Abstract
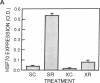
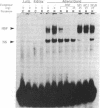
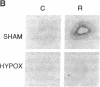
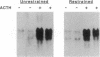
The induction of heat shock proteins (HSP) by cellular stress and the activation of the hypothalamic-pituitary-adrenal axis by physiologic stress are biological responses that aid in the maintenance of cellular and organismal homeostasis, respectively. In this report, restraint stress, known to activate the hypothalamic-pituitary-adrenal axis, is shown to induce expression of HSP70 mRNA selectively in the adrenal cortex of the rat. Restraint-induced HSP70 expression in the adrenals is rapid and is preceded by the activation of a protein factor capable of binding to the heat shock transcriptional control element. The ability of restraint to induce HSP70 expression in the adrenal is virtually eliminated in hypophysectomized rats but can be restored by the exogenous administration of adrenocorticotropic hormone. The magnitude of this induction declines as a function of increasing age, which may contribute to a reduced stress tolerance by aged animals. These results support a role for HSP70 in the physiologic stress response mediated by the hypothalamic-pituitary-adrenal axis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake M. J., Fargnoli J., Gershon D., Holbrook N. J. Concomitant decline in heat-induced hyperthermia and HSP70 mRNA expression in aged rats. Am J Physiol. 1991 Apr;260(4 Pt 2):R663–R667. doi: 10.1152/ajpregu.1991.260.4.R663. [DOI] [PubMed] [Google Scholar]
- Blake M. J., Gershon D., Fargnoli J., Holbrook N. J. Discordant expression of heat shock protein mRNAs in tissues of heat-stressed rats. J Biol Chem. 1990 Sep 5;265(25):15275–15279. [PubMed] [Google Scholar]
- Blake M. J., Nowak T. S., Jr, Holbrook N. J. In vivo hyperthermia induces expression of HSP70 mRNA in brain regions controlling the neuroendocrine response to stress. Brain Res Mol Brain Res. 1990 Jun;8(1):89–92. doi: 10.1016/0169-328x(90)90014-5. [DOI] [PubMed] [Google Scholar]
- Bradbury M. J., Cascio C. S., Scribner K. A., Dallman M. F. Stress-induced adrenocorticotropin secretion: diurnal responses and decreases during stress in the evening are not dependent on corticosterone. Endocrinology. 1991 Feb;128(2):680–688. doi: 10.1210/endo-128-2-680. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie R. W., White F. P. Characterization of the synthesis and accumulation of a 71-kilodalton protein induced in rat tissues after hyperthermia. Can J Biochem Cell Biol. 1983 Jun;61(6):438–446. doi: 10.1139/o83-059. [DOI] [PubMed] [Google Scholar]
- Deguchi Y., Negoro S., Kishimoto S. Age-related changes of heat shock protein gene transcription in human peripheral blood mononuclear cells. Biochem Biophys Res Commun. 1988 Dec 15;157(2):580–584. doi: 10.1016/s0006-291x(88)80289-4. [DOI] [PubMed] [Google Scholar]
- Edington B. V., Hightower L. E. Induction of a chicken small heat shock (stress) protein: evidence of multilevel posttranscriptional regulation. Mol Cell Biol. 1990 Sep;10(9):4886–4898. doi: 10.1128/mcb.10.9.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edington B. V., Whelan S. A., Hightower L. E. Inhibition of heat shock (stress) protein induction by deuterium oxide and glycerol: additional support for the abnormal protein hypothesis of induction. J Cell Physiol. 1989 May;139(2):219–228. doi: 10.1002/jcp.1041390202. [DOI] [PubMed] [Google Scholar]
- Engeland W. C., Shinsako J., Winget C. M., Vernikos-Danellis J., Dallman M. F. Circadian patterns of stress-induced ACTH secretion are modified by corticosterone responses. Endocrinology. 1977 Jan;100(1):138–147. doi: 10.1210/endo-100-1-138. [DOI] [PubMed] [Google Scholar]
- Fargnoli J., Kunisada T., Fornace A. J., Jr, Schneider E. L., Holbrook N. J. Decreased expression of heat shock protein 70 mRNA and protein after heat treatment in cells of aged rats. Proc Natl Acad Sci U S A. 1990 Jan;87(2):846–850. doi: 10.1073/pnas.87.2.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fluharty S. J., Snyder G. L., Zigmond M. J., Stricker E. M. Tyrosine hydroxylase activity and catecholamine biosynthesis in the adrenal medulla of rats during stress. J Pharmacol Exp Ther. 1985 Apr;233(1):32–38. [PubMed] [Google Scholar]
- Fornace A. J., Jr, Alamo I., Jr, Hollander M. C., Lamoreaux E. Induction of heat shock protein transcripts and B2 transcripts by various stresses in Chinese hamster cells. Exp Cell Res. 1989 May;182(1):61–74. doi: 10.1016/0014-4827(89)90279-6. [DOI] [PubMed] [Google Scholar]
- Goldenberg C. J., Luo Y., Fenna M., Baler R., Weinmann R., Voellmy R. Purified human factor activates heat shock promoter in a HeLa cell-free transcription system. J Biol Chem. 1988 Dec 25;263(36):19734–19739. [PubMed] [Google Scholar]
- Hightower L. E. Cultured animal cells exposed to amino acid analogues or puromycin rapidly synthesize several polypeptides. J Cell Physiol. 1980 Mar;102(3):407–427. doi: 10.1002/jcp.1041020315. [DOI] [PubMed] [Google Scholar]
- Holmgren R., Corces V., Morimoto R., Blackman R., Meselson M. Sequence homologies in the 5' regions of four Drosophila heat-shock genes. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3775–3778. doi: 10.1073/pnas.78.6.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Sato A., Sato Y., Suzuki H. Increases in adrenal catecholamine secretion and adrenal sympathetic nerve unitary activities with aging in rats. Neurosci Lett. 1986 Sep 12;69(3):263–268. doi: 10.1016/0304-3940(86)90491-x. [DOI] [PubMed] [Google Scholar]
- Kant G. J., Mougey E. H., Meyerhoff J. L. Diurnal variation in neuroendocrine response to stress in rats: plasma ACTH, beta-endorphin, beta-LPH, corticosterone, prolactin and pituitary cyclic AMP responses. Neuroendocrinology. 1986;43(3):383–390. doi: 10.1159/000124553. [DOI] [PubMed] [Google Scholar]
- Kubo T., Towle C. A., Mankin H. J., Treadwell B. V. Stress-induced proteins in chondrocytes from patients with osteoarthritis. Arthritis Rheum. 1985 Oct;28(10):1140–1145. doi: 10.1002/art.1780281010. [DOI] [PubMed] [Google Scholar]
- Landfield P. W., Waymire J. C., Lynch G. Hippocampal aging and adrenocorticoids: quantitative correlations. Science. 1978 Dec 8;202(4372):1098–1102. doi: 10.1126/science.715460. [DOI] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Liu A. Y., Lin Z., Choi H. S., Sorhage F., Li B. Attenuated induction of heat shock gene expression in aging diploid fibroblasts. J Biol Chem. 1989 Jul 15;264(20):12037–12045. [PubMed] [Google Scholar]
- Munck A., Guyre P. M., Holbrook N. J. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984 Winter;5(1):25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- O'Malley K., Mauron A., Barchas J. D., Kedes L. Constitutively expressed rat mRNA encoding a 70-kilodalton heat-shock-like protein. Mol Cell Biol. 1985 Dec;5(12):3476–3483. doi: 10.1128/mcb.5.12.3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odio M., Brodish A. Age-related adaptation of pituitary-adrenocortical responses to stress. Neuroendocrinology. 1989 Apr;49(4):382–388. doi: 10.1159/000125142. [DOI] [PubMed] [Google Scholar]
- Ottenweller J. E., Tapp W. N., Pitman D. L., Natelson B. H. Interactions among the effects of aging, chronic disease, and stress on adrenocortical function in Syrian hamsters. Endocrinology. 1990 Jan;126(1):102–109. doi: 10.1210/endo-126-1-102. [DOI] [PubMed] [Google Scholar]
- Pavlov E. P., Harman S. M., Chrousos G. P., Loriaux D. L., Blackman M. R. Responses of plasma adrenocorticotropin, cortisol, and dehydroepiandrosterone to ovine corticotropin-releasing hormone in healthy aging men. J Clin Endocrinol Metab. 1986 Apr;62(4):767–772. doi: 10.1210/jcem-62-4-767. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell. 1982 Sep;30(2):517–528. doi: 10.1016/0092-8674(82)90249-5. [DOI] [PubMed] [Google Scholar]
- Perlmutter D. H., Schlesinger M. J., Pierce J. A., Punsal P. I., Schwartz A. L. Synthesis of stress proteins is increased in individuals with homozygous PiZZ alpha 1-antitrypsin deficiency and liver disease. J Clin Invest. 1989 Nov;84(5):1555–1561. doi: 10.1172/JCI114332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth G. S. Reduced glucocorticoid binding site concentration in cortical neuronal perikarya from senescent rats. Brain Res. 1976 May 7;107(2):345–354. doi: 10.1016/0006-8993(76)90231-6. [DOI] [PubMed] [Google Scholar]
- Sapolsky R. M., Krey L. C., McEwen B. S. Corticosterone receptors decline in a site-specific manner in the aged rat brain. Brain Res. 1983 Dec 19;289(1-2):235–240. doi: 10.1016/0006-8993(83)90024-0. [DOI] [PubMed] [Google Scholar]
- Sapolsky R. M., Krey L. C., McEwen B. S. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev. 1986 Aug;7(3):284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Sorger P. K., Lewis M. J., Pelham H. R. Heat shock factor is regulated differently in yeast and HeLa cells. Nature. 1987 Sep 3;329(6134):81–84. doi: 10.1038/329081a0. [DOI] [PubMed] [Google Scholar]
- Sorger P. K., Pelham H. R. Purification and characterization of a heat-shock element binding protein from yeast. EMBO J. 1987 Oct;6(10):3035–3041. doi: 10.1002/j.1460-2075.1987.tb02609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vass K., Welch W. J., Nowak T. S., Jr Localization of 70-kDa stress protein induction in gerbil brain after ischemia. Acta Neuropathol. 1988;77(2):128–135. doi: 10.1007/BF00687422. [DOI] [PubMed] [Google Scholar]
- Wu B. J., Kingston R. E., Morimoto R. I. Human HSP70 promoter contains at least two distinct regulatory domains. Proc Natl Acad Sci U S A. 1986 Feb;83(3):629–633. doi: 10.1073/pnas.83.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimarino V., Wu C. Induction of sequence-specific binding of Drosophila heat shock activator protein without protein synthesis. 1987 Jun 25-Jul 1Nature. 327(6124):727–730. doi: 10.1038/327727a0. [DOI] [PubMed] [Google Scholar]