Abstract
Hypoxia is an important and influential factor in development. The embryonic kidney is exposed to a hypoxic environment throughout its development. The Wnt/β-catenin pathway plays vital roles in the differentiation and self-renewal of metanephrogenic mesenchymal stem cells (MMSCs) from which the kidney is derived. Thus, we hypothesized that hypoxia can regulate the differentiation and pluripotency of MMSCs through the Wnt/β-catenin pathway. To test this hypothesis, MMSCs from rats at embryonic day 18.5 were cultured in normoxic (21% O2) and hypoxic (1% O2) conditions. The effects of hypoxia on differentiation, stemness, proliferation, and apoptosis of cultured MMSCs and on the activity of the Wnt/β-catenin pathway were tested. Our results revealed that the hypoxic condition increased the number of epithelial cells (E-cadherin+ or CK18+) as well the expression of markers of renal tubule epithelia cells (CDH6, Aqp1, and OPN), decreased the number and proliferation of stem cells (SIX-2+ or CITED1+), and induced apoptosis. Additionally, hypoxia reduced the expression of Wnt4 as well as its downstream molecules β-catenin, LEF-1, and Axin2. Activation of the Wnt/β-catenin pathway by LiCl or BIO modified the effects of hypoxia on the differentiation and self-renewal of MMSCs. Thus, we concluded that hypoxia induces the differentiation and inhibits the self-renewal of MMSCs by inhibiting the Wnt/β-catenin pathway. The observations further our understanding of the effects of hypoxia on kidney.
1. Introduction
Prenatal hypoxia is a common complication during pregnancy, which leads to negative foetal outcomes such as birth defects [1]. It has been reported that foetal hypoxia decreases nephron numbers and kidney weight [2]. However, it has been shown that hypoxia stimulates ureteric bud branching in vitro [3]. In this regard, an appropriately low O2 tension is necessary for embryonic development. Because the effect of hypoxia on development of the kidney is controversial, understanding the kidney's responses to oxygen deprivation is critical.
Coordinated interactions between the embryo and its environment are critical for development. Early-stage mammalian embryos develop in hypoxic environments and live at low oxygen concentrations (ranging from 2% to 9%) throughout foetal development [4, 5]. A hypoxic environment represents the physiological growing conditions of embryonic stem cells. Many studies have indicated that hypoxia influences development of the embryo by regulating the differentiation and self-renewal (including the maintenance of stemness and proliferation) of stem cells. In some organs, such as the lung, nervous system and heart, hypoxia induces the differentiation of stem cells into mature cells [6–8]. For other cells such as cochlear spiral ganglion stem cells, human mesenchymal stem cells, and adipose-derived mesenchymal stem cells, hypoxia helps to maintain stemness to preserve the stem cell pool [9–11]. The kidney can exist at 1% O2 or even lower oxygen tension, owing to atypical blood vessel networks [12]. Oxygen tension may have a profound influence on the development of the kidney.
The development of the mammalian kidney is guided by reciprocal inductive interactions between the ureteric bud (UB, giving rise to the collecting duct system) and the metanephric mesenchyme (giving rise to all other renal epithelial cells) [13]. In rats, the metanephric kidney develops at embryonic day 12, and this is followed by the ingrowth of the UB into the metanephric blastema, inducing the metanephric mesenchymal stem cells (MMSCs) at the bud tips to condense around the UB tips. Subsequently, the condensed cells undergo a mesenchymal-epithelial transition (MET), thus forming epithelial vesicles, and this is followed by sequential differentiation into functional nephrons [14, 15]. The balance between the differentiation and self-renewal of MMSCs is essential for development of the kidney. However, the effects of hypoxia on the differentiation and self-renewal of MMSCs have been unclear.
Among many other signalling pathways, the Wnt/β-catenin pathway is considered to play a critical role in development of the kidney. Upregulation of Wnt4, which mediates epithelialization of the metanephric mesenchyme, has been detected after exposure of mesenchymal stem cells to hypoxia [16]. Disruption of Wnt4 impairs MET in the embryonic kidney; conversely, coculture of the mesenchyme with cells expressing Wnt4 induces tubulogenesis in organ culture [17, 18]. In contrast, inhibition of β-catenin (downstream of Wnt pathway) induces tubulogenesis [19] and constitutive expression of stabilized β-catenin blocks MET in cultured MMSCs [20, 21]. Thus, we hypothesized that hypoxia may regulate the differentiation and self-renewal of MMSCs by a Wnt4-dependent pathway. To test this hypothesis, we used hypoxic culture of isolated MMSCs to address the effects of hypoxia on development of the kidney. In embryonic kidney, the transcription factor sine oculis homeobox homolog 2 (SIX-2) and aspartic acid rich C terminal domain 1 (CITED1) that are essential for self-renewal are considered as markers of MMSCs [14, 22]. E-cadherin and cytokeratin 18 (CK18) are markers of mature epithelial cells; K- cadherin (CDH6) is an early marker of epithelial commitment of nephron progenitors; aquaporin 1 (Aqp1) and osteopontin (OPN) are a marker of renal tubule epithelia cells [23–26]. Our results demonstrated that the number of E-cadherin- or CK18-positive cells was increased, the expression of CDH6, Aqp1, and OPN was elevated, and the number of SIX-2- or CITED1-positive cells was decreased in hypoxia-exposed MMSCs. Additionally, proliferation of MMSCs was inhibited, and apoptosis was promoted by hypoxia. This means that hypoxia promoted the differentiation and inhibited the self-renewal of MMSCs. Furthermore, we found that hypoxia inhibited the Wnt4/β-catenin pathway. Activation of Wnt/β-catenin with lithium chloride (LiCl) or 6-bromoindirubin-3-oxime (BIO) attenuated the effect of hypoxia. Thus, we conclude that hypoxia promotes the differentiation and depresses the self-renewal of MMSCs by inhibiting the Wnt4/β-catenin pathway.
2. Materials and Methods
2.1. Animals
Twenty timed-pregnant female Sprague-Dawley rats were provided by the Animal Center of Shanghai Medical College, Fudan University (Shanghai, China). At embryonic days 18-19, the age of the embryos was counted from the day of the vaginal plug. This study was performed in strict accordance with the Guidelines on the Care and Use of Laboratory Animals issued by the Chinese Council on Animal Research and the Guidelines of Animal Care. The protocol was approved by the Committee on the Ethics of Animal Experiments of Fudan University.
2.2. Metanephric Mesenchymal Stem Cell (MMSC) Culture
The primary MMSC cultures were prepared as previously described [27]. Briefly, rat embryonic kidney rudiments were microdissected at embryonic days 18-19 (E18-E19). The ureteric bud was removed from isolated rudiments, and the remaining mesenchyme was incubated in 0.05% trypsin/0.5 mM EDTA for 5 minutes on ice. Foetal calf serum was added at a concentration of 50% to inactivate trypsin. The suspension was mechanically dissociated by gentle aspiration with a Pasteur pipette. The single-cell suspension was centrifuged at low speed (1500 rpm) for 5 minutes and plated at densities of 2 × 105 cells/ml on 30 mm culture dishes or 96-well plates or 3-well chamber slide system in Dulbecco's modified Eagle's medium (DMEM)/F12 (1 : 1) including 10% foetal calf serum and grown at 37°C, 5% CO2, and 95% air (approximately 21% O2). After attachment, cells were treated with 1% O2 in a variable oxygen control incubators (Thermo Scientific) which could generate hypoxic conditions and monitor real-time O2 sensitively or maintained at 21% O2 for 3 days. To activate the Wnt/β-catenin pathway, LiCl (20 mM) or BIO (5 nM) was added to the culture medium.
2.3. Western Blotting
Western blotting was performed to examine CITED1, SIX-2, E-cadherin, and the related proteins in the Wnt/β-Catenin signalling pathway (Wnt4, β-catenin, and LEF1). Briefly, after culture under hypoxic or normoxic conditions for 3 days, MMSCs were collected, and protein was extracted. The proteins were separated by 12.5% SDS–PAGE, electrotransferred to a PVDF membrane (Millipore, USA), and subsequently blocked with 5% (w/v) fat-free milk in TBST for 1.5 h at room temperature. The membranes were blotted sequentially with primary antibodies, including anti-GAPDH antibody (1 : 5000, Sigma-Aldrich, Catalog number SAB2701825), anti-SIX-2 antibody (1 : 1000; Proteintech, Catalog number 11562-1-AP), anti-CITED1 antibody (1 : 1000; Abcam, Catalog number ab213429), anti-E-cadherin antibody (1 : 5000; BD, Catalog number 610181), anti-Wnt4 antibody (1 : 600; Abcam, Catalog number ab91226), anti-β-catenin antibody (1 : 1000; Proteintech, Catalog number 51067-2-AP), anti-LEF1 antibody (1 : 1000; Cell Signalling Technology, Catalog number 2230), and anti-HIF1α antibody (1 : 1000; Cell Signalling Technology, Catalog number 14179), and then with secondary antibodies (1 : 5000; Sigma-Aldrich, Catalog numbers RABHRP1 and RABHRP2) conjugated with horseradish peroxidase. The last step of western blotting antibody detection was performed with western blot chemiluminescent HRP substrate reagent (Thermo Fisher). The expression levels of different proteins were normalized to those of the internal control (GAPDH) [28].
2.4. Real-Time PCR
The total RNA was isolated from MMSCs using TRIzol (Life Technologies, USA). The RNA levels of SIX-2, Wnt4, LEF1, Axin2, PKG1, Glut1, CDH6, Aqp1, and OPN were detected with a SYBR-Green RT-qPCR kit (Takara, Japan) according to the manufacturer's instructions (Sangon Biotech, China). The levels of mRNA expression were normalized to those of the housekeeping gene (GAPDH). Simple relative quantification of target gene expression normalized to GAPDH was performed using the 2−ΔΔCt method [29]. The primer sequences are shown in Table 1.
Table 1.
PCR primer sequence and size.
| Gene name | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| Wnt4 | CTGGAGAAGTGTGGCTGTGA | AAAGGACTGTGAGAAGGCTACG |
| β-Catenin | CTTACGGCAATCAGGAAAGC | GACAGACAGCACCTTCAGCA |
| LEF-1 | GCATCCCTCATCCAGCAA | GGCTCCTGTTCCTTTCTCTGT |
| Axin2 | ACGGAATACGAAAGGCACAG | ACGCTCACTCTCCAACATCC |
| SIX-2 | GCCAAGGAAAGGGAGAAC | CTGTGTAGGGAAGGCAACC |
| PKG1 | AAGACGGCAAGCATGAAGCT | CCCTTCTGTCCCTGTAAAGGTTT |
| Glut1 | GCTTCCTGCTCATCAATCGT | CTGCCGACCCTCTTCTTTC |
| CDH6 | ATGACAATCCTCCTCGCTTC | TTTCTCCCACATCAGCATCA |
| Aqp1 | CCGCAACTTCTCAAACCACT | CATCCAGGTCATACTCCTCCA |
| OPN | AAGCGTGGAAACACACAGC | TTTGGAACTCGCCTGACTG |
| GAPDH | GGGTGTGAACCACGAGAAAT | ACTGTGGTCATGAGCCCTTC |
2.5. Flow Cytometry
Cells were harvested from the culture dishes using trypsin EDTA and fixed with 4% paraformaldehyde, pH 7.4, dissolved in PBS. Single cells for flow cytometry (FCM) were incubated for 30 min with their respective primary antibodies (1 μl for 106 cells, CK18: Abcam, Catalog number ab82254) and subsequently washed with PBS supplemented with 1% FBS. After removing the primary antibody by washing twice, samples were reincubated with either a FITC-, Cy3, or Alexa flour 594-conjugated secondary antibody at a dilution of 1 : 500 using an exposure time of 20 min. Apoptosis was detected using Annexin V-FITC/PI staining kit (Beyotime Institute of Biotechnology, China) according to the manufacturer's instructions. Before starting FCM, cells were again rinsed twice with wash buffer. FCM was carried out using a FACSCalibur (Becton Dickinson, Heidelberg, Germany).
2.6. Immunofluorescence
The MMSCs (2 × 105 cells/ml in 500 μl complete medium) were seeded into each well of chamber slide system. After 24 hours of normoxic incubation, the chamber was removed, and cells were fixed with methanol for 10 minutes. The cells were preincubated with 5% normal goat serum to block nonspecific binding and then incubated with primary antibodies (1 : 100 rabbit anti-SIX-2 or CITED1 and 1 : 100 mouse anti-α-SMA, Sigma-Aldrich, Catalog number A5228) at 4°C overnight. Extensive washing was followed by incubation with FITC- or Cy3-conjugated secondary antibody (1 : 200) for 1 h at room temperature. Slides were mounted with antifade mounting medium (Beyotime Institute of Biotechnology, China) with DAPI (4′,6-diamidino-2-phenylindole). Images were acquired with a confocal laser scanning microscope (Zeiss 510).
2.7. TUNEL and EdU Staining
The MMSCs (2 × 105 cells/ml in 500 μl complete medium) were seeded into each well of chamber slide system and incubated under hypoxic or normoxic condition for 3 days. Apoptosis was detected using a TUNEL apoptosis assay kit (KeyGen Biotech, China). Cells growing on slides were fixed in 1% formaldehyde for 30 min, permeabilized (using 1% Triton-X100 in PBS) for 10 min, and rewashed before application of TUNEL reagents diluted to 50% with TUNEL dilution buffer. After washing, positive staining was detected with label solution. Proliferation of cells was detected using keyFluor488 Click-iT EdU kit (Beyotime Institute of Biotechnology, China). Cells were incubated with 10 μM EdU for 1 hour prior to being harvested and then fixed and permeabilized as described above. For EdU staining, the slides were incubated with Click-iT reaction cocktail containing Alexa Fluor 488 for 30 min at room temperature according to the manufacturer's instructions.
2.8. Cell Viability Assay
The effect of hypoxia on the viability of MMSCs was determined using AlamarBlue cell viability assay kit (Beyotime Institute of Biotechnology, China) as previously described. Briefly, the MMSCs (1 × 104 cells/well in 100 μl complete medium) were seeded into 96-well cell plates. After 72 hours of hypoxic or normoxic incubation, 10 μl AlamarBlue ready-to-use solution was added to each well and the plates were incubated at 37°C for a further 2 hours. The absorbencies with wavelengths set at 570/600 nm were measured by SpectraMax fluorescence multiwell plate reader. Breeding ratio (%) = (117216 × A570 sample − 80586 × A600 sample)/(117216 × A570 control − 80586 × A600 control) × 100%.
2.9. Statistical Analysis
Data are presented as means ± SEM and were analysed using SPSS software. Statistical analyses including the independent t-test (comparison of two groups) and one-way ANOVA followed by Tukey's post hoc test (comparison of more than two groups) were regarded as significant when p < 0.05.
3. Results
3.1. Characterization of the Isolated MMSCs
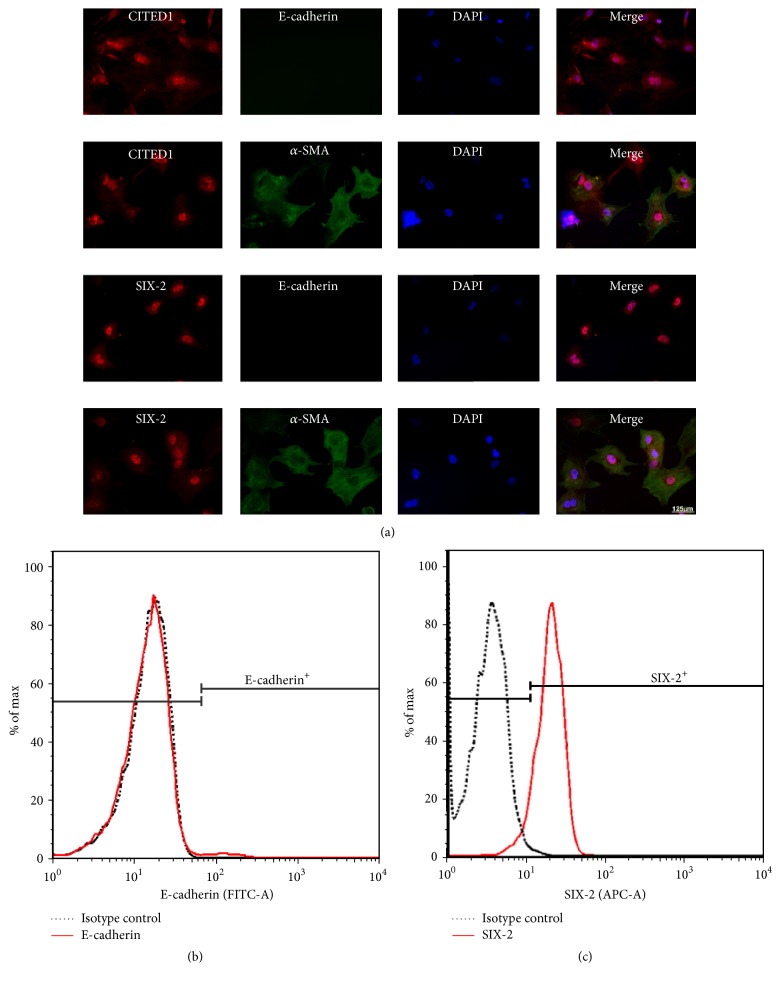
To verify that the cells isolated using our methods were MMSCs, immunofluorescence double staining was applied to test coexpression of stem cell and mesenchymal cell markers in these cells. Coexpression of stem cell markers (CITED1 and SIX-2) with a mesenchymal cell marker (α-SMA) was detected in most of isolated cells. In contrast, no significant expression of the epithelial cell marker (E-cadherin) was observed (Figure 1(a)). This result was confirmed by flow cytometry: the percentage of E-cadherin positive cells among the fresh isolated MMSCs was less than 1%, while percentage of SIX-2 positive cells was more than 90% (Figures 1(b) and 1(c)). Therefore, our results underline that the cells isolated from rat embryo kidney tissue represented specific metanephric mesenchymal stem cells.
Figure 1.
The isolated cells were identified as MMSCs by immunofluorescence double staining and flow cytometry. (a) The isolated cells were plated on chamber slide system and cultures in 21% O2 for 24 h, and then the cells were stained by immunofluorescence. The isolated cells coexpressed SIX-2 or CITED1 (markers of metanephric stem cell) and α-SMA (marker of mesenchymal cells). Both SIX-2 and CITED1 were located in the nucleus. α-SMA was expressed in cytoplasm. No E-cadherin (marker of epithelium) positive cells were observed. Bar is equal to 125 μm. (b)-(c) As the flow cytometry results shown, the percentage of E-cadherin cells is less than 1% and that of SIX-2 positive cells is over 90%. These results confirmed that the isolated cells were MMSCs.
3.2. Effect of Different O2 Tension on the Differentiation and Stemness of the MMSCs
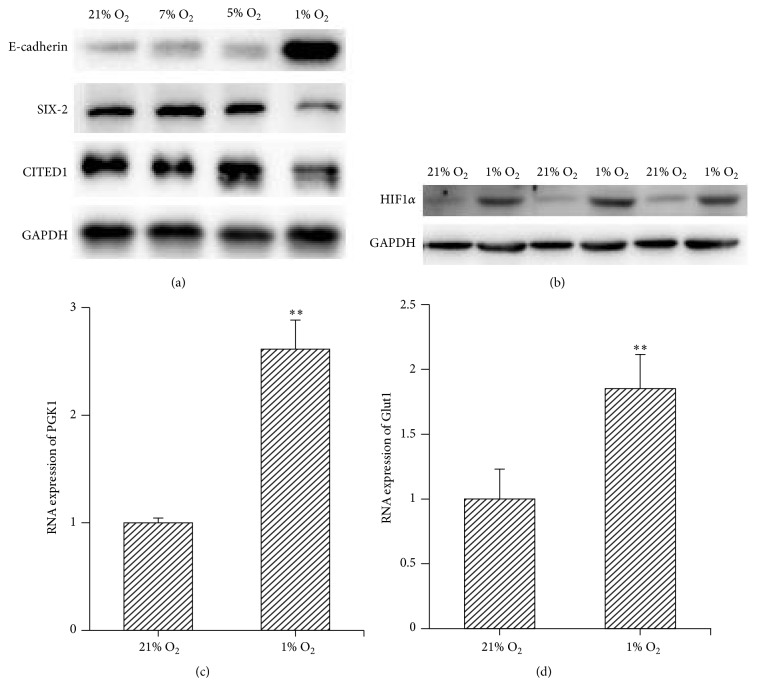
To select a proper O2 tension to investigate the effect of hypoxia on the MMSCs and its underline mechanism, we cultured the fresh isolated MMACs under 1%, 5%, 7%, and 21% O2 conditions for 3 days, respectively. The expression of E-cadherin, SIX-2, and CITED1 was measured by western blotting. The results show that the expression of E-cadherin, SIX-2, and CITED1 was not altered by 5% or 7% O2, compared with 21% O2. However, the expression of E-cadherin was increased and the levels of SIX-2 and CITED1 were decreased by 1% O2 culture (Figure 2(a)). Additionally, the hypoxia condition was confirmed by the elevated expression of hypoxia-inducible factors 1α (HIF1α) and its targets genes PKG1 and Glut1 under 1% O2 condition (Figures 2(b)–2(d)). Thus, we considered 1% O2 is optimal to research the effect of hypoxic condition on the MMSCs.
Figure 2.
Effect of different O2 tensions on the differentiation and stemness of the MMSCs. (a) The MMSCs were cultured under 1%, 5%, 7%, and 21% O2 conditions for 3 days, respectively. The expression of E-cadherin, SIX-2, and CITED1 was measured by western blotting. The results show that the expression of E-cadherin, SIX-2, and CITED1 was not altered by 5% or 7% O2, compared with 21% O2. However, the expression of E-cadherin was increased and the levels of SIX-2 and CITED1 were decreased by 1% O2 culture. (b)–(d) The hypoxia environment under 1% O2 culture was confirmed by the elevated expression of HIF1α, PGK1, and Glut1; ∗∗p < 0.01; n = 3.
3.3. Hypoxia Inhibited the Wnt4/β-Catenin Pathway
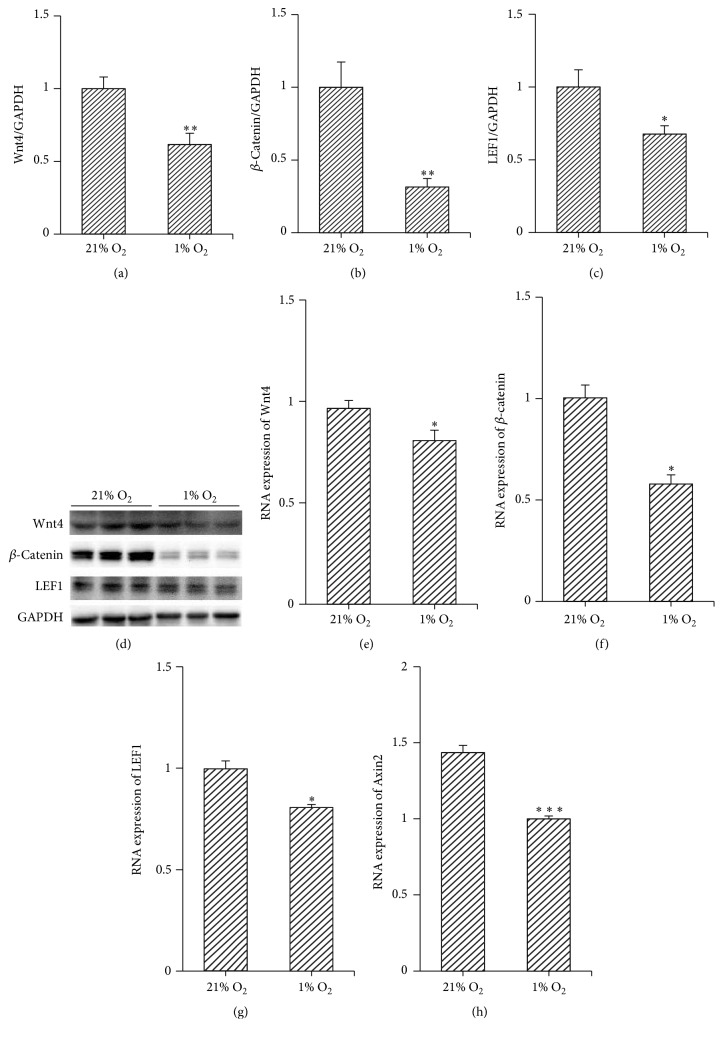
The Wnt signalling pathway is an essential intercellular pathway controlling differentiation. To identify the effect of hypoxia on the Wnt signalling pathway, the expression of Wnt4 mRNA as well as of β-catenin, transcription factor LEF1, and target gene Axin2 downstream of the Wnt signalling pathway was analysed by western blotting and/or real-time quantitative PCR. The results showed that both protein and mRNA levels of Wnt4, β-catenin, and LEF1 were decreased in 3 days after hypoxia culture (Figures 3(a)–3(g)). Additionally, RNA levels of Axin2 were also attenuated by 3 days of hypoxic culture (Figure 3(h)). These data revealed that hypoxia inhibited the Wnt4/β-catenin pathway.
Figure 3.
Hypoxia inhibited Wnt/β-catenin pathway. Expressions of Wnt4 and downstream molecules in the Wnt pathway, β-catenin, LEF1, and Axin2, were measured by western blotting and/or RT-qPCR, 3 days after hypoxic or normoxic culture. Hypoxia decreased the expression of these molecules. (a)–(d) Protein levels of Wnt4, β-catenin, and LEF1 were decreased by hypoxic culture; ∗p < 0.05; ∗∗p < 0.01; n = 6. (e)–(h) RNA levels of Wnt4, β-catenin, LEF1, and Axin2 were also reduced by hypoxic culture; ∗p < 0.05; ∗∗∗p < 0.001; n = 3. Data are represented as mean ± SD.
3.4. Treatment with Either LiCl or BIO Activated the Wnt/β-Catenin Pathway
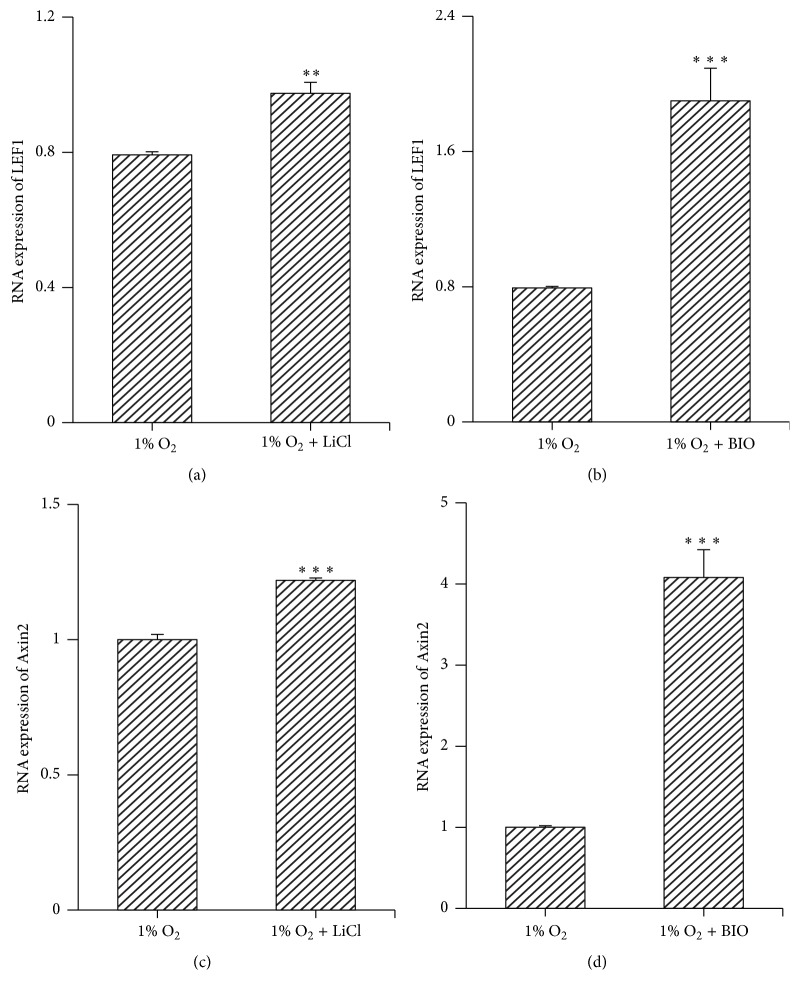
To evaluate the role of the Wnt/β-catenin pathway in mediating the effects of hypoxia, MMSCs were treated with Wnt pathway activators LiCl or BIO and subjected to hypoxia. After treatment with either LiCl (20 mM) or BIO (5 nM), the observed upregulation of LEF1 and Axin2 suggested activation of the Wnt signalling pathway (Figure 4).
Figure 4.
The Wnt/β-catenin pathway was stimulated by either treatment of LiCl or BIO. Cells were cultured in complete medium with LiCl (20 mM) or BIO (5 nM) and exposed to hypoxic conditions for 3 days. The expression of LEF-1 and Axin2 was measured by RT-qPCR. (a)–(d) Either LiCl or BIO increased the RNA levels of LEF-1 and Axin2, which suggested stimulation of the Wnt/β-catenin pathway. ∗∗p < 0.01; ∗∗∗p < 0.001; n = 3. Data are represented as mean ± SD.
3.5. Hypoxia Stimulated the Differentiation of MMSCs by Inhibiting the Wnt/β-Catenin Pathway
3.5.1. Hypoxia Stimulated the Differentiation of MMSCs
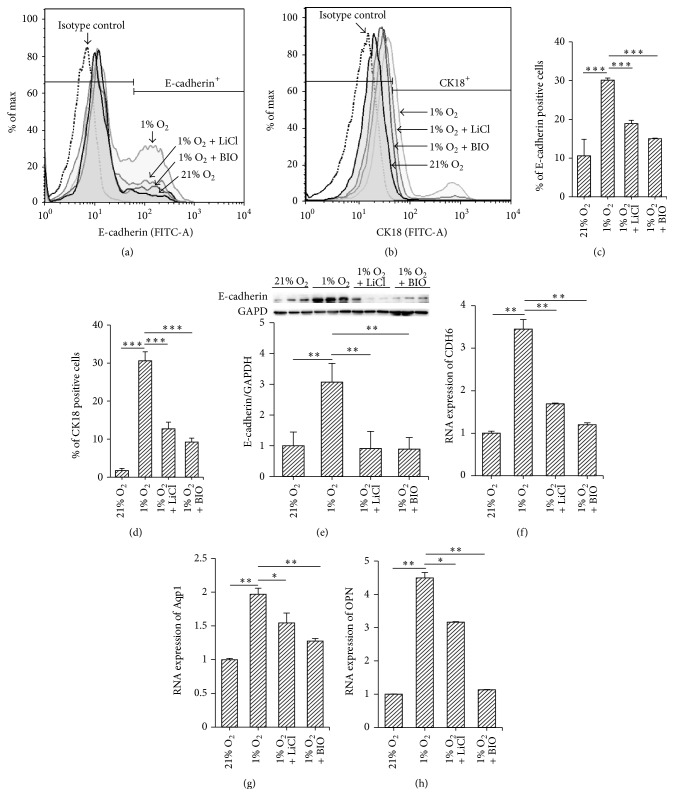
We performed flow cytometry of MMSCs under hypoxic conditions (1% O2) in comparison to conventional cell culture conditions (21% O2) for 3 consecutive days. Many more E-cadherin- or CK18-positive cells were observed among cells exposed to hypoxia, compared with those subjected to normoxic conditions (E-cadherin: 30.1 ± 0.56% versus 10.6 ± 4.3%; CK18: 30.6 ± 2.4% versus 1.7 ± 0.6%, both n = 3, p < 0.001) (Figures 5(a)–5(d)). Additionally, we performed western blotting to explore the expression of E-cadherin in protein extracted from hypoxic and normoxic treated MMSCs. The protein level of E-cadherin was increased by hypoxic culture for 3 days (increased by 46 ± 5%, n = 6, p < 0.01) (Figure 5(e)). The RNA levels of CDH6, Aqp1, and OPN were also increased by hypoxic culture (Figures 5(f)–5(h)).
Figure 5.
Stimulation of the Wnt/β-catenin pathway inhibited the differentiation of hypoxia-conditioned MMSCs. After MMSCs were cultured under normoxic (21% O2), hypoxic (1% O2) conditions or treated with LiCl (20 mM) or BIO (5 nM) for 3 days, detection of epithelial cell-related cell surface markers (E-cadherin or CK-18) was performed via flow cytometry. (a)-(b) Results of flow cytometry show that the number of epithelial cells was increased in hypoxia-cultured cells compared with those in normoxic conditions. Activating the Wnt/β-catenin pathway with LiCl or BIO attenuated the facilitatory effect of hypoxia on differentiation, as evidenced by a decreased percentage of E-cadherin and CK18-positive cells among LiCl- or BIO-treated cells. (c)-(d) Group data from flow cytometry. The data are shown as the mean ± SD. ∗∗∗p < 0.001, n = 3. (e) Expression of E-cadherin was detected by western blotting. The results show that 1% O2 increased and treatment with LiCl and BIO decreased the protein level of E-cadherin; ∗∗p < 0.01; n = 6. (f)–(h) Expression of CDH6, Aqp1, and OPN was detected by qRT-PCR; the result was consistent with that of E-cadherin; ∗p < 0.05; ∗∗p < 0.01; n = 3. Data are represented as mean ± SD.
3.5.2. Activation of the Wnt/β-Catenin Pathway Attenuated the Effect of Hypoxia on Differentiation of MMSCs
Flow cytometry showed that the percentage of E-cadherin and CK18-positive cells was reduced by treatment of LiCl or BIO compared with those exposed only to hypoxia (LiCl or BIO + hypoxia versus hypoxia: E-cadherin, 18.9 ± 0.85% and 15.0 ± 0.14% versus 30.1 ± 0.56%; CK18, 12.7 ± 1.77% and 9.25 ± 1.01% versus 30.6 ± 2.36%; n = 3, all p < 0.001) (Figures 5(a)–5(d)). This result was confirmed by reduced expressions of E-cadherin, CDH6, Aqp1, and OPN detected by western blotting or PCR (Figures 5(e)–5(h)), which suggested that activation of the Wnt/β-catenin pathway abrogated the differentiation of MMSCs induced by hypoxia.
3.6. Hypoxia Reduced the Stemness Phenotype of MMSCs by Inhibiting the Wnt/β-Catenin Pathway
3.6.1. Hypoxia Reduced the Stemness Phenotype of MMSCs
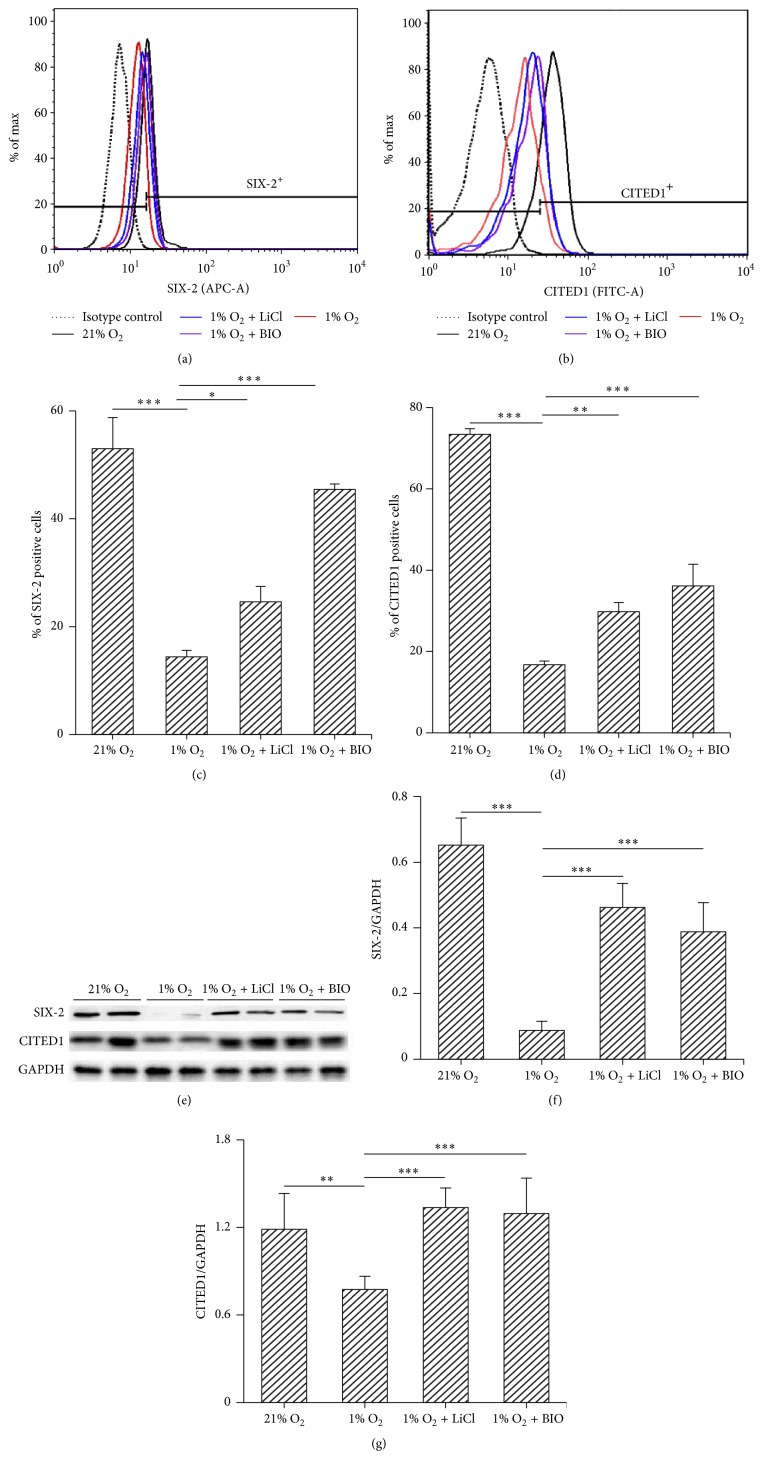
To investigate the effect of hypoxia on stemness of MMSCs, flow cytometry was applied to count SIX-2- and CITED1-positive cells among isolated MMSCs that were subjected to hypoxic or normoxic conditions for 3 days. The result revealed that the percentage of SIX-2 and CITED1-positive cells was significantly decreased by hypoxic culture (hypoxia versus normoxia: 14.4 ± 1.2% versus 53 ± 5.8% and 16.8 ± 0.9% versus 73.4 ± 1.4%, n = 3, both p < 0.001) (Figures 6(a)–6(d)). To confirm the negative effect of hypoxia on stemness, western blotting was performed to detect the expression of CITED1 and SIX-2 in hypoxia-cultured cells. The expressions of CITED1 and SIX-2 were decreased by hypoxic culture for 3 days (Figures 6(e)–6(g)).
Figure 6.
Stimulation of the Wnt/β-catenin pathway helps to maintain the stemness of MMSCs. MMSCs were treated as in Figure 4. Identification of stem cell-related cell surface markers (SIX-2 and CITED1) was performed via flow cytometry. (a)-(b) Results of flow cytometry show that the number of stem cells was decreased in hypoxia-cultured cells compared with those under normoxic conditions. Activating the Wnt/β-catenin pathway with LiCl or BIO attenuated the negative effect of hypoxia on stemness, as evidenced by an increased percentage of SIX-2 and CITED1-positive cells among LiCl- or BIO-treated cells. (c)-(d) Group data from flow cytometry. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; n = 3. Data are represented as mean ± SD. (e)–(g) Expressions of SIX-2 and CITED1 were detected by western blotting. The results show that treatment with LiCl and BIO increased the protein level of SIX-2 and CITED1; ∗∗p < 0.01; ∗∗∗p < 0.001; n = 6. Data are represented as mean ± SD.
3.6.2. Activation of the Wnt/β-Catenin Pathway Attenuated the Effect of Hypoxia on Stemness of MMSCs
Flow cytometry showed that the percentage of SIX-2 and CITED1-positive cells was increased by treatment of LiCl or BIO (LiCl or BIO + hypoxia versus hypoxia: SIX-2, 24.6 ± 2.85% and 45.47 ± 0.99% versus 14.4 ± 1.21%, n = 3, p < 0.05, p < 0.001; CITED1, 29.8 ± 2.25% and 36.1 ± 5.34% versus 16.77 ± 0.91%, n = 3, p < 0.01, p < 0.001, resp.) (Figures 6(a)–6(d)). This result was confirmed by elevated protein levels of SIX-2 and CITED1 detected by western blotting (Figures 6(e)–6(g)), which suggested that activation of the Wnt/β-catenin pathway mediated depressant effect of hypoxia on the stemness of MMSCs.
3.7. Hypoxia Inhibited Proliferation and Induced Apoptosis of MMSCs by Inhibiting the Wnt/β-Catenin Pathway
3.7.1. Hypoxia Inhibited Proliferation and Induced Apoptosis of MMSCs
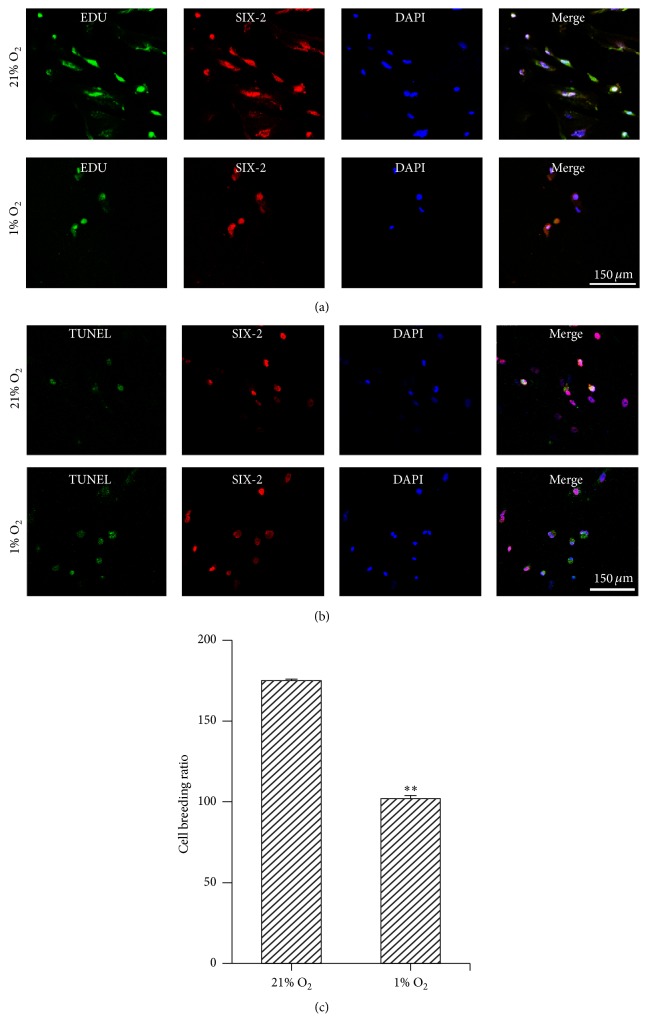
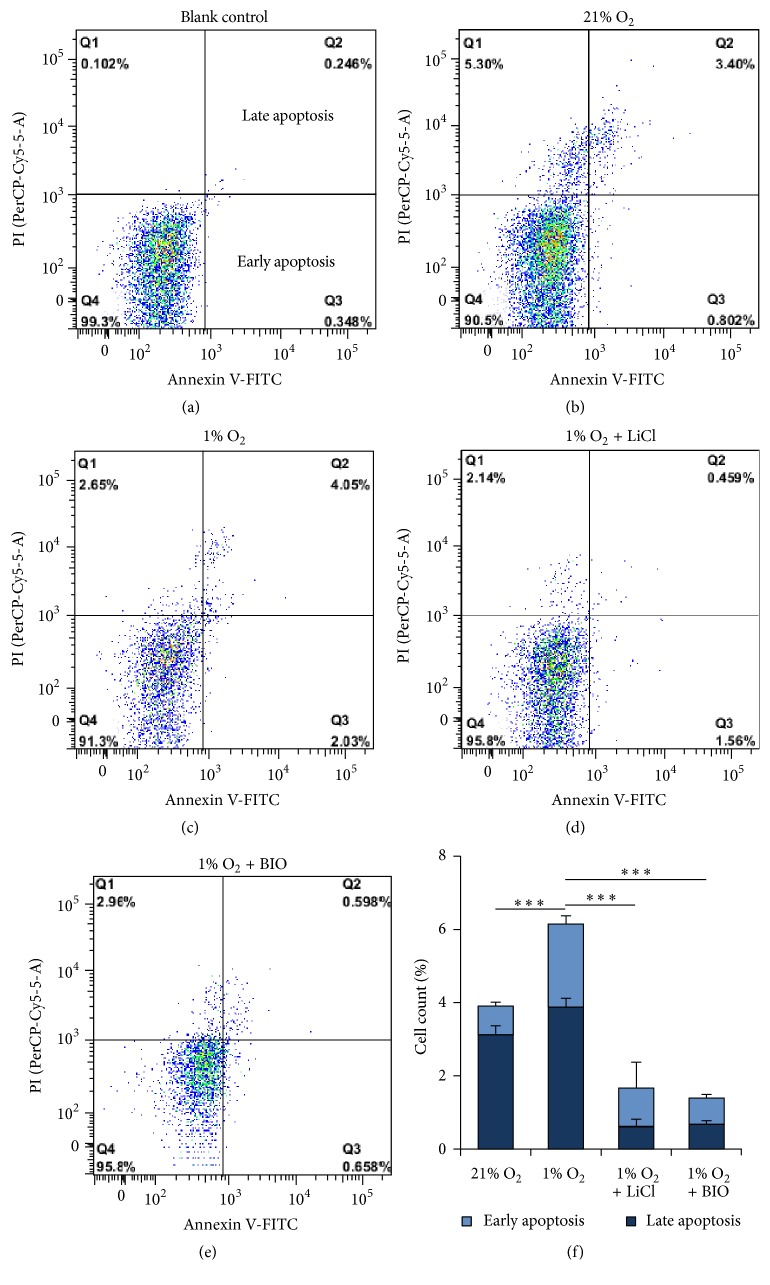
Proliferation of MMSCs was determined by Edu and Alamar Blue staining. MMSCs were suspended in serum-free media and plated onto chamber slides and 96-well plates. After hypoxic incubation for 3 days, the number of EdU-positive cells growing on the slide was counted, and the absorbance at the corresponding wavelength was measured for Alamar Blue staining. Our results showed that the number of EdU-positive cells and the breeding ratio were decreased in hypoxia-cultured MMSCs (breeding ratio under hypoxic versus normoxic conditions: 1.02 ± 1.92 versus 1.75 ± 0.82, n = 6, p < 0.01) (Figures 7(a) and 7(c)). Apoptosis of MMSCs was measured by TUNEL staining and flow cytometry. The results showed that the number of TUNEL-positive cells increased among hypoxia-cultured MMSCs (Figure 7(b)). As the flow cytometry showed, cells undergoing early and late apoptosis were both increased in hypoxia-cultured MMSCs (the early plus late apoptotic rate under hypoxic versus normoxic conditions: 6.14 ± 0.32% versus 3.91 ± 0.29%, n = 3, p < 0.01) (Figure 8).
Figure 7.
Hypoxia inhibited proliferation and elevated apoptosis of MMSCs. The MMSCs seeded in chamber slide system were incubated under hypoxic or normoxic condition for 3 days. (a) Proliferation of MMSCs was tested for by EdU staining. The number of EdU-positive cells was decreased under hypoxic conditions. (b) TUNEL staining showed that TUNEL-labelled SIX-2-positive cells were increased under hypoxic conditions. This also indicates that apoptosis mainly occurred in MMSCs. Bar is equal to 150 μm. (c) The MMSCs seeded in 96-well plate were exposed to hypoxic or normoxic condition for 3 days. Breeding ratio of cells was measured by Alamar Blue staining; the data show that hypoxia depressed breeding of MMSCs. ∗∗p < 0.01; n = 6. Data are represented as mean ± SD.
Figure 8.
Stimulation of the Wnt/β-catenin pathway inhibited the apoptosis of MMSCs. Cells were treated as in Figure 4. The number of apoptotic MMSCs was detected by Annexin V-FITC/PI staining via flow cytometry. (a) Blank control. (b)–(e) Results of flow cytometry show that the percentage of cells undergoing early and late apoptosis (quadrants Q3 and Q2) among hypoxia-conditioned cells was larger than that in the control condition. Activating the Wnt/β-catenin pathway with LiCl or BIO decreased the percentage of apoptotic cells. (f) Group data from flow cytometry; ∗∗∗p < 0.001; n = 3. Data are represented as mean ± SD.
3.7.2. Activation of the Wnt/β-Catenin Pathway Attenuated the Effect of Hypoxia on Apoptosis of MMSCs
Flow cytometry showed that hypoxia-induced apoptosis of MMSCs was also inhibited by LiCl or BIO (LiCl or BIO + hypoxia versus hypoxia: 1.40 ± 0.20% and 1.67 ± 0.5% versus 6.14 ± 0.32%, n = 3, both p < 0.01) (Figure 8). These results indicated that stimulation of the Wnt/β-catenin pathway promoted the renewal of MMSCs.
Collectively, these findings support our hypothesis that hypoxia facilitates the differentiation and suppresses the renewal of MMSCs by inhibiting the Wnt/β-catenin signalling pathway.
4. Discussion
Hypoxia is a critical microenvironmental factor for normal embryonic development. Mammalian embryonic development occurs at low intrauterine O2 levels ranging from 2% to 9% [5]. Particularly in the embryonic kidney, oxygen tension can be as low as 1% [12]. Even in adult kidneys, which exhibit unique vascular supply, the kidney medulla and papilla experience oxygen tensions as low as 1%, a relatively hypoxic environment when compared to other tissues [30]. However, there is little evidence regarding the role of hypoxia in embryonic kidney development. To explore a more “physiological hypoxia” for the MMSCs, we compared the effects of different O2 tension (1%, 5%, and 7%) on differentiation and self-renewal of the MMSCs. We found that unlike 1% O2, 5% and 7% O2 had no effects on either differentiation or self-renewal of the MMSCs. Additionally, the metabolic/molecular biology studies performed on cell lines show that mitochondrial respiration can provide sufficient amount of energy under 1% O2 to permit cell proliferation [31]. Thus, we applied 1% O2 to research the effect of hypoxic condition on the MMSCs. We demonstrated that hypoxia promotes the differentiation and attenuates the stemness of MMSCs through blocking of the Wnt/β-catenin pathway.
The effect of hypoxia on the differentiation of stem cells varies among individual tissues. Hypoxia is known to promote cell differentiation and development in many tissues. It has been reported that hypoxia increases formation of terminal branches during tracheal development [32] and enhances the differentiation of mouse embryonic stem cells into distal lung cells [6]. Low oxygen increases the differentiation of neural progenitor/stem cells into mature neurons [7, 33, 34]. Hypoxic conditions increase the yield of cardiomyocytes from mouse embryonic stem cells [8]. In contrast, low O2 tension has been shown to inhibit the differentiation of human embryonic stem cells [35]. In the kidney, hypoxic conditions (5% O2) induce ureteric bud branching during kidney development in vitro organ culture [3, 5]. However, the effect of hypoxia on the differentiation of MMSCs was previously unclear. Our results revealed that hypoxia (1% O2) induced the differentiation of mesenchymal cell progenitors into epithelial cells, as evidenced by increased expression of the epithelial cell marker E-cadherin and an increased number of E-cadherin- and CK18-positive cells. It seems that hypoxia may induce ureteric bud branching through stimulating the differentiation of MMSCs. However, we found that hypoxic conditions (1% O2) inhibited ureteric bud branching in vitro organ culture [36]. The reason for this contradiction may be attributed to 1% O2 that we applied exceeding the tolerance of organ to hypoxia. Thus, further investigation will be needed to explore the effect of hypoxia on development of kidney.
In addition to inducing differentiation, maintaining stemness and proliferation of stem cells to preserve an available pool of undifferentiated progenitors is also important for development. Some reports have demonstrated that hypoxic culture (1–4% O2) maintains a higher level of undifferentiated human embryonic stem cells [4, 37]. A reduced-O2 environment has also been demonstrated to have positive effects on the maintenance of stemness of murine stem cells [38]. Moreover, a study has reported that hypoxia is not beneficial for maintaining human embryonic stem cells in the undifferentiated state [39]. Another study has demonstrated that mouse embryonic stem cells lose self-renewal activity under in vitro hypoxia [40]. Our present results showed that hypoxia exposure reduced the expression of SIX-2 and CITED1 and the number of SIX-2-positive cells. Additionally, proliferation was depressed and apoptosis was increased by hypoxia. Interestingly, apoptosis mainly occurred in SIX-2-positive cells but not in transformed epithelial cells. Because there are more MMSCs differentiated into epithelium and fewer regenerative cells to compensate in the stem cell pool, it is not surprising that the percentage of MMSCs was decreased after hypoxic culture. The results suggested that the significance of hypoxia for development lies in its potential to stimulate differentiation. There are probably other elements that help in promoting renewal of MMSCs in vivo, which will require further efforts to explore.
The development of the kidney is a developmental process in which mesenchymal to epithelial transition (MET) occurs. A mesenchymal stem cell pool responds to paracrine and autocrine factors that regulate whether cells remain within the niche or epithelialize. Among these factors, Wnt4 is considered to be a critical regulator in this process. Our investigation found that hypoxic culture inhibited Wnt/β-catenin, as evidenced by the attenuated level of Wnt4 and its downstream molecules β-catenin, LEF1, and Axin2. Previous investigations have revealed that stimulation of the Wnt/β-catenin signalling pathway in stem cells represses differentiation and maintains a state of self-renewal [41, 42]. To test whether hypoxia promoted the differentiation and inhibited the self-renewal of MMSCs by blocking the Wnt pathway, LiCl or BIO-conditioned media were used to stimulate Wnt signalling simultaneously with hypoxic exposure. Our results suggested that activation of the Wnt pathway neutralized the effect of hypoxia on MMSCs. In contrast, many studies have revealed that stabilization of β-catenin induces nephron differentiation [43]. However, a previous study has reported that LiCl elicits the early stages of epithelial differentiation, but the process fails to progress into the later stages of nephrogenesis [44]. Further, another study has observed that Wnt signalling acts transiently in inducing MET and that downregulation of β-catenin activity is essential for the fully epithelialized state of the renal vesicle [20]. In this context, the effects of Wnt/β-catenin on the differentiation of MMSCs may be distinct at different stages of development. Our data were collected from MMSCs harvested from embryonic kidney at embryonic days 18-19, which is near birth. A simple explanation is that hypoxia inhibited the Wnt/β-catenin pathway, whose activation blocks the differentiation of MMSCs at the late embryo stage in rats. Further studies may be required to explore the effects of hypoxia on the differentiation of MMSCs as well as on the Wnt pathway at early embryo stages.
Differentiation is a major hurdle for the successful translation of stem cell research to clinical applications. The ability of hypoxic preconditioning to induce the differentiation of stem cells toward mature cell lineages provides support for the application of hypoxic pretreatment before transplantation of stem cells. Indeed, accumulating evidence is suggesting that transplantation of hypoxia-preconditioned stem cells is more effective in tissue repair. It has been shown that the transplantation of hypoxia-preconditioned mesenchymal stem cells (MSCs) enhances vessel formation and skeletal muscle fibre regeneration after hindlimb ischemia and stimulates angiogenesis and neurogenesis after cerebral ischemia [16, 45]. It has also been demonstrated that intrastriatal transplantation of hypoxia-induced human MSCs (hMSCs) can more efficaciously ameliorate behavioural deficits of parkinsonian rats than normoxia-induced hMSCs [46]. Additionally, therapeutic applications of MSCs to treat heart failure, limb ischemia, and so forth are currently in Phase I (safety studies) or Phase II (proof of concept for efficacy in human patients) clinical trials [47, 48]. A Phase I clinical trial using MSCs for treating acute kidney injury (AKI) is ongoing [49]. Our previous experiments have revealed that hypoxic preconditioning enhances the therapeutic effects of bone marrow mesenchymal stem cells for treatment of AKI [50]. However, limitations still exist in the understanding of how hypoxic-preconditioned MSCs exert their renoprotective effects. Our current results indicate that the elevated therapeutic activity of MSCs may be attributed to the ability of hypoxia to promote differentiation. Although hypoxia increased apoptosis, the overall percentage of cells undergoing apoptosis is not high, being lower than 7% even under hypoxia. Thus, we concluded that the therapeutic potential of MMSCs could be enhanced by hypoxic precondition.
In summary, the observations herein further our understanding of the effects of hypoxia on kidney development by demonstrating that hypoxic conditions stimulate the differentiation of MMSCs by inhibiting canonical Wnt signalling. Additionally, this study aids in understanding the therapeutic potential and possible clinical impact of hypoxia-preconditioned MMSCs.
Competing Interests
The authors declared that there are no competing interests.
Authors' Contributions
Shaopeng Liu and Nana Song performed experiments and data analysis and prepared the manuscript. Jianqiang He and Xiaofang Yu performed data analysis and discussion of the research progress at different stages. Jia Guo and Xiaoyan Jiao performed experiments. Xiaoqiang Ding and Jie Teng designed the project, performed data interpretation, and edited the manuscript prior to submission. All authors reviewed the manuscript. Shaopeng Liu and Nana Song (co-first authors) contributed equally to this work.
Funding
This work was funded by the Major State Basic Research Development Program of China (973 Program: 2011CB944000), Shanghai Key Laboratory of Kidney and Blood Purification, Shanghai Science and Technology Commission (14DZ2260200), the National Natural Science Foundation of China (81570600), and Zhenjiang Science and Technology Project (SH2012022).
References
- 1.Webster W. S., Abela D. The effect of hypoxia in development. Birth Defects Research Part C - Embryo Today: Reviews. 2007;81(3):215–228. doi: 10.1002/bdrc.20102. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez-Rodriguez P., Jr., Tong W., Xue Q., Li Y., Hu S., Zhang L. Fetal hypoxia results in programming of aberrant angiotensin II receptor expression patterns and kidney development. International Journal of Medical Sciences. 2013;10(5):532–538. doi: 10.7150/ijms.5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akimoto T., Hammerman M. R., Kusano E. Low ambient O2 enhances ureteric bud branching in vitro. Organogenesis. 2005;2(1):17–21. doi: 10.4161/org.2.1.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okazaki K., Maltepe E. Oxygen, epigenetics and stem cell fate. Regenerative medicine. 2006;1(1):71–83. doi: 10.2217/17460751.1.1.71. [DOI] [PubMed] [Google Scholar]
- 5.Tsuji K., Kitamura S., Makino H. Hypoxia-inducible factor 1α regulates branching morphogenesis during kidney development. Biochemical and Biophysical Research Communications. 2014;447(1):108–114. doi: 10.1016/j.bbrc.2014.03.111. [DOI] [PubMed] [Google Scholar]
- 6.Pimchanok P., Shimon L., Collin T. S., Gregg J., Edward S. S., Peter I. L. Hypoxia enhances differentiation of mouse embryonic stem cells into definitive endoderm and distal lung cells. Stem Cells and Development. 2015;24(5):663–676. doi: 10.1089/scd.2014.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mondragon-Teran P., Lye G. J., Veraitch F. S. Lowering oxygen tension enhances the differentiation of mouse embryonic stem cells into neuronal cells. Biotechnology Progress. 2009;25(5):1480–1488. doi: 10.1002/btpr.248. [DOI] [PubMed] [Google Scholar]
- 8.Bauwens C., Yin T., Dang S., Peerani R., Zandstra P. W. Development of a perfusion fed bioreactor for embryonic stem cell-derived cardiomyocyte generation: oxygen-mediated enhancement of cardiomyocyte output. Biotechnology and Bioengineering. 2005;90(4):452–461. doi: 10.1002/bit.20445. [DOI] [PubMed] [Google Scholar]
- 9.Chen H.-C., Lee J.-T., Shih C.-P., et al. Hypoxia Induces a Metabolic Shift and Enhances the Stemness and Expansion of Cochlear Spiral Ganglion Stem/Progenitor Cells. BioMed Research International. 2015;2015 doi: 10.1155/2015/359537.359537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fotia C., Massa A., Boriani F., Baldini N., Granchi D. Hypoxia enhances proliferation and stemness of human adipose-derived mesenchymal stem cells. Cytotechnology. 2015;67(6):1073–1084. doi: 10.1007/s10616-014-9731-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saller M. M., Prall W. C., Docheva D., et al. Increased stemness and migration of human mesenchymal stem cells in hypoxia is associated with altered integrin expression. Biochemical and Biophysical Research Communications. 2012;423(2):379–385. doi: 10.1016/j.bbrc.2012.05.134. [DOI] [PubMed] [Google Scholar]
- 12.Simon M. C., Keith B. The role of oxygen availability in embryonic development and stem cell function. Nature Reviews Molecular Cell Biology. 2008;9(4):285–296. doi: 10.1038/nrm2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costantini F., Kopan R. Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Developmental Cell. 2010;18(5):698–712. doi: 10.1016/j.devcel.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Self M., Lagutin O. V., Bowling B., et al. Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. The EMBO Journal. 2006;25(21):5214–5228. doi: 10.1038/sj.emboj.7601381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freeburg P. B., Abrahamson D. R. Hypoxia-inducible factors and kidney vascular development. Journal of the American Society of Nephrology. 2003;14(11):2723–2730. doi: 10.1097/01.asn.0000092794.37534.01. [DOI] [PubMed] [Google Scholar]
- 16.Leroux L., Descamps B., Tojais N. F., et al. Hypoxia preconditioned mesenchymal stem cells improve vascular and skeletal muscle fiber regeneration after ischemia through a Wnt4-dependent pathway. Molecular Therapy. 2010;18(8):1545–1552. doi: 10.1038/mt.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kispert A., Vainio S., McMahon A. P. Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development. 1998;125(21):4225–4234. doi: 10.1242/dev.125.21.4225. [DOI] [PubMed] [Google Scholar]
- 18.Stark K., Vainio S., Vassileva G., McMahon A. P. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature. 1994;372(6507):679–683. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- 19.Tanigawa S., Wang H., Yang Y., et al. Wnt4 induces nephronic tubules in metanephric mesenchyme by a non-canonical mechanism. Developmental Biology. 2011;352(1):58–69. doi: 10.1016/j.ydbio.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park J.-S., Valerius M. T., McMahon A. P. Wnt/β-catenin signaling regulates nephron induction during mouse kidney development. Development. 2007;134(13):2533–2539. doi: 10.1242/dev.006155. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt-Ott K. M., Masckauchan T. N. H., Chen X., et al. β-catenin/TCF/Lef controls a differentiation-associated transcriptional program in renal epithelial progenitors. Development. 2007;134(17):3177–3190. doi: 10.1242/dev.006544. [DOI] [PubMed] [Google Scholar]
- 22.Pietilä I., Vainio S. J. Kidney development: an overview. Nephron—Experimental Nephrology. 2014;126(2):40–44. doi: 10.1159/000360659. [DOI] [PubMed] [Google Scholar]
- 23.Yang G., Cheng Q.-L., Li C.-L., et al. Isolation and identification of rat kidney stem cells. Journal of Sichuan University (Medical Science Edition) 2015;46(5):667–672. [PubMed] [Google Scholar]
- 24.Murphy A. J., Pierce J., de Caestecker C., et al. CITED1 confers stemness to wilms tumor and enhances tumorigenic responses when enriched in the nucleus. Oncotarget. 2014;5(2):386–402. doi: 10.18632/oncotarget.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Georgas K., Rumballe B., Wilkinson L., et al. Use of dual section mRNA in situ hybridisation/immunohistochemistry to clarify gene expression patterns during the early stages of nephron development in the embryo and in the mature nephron of the adult mouse kidney. Histochemistry and Cell Biology. 2008;130(5):927–942. doi: 10.1007/s00418-008-0454-3. [DOI] [PubMed] [Google Scholar]
- 26.Crisi G. M., Marconi S. A., Rockwell G. F., Braden G. L., Campfield T. J. Immuno-localization of CD44 and osteopontin in developing human kidney. Pediatric Research. 2009;65(1):79–84. doi: 10.1203/PDR.0b013e31818912b7. [DOI] [PubMed] [Google Scholar]
- 27.Herzlinger D., Koseki C., Mikawa T., Al-Awqati Q. Metanephric mesenchyme contains multipotent stem cells whose fate is restricted after induction. Development. 1992;114(3):565–572. doi: 10.1242/dev.114.3.565. [DOI] [PubMed] [Google Scholar]
- 28.Lyu Z., Mao Z., Wang H., et al. MiR-181b targets Six2 and inhibits the proliferation of metanephric mesenchymal cells in vitro. Biochemical and Biophysical Research Communications. 2013;440(4):495–501. doi: 10.1016/j.bbrc.2013.09.059. [DOI] [PubMed] [Google Scholar]
- 29.Livak K. J., Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Brezis M., Rosen S., Silva P., Epstein F. H. Renal ischemia: a new perspective. Kidney International. 1984;26(4):375–383. doi: 10.1038/ki.1984.185. [DOI] [PubMed] [Google Scholar]
- 31.Guzy R. D., Schumacker P. T. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Experimental Physiology. 2006;91(5):807–819. doi: 10.1113/expphysiol.2006.033506. [DOI] [PubMed] [Google Scholar]
- 32.Jarecki J., Johnson E., Krasnow M. A. Oxygen regulation of airway branching in Drosophila is mediated by Branchless FGF. Cell. 1999;99(2):211–220. doi: 10.1016/S0092-8674(00)81652-9. [DOI] [PubMed] [Google Scholar]
- 33.Kim T.-S., Misumi S., Jung C.-G., et al. Increase in dopaminergic neurons from mouse embryonic stem cell-derived neural progenitor/stem cells is mediated by hypoxia inducible factor-1α. Journal of Neuroscience Research. 2008;86(11):2353–2362. doi: 10.1002/jnr.21687. [DOI] [PubMed] [Google Scholar]
- 34.Binh N. H., Aoki H., Takamatsu M., et al. Time-sensitive effects of hypoxia on differentiation of neural stem cells derived from mouse embryonic stem cells in vitro. Neurological Research. 2014;36(9):804–813. doi: 10.1179/1743132814Y.0000000338. [DOI] [PubMed] [Google Scholar]
- 35.Ezashi T., Das P., Roberts R. M. Low O2 tensions and the prevention of differentiation of hES cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(13):4783–4788. doi: 10.1073/pnas.0501283102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaopeng L., Nana S., Jia G., Xiaofang Y., Xiaoqiang D. Effect of hypoxia on differentiation of metanephric mesenchymal stem cells. Hong Kong Journal of Nephrology. 2015;17(2):p. S75. doi: 10.1016/j.hkjn.2015.09.090. [DOI] [Google Scholar]
- 37.Chen H.-F., Kuo H.-C., Lin S.-P., Chien C.-L., Chiang M.-S., Ho H.-N. Hypoxic culture maintains self-renewal and enhances embryoid body formation of human embryonic stem cells. Tissue Engineering—Part A. 2010;16(9):2901–2913. doi: 10.1089/ten.tea.2009.0722. [DOI] [PubMed] [Google Scholar]
- 38.Gibbons J., Hewitt E., Gardner D. K. Effects of oxygen tension on the establishment and lactate dehydrogenase activity of murine embryonic stem cells. Cloning and Stem Cells. 2006;8(2):117–122. doi: 10.1089/clo.2006.8.117. [DOI] [PubMed] [Google Scholar]
- 39.Chen H.-F., Kuo H.-C., Chen W., Wu F.-C., Yang Y.-S., Ho H.-N. A reduced oxygen tension (5%) is not beneficial for maintaining human embryonic stem cells in the undifferentiated state with short splitting intervals. Human Reproduction. 2009;24(1):71–80. doi: 10.1093/humrep/den345. [DOI] [PubMed] [Google Scholar]
- 40.Lee H.-J., Kim K.-W. Suppression of HIF-1α by valproic acid sustains self-renewal of mouse embryonic stem cells under hypoxia in vitro. Biomolecules & Therapeutics. 2012;20(3):280–285. doi: 10.4062/biomolther.2012.20.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willert K., Brown J. D., Danenberg E., et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423(6938):448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 42.Scheller E. L., Chang J., Wang C. Y. Wnt/β-catenin inhibits dental pulp stem cell differentiation. Journal of Dental Research. 2008;87(2):126–130. doi: 10.1177/154405910808700206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuure S., Popsueva A., Jakobson M., Sainio K., Sariola H. Glycogen synthase kinase-3 inactivation and stabilization of β-catenin induce nephron differentiation in isolated mouse and rat kidney mesenchymes. Journal of the American Society of Nephrology. 2007;18(4):1130–1139. doi: 10.1681/ASN.2006111206. [DOI] [PubMed] [Google Scholar]
- 44.Davies J. A., Garrod D. R. Induction of early stages of kidney tubule differentiation by lithium ions. Developmental Biology. 1995;167(1):50–60. doi: 10.1006/dbio.1995.1006. [DOI] [PubMed] [Google Scholar]
- 45.Wei L., Fraser J. L., Lu Z.-Y., Hu X., Yu S. P. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiology of Disease. 2012;46(3):635–645. doi: 10.1016/j.nbd.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y., Yang J., Li H., et al. Hypoxia promotes dopaminergic differentiation of mesenchymal stem cells and shows benefits for transplantation in a rat model of Parkinson's disease. PLoS ONE. 2013;8(1) doi: 10.1371/journal.pone.0054296.e54296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Philippe B., Luc S., Valérie P.-B., Jérôme R., Alessandra B.-R., Louis C. Culture and use of mesenchymal stromal cells in phase i and II clinical trials. Stem Cells International. 2010;2010:8. doi: 10.4061/2010/503593.503593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trounson A., Thakar R. G., Lomax G., Gibbons D. Clinical trials for stem cell therapies. BMC Medicine. 2011;9, article 52 doi: 10.1186/1741-7015-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morigi M., Benigni A. Mesenchymal stem cells and kidney repair. Nephrology Dialysis Transplantation. 2013;28(4):788–793. doi: 10.1093/ndt/gfs556. [DOI] [PubMed] [Google Scholar]
- 50.Yu X., Lu C., Liu H., et al. Hypoxic preconditioning with cobalt of bone marrow mesenchymal stem cells improves cell migration and enhances therapy for treatment of ischemic acute kidney injury. PLoS ONE. 2013;8(5) doi: 10.1371/journal.pone.0062703.e62703 [DOI] [PMC free article] [PubMed] [Google Scholar]