Abstract
We examined whether protein- and food-intake restrictions modulate the oxidized/reduced state of plasma albumin in Sprague-Dawley rats. Rats were fed a 3%, 5%, 10% or 20% casein diet for 2 weeks. The plasma albumin concentration significantly decreased with decreasing protein intake. However, no significant difference in plasma albumin concentration was seen between rats fed the 5% or 10% casein diet. In rats fed the 5% casein diet, the percentage of mercaptalbumin within total plasma albumin was significantly lower and that of nonmercaptalbumin-1 was significantly higher than in rats fed the 10% casein diet. In experiments with food-intake restriction for 2 weeks, rats were fed 50% or 75% of the amount of a 20% casein diet consumed by control rats. The percentage of mercaptalbumin was significantly lower and that of nonmercaptalbumin-2 was significantly higher in rats with food-intake restriction than in control rats. When rats with malnutrition were refed with the 20% casein diet ad libitum, the percentage of mercaptalbumin rapidly increased. The change in the percentage of mercaptalbumin was correlated with the plasma transthyretin concentration. These results indicate that the oxidized/reduced state of plasma albumin may be applied as a sensitive marker of nutritional status reflecting dietary pattern.
Keywords: reduced albumin, insufficient diet, malnutrition, transthyretin, rats
Introduction
Albumin is one of the major proteins synthesized in the liver and is the most abundant protein in the plasma of healthy individuals. Plasma albumin performs important metabolic functions by regulating colloid osmotic pressure and transporting free fatty acids, bilirubin and many drugs.(1,2) There are many factors regulating the level of plasma albumin under various conditions. Meal stimulation is one of the most important factors in the regulation of albumin synthesis.(3–5) Food intake activates the synthesis of albumin in healthy subjects.(3) In particular, the protein content and quality of a meal influence the rate of albumin synthesis.(4,5) Plasma albumin is one of the most common parameters used in evaluating nutritional status. Hypoalbuminemia is an independent risk factor for mortality in older persons(6) and in patients with various pathological conditions.(7–9) On the other hand, human albumin has a long biological half-life.(10) Because of this, hypoalbuminemia would present only after a long period of continuous malnutrition. Thus, the level of plasma albumin might be a poor indicator for short-term nutritional assessment.
Human albumin is a single-chain polypeptide composed of 585 amino acid residues.(11) The molecule contains 35 cysteine residues and forms 17 intramolecular disulfide bonds. Thus, albumin has a single free cysteine residue at the 34th position from the N-terminal. Because this cysteine residue is susceptible to oxidation, albumin displays microheterogeneity, including reduced and oxidized forms.(12) The reduced form of human albumin is referred to as human mercaptalbumin (HMA) and the oxidized form is referred to as human nonmercaptalbumin-1 (HNA-1) or HNA-2. HMA has a free thiol in the cysteine residue at the 34th position from the N-terminal. The cysteine residue in HNA-1 forms a disulfide bond with small thiol compounds such as cysteine. The cysteine residue in HNA-2 is more highly oxidized to sulfinic (-SO2H) or sulfonic (-SO3H) acid. In a healthy person, HMA accounts for 70% to 80%, HNA-1 accounts for 20% to 30%, and HNA-2 accounts for approximately 2% to 5% of the total plasma albumin.(12) Most diseases are accompanied by oxidative stress. The redox state of albumin is believed to change under disease conditions. In fact, previous studies have indicated that the HMA fraction was decreased during the course of several pathophysiological conditions such as diabetes mellitus,(13) diabetic nephropathy,(14) chronic liver failure(15) and chronic kidney disease(16) as well as in dialysis patients.(17)
Plasma albumin has become an indispensable factor for evaluating nutritional status. However, the redox state of plasma albumin under conditions of malnutrition caused by insufficient food intake has not yet been reported. The objective of this study was to investigate the ratio of oxidized/reduced plasma albumin in rats with restricted protein intake or food consumption. In addition, the redox state of plasma albumin and the levels of plasma transthyretin, a protein with rapid turnover, were investigated in rats recovering from malnutrition.
Materials and Methods
Animal experiments (Experiments 1–3)
The animal facilities and protocols were reviewed and approved by the Institutional Animal Care and Use Committee at Kyoto Prefectural University. Male Sprague-Dawley rats (weight, approximately 80 g) were housed under a 12-h light/dark cycle with ad libitum access to a commercial diet and water. After an acclimatization period of 5 days, rats were fed one of the experimental diets. Dietary intake and body weight were measured every day during the experimental period. Blood was drawn from the tail vein and centrifuged to obtain plasma. The plasma samples were stored at −70°C until analysis. The protocols of the animal experiments were as follows.
Experiment 1: Rats were divided into 4 groups and fed a 20% (20C group, n = 7), 10% (10C group, n = 6), 5% (5C group, n = 6) or 3% (3C group, n = 6) casein diet (Table 1) for 2 weeks. Rats were given ad libitum access to the diet and drinking water.
Table 1.
Composition of casein diets (g/kg diet)
| Component | 20% | 10% | 5% | 3% |
|---|---|---|---|---|
| Casein | 200 | 100 | 50 | 30 |
| Cornstarch | 457 | 523 | 557 | 570 |
| Sucrose | 228 | 262 | 278 | 285 |
| Rapeseed oil | 35 | 35 | 35 | 35 |
| Soybean oil | 15 | 15 | 15 | 15 |
| Cellulose | 20 | 20 | 20 | 20 |
| Vitamin mixture§ | 10 | 10 | 10 | 10 |
| Mineral mixture¶ | 35 | 35 | 35 | 35 |
§AIN-76 vitamin mixture. ¶AIN-76 mineral mixture.
Experiment 2: All rats were fed a 20% casein diet. In the control group (n = 5), rats were fed the experimental diet ad libitum. In the other groups, food intake was restricted: over a period of 2 weeks, rats in the 75R group (n = 5) and the 50R group (n = 6) were given 75% or 50%, respectively, of the amount of food consumed by rats in the control group on the previous day.
Experiment 3: Rats were fed a 3% casein diet (Table 1) for 3 weeks and then refed with a 20% casein diet ad libitum for 3 days (n = 6). In addition, another group of rats were given 50% of the amount of food consumed by normal rats for 3 weeks and then refed with a 20% casein diet ad libitum for 3 days (n = 6).
Measurement of plasma albumin and transthyretin
The plasma concentration of albumin was measured with BCG-L kits (Cerotec, Sapporo, Japan) using a CL-8000 autoanalyzer (Shimadzu, Kyoto, Japan). The plasma concentration of transthyretin was measured with a Ttr (Rat) ELISA kit (Abnova, Taipei, Taiwan) according to the manufacturer’s instructions.
Analysis of the oxidized/reduced state of albumin
High-performance liquid chromatography (HPLC) was performed as described previously.(18) The HPLC system consisted of a #3023 autosampler, #3101 pumps and a #3213 fluorescence detector (excitation wavelength, 280 nm; emission wavelength, 380 nm) in conjunction with a S-MC system controller (all from Shiseido, Tokyo, Japan). An ES-502N ion-exchange column (Shodex-Asahipak; Showa Denko K.K., Kawasaki, Japan) was used. Measurements were carried out by solvent gradient elution with increasing ethanol concentration from 0% to 10% in 50 mM sodium acetate and 400 mM sodium sulfate (pH 4.85) at a flowrate of 1.0 ml/min.
Measurement of plasma amino acids
Plasma samples were mixed with an equal volume of 10% trichloroacetic acid, and were centrifuged at 21,500 × g for 15 min at 4°C. The supernatant was filtrated through 0.20 µm PVDF filters (Thomson Instrument Company, Oceanside, CA), and the filtrate was applied to an amino acid analyzer (L-8900, Hitachi High-Technologies, Tokyo, Japan).
Statistical analysis
Data are expressed as the mean ± SEM. Statistical analysis for multiple comparisons was performed using one-way analysis of variance followed by a Tukey-Kramer post hoc test. Comparison of mean values at baseline and the indicated times was performed with a paired t test. The relationship between the percentage of reduced albumin and transthyretin concentration was evaluated using Spearman’s correlation coefficient by rank test. Data analysis was performed using Statcel3 software (Oms Publishing, Tokyo, Japan) and values of p<0.05 were considered to be statistically significant.
Results
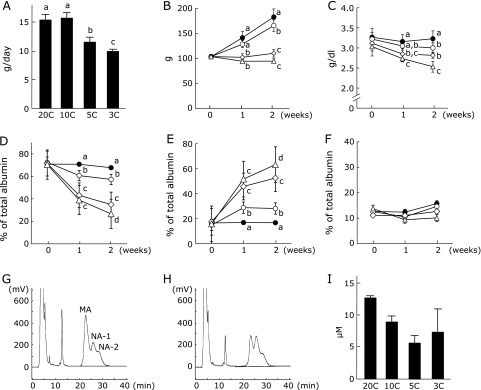
In Experiment 1, no significant difference in food intake was seen between the 20C and 10C groups (Fig. 1A). Food intake in both the 5C and 3C groups was significantly decreased when compared with the 20C group. Body weight in both the 20C and 10C groups increased steadily over the experimental period, but the final body weight in the 10C group was significantly lower than that in the 20C group (Fig. 1B). In the 5C and 3C groups, increases in body weight were not seen during the experimental period. The plasma albumin concentration decreased with decreasing protein intake (Fig. 1C) and was significantly higher in the 20C group than in the other groups. However, no significant difference in plasma albumin concentration was seen between the 10C and 5C groups. The percentage of mercaptalbumin (MA) also decreased with decreasing protein intake (Fig. 1D). The percentage of MA in the 10C group was significantly higher than in the 5C group from 1 week after the start of the experiment. On the other hand, the percentage of nonmercaptalbumin-1 (NA-1) increased with decreasing protein intake (Fig. 1E) and was significantly lower in the 20C group than in the other groups. No significant difference in the percentage of NA-2 was seen among the groups (Fig. 1F). Typical HPLC profiles at 2 weeks after the start of the experiment showed that the percentage of MA and NA-1 decreased and increased, respectively, in the 5C group when compared with the 10C group (Fig. 1G and H). The plasma cyst(e)ine concentration at 2 weeks after the start of the experiment decreased with decreasing protein intake (Fig. 1I).
Fig. 1.
Food intake, body weight, plasma albumin concentration and oxidized/reduced ratio of plasma albumin in rats fed protein-restricted diets. Rats were fed experimental diets containing 20% (20C group, n = 7, closed circles), 10% (10C group, n = 6, open circles), 5% (5C group, n = 6, open squares) or 3% (3C group, n = 6, open triangles) casein. Dietary intake (A) and body weight (B) were measured during the experimental period. Blood was drawn from the tail vein every week. Plasma albumin concentrations were monitored (C). The percentages of MA (D), NA-1 (E) and NA-2 (F) within total albumin were analyzed by HPLC. HPLC was performed using an ES-502N ion-exchange column with an increasing ethanol concentration from 0% to 10% in 50 mM sodium acetate and 400 mM sodium sulfate buffer (pH 4.85). Values represent the mean ± SEM. Means not sharing the same letter are significantly different from each other (p<0.05). Typical HPLC profiles of plasma albumin from rats fed a 10% (G) or 5% (H) casein diet for 2 weeks are shown. The plasma cyst(e)ine concentration at 2 weeks after the start of the experiment were analyzed by an amino acid analyzer (n = 3) (I).
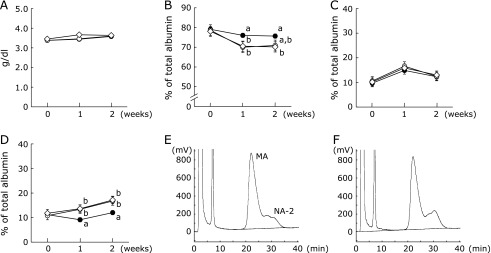
In Experiment 2, food intake in the control, 75R and 50R groups was 16.0 ± 0.7 g/day, 12.0 ± 0.0 g/day and 8.3 ± 0.0 g/day, respectively. Increases in body weight were suppressed in the 75R and 50R groups when compared with the control group (control: 198.4 ± 9.0 g; 75R: 157.4 ± 2.2 g; 50R: 113.1 ± 1.6 g). However, no significant difference in plasma albumin concentration was seen among the groups (Fig. 2A). On the other hand, the percentage of MA in both the 75R and 50R groups was significantly decreased at 1 week after the start of the experiment when compared with the control group (Fig. 2B). No significant difference in the percentage of NA-1 was seen among the groups during the experimental period (Fig. 2C). The percentage of NA-2 in both the 75R and 50R groups was significantly increased from 1 week after the start of the experiment when compared with the control group (Fig. 2D). Typical HPLC profiles at 2 weeks after the start of the experiment showed that the percentage of NA-2 was increased in the 75R group when compared with the control group (Fig. 2E and F).
Fig. 2.
Body weight, plasma albumin concentration and oxidized/reduced ratio of plasma albumin in rats with restricted food intake. Rats were fed a 20% casein diet. Rats in the control group (n = 6, closed circles) were fed ad libitum. The other rats were fed 75% (75R group, n = 6, open circles) or 50% (50R group, n = 6, open squares) of the amount of food consumed by rats in the control group on the previous day. Blood was drawn from the tail vein every week. Plasma albumin concentrations were monitored (A). The percentages of MA (B), NA-1 (C) and NA-2 (D) within total albumin were analyzed by HPLC. The HPLC conditions were the same as those in Fig. 1. Values represent the mean ± SEM. Means not sharing the same letter are significantly different from each other (p<0.05). Typical HPLC profiles of plasma albumin from rats in the control (E) and 75R (F) groups at 2 weeks after the start of the experiment are shown.
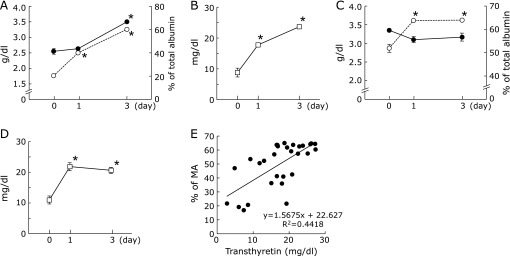
In Experiment 3, severe hypoalbuminemia was observed after protein restriction for 3 weeks. The plasma albumin concentration significantly increased with the feeding of the 20% casein diet for 3 days (Fig. 3A). On the other hand, the percentage of MA significantly increased after 1 day of refeeding with the 20% casein diet. Similarly, the plasma transthyretin concentration was significantly increased after 1 day of refeeding (Fig. 3B).
Fig. 3.
Plasma albumin concentration, percentage of reduced albumin and plasma transthyretin concentration in refed rats. Rats (n = 6) were fed a 3% casein diet for 3 weeks and then refed with a 20% casein diet ad libitum (A, B). Other rats (n = 6) were fed for 3 weeks a 20% casein diet restricted to 50% of the amount of food consumed by the control rats as described in Fig. 2 and then refed with a 20% casein diet ad libitum (C, D). Blood was drawn from the tail vein. Plasma albumin concentrations were monitored (A, C, closed circles) and the percentages of MA within total albumin were analyzed by HPLC (A, C, open circles). The HPLC conditions were the same as those in Fig. 1. Plasma transthyretin concentrations were also monitored (B, D). Values represent the mean ± SEM. *p<0.05 as compared with before refeeding (0 day). The relationship between the percentages of MA and transthyretin concentrations is shown (E).
After abolishing the 3-week 50% food restriction, there were no changes in plasma albumin concentration (Fig. 3C). However, both the percentage of MA and plasma transthyretin concentration were significantly increased after 1 day of refeeding with the 20% casein diet ad libitum (Fig. 3C and D).
The relationship between the percentage of MA and transthyretin concentration, as determined from the experiments on both protein- and food-intake restriction, is shown in Fig. 3E. A significant correlation was observed between the percentage of MA and transthyretin concentration (p<0.01).
Discussion
It is known that the oxidized/reduced state of plasma albumin changes in patients with chronic diseases in which oxidative stress is involved. The percentage of reduced albumin decreases progressively with increased severity of disease.(14–17) To the best of our knowledge, the present results comprise the first reported evidence that the oxidized/reduced state of plasma albumin changes in malnutrition caused by insufficient food consumption.
In the experiment with protein restriction for 2 weeks, there was no difference in plasma albumin concentration between the 10C and 5C groups. On the other hand, both the percentage of MA and body weight were obviously lower in the 5C group than in the 10C group 1 week after the start of the experiment. The difference in body weight was remarkable because growing rats were used in the present study. However, in clinical practice, it would be difficult to assess nutritional condition based on changes in body weight because edema is frequently observed in patients with hypoalbuminemia.(19) The present data suggest that an increase in NA-1 is a reliable and precise marker of malnutrition caused by insufficient protein intake.
In the experiments with food-intake restriction, there was no difference in plasma albumin concentration among any of the groups, which was consistent with the findings of a previous study.(20) On the other hand, the percentage of MA was significantly lower in rats with food-intake restriction than in the control rats. Unexpectedly, no significant difference in the oxidized/reduced state of plasma albumin was seen between the 75R and 50R groups. However, the increase in NA-2 should be recognized as an indicator of the risk of malnutrition caused by decreased food intake.
In the experiments involving recovery from protein deficiency or protein-energy deficiency, the percentage of MA rapidly increased with the feeding of the control diet ad libitum; similar results were obtained for the transthyretin concentration. Compared with albumin, transthyretin is a better marker of nutritional status because it has a short half-life and its concentration reflects the current nutritional condition of patients.(21) However, the concentration of plasma transthyretin may be affected by pathophysiological conditions such as inflammation and liver or kidney diseases.(22,23) It is important for nutrition assessments to be evaluated using several markers. The percentage of MA may be one reliable marker for judging the effects of nutritional interventions in individual patients.
In the present study, an increase in the percentage of NA-1 and NA-2 was induced by protein deficiency and protein-energy deficiency, respectively. The mechanism through which food intake regulates the oxidized/reduced state of plasma albumin remains unclear. The plasma cyst(e)ine concentration decreased in rats fed the low-protein diets. Cys is a major low molecular weight thiol in plasma and is a precursor for glutathione synthesis. Cys is important to maintain adequate redox status.(24) It was reported that the plasma concentrations of lipid peroxidation products and lipid hydroperoxides were increased in children with kwashiorkor caused by protein deficiency.(25) Furthermore, when protein intake was decreased, the intracellular concentration of reduced glutathione decreased in rats(26) and patients with malnutrition.(27) The increase in the percentage of NA-1 in rats fed the low-protein diets may be associated with a decrease in antioxidants. In higher oxidation states, MA is oxidized to NA-2, which is an irreversible form of oxidized albumin.(12) The energy deficiency caused by food-intake restriction may induce higher oxidation states than protein deficiency. The increase in the percentage of NA-2 caused by food-intake restriction rapidly decreased when the restriction was removed. Previous studies showed that the oxidized albumin was rapidly incorporated into cells and degraded.(28,29) Sufficient protein and energy intake may recover the percentage of MA through not only the activation of albumin synthesis, but also the reduction of NA-1 and the enhancement of oxidized albumin clearance.
A recent study indicated that the plasma level of cysteinylated proteins is inversely associated with protein and energy intake in hemodialysis patients.(30) The cysteine residue at the 34th position in albumin forms a disulfide bond with cysteine, homocysteine, cysteinylglycine and so on.(31) Future studies are needed to determine the detailed structures of oxidized albumin in conditions of malnutrition and to clarify the regulatory mechanisms of the oxidized/reduced state of plasma albumin. Nonetheless, the results of the present study suggest that the oxidized/reduced state of plasma albumin is a useful marker for dietary assessment. A recent study reported that serum carnitine concentration in patients receiving long-term enteral nutrition or total parenteral nutrition was significantly low, even if serum albumin concentration was normal.(32) The carnitine has a protective effect on the thiol groups in plasma proteins against oxidative damage.(33) A decrease in the percentage of MA in plasma may be induced by the carnitine deficiency in patients with complete artificial feeding. In several patients with disease, the decrease in the percentage of reduced albumin in plasma seemed to be influenced, at least in part, by dietary management. As such, appropriate dietary intervention may correct the decrease in the percentage of reduced albumin in plasma of patients with malnutrition.
Acknowledgments
This research was supported in part by JSPS KAKENHI Grant Number 15K00831.
Abbreviations
- HMA
human mercaptalbumin
- HNA
human nonmercaptalbumin
- HPLC
high-performance liquid chromatography
- MA
mercaptalbumin
- NA
nonmercaptalbumin
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Doweiko JP, Nompleggi DJ. Role of albumin in human physiology and pathophysiology. JPEN J Parenter Enteral Nutr. 1991;15:207–211. doi: 10.1177/0148607191015002207. [DOI] [PubMed] [Google Scholar]
- 2.Doweiko JP, Nompleggi DJ. Interactions of albumin and medications. JPEN J Parenter Enteral Nutr. 1991;15:212–214. doi: 10.1177/0148607191015002212. [DOI] [PubMed] [Google Scholar]
- 3.De Feo P, Horber FF, Haymond MW. Meal stimulation of albumin synthesis: a significant contributor to whole body protein synthesis in humans. Am J Physiol Endocrinol Metab. 1992;263 (4 Pt 1):E794–E799. doi: 10.1152/ajpendo.1992.263.4.E794. [DOI] [PubMed] [Google Scholar]
- 4.Caso G, Scalfi L, Marra M, et al. Albumin synthesis is diminished in men consuming a predominantly vegetarian diet. J Nutr. 2000;130:528–533. doi: 10.1093/jn/130.3.528. [DOI] [PubMed] [Google Scholar]
- 5.Caso G, Feiner J, Mileva I, et al. Response of albumin synthesis to oral nutrients in young and elderly subjects. Am J Clin Nutr. 2007;85:446–451. doi: 10.1093/ajcn/85.2.446. [DOI] [PubMed] [Google Scholar]
- 6.Corti MC, Guralnik JM, Salive ME, Sorkin JD. Serum albumin level and physical disability as predictors of mortality in older persons. JAMA. 1994;272:1036–1042. [PubMed] [Google Scholar]
- 7.Gibbs J, Cull W, Henderson W, Daley J, Hur K, Khuri SF. Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the National VA Surgical Risk Study. Arch Surg. 1999;134:36–42. doi: 10.1001/archsurg.134.1.36. [DOI] [PubMed] [Google Scholar]
- 8.Barchel D, Almoznino-Sarafian D, Shteinshnaider M, Tzur I, Cohen N, Gorelik O. Clinical characteristics and prognostic significance of serum albumin changes in an internal medicine ward. Eur J Intern Med. 2013;24:772–778. doi: 10.1016/j.ejim.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Jellinge ME, Henriksen DP, Hallas P, Brabrand M. Hypoalbuminemia is a strong predictor of 30-day all-cause mortality in acutely admitted medical patients: A prospective, observational, cohort study. PLoS One. 2014;9:e105983. doi: 10.1371/journal.pone.0105983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beeken WL, Volwiler W, Goldsworthy PD, et al. Studies of I131-albumin catabolism and distribution in normal young male adults. J Clin Invest. 1962;41:1312–1333. doi: 10.1172/JCI104594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dugaiczyk A, Law SW, Dennison OE. Nucleotide sequence and the encoded amino acids of human serum albumin mRNA. Proc Natl Acad Sci U S A. 1982;79:71–75. doi: 10.1073/pnas.79.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oettl K, Marsche G. Redox state of human serum albumin in terms of ctsteine-34 in health and disease. Methods Enzymol. 2010;474:181–195. doi: 10.1016/S0076-6879(10)74011-8. [DOI] [PubMed] [Google Scholar]
- 13.Oettl K, Reibnegger G, Schmut O. The redox state of human serum albumin in eye diseases with and without complications. Acta Ophthalmol. 2011;89:e174–e179. doi: 10.1111/j.1755-3768.2009.01824.x. [DOI] [PubMed] [Google Scholar]
- 14.Medina-Navarro R, Corona-Candelas I, Barajas-González S, Díaz-Flores M, Durán-Reyes G. Albumin antioxidant response to stress in diabetic nephropathy progression. PLoS One. 2014;9:e106490. doi: 10.1371/journal.pone.0106490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watanabe A, Matsuzaki S, Moriwaki H, Suzuki K, Nishiguchi S. Problems in serum albumin measurement and clinical significance of albumin microheterogeneity in cirrhotics. Nutrition. 2004;20:351–357. doi: 10.1016/j.nut.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Matsuyama Y, Terawaki H, Terada T, Era S. Albumin thiol oxidation and serum protein carbonyl formation are progressively enhanced with advancing stages of chronic kidney disease. Clin Exp Nephrol. 2009;13:308–315. doi: 10.1007/s10157-009-0161-y. [DOI] [PubMed] [Google Scholar]
- 17.Lim PS, Jeng Y, Wu MY, et al. Serum oxidized albumin and cardiovascular mortality in normoalbuminemic hemodialysis patients: a cohort study. PLoS One. 2013;8:e70822. doi: 10.1371/journal.pone.0070822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuwahata M, Kubota H, Katsukawa M, et al. Effect of branched-chain amino acid supplementation on the oxidized/reduced state of plasma albumin in rats with chronic liver disease. J Clin Biochem Nutr. 2012;50:67–71. doi: 10.3164/jcbn.11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coulthard MG. Oedema in kwashiorkor is caused by hypoalbuminaemia. Paediatr Int Child Health. 2015;35:83–89. doi: 10.1179/2046905514Y.0000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hubert MF, Laroque P, Gillet JP, Keenan KP. The effect of diet, ad libitum feeding, and moderate and severe dietary restriction on body weight, survival, clinical pathology parameters, and cause of death in control Sprague-Dawley rats. Toxicol Sci. 2000;58:195–207. doi: 10.1093/toxsci/58.1.195. [DOI] [PubMed] [Google Scholar]
- 21.Bernstein LH, Leukhardt-Fairfield CJ, Pleban W, Rudolph R. Usefulness of data on albumin and prealbumin concentrations in determining effectiveness of nutritional support. Clin Chem. 1989;35:271–274. [PubMed] [Google Scholar]
- 22.Caccialanza R, Palladini G, Klersy C, et al. Serum prealbumin: an independent marker of short-term energy intake in the presence of multiple-organ disease involvement. Nutrition. 2013;29:580–582. doi: 10.1016/j.nut.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Yang HT, Yim H, Cho YS, et al. Serum transthyretin level is associated with clinical severity rather than nutrition status in massively burned patients. JPEN J Parenter Enteral Nutr. 2014;38:966–972. doi: 10.1177/0148607113499588. [DOI] [PubMed] [Google Scholar]
- 24.Yin J, Ren W, Yang G, et al. l-Cysteine metabolism and its nutritional implications. Mol Nutr Food Res. 2016;60:134–146. doi: 10.1002/mnfr.201500031. [DOI] [PubMed] [Google Scholar]
- 25.Lenhartz H, Ndasi R, Anninos A, et al. The clinical manifestation of the kwashiorkor syndrome is related to increased lipid peroxidation. J Pediatr. 1998;132:879–881. doi: 10.1016/s0022-3476(98)70324-5. [DOI] [PubMed] [Google Scholar]
- 26.Hum S, Koski KG, Hoffer LJ. Varied protein intake alters glutathione metabolism in rats. J Nutr. 1992;122:2010–2018. doi: 10.1093/jn/122.10.2010. [DOI] [PubMed] [Google Scholar]
- 27.Reid M, Badaloo A, Forrester T, et al. In vivo rates of erythrocyte glutathione synthesis in children with severe protein-energy malnutrition. Am J Physiol Endocrinol Metab. 2000;278:E405–E412. doi: 10.1152/ajpendo.2000.278.3.E405. [DOI] [PubMed] [Google Scholar]
- 28.Bito R, Hino S, Baba A, Tanaka M, Watabe H, Kawabata H. Degradation of oxidative stress-induced denatured albumin in rat liver endothelial cells. Am J Physiol Cell Physiol. 2005;289:C531–C542. doi: 10.1152/ajpcell.00431.2004. [DOI] [PubMed] [Google Scholar]
- 29.Iwao Y, Anraku M, Hiraike M, et al. The structural and pharmacokinetic properties of oxidized human serum albumin, advanced oxidation protein products (AOPP) Drug Metab Pharmacokinet. 2006;21:140–146. doi: 10.2133/dmpk.21.140. [DOI] [PubMed] [Google Scholar]
- 30.Fanti P, Giustarini D, Rossi P, et al. Dietary intake of proteins and calories is inversely associated with the oxidation state of plasma thiols in end-stage renal disease patients. J Ren Nutr. 2015;25:494–503. doi: 10.1053/j.jrn.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zinellu A, Sotgia S, Scanu B, et al. N- and S-homocysteinylation reduce the binding of human serum albumin to catechins Eur J Nutr 2015. DOI: 10.1007/s00394-015-1125-5. [DOI] [PubMed] [Google Scholar]
- 32.Iwamoto J, Honda A, Miyamoto Y, et al. Serum carnitine as an independent biomarker of malnutrition in patients with impaired oral intake. J Clin Biochem Nutr. 2014;55:221–227. doi: 10.3164/jcbn.14-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kolodziejczyk J, Saluk-Juszczak J, Wachowicz B. l-Carnitine protects plasma components against oxidative alterations. Nutrition. 2011;27:693–699. doi: 10.1016/j.nut.2010.06.009. [DOI] [PubMed] [Google Scholar]