Abstract
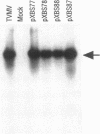
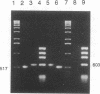
Inoculation of Nicotiana tabacum plants with RNA transcribed in vitro from a variant (pXBS8) of a cloned full-length DNA copy of tobacco vein mottling virus (TVMV) RNA resulted in attenuation of the vein mottling and blotching symptoms typically produced by transcripts of cloned wild-type cDNA (pXBS7). Similar amounts of virus were detected by ELISA (using anti-TVMV coat protein serum) in systemically infected leaves of plants inoculated with pXBS7 or pXBS8 transcripts. pXBS8 was shown to contain a 58-nucleotide segment in the sequence corresponding to the 3'-terminal untranslated region of TVMV RNA that was not present in pXBS7. This segment resulted in the appearance in pXBS8 transcripts of four adjacent direct repeats of a 14-nucleotide sequence, AUAAUUAUAUAUAU, that is present in the 3'-untranslated region of TVMV RNA, with two additional nucleotides (AU) between the first and second repeats. Insertion of restriction fragments containing the segment into pXBS7 and inoculation of plants with transcripts of the chimeric construct (pXBS78) resulted in the attenuated-symptom phenotype and was not accompanied by a reduced accumulation of virus in the plant as determined by ELISA and Northern blot analysis. When the extra nucleotides were removed from the variant clone, symptoms induced by transcripts of the cDNA (pXBS87) resembled those induced by wild-type transcripts. The results indicate that a noncoding region of the genome can have a direct effect on the induction of disease symptoms by an RNA virus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
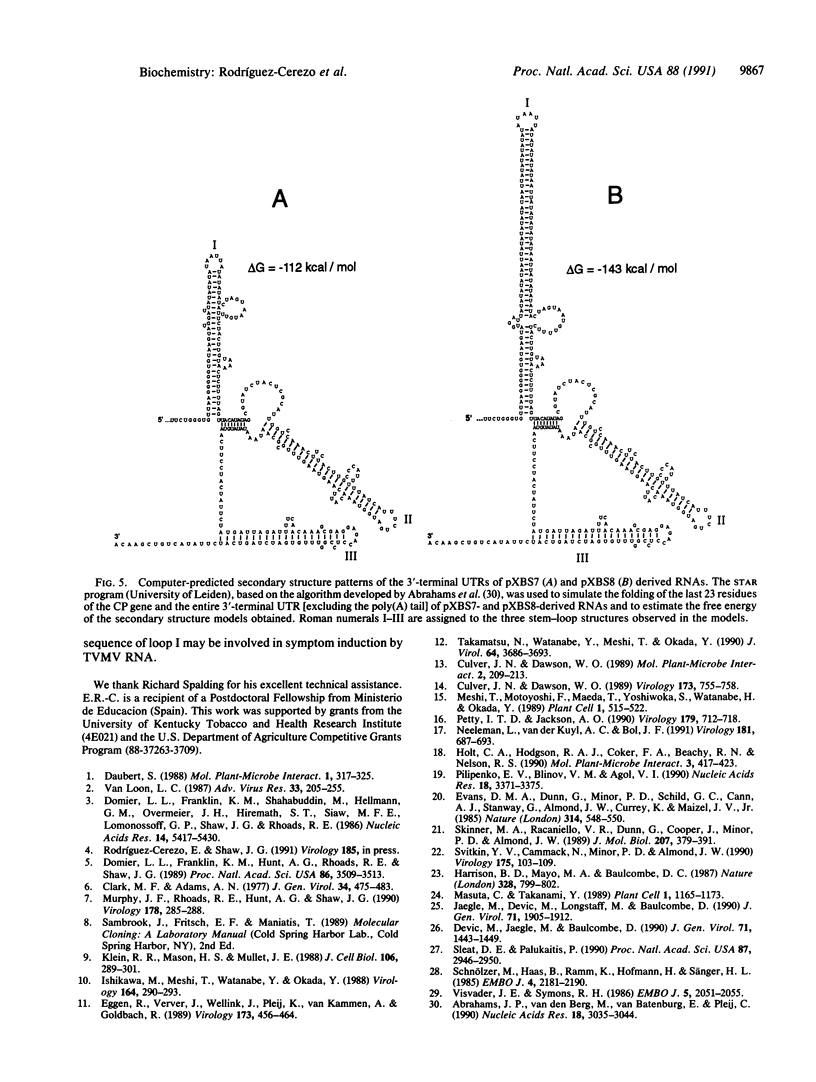
- Abrahams J. P., van den Berg M., van Batenburg E., Pleij C. Prediction of RNA secondary structure, including pseudoknotting, by computer simulation. Nucleic Acids Res. 1990 May 25;18(10):3035–3044. doi: 10.1093/nar/18.10.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. F., Adams A. N. Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J Gen Virol. 1977 Mar;34(3):475–483. doi: 10.1099/0022-1317-34-3-475. [DOI] [PubMed] [Google Scholar]
- Culver J. N., Dawson W. O. Tobacco mosaic virus coat protein: an elicitor of the hypersensitive reaction but not required for the development of mosaic symptoms in Nicotiana sylvestris. Virology. 1989 Dec;173(2):755–758. doi: 10.1016/0042-6822(89)90592-8. [DOI] [PubMed] [Google Scholar]
- Daubert S. Sequence determinants of symptoms in the genomes of plant viruses, viroids, and satellites. Mol Plant Microbe Interact. 1988 Nov-Dec;1(8):317–325. doi: 10.1094/mpmi-1-317. [DOI] [PubMed] [Google Scholar]
- Devic M., Jaegle M., Baulcombe D. Cucumber mosaic virus satellite RNA (strain Y): analysis of sequences which affect systemic necrosis on tomato. J Gen Virol. 1990 Jul;71(Pt 7):1443–1449. doi: 10.1099/0022-1317-71-7-1443. [DOI] [PubMed] [Google Scholar]
- Domier L. L., Franklin K. M., Hunt A. G., Rhoads R. E., Shaw J. G. Infectious in vitro transcripts from cloned cDNA of a potyvirus, tobacco vein mottling virus. Proc Natl Acad Sci U S A. 1989 May;86(10):3509–3513. doi: 10.1073/pnas.86.10.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domier L. L., Franklin K. M., Shahabuddin M., Hellmann G. M., Overmeyer J. H., Hiremath S. T., Siaw M. F., Lomonossoff G. P., Shaw J. G., Rhoads R. E. The nucleotide sequence of tobacco vein mottling virus RNA. Nucleic Acids Res. 1986 Jul 11;14(13):5417–5430. doi: 10.1093/nar/14.13.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggen R., Verver J., Wellink J., Pleij K., van Kammen A., Goldbach R. Analysis of sequences involved in cowpea mosaic virus RNA replication using site-specific mutants. Virology. 1989 Dec;173(2):456–464. doi: 10.1016/0042-6822(89)90558-8. [DOI] [PubMed] [Google Scholar]
- Evans D. M., Dunn G., Minor P. D., Schild G. C., Cann A. J., Stanway G., Almond J. W., Currey K., Maizel J. V., Jr Increased neurovirulence associated with a single nucleotide change in a noncoding region of the Sabin type 3 poliovaccine genome. Nature. 1985 Apr 11;314(6011):548–550. doi: 10.1038/314548a0. [DOI] [PubMed] [Google Scholar]
- Holt C. A., Hodgson R. A., Coker F. A., Beachy R. N., Nelson R. S. Characterization of the masked strain of tobacco mosaic virus: identification of the region responsible for symptom attenuation by analysis of an infectious cDNA clone. Mol Plant Microbe Interact. 1990 Nov-Dec;3(6):417–423. doi: 10.1094/mpmi-3-417. [DOI] [PubMed] [Google Scholar]
- Ishikawa M., Meshi T., Watanabe Y., Okada Y. Replication of chimeric tobacco mosaic viruses which carry heterologous combinations of replicase genes and 3' noncoding regions. Virology. 1988 May;164(1):290–293. doi: 10.1016/0042-6822(88)90648-4. [DOI] [PubMed] [Google Scholar]
- Jaegle M., Devic M., Longstaff M., Baulcombe D. Cucumber mosaic virus satellite RNA (Y strain): analysis of sequences which affect yellow mosaic symptoms on tobacco. J Gen Virol. 1990 Sep;71(Pt 9):1905–1912. doi: 10.1099/0022-1317-71-9-1905. [DOI] [PubMed] [Google Scholar]
- Klein R. R., Mason H. S., Mullet J. E. Light-regulated translation of chloroplast proteins. I. Transcripts of psaA-psaB, psbA, and rbcL are associated with polysomes in dark-grown and illuminated barley seedlings. J Cell Biol. 1988 Feb;106(2):289–301. doi: 10.1083/jcb.106.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuta C., Takanami Y. Determination of sequence and structural requirements for pathogenicity of a cucumber mosaic virus satellite RNA (Y-satRNA). Plant Cell. 1989 Dec;1(12):1165–1173. doi: 10.1105/tpc.1.12.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshi T., Motoyoshi F., Maeda T., Yoshiwoka S., Watanabe H., Okada Y. Mutations in the tobacco mosaic virus 30-kD protein gene overcome Tm-2 resistance in tomato. Plant Cell. 1989 May;1(5):515–522. doi: 10.1105/tpc.1.5.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. F., Rhoads R. E., Hunt A. G., Shaw J. G. The VPg of tobacco etch virus RNA is the 49-kDa proteinase or the N-terminal 24-kDa part of the proteinase. Virology. 1990 Sep;178(1):285–288. doi: 10.1016/0042-6822(90)90405-g. [DOI] [PubMed] [Google Scholar]
- Neeleman L., van der Kuyl A. C., Bol J. F. Role of alfalfa mosaic virus coat protein gene in symptom formation. Virology. 1991 Apr;181(2):687–693. doi: 10.1016/0042-6822(91)90902-n. [DOI] [PubMed] [Google Scholar]
- Petty I. T., Jackson A. O. Mutational analysis of barley stripe mosaic virus RNA beta. Virology. 1990 Dec;179(2):712–718. doi: 10.1016/0042-6822(90)90138-h. [DOI] [PubMed] [Google Scholar]
- Pilipenko E. V., Blinov V. M., Agol V. I. Gross rearrangements within the 5'-untranslated region of the picornaviral genomes. Nucleic Acids Res. 1990 Jun 11;18(11):3371–3375. doi: 10.1093/nar/18.11.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnölzer M., Haas B., Raam K., Hofmann H., Sänger H. L. Correlation between structure and pathogenicity of potato spindle tuber viroid (PSTV). EMBO J. 1985 Sep;4(9):2181–2190. doi: 10.1002/j.1460-2075.1985.tb03913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M. A., Racaniello V. R., Dunn G., Cooper J., Minor P. D., Almond J. W. New model for the secondary structure of the 5' non-coding RNA of poliovirus is supported by biochemical and genetic data that also show that RNA secondary structure is important in neurovirulence. J Mol Biol. 1989 May 20;207(2):379–392. doi: 10.1016/0022-2836(89)90261-1. [DOI] [PubMed] [Google Scholar]
- Sleat D. E., Palukaitis P. Site-directed mutagenesis of a plant viral satellite RNA changes its phenotype from ameliorative to necrogenic. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2946–2950. doi: 10.1073/pnas.87.8.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkin Y. V., Cammack N., Minor P. D., Almond J. W. Translation deficiency of the Sabin type 3 poliovirus genome: association with an attenuating mutation C472----U. Virology. 1990 Mar;175(1):103–109. doi: 10.1016/0042-6822(90)90190-3. [DOI] [PubMed] [Google Scholar]
- Takamatsu N., Watanabe Y., Meshi T., Okada Y. Mutational analysis of the pseudoknot region in the 3' noncoding region of tobacco mosaic virus RNA. J Virol. 1990 Aug;64(8):3686–3693. doi: 10.1128/jvi.64.8.3686-3693.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvader Jane E., Symons Robert H. Replication of in vitro constructed viroid mutants: location of the pathogenicity-modulating domain of citrus exocortis viroid. EMBO J. 1986 Sep;5(9):2051–2055. doi: 10.1002/j.1460-2075.1986.tb04465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon L. C. Disease induction by plant viruses. Adv Virus Res. 1987;33:205–255. doi: 10.1016/s0065-3527(08)60319-x. [DOI] [PubMed] [Google Scholar]