Abstract
Owing to its distinctive electrochemical properties with interconvertible multiple oxidation states, iron plays a significant role in various physiologically important functions such as respiration, oxygen transport, energy production, and enzymatic reactions. This redox activity can also potentially produce cellular damage and death, and numerous diseases are related to iron overload resulting from the dysfunction of the iron regulatory system. In this case, “free iron” or “labile iron,” which refers to iron ion weakly bound or not bound to proteins, causes aberrant production of reactive oxygen species. With the aim of elucidating the variation of labile iron involved in pathological processes, some chemical tools that can qualitatively and/or quantitatively monitor iron have been utilized to investigate the distribution, accumulation, and flux of biological iron species. Since iron ions show unique reactivity depending on its redox state, i.e., Fe2+ or Fe3+ (or transiently higher oxidative states), methods for the separate detection of iron species with different redox states are preferred to understand its physiological and pathological roles more in detail. The scope of this review article covers from classical chromogenic to newly emerging chemical tools for the detection of Fe ions. In particular, chemical tools applicable to biological studies will be presented.
Keywords: iron, fluorescenct probe, chromogenic probe, imaging
Introduction
An adult human accumulates ca. 3–4 g of iron in his body, this amount being prominently high as compared to other transition metals. Iron is involved in a considerable number of physiologically important processes such as respiration, oxygen transport, signal transduction, and enzymatic reactions in a biological system(1–3) relying on its ability to activate molecular oxygen. Activation of oxygen means generation of reactive oxygen species (ROS).(4) Under physiologically normal conditions, the rate between production and consumption/detoxification of ROS is strictly controlled to exploit the beneficial aspects of ROS. Dysfunction of the iron homeostasis process could seriously affect the balanced ROS metabolism leading to the aberrant production of ROS, thus, resulting in cell damage and death.(4) In particular, the Fenton reaction has been known as one of the most harmful reactions in a biological system where Fe2+ acts as an activator to generate highly toxic hydroxyl radicals (or highly reactive chemical species) that can induce DNA damage and peroxidation of lipids.(5–7) Thus, an excess of iron has been reported to be highly relevant to serious diseases, such as cancer,(8,9) and neurodegenerative diseases including Alzheimer and Parkinson, where deposits of iron are often observed in patients suffering these diseases.(10–12) Moreover, some indexes relevant to biological iron such as the total serum iron concentration, total iron binding capacity, and the unsaturated iron binding capacity have been clinically used for diagnosing hemochromatosis and anemia.(13) Since iron has multiple oxidation states, Fe2+ or Fe3+ (or transiently higher oxidative states), and shows unique reactivity depending on the redox state, utilizing analytical methods for separate detection of iron at different redox states is essential to deeply understand the physiological and pathological roles of this metal ion.
This review article focuses on not only classical but also emerging chemical tools for detecting Fe ions. In particular, chemical tools applicable to biological studies will be presented.
Chromogenic Chelators for Colorimetric Methods
Metal complexes with chelators sometimes show characteristic intense colors (i.e., absorption in the visible region) attributed to their metal to ligand charge transfer (MLCT) and/or ligand to metal charge transfer (LMCT) band. The color changes are generally dependent on the metal concentration, thereby enabling accurate quantification of metal ions on the basis of molar absorptivity of the complexes. In this context, colorimetric methods using chromogenic chelators have been widely used to quantitatively determine the concentration of iron ions. Although tissues and cells need to be homogenized prior to measurements, colorimetric methods enable easy and precise quantification with conventional instruments such as UV–vis spectrometers and microplate readers. In this section, we present several chromogenic chelators for the selective detection and quantification of iron ions.
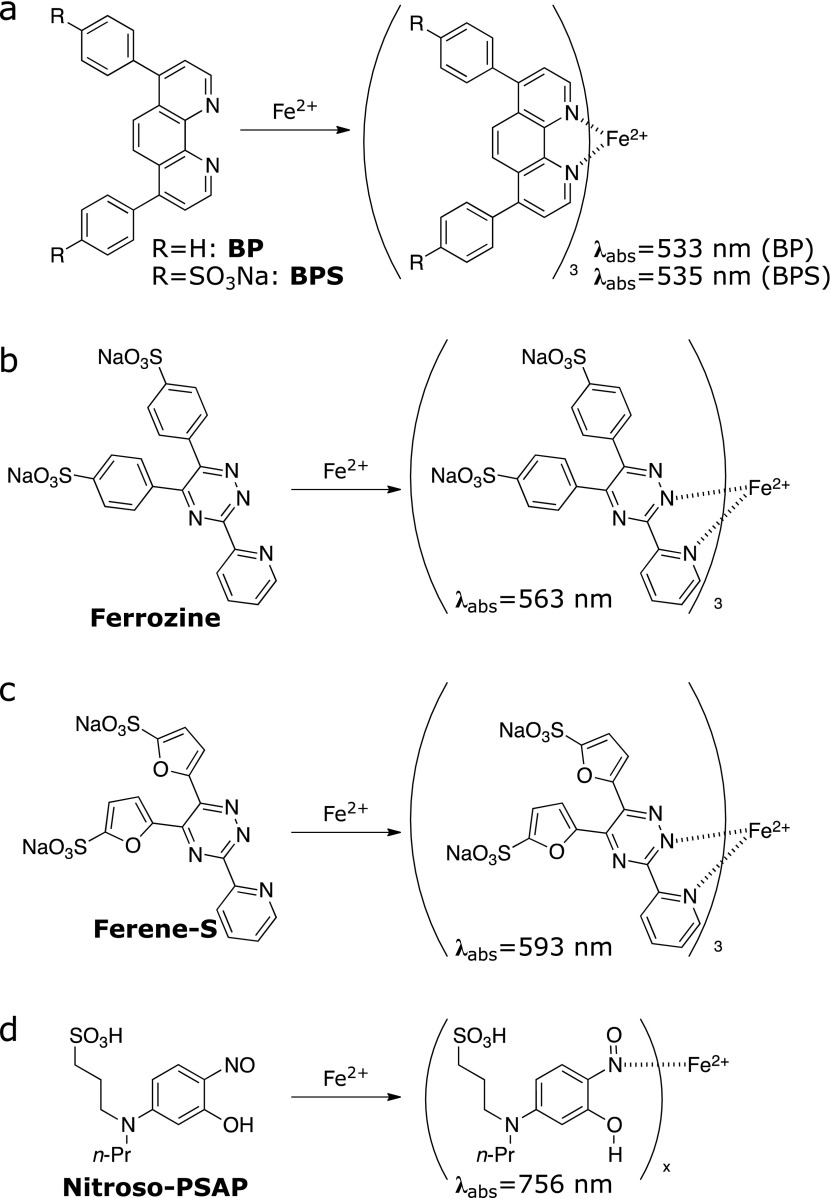
Bathophenanthroline (BP) and bathophenanthroline disulfonic acid disodium salt (BPS) (Fig. 1a)
Fig. 1.
Chemical tools for the colorimetric detection of Fe ions. (a) BP and BPS, (b) Ferrozine, (c) Ferene-S, and (d) Nitroso-PSAP.
4,7-Diphenyl-1,10-phenanthroline, known as BP,(14) has been developed as an indicator of Fe2+. BP showed an improved molar absorptivity of 22,400 cm−1 M−1 at 533 nm upon formation of a 3:1 complex with Fe2+ as compared to its parent compound 1,10-phananthroline (11,100 cm−1 M−1). In the first report on colorimetric methods with BP, total iron in aqueous samples was reduced by hydroxylamine followed by extraction with water-insoluble alcohols such as isoamyl and n-hexyl alcohols. The optical density (OD) or absorbance at 533 nm of the ferrous complex extracted into the alcohol fraction was measured and compared with standard solutions to determine the exact concentration of Fe2+ ions. The detection range with a cuvette of 1 cm path length was 0.1–1 ppm (1.8–18 µM). The BP method had been employed as standard method to determine the concentration of serum iron by the International Committee for Standardization in Haematology (ICSH), although it is not recommended at present.(15,16)
BPS was produced by the introduction of two hydrophilic sulfonates on BP. Since it is the water-soluble analogue of BP, it enables direct application to aqueous sample without the need of extraction processes.(17) Introduction of two hydrophilic sulfonates on BP enhances water-solubility. Sulfonation and its application in aqueous samples did not alter its molar absorptivity (22,140 cm−1 M−1), color (λabs = 535 nm), and sensitivity characteristics. It is noted that the presence of excess copper ion may interfere with quantification of iron via formation of a 2:1 complex with BP or BPS (λabs = 425 nm) in the presence of reductants such as ascorbate and hydroxylamine.
Ferrozine and Ferene-S (Fig. 1b and c)
3-(Pyridin-2-yl)-5,6-bis(4-sulfophenyl)-1,2,4-triazine disodium salt, known as Ferrozine,(18) is a highly water-soluble chromogenic chelator of Fe2+. Ferrozine has one pyridyl group and two phenylsulfonate groups on a 1,2,4-triazine core. The two sulfonate groups provide high water-solubility characteristics and allow utilization in aqueous samples. The chelating motif of the pyridyl-tetrazine core forms a 3:1 complex with Fe2+ having an intense color (absorption centered at 563 nm) with high molar absorptivity (27,900 cm−1 M−1). The presence of five equivalents of cobalt (Co2+) and copper (Cu+) introduces 5% and 15% error, respectively. 3-(Pyridin-2-yl)-5,6-bis(5-sulfofuran-2-yl)-1,2,4-triazine disodium salt, known as Ferene-S, is an analogue of Ferrozine where the 4-sulfonylphenyl groups are substituted with 5-sulfonylfuryl groups.(19) Ferene-S presents absorption maximum at 593 nm and a molar absorption of 34,500 cm−1 M−1, thereby improving sensitivity up to ca. 1.3-fold compared to Ferrozine. The selectivity and limitations are similar to those of Ferrozine. Quantitative iron detection kits based on Ferrozine or Ferene-S are now commercially available and recommended as standard methods of iron quantification by ICSH and the Clinical and Laboratory Standards Institute (CLSI).(15,20)
Nitroso-PSAP (Fig. 1d)
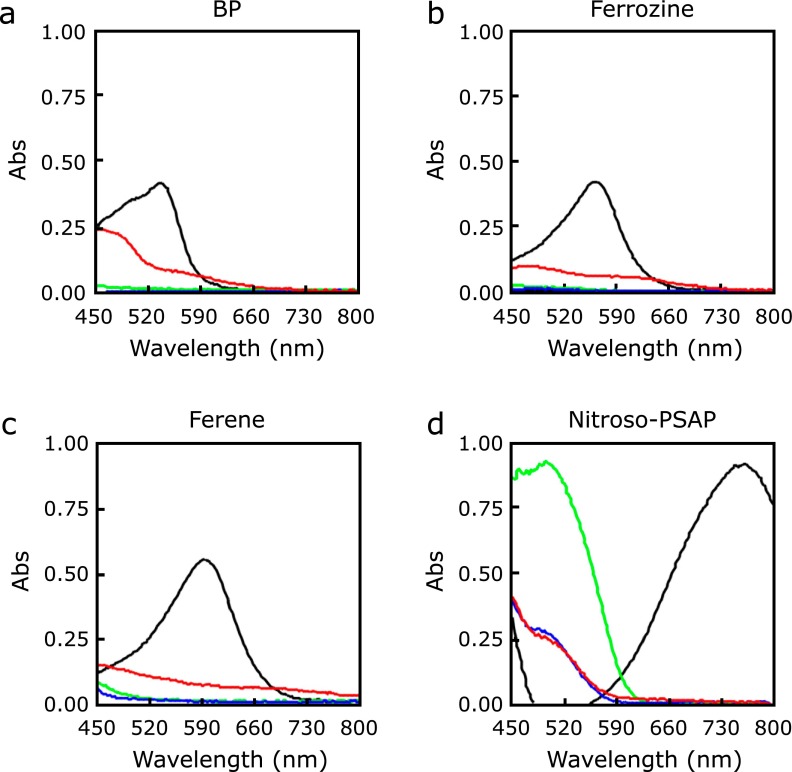
A series of nitrosophenol derivatives had been known to show absorption maxima in the near infrared region upon binding to Fe2+ ion. 2-Nitroso-5-(N-propyl-N-sulfopropylamino)phenol, known as Nitroso-PSAP, shows higher water-solubility and selectivity to Fe2+ ion as compared to other transition metal ions.(21,22) The colorimetric response to Fe2+ ion via formation of complex is highly specific (i.e., unique absorption wavelength at 756 nm) with high molar absorptivity (45,000 cm−1 M−1). This response can be clearly differentiated from those showed by the complexes with other metal ions such as Co2+ (493 nm), Cu2+ (430 nm), and Ni2+ (391 nm). This large separation of the absorption band enables selective and accurate quantification of Fe2+ ion in aqueous samples in the presence of other metal ions. Thus, Nitroso-PSAP shows a low error of only 7% in the presence of Cu2+. Recently, Ito et al.(23) reported a well-established assay system of non-transferrin-bound iron (NTBI) by exploiting the Nitroso-PSAP system. In this report, they compared the spectral changes of Nitroso-PSAP, BP, Ferene-S, and Ferrozine when forming complexes with Fe2+, Cu2+, Co2+, and Ni2+ to decide the best chromogenic chelator for their iron detection system (Fig. 2).
Fig. 2.
Absorbance spectrum of iron-chelating chromogenic chelators complexed with metal ions (cited from ref. (23): Clin Chim Acta, 2014; 437: 129–135). Black: Fe2+, red: Cu2+, green: Co2+, blue: Ni2+. (a) BP, (b) Ferrozine, (c) Ferene, and (d) Nitroso-PSAP.
All the colorimetric methods described above are well-established and accurate methods for quantification of Fe2+ in aqueous samples without the need of expensive instruments such as atomic absorption (AAS) and inductive-coupled plasma mass spectrometers (ICP-MS) and radioisotopes (59Fe). However, these colorimetric methods require treatment of the sample with reductant, surfactant, and/or acid agents to prepare a homogenous solution, thereby making it hard to apply to living samples.
Fluorescent Probes
Unlike colorimetry, fluorescent probes are capable of monitoring metal ions in cuvette and in living cells/animals. Fluorescent probes are categorized into three types depending on the mode of signal change: turn-on, turn-off, and ratiometric probes. Turn-on and turn-off probes show increase and decrease in fluorescence signal against the targets (iron), respectively. Ratiometric probes provide shifts in emission or excitation wavelength and readout of ratio between two intensities. Generally, transition metal ions having unoccupied d-orbitals exhibit strong fluorescence-quenching effects due to their paramagnetic effect.(24) Therefore, both Fe3+ and Fe2+ act as quenchers of fluorescence, and the majority of early fluorescent probes for iron ions exhibit turn-off responses resulting from the quenching effect of the metal ions. Ratiometric probes provide shifts in emission or excitation wavelength and ratio readout between two intensities. In this section, we present the progress in the development of fluorescent probes for Fe3+ and Fe2+ ranging from early turn-off probes to emerging turn-on and ratiometric probes.
Early fluorescent probes with turn-off response
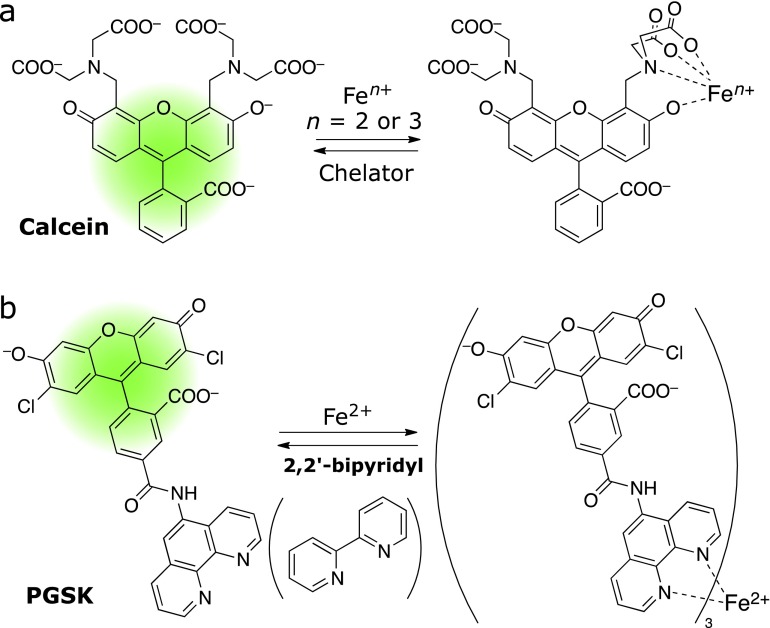
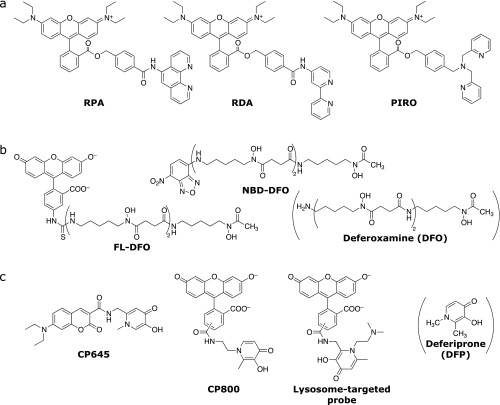
The first fluorescent probe of Fe ion is calcein. Breuer et al.(25,26) found that calcein and its acetoxymethyl ester (calcein-AM) (Fig. 3a) can work as turn-off fluorescent probes of Fe ion. These probes consist of fluorescein as a fluorophore and two iminodiacetate arms as a metal ion receptor. The fluorescence signal decreased to 46% upon Fe ion binding to the metal receptor of calcein. However, this probe cannot distinguish between Fe2+ and Fe3+ since Fe2+ is readily oxidized to Fe3+ upon binding to calcein. These authors explored cell-based fluorometry with calcein-AM to monitor the uptake of Fe ion by K562 cell and succeeded in observing attenuation of fluorescence signal as a result of metal binding to calcein and subsequent recovery of the signal by loading salycylaldehyde-isonicotinoyl-hydrazone (SIH)(27) or BIP (2,2'-bipyridyl),(25) which remove Fe3+ and/or Fe2+ from calcein. These experiments represent the first success to trace intracellular iron flux by fluorometry.(28) PhenGreen-SK (PGSK), which is composed of 2',7'-dichlorofluorecein tethered with 1,10-phenanthroline, was subsequently proposed as a Fe2+-selective chelator. PGSK forms a 3:1 complex with Fe2+ and exhibits turn-off response (Fig. 3b). Rauen and Petrat performed fluorescence imaging experiments with PGSK and revealed that “chelatable iron ions” or “labile irons” are present in living hepatocytes. The binding affinity of PGSK to Fe2+ is much stronger than calcein and too strong to dissociate Fe2+ from the chelating moiety of PGSK by other iron chelator, and therefore, calcein has been favored to study cytoplasmic iron pools.(29) The endogenous labile iron ions were found to exist as Fe2+ rather than Fe3+ by using a Fe2+-selective chelator, 2,2'-bipyridyl.(30) Petrat et al.(31) reported RPA {rahodamine B-[(1,10-phenanthroline-5-yl)aminocarbonyl]benzyl ester}, an analogue of PGSK, which can localize the mitocondria and monitor the mitochondrial flux of Fe2+ (Fig. 4). Although RPA is a turn-off type fluorescent probe, they succeeded in detecting accumulative iron in mitochondria caused by inhibition of heme synthesis. Remarkably, this study indicates that Cu2+ can also induce quenching of RPA. These authors also reported utilization of RDA and PIRO (Fig. 4a), new probes bearing 2,2'-bipyridyl and dipyridylmethylamine, respectively, which were designed to monitor mitochondrial Fe2+ as analogues of RPA.(32) RDA showed improved affinity to Fe2+ as compared to RPA, while that of PIRO was not intense enough to detect endogenous Fe2+ in mitochondria.
Fig. 3.
Classical turn-off fluorescent probes of iron ions. (a) Calcein and (b) Phen-Green SK (PGSK).
Fig. 4.
Turn-off fluorescent probes applicable to live cell imaging. (a) Mitochondria-targeted probes. (b) Siderophore-based probes. (c) Deferiprone-based probes.
Calcein, calcein-AM, and PGSK are commercially available at present and widely used to monitor labile iron in living systems by fluorescence microscopy and fluorometry. For the use of such turn-off type fluorescent probes, it is necessary to distinguish positive from false-positive responses owing to photo-bleaching and leakage of the probe from cells. Moreover, both probes show similar turn-off responses to other metal ions such as Ni2+, Cu2+, and Co2+, among others.
A large number of probes for Fe3+ have been reported, although the majority of them show turn-off type response. Several probes have iron-binding ligands derived from siderophores, which are natural iron-chelating compounds secreted by plants and bacteria to uptake insoluble Fe3+ from soil. FL-DFO(33) and NBD-DFO(34) (Fig. 4b) have desferrioxamine (DFO) motifs for iron binding. DFO is a highly selective and strong siderophore to Fe3+, which makes these probes highly selective and sensitive to this ion.
3-Hydroxy-1,2-dimethylpyridin-4-one, known as deferiprone (DFP), is clinically used as an iron-exporting agent(35) and utilized to develop several turn-off probes. A series of Fe3+ probes consisting of coumarin fluorophore conjugated with a DFP motif have been reported. Among them, the cell membrane permeable analogues (CP645 and CP800, Fig. 4c), have quenching efficiencies for Fe3+ of 93% and 66%, respectively.(36) These two probes showed significant quenching characteristics in live cell applications using hepatocyte cells. Turn-off responses were also observed against Fe2+ because of acceleration of oxidation (from Fe2+ to Fe3+) upon chelation, thereby indicating that this type of probes with DFP as a metal receptor enable to measure total labile iron concentrations. The same group reported a derivative of CP800 to monitor lysosomal labile iron (Fig. 4c).(37)
The main drawback of the siderophore-inspired probes described above is their turn-off readout, which causes low spatial resolution while imaging and false positive derived from photo bleaching and leakage of the probes, as noted above.
Turn-on fluorescent probes of Fe3+
Unlike turn-off fluorescent probes, turn-on fluorescent probes for aqueous Fe3+ are still limited. Hua and Wang(38) reported the first turn-on probe of Fe3+ that can work in aqueous media (Fig. 5, Fe3+-1). This probe consists of two cyclic polyether groups attached on a 7-aminocoumarin moiety. This probe showed a 15-fold increase in fluorescence at 412 nm (λex = 336 nm), with the response being highly selective to Fe3+. The affinity (Ka) toward Fe3+ was calculated to be 1.5 × 105 M−1 in the form of 1:1 complex. This probe also showed a minor increase in fluorescence (2.5-fold) upon Cu2+ with a Ka of 2.4 × 103 M−1, which can be considered negligible compared to its response against Fe3+. This probe requires UV light (336 nm) for excitation.
Fig. 5.
Turn-on and ratiometric fluorescent probes of Fe3+ applicable to live cell imaging.
BODIPY-based turn-on fluorescent probe for Fe3+ (Fig. 5, Fe3+-2) was reported by Bricks et al.(39) They utilized a macrocycle (3-aza-10-oxa-1,7-dithiacyclododecane) as a Fe3+-binding receptor, which was attached on the meso-position of the BODIPY dye. The probe showed over 500-fold increase in its fluorescence intensity at 512 nm (λex = 470 nm) upon addition of Fe3+ or Cu+ in acetonitrile, this increase being 15-fold in weakly acidic aqueous solution (pH 5.1 or 5.8) or non-buffered water. Unlike the aqueous system, the response was not selective in acetonitrile. The stoichiometry between the probe and Fe3+ was 1:1, with Ka = 1.6 × 104, 1.0 × 104, 1.3 × 104 M−1 in MOPS buffer (pH 5.1), tris HCl buffer (pH 5.8), and water, respectively. This probe can be excited by visible wavelength for detection, although no biological application was demonstrated.
Controlling the equilibrium between spirocycle and open-quinoid structures of the rhodamine derivative has been established (summarized in Fig. 5, right column) as a reliable strategy to design fluorescent probes for Fe3+.(40) Xiang and Tong(41) (Fe3+-3) first employed rhodamine spirolactam to develop a turn-on fluorescent probe by tethering two rhodamine B spirolactams with a 3-aza-pentylene linker. The spirocyclic structure of rhodamine B was dominant in aqueous media within a neutral pH range (5.0–9.0), resulting in no visible absorption and fluorescence. Upon binding to Fe3+, the spirolactam opened via coordination of oxygen or nitrogen atoms to Fe3+. Probe Fe3+-3 responded selectively to Fe3+ in neutral aqueous buffer showing a fluorescence enhancement rate of 114-fold at 572 nm (λex = 510 nm). The fluorescence response was reversible by addition of EDTA. However, the affinity of the probe was not high enough for cellular applications (Ka = 3.1 × 103 M−1). Once the design strategy using rhodamine spirolactam was established, a number of fluorescent probes having different chelating motifs emerged on the basis of similar strategies. Since a good review covering all these probes has been recently published elsewhere,(40) herein we focused on examples that are applicable to aqueous media (<5% organic co-solvent) and imaging studies.
Zhang et al.(42) reported rhodamine B hydrazone-spirolactam (FD1) and its cellular application. Under excitation at 510 nm, the addition of Fe3+ to FD1 in aqueous buffer resulted in up to 112-fold increase in fluorescence at 582 nm. The binding stoichiometry was determined to be 1:1 with a binding constant of Ka = 2.3 × 104 M−1. Imaging studies of pig iliac artery endothelium cells revealed that FD1 was able to detect intracellular Fe3+. Kang et al.(43) utilized Fe3+-mediated hydrolysis of a Shiff base combined with the spirolactam strategy. They designed and synthesized FS-1 having rhodamine 6G spirolactam tethered with a Shiff base unit, which was readily hydrolyzed in the presence of Fe3+. FS-1 worked in a 5% MeCN aqueous solution showing fluorescence enhancement with good contrast and selectivity and was successfully applied to imaging of HepG2 (hepatocarcinoma) cells. Wang et al.(44) pointed out that the low water solubility of the parent fluorophore (variants of rhodamine spirolactam) prevented the probes from being used in biological samples. It was the main drawback of controlling spirolactam strategy. Thus, they developed a good water-compatible probe Fe3+-4 from a rhodamine 6G derivative (Rh6G-LEDA), which works as a Fe3+ probe via the spirolactam mechanism, conjugated with polyethylene glycol (PEG)-coated Fe3O4 nanoparticles (NP) to enhance the biological utility of the spirolactam-rhodamine variants. The probe-loaded NP showed efficient fluorescence responses (up to 56-fold increase at 545 nm) against Fe3+ selectively with highly improved affinity (Ka = 5.0 × 106 M−1). They demonstrated that the probe-loaded NP worked in HeLa cells to detect Fe3+. Recently, further efforts to improve the rhodamine spirolactam-based fluorescent probes regarding to sensitivity, water-solubility, and biocompatibility have been made to achieve live cell applications. These efforts have resulted in the onset of a number of probes for Fe3+ with similar structure. Thus, Bordini et al.(45) appended a 2-hydroxybenzilideneaminoethyl group at the spirolactam to form Rhodenal, which shows color change to Fe3+ in 50% ethanolic aqueous solutions. They successfully explored live cell imaging study. Wu et al.(46) presented P1, a similar probe than Rhodenal, in which the phenyl group of benzylidene moiety was replaced with a naphtyl group. It is noteworthy that P1 was able to detect Fe3+ not only in live cells but also in zebrafish. In the case of RD-1 developed by Ji et al.(47), a pyridylmethylaminomethylcarbonyl group was introduced as an iron receptor that allowed use of this probe in aqueous system and imaging study. Yan and coworkers(48) attached a 1,2,3-triazine structure bearing a 2-pyridylamino substituent on the spirolactum to improve water compatibility affording RC2P. RC2P worked in pure aqueous solutions (without organic co-solvent) and successfully detected Fe3+ in living HL7702 cells by fluorescence imaging.
Pyochelin is a different type of siderophore produced by Pseudomonas aeruginosa (P. aeruginosa) and common to several pathogenic bacterial strains. Noël et al.(49) exploited pyochelin as a receptor of Fe3+ by conjugating it with 4-nitro-benzo[1,2,5]oxadiazole (NBD). Although the details on the functional mechanism remain unclear, two types of pyochelin-conjugated NBD (Pyochelin-NBD-1 and Pyochelin-NBD-2, Fig. 5) showed ca. 3-fold increase in fluorescence at 545 nm in aqueous buffer. The affinities were measured to be K = 6.3 × 1010 M−2 and K = 2.6 × 1019 M−3 with 2:1 and 3:1 binding stoichiometries, respectively, for each probe. In their fluorescence imaging study using P. aeruginosa, the iron uptake was exclusively detected in the bacteria strain expressing the pyochelin receptor.
BOD-NHOH is a reaction-based fluorescent probe of Fe3+ developed by Wang et al.(50) Hydroxylaminomethyl group at meso-position of the BODIPY moiety can act as a fluorescence quencher via photo-induced electron transfer, which allows nonfluorescent BOD-NHOH. Oxidative cleavage of the hydroxylamine was mediated by Fe3+ to provide turn-on readout of fluorescence. The oxidative process was selective to Fe3+ over any other transition metals except for Cu2+ (i.e., it showed a few folds increase in fluorescence). BOD-NHOH exhibited brighter fluorescence signals with increasing Fe3+ concentrations in living MCF-7 cells with cytosolic distribution.
For the development of selective ratiometric fluorescent probes for iron, Goel et al.(51) designed and synthesized naphttho[2,1-b]-[1,10]-phenanthroline (NAP) derivatives. Among them, NAP-3, comprising a 1,10-phenanthroline moiety as a Fe3+ chelator and a conjugated donor–acceptor as a chromophore, was selected as the best candidate (Fig. 5). Chelation with Fe3+ caused ratiometric response. Originally, NAP-3 showed an emission peak at 544 nm upon excitation with 365 nm, while the emission peak shifted to 605 nm upon binding to Fe3+. The ratio between emission intensities at 605 and 544 nm (I605/I544) changed linearly against the Fe3+ concentration. The binding stoichiometry and affinity were determined to be 2:1 and Ka = 9.36 × 1010 M−2, respectively. They succeeded in monitoring Fe3+ by ratiometric imaging of HepG2 cells using the two wavelength regions. Moreover, a ratiometric-imaging study with NAP-3 enabled detection of accumulated Fe3+ in ftn-1-silenced C. elegans in which mRNA level of ferritin, an iron-storage protein,was transiently suppressed.
Turn-on Fluorescent Probes for Fe2+
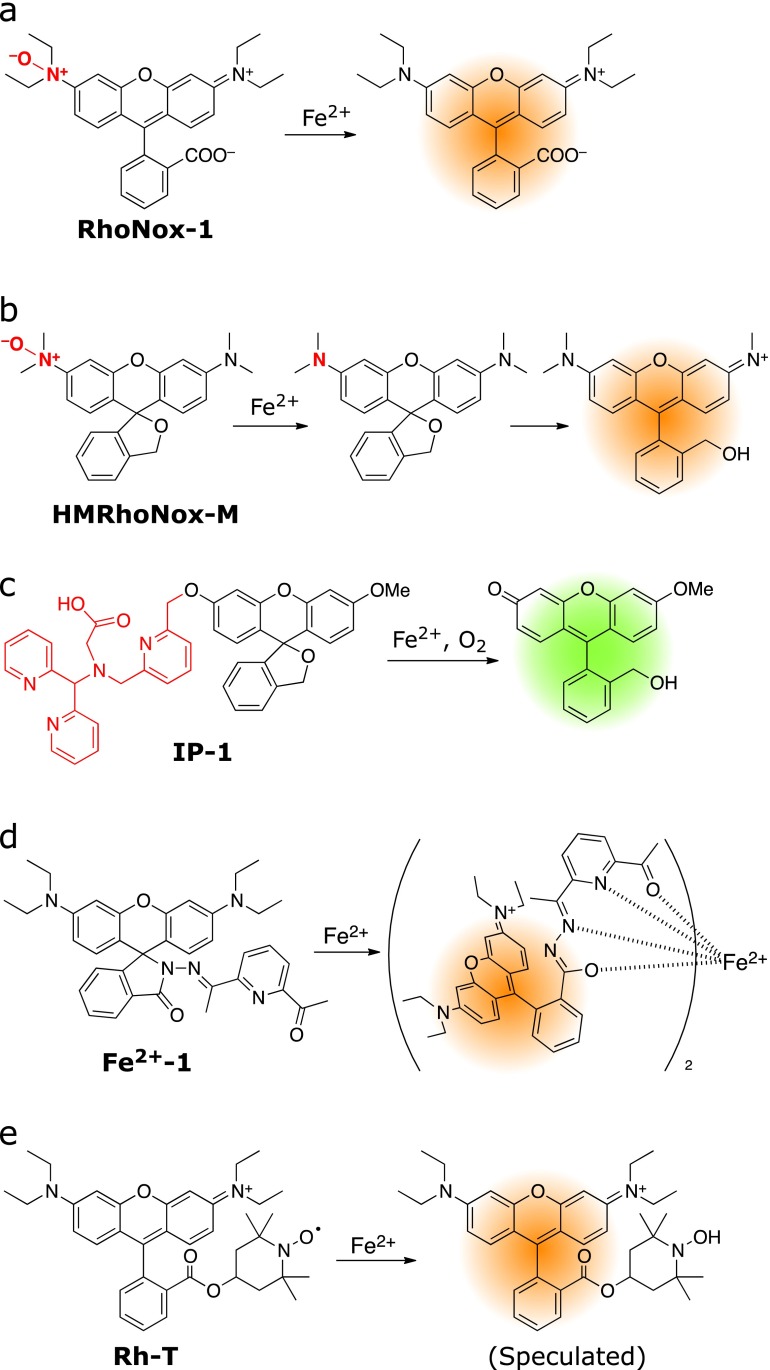
Unlike the large number of Fe3+ probes mentioned above, only a limited number of Fe2+-selective fluorescent probes have been reported.(52) As described above, the early Fe2+ fluorescent probes consist of fluorophore tethered with a chelator which exhibit turn-off response. For fluorescence intensity-based sensing, a turn-on response is more intuitive and provides improved contrast over turn-off response. However, it is principally hard to achieve turn-on response by the chelation-based strategy since iron ion itself shows strong fluorescence quenching property due to its paramagnetic effect. Indeed, detection mechanism of all the turn-off probes underlies recruiting such the fluorescence-quenching metal ion to fluorophore by chelation. Alternatively, another strategy relying not on chelation but Fe2+-selective chemical reactions should be necessary to avoid the Fe ion-mediated fluorescence quenching and to gain turn-on response. In the past few years, several turn-on fluorescent probes for Fe2+ on the basis of Fe2+-triggered chemical reactions have emerged enabling imaging studies and biological applications. The first one was reported by our group in 2013 as RhoNox-1 (Fig. 6a).(53) RhoNox-1 consists of rhodamine B modified with N-oxide, which keeps its emission to a considerably low level. Fe2+ reduced and de-oxygenate the N-oxide selectively, leading to an increase in florescence (30-fold, 575 nm). The response did not occur against other metal ions including Fe3+. The live cell imaging study revealed that RhoNox-1 detected artificially loaded overload of Fe2+ and endogenous level of labile Fe2+. Co-staining experiments showed that RhoNox-1 mainly localized at the Golgi apparatus with a small fraction of endoplasmic reticulum (ER). Recently, RhoNox-1 has been applied to staining frozen sections of proximal renal tubule in Fe-NTA (nitrilotriacetic acid)-induced carcinogenesis model rats(54) and to a cellular model of aged-macular disease to reveal light-induced fluctuation of Fe2+ ions in photoreceptor cells.(55) We also developed a series of N-oxide-based fluorescent probes by controlling spirocyclization of hydroxymethylrhodamine and hydroxymethylrhodol scaffolds (Fig. 6b).(56) Compared with RhoNox-1, HMRhoNox-M exhibited an improved turn-on contrast and succeeded in monitoring accumulated Fe2+ in lysosomes via transferrin-induced endocytosis.
Fig. 6.
Recent Fe2+-selective turn-on fluorescent probes applicable to live cell imaging study.
Chang et al.(57) also developed a reaction-based fluorescent probe for Fe2+, IP-1 (Fig. 6c). In the structure of IP-1, fluorescein derivative was coupled with a chelator that can activate Fe2+ for the oxidative benzylic C–O bond cleavage reaction. IP-1 monitored labile Fe2+ in living cells as well as accumulation of Fe2+ induced by a biological stimulation with an iron regulatory hormone (hepcidin), which degraded the iron exporter (ferroportin). It is noted that the probe responded to Co2+ with higher sensitivity than Fe2+, and therefore control experiments to exclude the possibility of involvement of Co2+ need to be provided.
Hou et al.(58) reported a turn-on probe for Fe2+ on the basis of chelation-induced spirocycle opening of rhodamine conjugated with diacetylpyridine hydrazone as a Fe2+ chelator (Fig. 6d, Fe2+-1). The probe Fe2+-1 showed a 2:1 binding ratio to Fe2+ concomitantly with a 60-fold increase in emission at 572 nm. Any other metal ions including Fe3+ did not interfere with the response to Fe2+, thereby indicating its high selectivity. Fluorescence imaging studies using RAW 264.7 macrophage cells revealed that the probe responded to Fe2+ in cells. However, relatively high loading of the probe was required for the imaging experiments, which might be due to low affinity of the probe.
Maiti et al.(59) reported Rh-T, a nitroxide radical-based fluorescent probe. Rh-T provides dual readouts of fluorescence and electron paramagnetic resonance (EPR) spectrum (Fig. 6e). Rh-T has a rhodamine B scaffold linked with 2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPO), which acts as a fluorescence quencher owing to its radical properties. The TEMPO moiety was reduced by Fe2+ selectively over other metal ions and ROS such as superoxide, nitric oxide, hydrogen peroxide, and NADH. The loss of the radical by Fe2+ resulted in the enhancement of the fluorescence signal as well as the disappearance of the EPR signal. Although Rh-T only showed a 2-fold increase in fluorescence in cuvette, imaging studies with primary fibrobrast cells demonstrated successful detection of severe increases of Fe2+ and endogenous levels of labile Fe2+ in mitochondria by the probe. Additionally, the probe had an ability to detect Fe2+ of cell suspension by EPR spectroscopy. All the turn-on Fe2+ probes described here are relying on the unique Fe2+-selective chemical reactions. In contrast to chelation-based probes, it should be noted that these reaction-based probes exhibit irreversible responses depending on the property of original chemical reactions. The irreversibility of the response of the probes is inapplicable to time-lapse monitoring of oscillation of intracellular iron by these probes.
The only example of ratiometric fluorescent probe for Fe2+ was reported by Li et al.(60) (Fig. 7). These authors developed a highly selective and ratiometric fluorescent probe (BDP-Cy-Tpy) by tethering BODIPY dyes to cyanine bearing terpyridine (Tpy) as a Fe2+ chelator. BDP-Cy-Tpy exhibited a set of excitation and emissions at 485/507 nm (λex/λem) and 569/635 nm derived from BODIPY and cyanine, respectively. Upon binding to Fe2+ at the Tpy moiety, the emission from the cyanine was dominantly quenched, whereas that of BODIPY remained unchanged. This indicates that the two fluorophore behaved independently. The ratio between the emission intensities at 507 and 635 nm revealed the concentration of Fe2+. They succeeded in ratiometric imaging of externally loaded Fe2+ and ascorbate-stimulated burst of intracellular Fe2+ in living cells. It is noted that the response mechanism basically depended on the quenching effect of Fe2+, and thus the same cautions paid with turn-off type probes should be applied here.
Fig. 7.
Ratiometric fluorescent probe for Fe2+.
In this review, we present chemical tools for optical detection of iron. Although the probes shown here basically provide optical readout that can be easily recognized by eye or fluorometry, we have to understand their chemical and physical properties well to avoid misunderstandings originated from false-positive/negative signals. For instance, chromogenic chelators and fluorescent probes described above are often susceptible to pH, decreasing their affinity when used under inappropriate pH conditions. When using these chemical tools for the detection of metal ions, we have to understand and duly consider the chemical properties of the probes, because there are not “perfect” chemical tools up to now. Moreover, compared to Ca2+ and Zn2+, fluorescent probes for Fe3+ and Fe2+ still remain immature. For the comprehensive elucidation of iron homeostasis, we must explore not only labile iron but also iron-containing species such as heme and iron proteins exhaustively, thereby requiring a diversity of chemical tools for detection of these molecules. In the particular case of iron, chemical tools applicable to in vivo studies as well as neuronal science are also of great interest and highly desired.
Chemists are pursuing the development of such chemical tools in recent years, but then chemists often do not know the utility of these tools. Corroborative work with biologists or scientists from other fields would expand the utility of these chemical tools to accelerate the improvements of life science and medicine. Furthermore, we must redouble our efforts to develop chemical tools able to respond to various target molecules as a key factor of life phenomenon.
Acknowledgments
This work was partially supported by JSPS KAKENHI (no. 25702050 for T.H.) and JST Adaptable and Seamless Technology transfer Program (A-STEP) (no. AS242Z01161P for T.H.).
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142:24–38. doi: 10.1016/j.cell.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 2.Theil EC, Goss DJ. Living with iron (and oxygen): questions and answers about iron homeostasis. Chem Rev. 2009;109:4568–4579. doi: 10.1021/cr900052g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ganz T. Systemic iron homeostasis. Physiol Rev. 2013;93:1721–1741. doi: 10.1152/physrev.00008.2013. [DOI] [PubMed] [Google Scholar]
- 4.Dixon SJ, Stockwell BR. The role of iron and reactive oxygen species in cell death. Nat Chem Biol. 2014;10:9–17. doi: 10.1038/nchembio.1416. [DOI] [PubMed] [Google Scholar]
- 5.Enami S, Sakamoto Y, Colussi AJ. Fenton chemistry at aqueous interfaces. Proc Natl Acad Sci USA. 2014;111:623–628. doi: 10.1073/pnas.1314885111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halliwell B, Gutteridge JM. Biologically relevant metal ion-dependent hydroxyl radical generation. An update. FEBS Lett. 1992;307:108–112. doi: 10.1016/0014-5793(92)80911-y. [DOI] [PubMed] [Google Scholar]
- 7.Imlay JA, Chin SM, Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988;240:640–642. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- 8.Torti SV, Torti FM. Iron and cancer: more ore to be mined. Nat Rev Cancer. 2013;13:342–355. doi: 10.1038/nrc3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toyokuni S. Role of iron in carcinogenesis: cancer as a ferrotoxic disease. Cancer Sci. 2009;100:9–16. doi: 10.1111/j.1349-7006.2008.01001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li K, Reichmann H. Role of iron in neurodegenerative diseases. J Neural Trans (Venna) 2016;123:389–399. doi: 10.1007/s00702-016-1508-7. [DOI] [PubMed] [Google Scholar]
- 11.Madsen E, Gitlin JD. Copper and iron disorders of the brain. Annu Rev Neurosci. 2007;30:317–337. doi: 10.1146/annurev.neuro.30.051606.094232. [DOI] [PubMed] [Google Scholar]
- 12.Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- 13.International Committee for Standardization in Haematology The measurement of total and unsaturated iron-binding capacity in serum. Br J Haematol. 1978;38:281–287. doi: 10.1111/j.1365-2141.1978.tb01044.x. [DOI] [PubMed] [Google Scholar]
- 14.Brandt WW, Smith GF. Polysubstituted 1,10-phenanthrolines and bipyridines as multiple range redox indicators. Anal Chem. 1949;21:1313–1319. [Google Scholar]
- 15.Beutler E, Blume KG, Kaplan JC, Löhr GW, Ramot B, Valentine WN. International Committee for Standardization in Haematology: recommended methods for red-cell enzyme analysis. Br J Haematol. 1977;35:331–340. doi: 10.1111/j.1365-2141.1977.tb00589.x. [DOI] [PubMed] [Google Scholar]
- 16.Iron Panel of the International Committee for Standardization in Haematology Revised recommendations for the measurements of the serum iron in human blood. Br J Haematol. 1990;75:615–616. doi: 10.1111/j.1365-2141.1990.tb07808.x. [DOI] [PubMed] [Google Scholar]
- 17.Blair D, Diehl H. Bathophenanthrolinedisulphonic acid and bathocuproinedisulphonic acid, water soluble reagents for iron and copper. Talanta. 1961;7:163–174. [Google Scholar]
- 18.Stookey LL. Ferrozine—a new spectrophotometric reagent for iron. Anal Chem. 1970;42:779–781. [Google Scholar]
- 19.Hennessy DJ, Reid GR, Smith FE, Thompson SL. Ferene—a new spectrophotometric reagent for iron. Can J Chem. 1984;62:721–724. [Google Scholar]
- 20.van Assendelft OW, Doumas BT, Fairbanks VF, Gunter EW, Nealon DA. Determination of Serum Iron, Total Iron-Binding Capacity and Percent Transferrin Saturation; Approved Standard. Clinical and Laboratory Standards Institute, Wayne, PA, 1998, Vol. 18, pp. C61-A. [Google Scholar]
- 21.Ohno N, Sakai T. Spectrophotometric determination of iron in boiler and well waters by flow injection analysis using 2-nitroso-5-(N-propyl-N-sulphopropylamino)phenol. Analyst. 1987;112:1127–1130. [Google Scholar]
- 22.Tôei K, Motomizu S, Korenaga T. Nitrosophenol and nitrosonaphthol derivatives as reagents for the spectrophotometric determination of iron and determination of micro-amounts in waters with 2-nitroso-5-dimethylaminophenol. Analyst. 1975;100:629–636. [Google Scholar]
- 23.Ito S, Ikuta K, Kato D, et al. Non-transferrin-bound iron assay system utilizing a conventional automated analyzer. Clin Chim Acta. 2014;437:129–135. doi: 10.1016/j.cca.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Lakowicz JR. Principles of Fluorescence Spectroscopy (3rd ed.) Springer; 2006. [Google Scholar]
- 25.Breuer W, Epsztejn S, Cabantchik ZI. Iron acquired from transferrin by K562 cells is delivered into a cytoplasmic pool of chelatable iron(II) J Biol Chem. 1995;270:24209–24215. doi: 10.1074/jbc.270.41.24209. [DOI] [PubMed] [Google Scholar]
- 26.Breuer W, Epsztejn S, Millgram P, Cabantchik IZ. Transport of iron and other transition metals into cells as revealed by a fluorescent probe. Am J Physiol . 1995;268 (6 Pt 1):C1354–C1361. doi: 10.1152/ajpcell.1995.268.6.C1354. [DOI] [PubMed] [Google Scholar]
- 27.Ponka P, Borová J, Neuwirt J, Fuchs O, Necas E. A study of intracellular iron metabolism using pyridoxal isonicotinoyl hydrazone and other synthetic chelating agents. Biochim Biophys Acta. 1979;586:278–297. doi: 10.1016/0304-4165(79)90100-4. [DOI] [PubMed] [Google Scholar]
- 28.Thomas F, Serratrice G, Béguin C, et al. Calcein as a fluorescent probe for ferric iron. Application to iron nutrition in plant cells. J Biol Chem. 1999;274:13375–13383. doi: 10.1074/jbc.274.19.13375. [DOI] [PubMed] [Google Scholar]
- 29.Kakhlon O, Cabantchik ZI. The labile iron pool: characterization, measurement, and participation in cellular processes. Free Radical Biol Med. 2002;33:1037–1046. doi: 10.1016/s0891-5849(02)01006-7. [DOI] [PubMed] [Google Scholar]
- 30.Petrat F, Rauen U, de Groot H. Determination of the chelatable iron pool of isolated rat hepatocytes by digital fluorescence microscopy using the fluorescent probe, phen green SK. Hepatology. 1999;29:1171–1179. doi: 10.1002/hep.510290435. [DOI] [PubMed] [Google Scholar]
- 31.Petrat F, Weisheit D, Lensen M, De Groot H, Sustmann R, Rauen U. Selective determination of mitochondrial chelatable iron in viable cells with a new fluorescent sensor. Biochem J. 2002;362 (Pt 1):137–147. doi: 10.1042/0264-6021:3620137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rauen U, Springer A, Weisheit D, et al. Assessment of chelatable mitochondrial iron by using mitochondrion-selective fluorescent iron indicators with different iron-binding affinities. Chembiochem. 2007;8:341–352. doi: 10.1002/cbic.200600311. [DOI] [PubMed] [Google Scholar]
- 33.Weizman H, Ardon O, Mester B, et al. Fluorescently-labeled ferrichrome analogs as probes for receptor-mediated, microbial iron uptake. J Am Chem Soc. 1996;118:12368–12375. [Google Scholar]
- 34.Lytton SD, Mester B, Libman J, Shanzer A, Cabantchik ZI. Monitoring of iron(III) removal from biological sources using a fluorescent siderophore. Anal Biochem. 1992;205:326–333. doi: 10.1016/0003-2697(92)90443-b. [DOI] [PubMed] [Google Scholar]
- 35.Hider RC, Liu ZD, Piyamongkol S. The design and properties of 3-hydroxypyridin-4-one iron chelators with high pFe3+ values. Transfus Sci. 2000;23:201–209. doi: 10.1016/s0955-3886(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 36.Ma Y, de Groot H, Liu Z, Hider RC, Petrat F. Chelation and determination of labile iron in primary hepatocytes by pyridinone fluorescent probes. Biochem J. 2006;395:49–55. doi: 10.1042/BJ20051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fakih S, Podinovskaia M, Kong X, Collins HL, Schaible U, Hider RC. Targeting the lysosome: fluorescent iron(III) chelators to selectively monitor endosomal/lysosomal labile iron pools. J Med Chem. 2008;51:4539–4552. doi: 10.1021/jm8001247. [DOI] [PubMed] [Google Scholar]
- 38.Hua J, Wang Y-G. A highly selective and sensitive fluorescent chemosensor for Fe3+ in physiological aqueous solution. Chem Lett. 2005;34:98–99. [Google Scholar]
- 39.Bricks JL, Kovalchuk A, Trieflinger C, et al. On the development of sensor molecules that display Fe(III)-amplified fluorescence. J Am Chem Soc. 2005;127:13522–13529. doi: 10.1021/ja050652t. [DOI] [PubMed] [Google Scholar]
- 40.Zhu H, Fan J, Wang B, Peng X. Fluorescent, MRI, and colorimetric chemical sensors for the first-row d-block metal ions. Chem Soc Rev. 2015;44:4337–4366. doi: 10.1039/c4cs00285g. [DOI] [PubMed] [Google Scholar]
- 41.Xiang Y, Tong A. A new rhodamine-based chemosensor exhibiting selective FeIII-amplified fluorescence. Org Lett. 2006;8:1549–1552. doi: 10.1021/ol060001h. [DOI] [PubMed] [Google Scholar]
- 42.Zhang M, Gao Y, Li M, et al. A selective turn-on fluorescent sensor for FeIII and application to bioimaging. Tetrahedron Lett. 2007;48:3709–3712. [Google Scholar]
- 43.Lee MH, Giap TV, Kim SH, Lee YH, Kang C, Kim JS. A novel strategy to selectively detect Fe(III) in aqueous media driven by hydrolysis of a rhodamine 6G Schiff base. Chem Commun (Camb) 2010;46:1407–1409. doi: 10.1039/b921526c. [DOI] [PubMed] [Google Scholar]
- 44.Wang B, Hai J, Liu Z, Wang Q, Yang Z, Sun S. Selective detection of iron(III) by rhodamine-modified Fe3O4 nanoparticles. Angew Chem Int Ed Engl. 2010;49:4576–4579. doi: 10.1002/anie.201001373. [DOI] [PubMed] [Google Scholar]
- 45.Bordini J, Calandreli I, Silva GO, et al. A rhodamine-B-based turn-on fluorescent sensor for biological iron(III) Inorg Chem Commun. 2013;35:255–259. [Google Scholar]
- 46.Wu K, Xiao H, Wang L, Yin G, Quan Y, Wang R. A rhodamine derivative as a highly sensitive chemosensor for iron(III) RSC Adv. 2014;4:39984–39990. [Google Scholar]
- 47.Ji S, Meng X, Ye W, et al. A rhodamine-based “turn-on” fluorescent probe for Fe3+ in aqueous solution. Dalton Trans. 2014;43:1583–1588. doi: 10.1039/c3dt52422a. [DOI] [PubMed] [Google Scholar]
- 48.Yan F, Zheng T, Shi D, et al. Rhodamine-aminopyridine based fluorescent sensors for Fe3+ in water: synthesis, quantum chemical interpretation and living cell application. Sensors Actuators B: Chem. 2015;215:598–606. [Google Scholar]
- 49.Noël S, Guillon L, Schalk IJ, Mislin GL. Synthesis of fluorescent probes based on the pyochelin siderophore scaffold. Org Lett. 2011;13:844–847. doi: 10.1021/ol1028173. [DOI] [PubMed] [Google Scholar]
- 50.Wang R, Yu F, Liu P, Chen L. A turn-on fluorescent probe based on hydroxylamine oxidation for detecting ferric ion selectively in living cells. Chem Commun (Camb) 2012;48:5310–5312. doi: 10.1039/c2cc31426f. [DOI] [PubMed] [Google Scholar]
- 51.Goel A, Umar S, Nag P, et al. A dual colorimetric-ratiometric fluorescent probe NAP-3 for selective detection and imaging of endogenous labile iron(III) pools in C. elegans. Chem Commun (Camb) 2015;51:5001–5004. doi: 10.1039/c4cc09798j. [DOI] [PubMed] [Google Scholar]
- 52.Carter KP, Young AM, Palmer AE. Fluorescent sensors for measuring metal ions in living systems. Chem Rev. 2014;114:4564–4601. doi: 10.1021/cr400546e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hirayama T, Okuda K, Nagasawa H. A highly selective turn-on fluorescent probe for iron(II) to visualize labile iron in living cells. Chem Sci. 2013;4:1250–1256. [Google Scholar]
- 54.Mukaide T, Hattori Y, Misawa N, et al. Histological detection of catalytic ferrous iron with the selective turn-on fluorescent probe RhoNox-1 in a Fenton reaction-based rat renal carcinogenesis model. Free Radic Res. 2014;48:990–995. doi: 10.3109/10715762.2014.898844. [DOI] [PubMed] [Google Scholar]
- 55.Imamura T, Hirayama T, Tsuruma K, Shimazawa M, Nagasawa H, Hara H. Hydroxyl radicals cause fluctuation in intracellular ferrous ion levels upon light exposure during photoreceptor cell death. Exp Eye Res. 2014;129:24–30. doi: 10.1016/j.exer.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 56.Niwa M, Hirayama T, Okuda K, Nagasawa H. A new class of high-contrast Fe(II) selective fluorescent probes based on spirocyclized scaffolds for visualization of intracellular labile iron delivered by transferrin. Org Biomol Chem. 2014;12:6590–6597. doi: 10.1039/c4ob00935e. [DOI] [PubMed] [Google Scholar]
- 57.Au-Yeung HY, Chan J, Chantarojsiri T, Chang CJ. Molecular imaging of labile iron(II) pools in living cells with a turn-on fluorescent probe. J Am Chem Soc. 2013;135:15165–15173. doi: 10.1021/ja4072964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hou G-G, Wang C-H, Sun J-F, Yang M-Z, Lin D, Li H-J. Rhodamine-based “turn-on” fluorescent probe with high selectivity for Fe2+ imaging in living cells. Biochem Biophys Res Commun. 2013;439:459–463. doi: 10.1016/j.bbrc.2013.08.092. [DOI] [PubMed] [Google Scholar]
- 59.Maiti S, Aydin Z, Zhang Y, Guo M. Reaction-based turn-on fluorescent probes with magnetic responses for Fe2+ detection in live cells. Dalton Trans. 2015;44:8942–8949. doi: 10.1039/c4dt03792h. [DOI] [PubMed] [Google Scholar]
- 60.Li P, Fang L, Zhou H, et al. A new ratiometric fluorescent probe for detection of Fe2+ with high sensitivity and its intracellular imaging applications. Chem Eur J. 2011;17:10520–10523. doi: 10.1002/chem.201101327. [DOI] [PubMed] [Google Scholar]