfoximine (BSO) at 2 mmol/kg (which results in depletion of kidney GSH) and the effect of increasing renal GSH by i.v. administration of reduced GSH (2 mmol/kg every 3 hr) on kidney function in glycerol-treated rats. Glycerol-injected rats treated with BSO showed significantly worse renal failure than did rats given glycerol alone, while administration of GSH resulted in significant amelioration of glycerol-induced acute renal failure [glycerol treatment alone, blood urea nitrogen (BUN) = 96 +/- 10 and creatinine = 2.5 +/- 0.4 mg/dl; BSO + glycerol treatment, BUN = 123 +/- 7 and creatinine = 3.5 +/- 0.1 mg/dl (n = 9, P less than 0.05); GSH + glycerol treatment, BUN = 78 +/- 10 and creatinine = 1.25 +/- 0.2 mg/dl (n = 8, P less than 0.05)]. In separate experiments 1,3-bis(chloroethyl)-1-nitrosourea (BCNU) [which interferes with the enzyme GSH reductase and prevents recycling of oxidized GSH (GSSG) into GSH] resulted in worsening of glycerol-induced acute renal failure similar to that produced by BSO. These functional differences between GSH-depleted and GSH-repleted rats were further substantiated by significant histological differences in tubular injury. Taken together, these results provide evidence for an important role of GSH in glycerol-induced acute renal failure.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreoli S. P., Mallett C. P., Bergstein J. M. Role of glutathione in protecting endothelial cells against hydrogen peroxide oxidant injury. J Lab Clin Med. 1986 Sep;108(3):190–198. [PubMed] [Google Scholar]
- Arrick B. A., Nathan C. F., Griffith O. W., Cohn Z. A. Glutathione depletion sensitizes tumor cells to oxidative cytolysis. J Biol Chem. 1982 Feb 10;257(3):1231–1237. [PubMed] [Google Scholar]
- Blaustein A., Deneke S. M., Stolz R. I., Baxter D., Healey N., Fanburg B. L. Myocardial glutathione depletion impairs recovery after short periods of ischemia. Circulation. 1989 Nov;80(5):1449–1457. doi: 10.1161/01.cir.80.5.1449. [DOI] [PubMed] [Google Scholar]
- Cotgreave I. A., Sandy M. S., Berggren M., Moldéus P. W., Smith M. T. N-acetylcysteine and glutathione-dependent protective effect of PZ51 (Ebselen) against diquat-induced cytotoxicity in isolated hepatocytes. Biochem Pharmacol. 1987 Sep 15;36(18):2899–2904. doi: 10.1016/0006-2952(87)90200-0. [DOI] [PubMed] [Google Scholar]
- Dethmers J. K., Meister A. Glutathione export by human lymphoid cells: depletion of glutathione by inhibition of its synthesis decreases export and increases sensitivity to irradiation. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7492–7496. doi: 10.1073/pnas.78.12.7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroshow J. H., Locker G. Y., Ifrim I., Myers C. E. Prevention of doxorubicin cardiac toxicity in the mouse by N-acetylcysteine. J Clin Invest. 1981 Oct;68(4):1053–1064. doi: 10.1172/JCI110328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle D., Clarke R., Kaplowitz N. Rapid oxidation in vitro of endogenous and exogenous glutathione in bile of rats. J Biol Chem. 1981 Mar 10;256(5):2115–2117. [PubMed] [Google Scholar]
- Gabow P. A., Kaehny W. D., Kelleher S. P. The spectrum of rhabdomyolysis. Medicine (Baltimore) 1982 May;61(3):141–152. doi: 10.1097/00005792-198205000-00002. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Bridges R. J., Meister A. Formation of gamma-glutamycyst(e)ine in vivo is catalyzed by gamma-glutamyl transpeptidase. Proc Natl Acad Sci U S A. 1981 May;78(5):2777–2781. doi: 10.1073/pnas.78.5.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980 Jul 15;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem. 1979 Aug 25;254(16):7558–7560. [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Translocation of intracellular glutathione to membrane-bound gamma-glutamyl transpeptidase as a discrete step in the gamma-glutamyl cycle: glutathionuria after inhibition of transpeptidase. Proc Natl Acad Sci U S A. 1979 Jan;76(1):268–272. doi: 10.1073/pnas.76.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman R. A., Hamilton R. W., Morse B. M., Penn A. S., Goldberg M. Nontraumatic rhabdomyolysis and acute renal failure. N Engl J Med. 1974 Oct 17;291(16):807–811. doi: 10.1056/NEJM197410172911601. [DOI] [PubMed] [Google Scholar]
- Guidet B. R., Shah S. V. In vivo generation of hydrogen peroxide by rat kidney cortex and glomeruli. Am J Physiol. 1989 Jan;256(1 Pt 2):F158–F164. doi: 10.1152/ajprenal.1989.256.1.F158. [DOI] [PubMed] [Google Scholar]
- Hagen T. M., Aw T. Y., Jones D. P. Glutathione uptake and protection against oxidative injury in isolated kidney cells. Kidney Int. 1988 Jul;34(1):74–81. doi: 10.1038/ki.1988.147. [DOI] [PubMed] [Google Scholar]
- Harlan J. M., Levine J. D., Callahan K. S., Schwartz B. R., Harker L. A. Glutathione redox cycle protects cultured endothelial cells against lysis by extracellularly generated hydrogen peroxide. J Clin Invest. 1984 Mar;73(3):706–713. doi: 10.1172/JCI111263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue D. P., Griffith K. L., McLean A. E. Hepatocytes in primary culture become susceptible to paracetamol injury after depletion of glutathione using DL-buthionine-SR-sulphoximine (BSO). Biochem Pharmacol. 1985 Dec 15;34(24):4341–4344. doi: 10.1016/0006-2952(85)90298-9. [DOI] [PubMed] [Google Scholar]
- Koffler A., Friedler R. M., Massry S. G. Acute renal failure due to nontraumatic rhabdomyolysis. Ann Intern Med. 1976 Jul;85(1):23–28. doi: 10.7326/0003-4819-85-1-23. [DOI] [PubMed] [Google Scholar]
- Kondo T., Dale G. L., Beutler E. Glutathione transport by inside-out vesicles from human erythrocytes. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6359–6362. doi: 10.1073/pnas.77.11.6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash L. H., Hagen T. M., Jones D. P. Exogenous glutathione protects intestinal epithelial cells from oxidative injury. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4641–4645. doi: 10.1073/pnas.83.13.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash L. H., Tokarz J. J. Oxidative stress in isolated rat renal proximal and distal tubular cells. Am J Physiol. 1990 Aug;259(2 Pt 2):F338–F347. doi: 10.1152/ajprenal.1990.259.2.F338. [DOI] [PubMed] [Google Scholar]
- Linas S. L., Shanley P. F., White C. W., Parker N. P., Repine J. E. O2 metabolite-mediated injury in perfused kidneys is reflected by consumption of DMTU and glutathione. Am J Physiol. 1987 Oct;253(4 Pt 2):F692–F701. doi: 10.1152/ajprenal.1987.253.4.F692. [DOI] [PubMed] [Google Scholar]
- McCoy R. N., Hill K. E., Ayon M. A., Stein J. H., Burk R. F. Oxidant stress following renal ischemia: changes in the glutathione redox ratio. Kidney Int. 1988 Apr;33(4):812–817. doi: 10.1038/ki.1988.72. [DOI] [PubMed] [Google Scholar]
- Meister A., Anderson M. E., Hwang O. Intracellular cysteine and glutathione delivery systems. J Am Coll Nutr. 1986;5(2):137–151. doi: 10.1080/07315724.1986.10720121. [DOI] [PubMed] [Google Scholar]
- Meister A. New aspects of glutathione biochemistry and transport--selective alteration of glutathione metabolism. Nutr Rev. 1984 Dec;42(12):397–410. doi: 10.1111/j.1753-4887.1984.tb02277.x. [DOI] [PubMed] [Google Scholar]
- Meister A. New developments in glutathione metabolism and their potential application in therapy. Hepatology. 1984 Jul-Aug;4(4):739–742. doi: 10.1002/hep.1840040431. [DOI] [PubMed] [Google Scholar]
- Meister A. Selective modification of glutathione metabolism. Science. 1983 Apr 29;220(4596):472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- Messana J. M., Cieslinski D. A., O'Connor R. P., Humes H. D. Glutathione protects against exogenous oxidant injury to rabbit renal proximal tubules. Am J Physiol. 1988 Nov;255(5 Pt 2):F874–F884. doi: 10.1152/ajprenal.1988.255.5.F874. [DOI] [PubMed] [Google Scholar]
- Mårtensson J., Steinherz R., Jain A., Meister A. Glutathione ester prevents buthionine sulfoximine-induced cataracts and lens epithelial cell damage. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8727–8731. doi: 10.1073/pnas.86.22.8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olerud J. E., Homer L. D., Carroll H. W. Serum myoglobin levels predicted from serum enzyme values. N Engl J Med. 1975 Sep 4;293(10):483–485. doi: 10.1056/NEJM197509042931006. [DOI] [PubMed] [Google Scholar]
- Olson C. E. Glutathione modulates toxic oxygen metabolite injury of canine chief cell monolayers in primary culture. Am J Physiol. 1988 Jan;254(1 Pt 1):G49–G56. doi: 10.1152/ajpgi.1988.254.1.G49. [DOI] [PubMed] [Google Scholar]
- Olson R. D., MacDonald J. S., vanBoxtel C. J., Boerth R. C., Harbison R. D., Slonim A. E., Freeman R. W., Oates J. A. Regulatory role of glutathione and soluble sulfhydryl groups in the toxicity of adriamycin. J Pharmacol Exp Ther. 1980 Nov;215(2):450–454. [PubMed] [Google Scholar]
- Paller M. S. Hemoglobin- and myoglobin-induced acute renal failure in rats: role of iron in nephrotoxicity. Am J Physiol. 1988 Sep;255(3 Pt 2):F539–F544. doi: 10.1152/ajprenal.1988.255.3.F539. [DOI] [PubMed] [Google Scholar]
- Paller M. S., Hoidal J. R., Ferris T. F. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984 Oct;74(4):1156–1164. doi: 10.1172/JCI111524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller M. S. Renal work, glutathione and susceptibility to free radical-mediated postischemic injury. Kidney Int. 1988 Apr;33(4):843–849. doi: 10.1038/ki.1988.75. [DOI] [PubMed] [Google Scholar]
- Rankin B. B., Wells W., Curthoys N. P. Rat renal peritubular transport and metabolism of plasma [35S]glutathione. Am J Physiol. 1985 Aug;249(2 Pt 2):F198–F204. doi: 10.1152/ajprenal.1985.249.2.F198. [DOI] [PubMed] [Google Scholar]
- Ratych R. E., Bulkley G. B. Free-radical-mediated postischemic reperfusion injury in the kidney. J Free Radic Biol Med. 1986;2(5-6):311–319. doi: 10.1016/s0748-5514(86)80030-7. [DOI] [PubMed] [Google Scholar]
- Reed D. J., Fariss M. W. Glutathione depletion and susceptibility. Pharmacol Rev. 1984 Jun;36(2 Suppl):25S–33S. [PubMed] [Google Scholar]
- Scaduto R. C., Jr, Gattone V. H., 2nd, Grotyohann L. W., Wertz J., Martin L. F. Effect of an altered glutathione content on renal ischemic injury. Am J Physiol. 1988 Nov;255(5 Pt 2):F911–F921. doi: 10.1152/ajprenal.1988.255.5.F911. [DOI] [PubMed] [Google Scholar]
- Sekura R., Meister A. Glutathione turnover in the kidney; considerations relating to the gamma-glutamyl cycle and the transport of amino acids. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2969–2972. doi: 10.1073/pnas.71.8.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S. V., Walker P. D. Evidence suggesting a role for hydroxyl radical in glycerol-induced acute renal failure. Am J Physiol. 1988 Sep;255(3 Pt 2):F438–F443. doi: 10.1152/ajprenal.1988.255.3.F438. [DOI] [PubMed] [Google Scholar]
- Singh A., Lee K. J., Lee C. Y., Goldfarb R. D., Tsan M. F. Relation between myocardial glutathione content and extent of ischemia-reperfusion injury. Circulation. 1989 Dec;80(6):1795–1804. doi: 10.1161/01.cir.80.6.1795. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Beutler E. The transport of oxidized glutathione from human erythrocytes. J Biol Chem. 1969 Jan 10;244(1):9–16. [PubMed] [Google Scholar]
- Suttorp N., Toepfer W., Roka L. Antioxidant defense mechanisms of endothelial cells: glutathione redox cycle versus catalase. Am J Physiol. 1986 Nov;251(5 Pt 1):C671–C680. doi: 10.1152/ajpcell.1986.251.5.C671. [DOI] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Zager R. A. Hypoperfusion-induced acute renal failure in the rat: an evaluation of oxidant tissue injury. Circ Res. 1988 Mar;62(3):430–435. doi: 10.1161/01.res.62.3.430. [DOI] [PubMed] [Google Scholar]