Abstract
The therapeutic effects of non-thermal plasma are expected in the medical fields, including hemostasis, vascularization, prevention of organ adhesion, and cell proliferation. Cancer is an internal enemy arising from normal tissue in the body. The prognosis of metastatic and recurrent cancers is still poor despite advances in medicine. To apply non-thermal plasma in cancer treatment is now on going. The mechanism of the proliferation-inhibitory effect of plasma is reactive nitrogen oxide species/reactive oxygen species production in cells. There are a number of problems to be overcome, such as existence of intrinsic reactive oxygen species/reactive nitrogen species scavengers and the shallow infiltration of plasma on tumor surface. The current reviews makes referral to the study results of plasma therapy clarified so far, the possibility of its application in the future.
Keywords: cancer therapy, apoptosis, aqueous non-thermal plasma, ROS
Introduction
Plasma is the 4th phase of substances following the solid, liquid, and gas phases, and it is constituted of gas containing electrons, cations, anions, neutral atoms, and neutral and charged molecules. Plasma can be generated by dissociating molecules to atoms in gas by loading energy to the gas and then ionizing the atoms into ions and electrons. Previous plasma is termed low-pressure plasma and its generation was limited to that in a vacuum container, but non-equilibrium atmospheric pressure plasma generated under atmospheric pressure at a low temperature has recently been developed, and it is now applied not only in the manufacturing and industrial fields but also the medical field, and plasma coagulation of the mucosa are performed.(1,2) Various therapeutic effects, such as inactivation of pathogens(3–5) wound healing,(6) blood coagulation,(7) and tissue sterilization,(7,8) and the ablation of cultured cancer cells, are also expected. Basic studies on application of plasma for cancer therapy have just started over the world.(6,9,10) To supplement it from standpoints of cancer researchers and clinicians, the current reviews makes referral to the study results of plasma therapy clarified so far, the possibility of its application in the future, and problems to be solved.
Current State of Cancer Medical Care
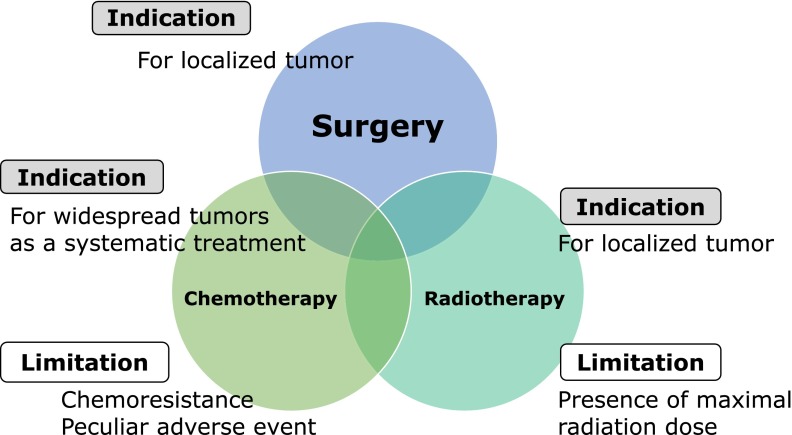
It is said that currently, approximately one in three people die of cancer in Japan. Cancer is an internal enemy arising from normal tissue in the body. However, it has not yet been fully understood why self-destroying neoplasm emerges in one’s own body. Cancer is also termed malignant tumor, but why is it ʻmalignantʼ? In contrast to cancer cells, ‘normal cells’ basically constitute the body. Normal cells start to proliferate when they repair an injury made in the body or cell proliferation is switched on in response to its necessity as a part of metabolism. When active proliferation becomes unnecessary, the switch is turned off and cells enter a quiescence state. Excluding some cells, such as the embryonic, blood, and bone marrow cells, basically, cells do not move to a site very far from the organ where they are originally present. On the other hand, how do cancer cells behave in these states? Cancer cells may also temporarily enter a dormant state, but their proliferation switch is basically on. Cancer cells metastasize to multiple organs and uncontrollable proliferation continues while gradually undermining physical strength of the body, that’s why cancer is ‘malignant’. Generally, cancer is treated with surgery, chemotherapy with anticancer drugs, and radiotherapy, and these are said to be the three major treatment methods of cancer. Regarding merits and demerits of the three major treatment modalities, when cancer is localized and resectable without markedly impairing the surrounding organs, cancer removal by ‘surgery’ is the most effective method. However, it is accompanied by surgical stress and may be difficult to perform when physical strength is markedly reduced. In contrast, when lesions are present in multiple organs throughout the body or diffusely scattered on the surface of the body cavities, such as abdominal and thoracic cavities, chemotherapy with anticancer drugs is performed. Recently, ‘molecular target drugs’ targeting specific intracellular molecules helping cancer cell proliferation appeared in chemotherapy. Anti-cancer drugs exhibit adverse effects damaging normal cells, in addition to cancer cells. Radiotherapy is effective against some types of cancer. Radiotherapy is local therapy sharpshooting cancer staying in a specific region, in principle. Its indication is considered when a tumor is localized to some extent but surgical removal en bloc is difficult. However, irradiation is not necessarily effective for all cancers, and there is variation in sensitivity (Fig. 1).
Fig. 1.
Indication and limitations of the three major cancer treatment modalities.
Moreover, the same region cannot be irradiated at a dose exceeding a specific dose, termed the maximum exposure dose. We fight against cancer by making full use of the ‘3 major treatment methods’, but cancer is difficult to control because it is the ‘internal enemy’, and cancer became the first place as a cause of death. Why is intrinsic eradication of cancer not possible? Except for cancer which widely expands in the body from the beginning and does not respond to treatment, many types of cancer disappear from the body after a series of treatments as if they were cured (remission). The problem is ‘recurrence’. Cancer remits only for a short time and it recurs after a specific time and then is treated again, repeating recurrence and remission, like a cat-and-mouse game. Cancer responds to anticancer drugs to some extent at the beginning, but the drugs gradually become ineffective and the cancer becomes highly malignant ‘resistant cancer’ spreading (metastasizing) throughout the body, i.e., ‘recurrence’ and accompanying ‘resistance’ are the hardest obstacles against overcoming cancer. When cancer therapy is performed, how to induce remission and when remission can be induced, how to prevent recurrence and how to deal with cancer which unfortunately recurred are important therapeutic strategies across various cancer types.
Effect of Plasma on Cancer Cells
The effect of plasma irradiation on mammalian cells was initially reported by Kieft et al.(11) in 2004, in which cell adhesion was actually inhibited by irradiation and resulted in induction of cell death. Since then, apoptotic cell death induced by plasma irradiation has been reported over the last decade in not only normal cells, such as fibroblasts and mammary gland epithelial cells, but also various cancer cells, such as brain tumor and colorectal, skin, lung, pancreatic, and ovarian cancer cells.(12–17) The therapeutic effect of plasma is not limited to a specific cancer type, and it is constant for all types of tumor. Unlike necrosis, which is passive cell death in response to external stimulation, apoptosis is active cell death. Apoptotic cell death is not accompanied by inflammation of the surrounding tissue, being considered as a mechanism managed and adjusted in the proliferation control system.
Why does plasma influence cell proliferation activity? It has been confirmed that various ions, radical species, and particle types with biologically strong actions are generated in gas-phase plasma. Inhibition of cell proliferation or action causing cell death is considered due to impairment of redox equilibrium of cells caused by reactive nitrogen oxide species (RNOS) including free radicals released from plasma.(18–20) When oxidative stress increases in cancer cells, gene mutation occurs, cell proliferation is promoted, and resistance to apoptosis is induced, increasing treatment resistance of cancer cells. However, when they are exposed to oxidative stress exceeding a specific threshold, the cell function is negatively controlled by oxidative damage, resulting in arrest of proliferation followed by induction of apoptosis. Actually, RNOS production increases in a manner dependent on the plasma irradiation time,(21) and cancellation of the plasma-induced antitumor effect by the addition of oxidative stress scavenger to the culture system has been reported.(22,23)
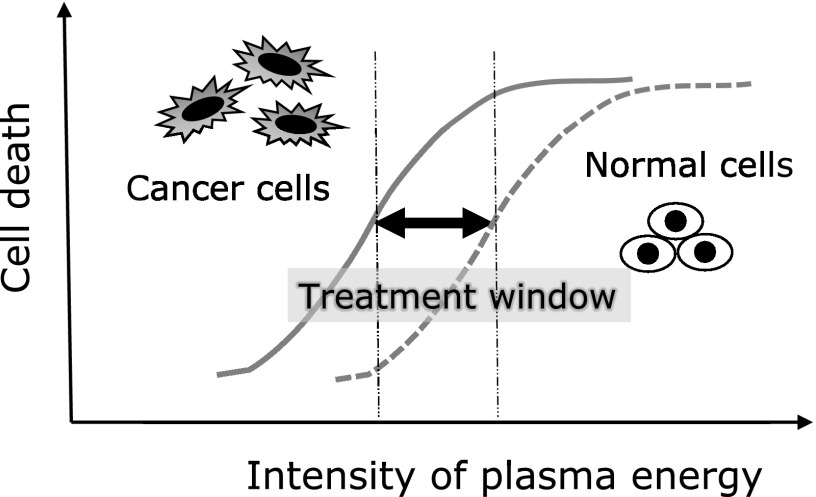
However, considering that it is a treatment for the body, a selective antitumor effect acting on only cancer cells with less toxicity to normal cells is desirable. Generally, anticancer drugs and radiotherapy are also toxic to normal cells, and these treatments utilize differences in the sensitivity between tumor and normal cells. The action is exhibited as an antitumor effect on cancer cells and an adverse effect on normal cells. Therefore, if treatment can be applied within a range in which tumor cells are sensitive but normal cells are less influenced, adverse effects can be reduced. According to several previous studies, interestingly, there is a specific selectivity in the cell proliferation-inhibitory effect of plasma: the inhibitory effect on tumor cells is stronger than that on normal cells. Zucker et al.(24) reported that when highly metastatic melanoma cells (skin cancer cells) and normal skin keratinocytes were directly irradiated with plasma for 10 s, apoptosis was 4.9 times more frequently observed in the former than in the latter after 24 h. In addition, tumor cell selectivity of plasma compared with that for normal cells was clarified by comparison between lung cancer and normal cells (embryonic fibroblasts and pulmonary epithelial cells) (Panngom et al.),(17) between glioma cells (a type of brain tumor) and astrocytes (Tanaka et al.),(25) and between ovarian cancer cells and fibroblasts (Iseki et al.).(12) Although it is still hypothetic, these study results suggest that although high-energy plasma irradiation is also toxic to normal cells, a specific optimum treatment range (treatment window) is present between normal and tumor cells (Fig. 2). If there is a difference in plasma sensitivity between normal and cancer cells, what is the underlying mechanism? The fundamental difference between cancer and normal cells may be based on the proliferation activity. For example, generally, when a space between normal cells is wide, cells proliferate to fill the space, and when cells become dense and closely contacted, the proliferation signal is turned off to stop further proliferation. This is an effect termed contact inhibition and a property of normal cells. In contrast, this control system does not act on cancer cells and they infinitely proliferate. In culture systems, when cancer cells proliferate and fill the dish, they repeat proliferation lying on top of one another and die due to malnutrition because the proliferation mechanism is constantly turned on in cancer cells, as described above. It is possible that the presence of these differences in the proliferation activity and apoptosis-inhibitory control system between cancer and normal cells influences changes in plasma sensitivity. Tanaka et al.(25) clarified that plasma-irradiated solution selectively inhibits cultured glioma cells of brain tumor on comparison of proliferation activity with that of astrocytes, and this mechanism is due to inhibition of AKT molecule, which serves as a hub of proliferation/survival signal transmission. Various molecular mechanisms may be identified in the future, in which normal cells represent all cells other than cancer cells. In previous studies, only 1–2 types of normal cells were picked up and a more marked inhibitory effect of plasma irradiation at a specific energy on proliferation of cancer cells was observed, i.e., normal cell death occurs when the energy is increased, and it is possible that some normal cells are more readily influenced by plasma than cancer cells. To apply plasma for treatment in consideration of this point, attention should be paid to the necessity of careful re-investigation.
Fig. 2.
Different sensitivity to plasma treatment between cancer and normal cells.
Is the proliferation-inhibitory effect via apoptosis the only influence of plasma irradiation on cells? Not only active proliferative ability but also various cell functions, such as abilities to infiltrate the surrounding tissue (invasive capacity), actively move for metastasis (motility), and tightly adhere to other cells to fix scaffolds and form metastatic lesions (adhesiveness), are used as indices of malignant transformation of cancer. Wang et al.(26) performed cell invasion assay using breast cancer cells and a transwell chamber. This assay applies the property of cells that cancer cells cultured on a membrane with small 8-µm pores cannot pass through the pores because the cell size is far larger than the pore size, but some cells with metastatic motility alter their morphology to an elongated shape and pass through the pores. The membrane is coated with a specific matrix (matrigel), and cells cannot reach the undersurface of the membrane unless they secrete a specific enzyme and dissolve the matrix, i.e., not only motility but also tissue-invasive capacity are observed by counting cells which moved to the undersurface of the membrane. These potentials can be compared by counting cells which could pass through the membrane out of a specific number of cells seeded on the membrane. The invasive capacity of plasma-irradiated cells decreased in a irradiation time (30–90 s)-dependent manner (decreased to about 30% by 90-s irradiation) compared with that of non-irradiated control cells. Kim et al.(13) also investigated the invasive capacity of colorectal cancer cells using a similar assay system and observed almost the same results. In these assays, the influence on cell motility was presented without strongly reflecting differences in proliferative ability because the time to assay after exposure to plasma was relatively short. The findings suggested that exposure to plasma at an energy level not inducing apoptosis reduces metastatic invasive capacity of cancer. Clarification of previously unknown effects of plasma on cancer cells is expected.
Antitumor Effect of Plasma-Irradiated Culture Medium on Ovarian Cancer Cells
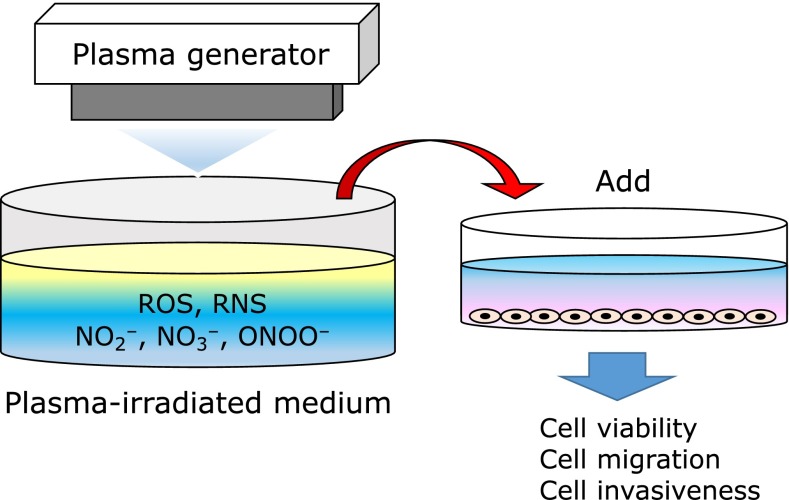
Active species in the gas phase generated by plasma irradiation are incorporated into fluid around tissue before they directly contact the biological tissue and affect the cells and tissue. Some points are still unclear with regard to how active species are generated in the liquid phase, but there is one new method to deal with it, termed in-liquid plasma (not a formal name, but I call it so). When a solution is irradiated with plasma, RNOS is produced. This is an indirect plasma treatment method in which plasma-irradiated solution (aqueous plasma) is added to the culture supernatant of separately cultured cells to expose them to RNOS without direct plasma irradiation (Fig. 3). This indirect plasma irradiation has been suggested to exhibit an effect comparable to that of direct irradiation and be sufficiently applicable for treatment.(17,18,25,27)
Fig. 3.
Indirect plasma treatment method: plasma-irradiated solution is added to the culture supernatant of separately cultured cells to expose them to RNOS without direct plasma irradiation.
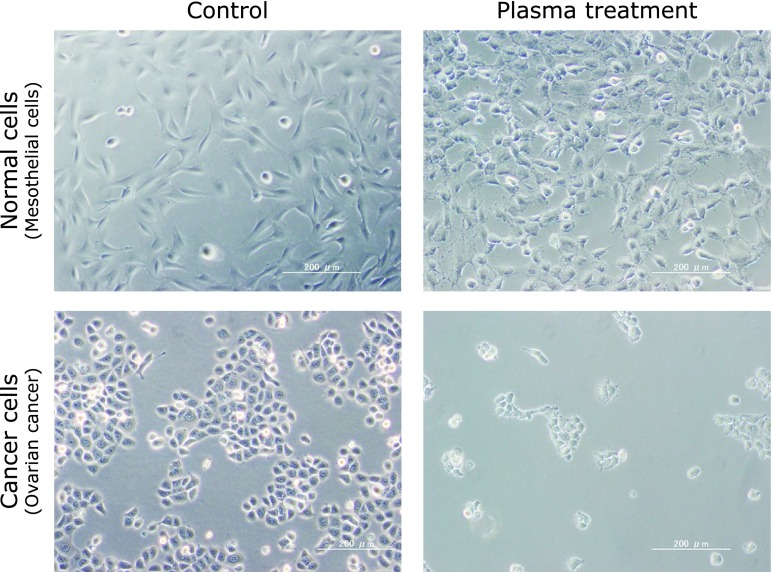
In the gynecological oncology field, the therapeutic effect of plasma-irradiated culture medium on ovarian cancer is expected. Ovarian cancer is one of the cancer types with the poorest prognosis among gynecological malignant tumors. Drug-resistant recurrence occurs in most cases of progressive ovarian cancer within 2 years even though remission was achieved by multidisciplinary treatment. Nagoya University Collaborative Study Group developed an original non-equilibrium atmospheric pressure plasma generator. When culture medium irradiated with plasma generated by this device was added to ovarian cancer cells, the irradiated culture medium exhibited an antitumor effect in a manner dependent on the exposure time.(23) Generally, normal cells around ovarian cancer are peritoneal mesothelial cells. The fluid was less toxic to normal cells (human mesothelial cells) and a selective antitumor effect was observed in only cancer cells, as described above (Fig. 4). In addition, signals of RNOS-induced reduction products were detected in ovarian cancer cells exposed to aqueous plasma, suggesting that apoptosis induction by increased intracellular RNOS is a cause of the toxicity. Interestingly, the proliferation-inhibitory effect of in-liquid plasma on an anticancer drug-resistant cell line derived from the original ovarian cancer cells was stronger than that on the parent cells. Therefore, in-liquid plasma therapy is expected to exhibit a therapeutic effect on ovarian cancer cells which acquired resistance to anticancer drugs. A treatment, intraperitoneal administration of aqueous plasma against ovarian cancer which expanded over the abdominal cavity and became resistant to anticancer drugs, might be realized in the future.
Fig. 4.
The representative images showing that plasma-irradiated solution exerts less anti-proliferative effects to normal cells, compared to cancer cells. Upper images: human mesothelial cells. Lower images: ovarian cancer cells.
Tumor-Inhibitory Effect of Plasma in Animal Model
The influence of plasma on cells appears to be a constant proliferation-inhibitory effect via apoptosis, although the application method differs: direct plasma irradiation and aqueous plasma. However, only a few studies on the tumor-inhibitory effect in animal models, which is a preliminary step before clinical application have been reported. Regarding the antitumor effect of plasma in animal models, tumor growth inhibition was reported by Vandamme in 2010.(28) They subcutaneously transplanted brain tumor cells into mice and applied DBD irradiation for 5 days to the ectopic tumor formed under the skin. The tumor size did not change at the beginning of irradiation compared with that in the non-irradiated control group, but the converted tumor volume was reduced to below 1/2 at the completion of irradiation. This difference increased with time, resulting in prolongation of the survival time by more than 30% in the treatment group compared with that in the control group. Brulle et al.(16) investigated the combined effect of DBD plasma and an anticancer drug, gemcitabine, on pancreatic cancer cells in a mouse orthotopic graft model. They applied plasma irradiation using a plasma gun to the tumor on days 14 and 24 after transplantation and administered concomitant gemcitabine 5 times during this period. The mice were euthanized on day 36 and the antitumor effect was evaluated. The tumor weight decreased by about 20% in the combination group compared with that in the group treated with gemcitabine alone. The tumor volume was also reduced to about 50% in the combination group compared with that in the group treated with plasma alone. Keidar et al.(29) investigated the antitumor effect of plasma using Plasma Jet in a mouse subcutaneous ectopic tumor model of urinary bladder cancer, and observed that the tumor markedly shrank and the mean survival time extended by about 9 days in the plasma treatment group compared with that in the untreated group (plasma treatment group: 33.5 days vs untreated group: 24.5 days). Other than the effect of direct plasma irradiation, does local injection of plasma-irradiated solution exhibit an effect? We prepared a nude mouse model of subcutaneous tumor formation in the thigh using anticancer drug-resistant ovarian cancer cells to investigate the antitumor effect of plasma-irradiated culture medium in vivo. The weight of anticancer drug-sensitive ovarian cancer cell tumor excised on day 28 after treatment initiation was reduced to 66% in the in-liquid plasma treatment group compared with that in the control group and that of anticancer drug-resistant ovarian cancer cells was also reduced to 52% compared with that in the control group.(23) Although the administration method was different: direct plasma irradiation and local administration of in-liquid plasma, the therapeutic tumor-shrinking effect on localized tumor was observed in the animal experimental model. Although it is necessary to accumulate various basic study results, plasma irradiation of localized superficial tumor present on the body or body cavity surface is sufficiently assumable treatment.
However, it is practically difficult to apply plasma irradiation to many tumors diffusely spreading all over the body cavity. The problem in actual clinical practice is the presence of many small lesions widely spreading over the body cavity, such as peritoneal metastasis of ovarian cancer, and either surgery or radiation is unlikely to be useful treatment. Anticancer drugs may be used, but when the tumor has acquired resistance, the efficacy is not satisfactory. There are various cavities in the body, and the abdominal cavity containing the stomach, intestine, liver, gall bladder, spleen, uterus, and ovary is the largest. The abdominal cavity is covered with the peritoneum, which often serves as a tumor bed of diffusely spreading digestive organ-derived and ovarian cancers. There is also the thoracic cavity holding the lung and medullary cavity holding the brain and spinal cord. The pathologies of cancer extensively spreading in these body cavities are termed peritoneal, pleural, and intramedullary dissemination, respectively. Intra-cavity treatment with aqueous plasma is thought to be one of the most effective modalities of plasma cancer therapy.
Possibility of Application of Plasma for Cancer Therapy
If plasma is indicated for cancer therapy, for what type of cancer is it effective and what are the problems? Direct plasma irradiation for local treatment is relatively similar to radiotherapy among the three major cancer therapies. Irradiation induces cell activation when the dose is very low, but it induces cell death at a dose higher than a specific level. Utilizing the fact that cancer cells are more sensitive to radiation compared with normal cells, the therapeutic dose range in which the therapeutic effect can be obtained while minimizing damage of normal cells is established. These properties are common to the apoptosis-inductivity of plasma, as described above. In-liquid plasma has a point in common with intra-cavity drug administration capable of extensively exposing cancer to drugs, such as intraperitoneal administration against ovarian cancer. In addition, there may be a cell death-inducing mechanism different from the conventional mechanism of anticancer drugs, for which an effect on anticancer drug-resistant cancer cells may be expected. Furthermore, previous basic studies clarified that plasma inhibits the motility and invasive capacity of cancer cells. Although it is still hypothetical, it may inhibit secondary metastasis of cancer cells which escaped from cell death.
Although plasma has these promising possibilities, problems to be overcome are also present. The mechanism of the proliferation-inhibitory effect of plasma is RNOS production in cells, as described, but reactive oxygen species (ROS) scavengers, such as glutathione, are abundantly present in body tissue. Oxidative stress is constantly generated in the body and biologics would not have advanced to this level without the development of the system removing the stress and protecting the body. Considering this, it is understood that the presence of scavengers is deep-rooted. Generally, the influence of plasma tends to decrease in animal experiments although a marked effect was observed in cell studies, and this may be due to the removal of plasma-induced ROS by ROS scavengers in vivo. Solution of this problem by improving the plasma irradiator and developing a method to temporarily inhibit scavengers is continuously searched for. Multiple applications and intra-cavity perfusion of in-liquid plasma are also being investigated on the assumption that the effect of single administration will be scavenged.
Another problem is the extent of tumor infiltration of plasma. According to a previous report, tissue permeability of plasma is about 50 µm from the superficial layer.(30) Since the penetration capability of RNOS generated by the current experimental plasma generator is shallow, it cannot readily reach the inner region of tumors which have already grown to a certain size. Can it be actually solved by increasing the plasma intensity? To apply high-energy plasma irradiation or in-liquid plasma, it is necessary to sufficiently confirm the absence of administration-related adverse events at the animal experiment level. In any case, it is necessary to further improve the plasma irradiation device through information exchange among physicians, physicists, and engineers.
Conclusion
The prognosis of metastatic and recurrent cancers is still poor despite advances in medicine, as described above. The environment to promote ‘plasma medical science’ will be developing from now on, and early creation of the new interdisciplinary area and preparation of a systematic research structure are important. Plasma medicine, which may cause a stir in the cancer therapy field with a sense of stagnation, is promising in the future.
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Fridman G, Peddinghaus M, Balasubramanian M, et al. Blood coagulation and living tissue sterilization by floating-electrode dielectric barrier discharge in air. Plasma Chem Plasma Process. 2006;26:425–442. [Google Scholar]
- 2.Kalghatgi SU, Fridman G, Cooper M, et al. Mechanism of blood coagulation by nonthermal atmospheric pressure dielectric barrier discharge plasma. Ieee Trans Plasma Sci. 2007;35:1559–1566. [Google Scholar]
- 3.Goree J, Liu B, Drake D, Stoffels E. Killing of S. mutans bacteria using a plasma needle at atmospheric pressure. IEEE Trans Plasma Sci. 2006;34:1317–1324. [Google Scholar]
- 4.Kolb JF, Mohamed AAH, Price RO, et al. Cold atmospheric pressure air plasma jet for medical applications. Appl Phys Lett. 2008;92:241501. [Google Scholar]
- 5.Stoffels E, Kieft IE, Sladek REJ, van den Bedem LJM, van der Laan EP, Steinbuch M. Plasma needle for in vivo medical treatment: recent developments and perspectives. Plasma Sources Sci T. 2006;15:S169–S180. [Google Scholar]
- 6.Fridman G, Friedman G, Gutsol A, Shekhter AB, Vasilets VN, Fridman A. Applied plasma medicine. Plasma Process Polym. 2008;5:503–533. [Google Scholar]
- 7.Fridman G, Shereshevsky A, Jost MM, et al. Floating electrode dielectric barrier discharge plasma in air promoting apoptotic behavior in melanoma skin cancer cell lines. Plasma Chem Plasma Process. 2007;27:163–176. [Google Scholar]
- 8.Shashurin A, Keidar M, Bronnikov S, Jurjus RA, Stepp MA. Living tissue under treatment of cold plasma atmospheric jet. Appl Phys Lett. 2008;93:181501. [Google Scholar]
- 9.Kong MG, Kroesen G, Morfill G, et al. Plasma medicine: an introductory review. New J Phys. 2009;11:115012. [Google Scholar]
- 10.Laroussi M. Low temperature plasma-based sterilization: overview and state-of-the-art. Plasma Process Polym. 2005;2:391–400. [Google Scholar]
- 11.Kieft IE, Broers JL, Caubet-Hilloutou V, Slaaf DW, Ramaekers FC, Stoffels E. Electric discharge plasmas influence attachment of cultured CHO K1 cells. Bioelectromagnetics. 2004;25:362–368. doi: 10.1002/bem.20005. [DOI] [PubMed] [Google Scholar]
- 12.Iseki S, Nakamura K, Hayashi M, et al. Selective killing of ovarian cancer cells through induction of apoptosis by nonequilibrium atmospheric pressure plasma. Appl Phys Lett. 2012;100:113702. [Google Scholar]
- 13.Kim CH, Bahn JH, Lee SH, et al. Induction of cell growth arrest by atmospheric non-thermal plasma in colorectal cancer cells. J Biotechnol. 2010;150:530–538. doi: 10.1016/j.jbiotec.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Haertel B, Hahnel M, Blackert S, Wende K, von Woedtke T, Lindequist U. Surface molecules on HaCaT keratinocytes after interaction with non-thermal atmospheric pressure plasma. Cell Biol Int. 2012;36:1217–1222. doi: 10.1042/CBI20120139. [DOI] [PubMed] [Google Scholar]
- 15.Kaushik NK, Attri P, Kaushik N, Choi EH. A preliminary study of the effect of DBD plasma and osmolytes on T98G brain cancer and HEK non-malignant cells. Molecules. 2013;18:4917–4928. doi: 10.3390/molecules18054917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brullé L, Vandamme M, Riès D, et al. Effects of a non thermal plasma treatment alone or in combination with gemcitabine in a MIA PaCa2-luc orthotopic pancreatic carcinoma model. PLoS One. 2012;7:e52653. doi: 10.1371/journal.pone.0052653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panngom K, Baik KY, Nam MK, Han JH, Rhim H, Choi EH. Preferential killing of human lung cancer cell lines with mitochondrial dysfunction by nonthermal dielectric barrier discharge plasma. Cell Death Dis. 2013;4:e642. doi: 10.1038/cddis.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalghatgi S, Kelly CM, Cerchar E, et al. Effects of non-thermal plasma on mammalian cells. PLoS One. 2011;6:e16270. doi: 10.1371/journal.pone.0016270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahn HJ, Kim KI, Kim G, Moon E, Yang SS, Lee JS. Atmospheric-pressure plasma jet induces apoptosis involving mitochondria via generation of free radicals. PLoS One. 2011;6:e28154. doi: 10.1371/journal.pone.0028154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arjunan KP, Friedman G, Fridman A, Clyne AM. Non-thermal dielectric barrier discharge plasma induces angiogenesis through reactive oxygen species. J R Soc Interface. 2012;9:147–157. doi: 10.1098/rsif.2011.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan X, Xiong Z, Zou F, et al. Plasma-induced death of HepG2 cancer cells: intracellular effects of reactive species. Plasma Process Polym. 2012;9:59–66. [Google Scholar]
- 22.Kang SU, Cho JH, Chang JW, et al. Nonthermal plasma induces head and neck cancer cell death: the potential involvement of mitogen-activated protein kinase-dependent mitochondrial reactive oxygen species. Cell Death Dis. 2014;5:e1056. doi: 10.1038/cddis.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Utsumi F, Kajiyama H, Nakamura K, et al. Effect of indirect nonequilibrium atmospheric pressure plasma on anti-proliferative activity against chronic chemo-resistant ovarian cancer cells in vitro and in vivo. PLoS One. 2013;8:e81576. doi: 10.1371/journal.pone.0081576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zucker SN, Zirnheld J, Bagati A, et al. Preferential induction of apoptotic cell death in melanoma cells as compared with normal keratinocytes using a non-thermal plasma torch. Cancer Biol Ther. 2012;13:1299–1306. doi: 10.4161/cbt.21787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka H, Mizuno M, Ishikawa K, et al. Plasma-activated medium selectively kills glioblastoma brain tumor cells by down regulating a survival signaling molecule, AKT kinase. Plasma Medicine. 2011;1:265–277. [Google Scholar]
- 26.Wang M, Holmes B, Cheng X, Zhu W, Keidar M, Zhang LG. Cold atmospheric plasma for selectively ablating metastatic breast cancer cells. PLoS One. 2013;8:e73741. doi: 10.1371/journal.pone.0073741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato T, Yokoyama M, Johkura K. A key inactivation factor of HeLa cell viability by a plasma flow. J Phys D Appl Phys. 2011;44:372001. [Google Scholar]
- 28.Vandamme M, Robert E, Dozias S, et al. Response of human glioma U87 xenografted on mice to non thermal plasma treatment. Plasma Medicine. 2011;1:27–43. [Google Scholar]
- 29.Keidar M, Walk R, Shashurin A, et al. Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. Br J Cancer. 2011;105:1295–1301. doi: 10.1038/bjc.2011.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Partecke LI, Evert K, Haugk J, et al. Tissue tolerable plasma (TTP) induces apoptosis in pancreatic cancer cells in vitro and in vivo. BMC Cancer. 2012;12:473. doi: 10.1186/1471-2407-12-473. [DOI] [PMC free article] [PubMed] [Google Scholar]