Abstract
Background
Until now, there has been no comprehensive long-term study in Germany on the development of extremely premature infants up to school age.
Methods
From October 2004 to September 2008, in the German federal state of Lower Saxony, 437 infants born at a gestational age less than 28 weeks were followed up at the ages of 2 and 5 years, and some at the age of 10 years. The 5-year follow-up data were collated with the peri- and neonatological parameters and compared with the 2– and 10-year follow-up data.
Results
The mortality of extremely premature infants was 25.1%. Among the five-year-olds studied, 14.1% showed cognitive impairment and 17.4% had cerebral palsy. 40.4% manifested abnormalities of speech or language, 33.1% had behavioral abnormalities, and 72.5% received therapeutic interventions. Infants in whom severe brain damage was diagnosed by ultrasonography shortly after birth were more likely to develop cerebral palsy (odds ratio [OR] 38.28, 99% confidence interval [12.55; 116.80]) and to have impaired cognitive development (OR 7.36 [2.52; 21.51]). The likelihood of cognitive impairment was also higher among infants whose mothers had a lower level of education (OR 3.83 [1.68; 8.77]). 73.1% (242 out of 331) of the two-year-olds were in the same category of cognitive function at the 5-year follow-up; 82.4% (65 out of 79) of the 5-year-olds were in the same category of cognitive function at the 10-year follow-up.
Conclusion
Many of these extremely premature infants had developmental disturbances, and many required therapeutic interventions. The risk factors revealed by this study may help identify patients who are in particular need of support, enabling targeted measures to be taken at the earliest possible stage in order to improve their cognitive and motor abilities. Nationwide, standardized follow-up at the age of 5 years would be desirable.
In Germany, approximately 0.6% of all infants are born earlier than 28 weeks’ gestation (1). These extremely premature infants constitute a high-risk population, but advances in obstetrics and neonatology have significantly improved their survival chances (2, 3). Today, approximately 80% of these children survive (4) compared to the survival rate of 30% in the late 1970s (5).
With increasing survival rates, the long-term developmental outcomes continue to gain in importance. Large international studies, such as EPICure (United Kingdom [UK] and Ireland) (6), EPIPAGE (France) (7) and the study of the Victorian Infant Collaborative Study Group (Australia) (8), indicate a high prevalence of developmental disorders. According to these studies, the proportion of children with cerebral palsy (CP)—a disorder of posture and movement caused by damage to the developing brain— ranges from 10 to 15%, the proportion of those with cognitive impairment (intelligence quotient [IQ] <70) between 15 and 20%, even up to 40% among infants born before 26 weeks‘ gestation.
In Germany, prospective long-term studies extending into school age are rare (9, 10). Nationwide multicenter follow-up data have not become available as yet. Based on the model of the follow-up examinations of the Hannover Premature Infant Long-Term Study (11), the Lower Saxony Longitudinal Study of Prematurity (Niedersächsisches Frühgeborenen-Nachuntersuchungsprojekt), a state-wide follow-up study of extremely premature infants, was initiated in 2004. The aim of this study was to provide information about survival and about the neurological and cognitive long-term outcome. Because of their relevance for clinical practice, this study focused on the following questions:
At what point in time can the further development of these premature infants be predicted with acceptable certainty?
Which peri- and neonatal risk factors have such a significant impact on long-term outcome that the affected infants require special monitoring and support?
Methods
Former extremely premature infants born between October 2004 and September 2008 were followed up at defined points in time (years 2, 5 and 10). The 5-year study data were combined with the relevant peri- and neonatal data and the follow-up data at ages 2 and 10 years. For the cross-sectional evaluation of motor development, a comparison sample of healthy term births in Lower Saxony kindergartens was analyzed which was described in detail elsewhere (12).
SPSS version 21 was used for statistical analysis. Apart from comparisons made using the chi-square test and Student‘s t-test or Mann–Whitney U test, the impact of relevant predictor variables on the development of the children was determined using a multiple logistic regression model. The significance level was set at p <0.001 and the relationships between independent variables and the dependent variable was reported as odds ratios (ORs) with a 99% confidence interval (CI). The cross-sectional comparison results were reported with a 95% CI.
Detailed information about the methodology is provided in the eBox.
eBOX. Supplementary information about methodology.
All Departments of Neonatology providing care for extremely premature infants in the German federal state of Lower Saxony have been participating in the project presented here. During the discharge discussion, all parents of children who have been born prematurely at <28 weeks’ gestation in Lower Saxony since October 2004 have been asked to visit any of the 11 regional Sociopediatric Centers for follow-up examinations. After obtaining the parents’ informed consent, which was prepared in consultation with the Federal Data Protection Commissioner, extremely premature infants born between October 2004 and September 2008 were followed up at years 2 after the calculated due date (consistent with the decision of the Federal Joint Committee [Gemeinsamer Bundesausschuss, G-BA], [26]) and at age 5 years (prior to start of schooling) and 10 years, based on a standardized concept. For the 10-year follow-up, no complete year groups could be included in the analysis because at the time of submission of the article only children of the first one and a half year groups had turned 10 years old.
At all 3 follow-up time points, the children were medically examined and sociodemographic and medical history data were obtained. For the 2-year-olds, the Mental Scale of the Bayley Scales of Infant Development II (Bayley II) (e1) was used. The 5-year-olds were examined by psychologists using a standardized intelligence test—the Kaufman Assessment Battery for Children (K-ABC) (14)—and two subtests (“understanding of sentences“ and “morphologic rule formation“) from a language test, the language development test for 3– to 5-year-olds (Sprachentwicklungstest, SETK 3–5) (e2). For the 10-year-olds, the Wechsler Intelligence Scales for Children – fourth edition (WISC IV) (e3) was used. Children with an intelligence quotient (IQ) or Mental Development Index (MDI) = 85 were categorized as normal, children with a score between 70 and 84 as below average and children with a score <70 as cognitively impaired. Children achieving a T value <40 in at least one subtest were regarded as having an abnormal language development. Behavioral data were obtained from the parents of both the 5– and 10-year-olds who completed the German version of the Child Behavior Checklist 4–18 (CBCL 4–18) (15). Behavior was evaluated as falling into the borderline range at total T values between 60 to 63; at T values >63, it was considered in the clinical range. The motor development of 5-year-olds was tested using a defined set of criteria which included motor skills such standing on one leg and hopping on one leg, tandem walking, ball catching, and climbing stairs. Children who did not achieve age-appropriate results for the tested criteria were considered as having abnormal motor function. At the 10-year follow-up, all children not diagnosed with cerebral palsy (CP) underwent a standardized motor function test, the Movement Assessment Battery for Children-2 (M-ABC-2) (e4) and a screening test, the Developmental Coordination Disorder Questionnaire—German (DCDQ-G) (e5). CP was diagnosed and classified according to the terminology of the Surveillance of Cerebral Palsy in Europe (e6). The assessment of educational attainment was based on the academic and professional training completed and the current profession of the mother. The classification into a high (= entrance qualification for a university of applied sciences) and lower level of education was based on the International Standard Classification of Education (ISCED) (e7).
Project management and analysis was performed at the Center for Quality and Management in Healthcare (ZQ), Medical Association of Lower Saxony.
Data sources
For the analyses, the study data were combined with the relevant peri- and neonatal data. Four children were excluded from the analyses due to congenital malformations or syndromes with developmental disorders independent of prematurity. During longitudinal analysis, the developmental data obtained at age 5 years were compared with those collected at ages 2 and 10 years for each of the study participants. For cross-sectional evaluation of motor development, a comparison sample of term births from Lower Saxony kindergartens was analyzed. For this purpose, stratification specifications were prepared for the comparison sample based on the demographic distribution of the premature infant sample, using a matching procedure. The selection of the control group (n = 305) was based on the following five demographic criteria: region, residential area, gender, parental educational background and migration background (12).
Statistical analyses
SPSS version 21 was used for statistical analysis. Apart from comparisons made using the chi-square test (for binary variables) or Student‘s t-test and Mann–Whitney U test (for normally and not normally distributed continuous variables, respectively), the impact of relevant predictor variables on the development of the children was determined using a multiple logistic regression model. Table 3 shows the results obtained using the method with parallel inclusion of the independent variables. The variables with the strongest correlation were then entered using step-wise forward selection, with the Wald test as the selection criterion. The regression model’s quality of fit was evaluated using the Hosmer-Lemeshow test; Nagelkerkes R2 is reported as measure of explained variance. The significance level was set at p <0.001 and the relationships between variables and the dependent variable was reported as odds ratios (ORs) with a 99% confidence interval (CI).
Results
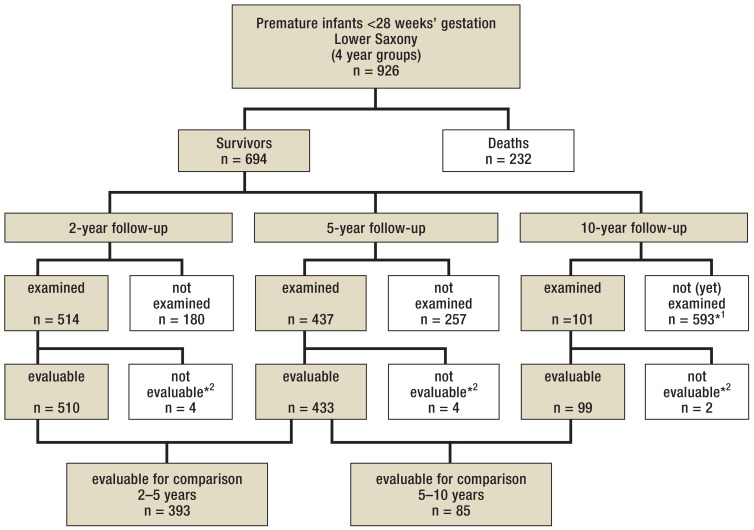
During the observation period, 249 076 children were born in Lower Saxony (13), 926 of these (0.4%) at <28 weeks’ gestation. Of these, 232 (25.1%) died while still hospitalized in the department of pediatrics and 2 (0.2%) after discharge before the age of 2 years.
All infants <23 weeks’ gestation died. Then, the survival rate continuously increased with gestational age: from 44.4% at 23 weeks’ gestation to 91.5 at 27 weeks’ gestation. Of the 694 surviving infants, 260 (37.4%) were born at 23 to 25 weeks’ gestation. Altogether 437 children (63.0% of the survivors) were followed up after 5 years (figure). As shown in Table 1, the relevant peri- and neonatal parameters of the analyzed group were not significantly different from those in the group of all survivors.
Figure.
Project participants
*1 Ongoing study: At the time of completion of this paper, the examination age was only reached by 1.5 year groups.
*2 Exclusion due to congenital malformations or syndromes independent of prematurity
Table 1. Comparison of demographic and clinical parameters.
|
Treated children |
Surviving children |
Evaluated children |
Lost to follow-up |
p | |
| (n = 926) | (n = 694) | (n = 433) | (n = 257) | ||
| Data at birth | |||||
| Weeks’ gestation | 25.5 ± 1.3 | 25.8 ± 1.2 | 25.7 ± 1.2 | 25.9 ± 1.1 | 0.190 |
| Birth weight (g) | 798 ± 207 | 832 ± 197 | 831 ± 199 | 837 ± 192 | 0.451 |
| Sex, male | 55.1% | 52.4% | 53.3% | 51.0% | 0.548 |
| Multiple births | 25.3% | 25.3% | 24.6% | 26.4% | 0.601 |
| Spontaneous delivery | 13.9% | 13.1% | 14.4% | 10.7% | 0.184 |
| pH value <7.1 | 4.2% | 3.4% | 3.3% | 3.6% | 0.888 |
| Neonatal care data | |||||
| Severe brain injury (IVH III/PVH/PVL) |
21.8% | 14.4% | 15.7% | 12.2% | 0.212 |
| Mechanical ventilation 2 weeks |
30.1% | 33.9% | 36.0% | 29.4% | 0.076 |
| Sepsis | 39.8% | 39.2% | 40.1% | 37.3% | 0.483 |
| Patent ductus arteriosus | 24.8% | 28.0% | 28.3% | 26.9% | 0.709 |
| Surgery for necrotizing enterocolitis | 5.5% | 3.8% | 3.4% | 4.2% | 0.622 |
IVH III, intraventricular hemorrhage grade 3; PVH,periventricular hemorrhage; PVL, periventricular leukomalacia
Development at age 5 years
The subtest results for cognition, speech and behavior are summarized in Table 2.
Table 2. Development at age 5 years.
| Subtest |
Results <28 weeks’ gestation total: n = 433 |
Results <26 weeks’ gestation: n = 172 |
Results 26–27 weeks’ gestation: n = 261 |
p | |||
| n | % | n | % | n | % | ||
|
Cognition (Test: K-ABC*1) |
384 | 100.0 | 153 | 100.0 | 231 | 100.0 | |
| normal (IQ ≥ 85) |
244 | 63.5 | 81 | 52.9 | 163 | 70.6 | <0.001 |
| below average cognitive performance (IQ 70–84) |
86 | 22.4 | 40 | 26.1 | 46 | 19.9 | |
| cognitively impaired (IQ <70) |
54 | 14.1 | 32 | 20.9 | 22 | 9.5 | |
|
Language (Test: SETK 3–5*2) |
376 | 100.0 | 151 | 100.0 | 225 | 100.0 | |
| normal (T value ≥ 40) |
224 | 59.6 | 73 | 48.4 | 151 | 67.1 | <0.001 |
| abnormal (T value <40) |
152 | 40.4 | 78 | 51.6 | 74 | 32.9 | |
|
Behavior (Parents’ questionnaire: CBCL 4–18*3) |
344 | 100.0 | 132 | 100.0 | 212 | 100.0 | |
| normal range (T value ≤ 59) |
230 | 66.9 | 86 | 65.1 | 144 | 67.9 | 0.406 |
| borderline range (T value 60–63) |
50 | 14.5 | 17 | 12.9 | 33 | 15.6 | |
| clinical range (T value ≥ 64) |
64 | 18.6 | 29 | 22.0 | 35 | 16.5 | |
|
Motor function (Set of criteria see eBox) |
430 | 100.0 | 170 | 100.0 | 260 | 100.0 | |
| normal | 194 | 45.2 | 55 | 32.4 | 139 | 53.5 | <0.001 |
| abnormal (according to set of criteria) | 161 | 37.4 | 68 | 40.0 | 93 | 35.7 | |
| CP / of these able to walk | 75 / 40 | 17.4 / 53.4 | 47 / 26 | 27.6 / 55.4 | 28 / 14 | 10.8 / 50.0 | |
*1 Kaufman Assessment Battery for Children
*2 Language development test for 3– to 5-year-old children
*3 Child Behavior Checklist/4 –18, IQ, intelligence quotient
On motor assessment, 194 premature infants (45.2%) were normal (table 2). However, 89.6% of the children in the comparison group of term births (cross-sectional comparison) were normal and only 10.4% showed problems in motor development. There was no case with CP (12).
The developmental assessment results were significantly different between the two analyzed gestational age groups in all categories, except for behavior (table 2).
Therapeutic interventions
Over their life span, 401 children (92.6%) received physiotherapy and other allied health services and/or early intervention. In 72.5% of these children (n = 314), these treatments were provided at age 5. Of those, treatments were newly initiated in 29.8% (n = 129) by the assessing health professionals at the 5-year follow-up.
Variables influencing the course of development
The univariate analysis identified the following parameters as highly significant factors (p<0.001) for the risk of developing CP:
severe brain injury (intraventricular hemorrhage [IVH] III, periventricular hemorrhage [PVH], periventricular leukomalacia [PVL])
mechanical ventilation for more than two weeks
<26 weeks’ gestation.
With regard to cognitive development (IQ <85 versus IQ = 85), birth weight <750g and lower maternal educational attainment were identified as further highly significant factors, apart from the three factors mentioned before.
The results of multiple logistic regression analysis are listed in Table 3. For the risk of developing CP, the factor “severe brain injury” (p<0.001) was also highly significant in the statistical model with stepwise addition of independent variables, which explains 50.2% of the total variance. In this model, the variable “lower maternal educational attainment” was confirmed as an important factor for cognitive development, apart from the expected high impact of severe brain injury.
Table 3. Results of multiple logistic regression analysis.
| Variable | OR | (99% CI) | p |
| Factors influencing cognitive development* | |||
| Severe brain injury (IVH III/PVH/PVL) | 7.36 | [2.52; 21.51] | 0.000 |
| Lower maternal educational attainment | 3.83 | [1.68; 8.77] | 0.000 |
| Birth weight <750g | 2.50 | [1.11; 5.63] | 0.004 |
| Mechanical ventilation >2 weeks | 1.96 | [0.91; 4.24] | 0.024 |
| Surgery for necrotizing enterocolitis | 1.58 | [0.22; 11.23] | 0.546 |
| Sex, male | 1.43 | [0.70; 2.91] | 0.197 |
| Sepsis | 1.27 | [0.61; 2.63] | 0.396 |
| <26 weeks’ gestation | 1.19 | [0.52; 2.72] | 0.587 |
| Multiple births | 1.14 | [0.50; 2.59] | 0.679 |
| Patent ductus arteriosus | 1.06 | [0.49; 2.29] | 0.853 |
| Spontaneous delivery | 0.83 | [0.28; 2.47] | 0.653 |
| Migration background, at least 1 parent | 0.65 | [0.30; 1.44] | 0.164 |
| Factors influencing the development of cerebral palsy | |||
| Severe brain injury (IVH III/PVH/PVL) | 38.28 | [12.55; 116.80] | 0.000 |
| Mechanical ventilation >2 weeks | 2.74 | [0.89; 8.41] | 0.021 |
| Sex, male | 1.81 | [0.63; 5.20] | 0.146 |
| <26 weeks’ gestation | 1.67 | [0.48; 5.74] | 0.287 |
| Birth weight <750 g | 1.37 | [0.41; 4.60] | 0.505 |
| Sepsis | 1.22 | [0.43; 3.48] | 0.624 |
| Surgery for necrotizing enterocolitis | 1.17 | [0.11; 12.78] | 0.867 |
| Lower maternal educational attainment | 1.14 | [0.40; 3.22] | 0.754 |
| Multiple births | 0.93 | [0.27; 3.17] | 0.877 |
| Migration background, at least 1 parent | 0.68 | [0.20; 2.27] | 0.404 |
| Spontaneous delivery | 0.67 | [0.15; 3.01] | 0.487 |
| Patent ductus arteriosus | 0.31 | [0.09; 1.14] | 0.021 |
*IQ <85 versus IQ = 85
IQ,intelligence quotient; IVH III, grade 3 intraventricular hemorrhage; 99% CI, 99% confidence interval; OR, odds ratio; PVH, periventricular hemorrhage; PVL, periventricular leukomalacia
Longitudinal comparison
The longitudinal developmental assessment is described in Table 4. In the cognition subtest, the results for the age between 2 and 5 years were nominally less stable compared to the age between 5 and 10 years (unchanged category in 73.1% (n = 242) versus 82.4% (n = 65). Positive category changes occurred significantly more frequently in the age between 2 and 5 years compared to the age between 5 and 10 years (17.8% [14.1; 22.3], [n = 59], versus 8.8% [4.3; 17.0], [n = 7]).
Table 4. Longitudinal assessment of development.
| n | % | % | |||
| Comparison at age 2 and 5 years (n = 393) | |||||
| Cognition (n = 331) | MDI*1/IQ*2 ≥ 85 | 168 | 50.8 | unchanged category |
73.1 |
| MDI*1/IQ*2 70–84 | 36 | 10.9 | |||
| MDI*1/IQ*2 <70 | 38 | 11.4 | |||
| MDI*1/IQ*2 70–84 → ≥ 85 | 40 | 12.1 | positive category change |
17.8 | |
| MDI*1/IQ*2 <70 → 70–84 | 19 | 5.7 | |||
| MDI*1/IQ*2 ≥ 85 → 70–84 | 27 | 8.2 | negative category change |
9.1 | |
| MDI*1/IQ*2 70–84 → <70 | 3 | 0.9 | |||
| Motor function (n = 389) | normal | 154 | 39.6 | unchanged category |
67.9 |
| abnormal*3 | 57 | 14.7 | |||
| Cerebral palsy (CP) | 53 | 13.6 | |||
| abnormal*3→ normal | 22 | 5.6 | positive category change |
7.4 | |
| CP → abnormal*3 | 7 | 1.8 | |||
| normal → abnormal*3 | 85 | 21.9 | negative category change |
24.7 | |
| abnormal*3→ CP | 11 | 2.8 | |||
| Comparison at age 5 and 10 years (n = 85) | |||||
| Cognition (n = 79) | IQ*2 ≥ 85 | 45 | 57.1 | unchanged category |
82.4 |
| IQ*2 70–84 | 11 | 13.9 | |||
| IQ*2 <70 | 9 | 11.4 | |||
| IQ*2 70–84 → ≥ 85 | 5 | 2.5 | positive category change |
8.8 | |
| IQ*2 <70 → 70–84 | 2 | 6.3 | |||
| IQ*2 ≥ 85 → 70–84 | 5 | 6.3 | negative category change |
8.8 | |
| IQ*2 70–84 → <70 | 2 | 2.5 | |||
| Motor function (n = 85) | normal | 28 | 32.9 | unchanged category |
72.8 |
| DCD*4 | 16 | 18.8 | |||
| CP | 18 | 21.1 | |||
| DCD*4 → normal | 19 | 22.4 | positive category change |
22.4 | |
| CP → DCD*4 | 0 | 0 | |||
| normal → DCD*4 | 2 | 2.4 | negative category change |
4.8 | |
| DCD*4 → CP | 2 | 2.4 | |||
| Behavior (n = 68) | normal range*5 | 32 | 47.1 | unchanged category |
57.4 |
| borderline range*6 | 1 | 1.5 | |||
| clinical range*7 | 6 | 8.8 | |||
| clinical range*7→ normal range*5 | 3 | 4.4 | positive category change |
19.1 | |
| borderline range*6→ normal range*5 | 7 | 10.3 | |||
| clinical range*7→ borderline range*6 | 3 | 4.4 | |||
| normal range*5→ borderline range*6 | 7 | 10.3 | negative category change |
23.5 | |
| borderline range*6→ clinical range*7 | 2 | 2.9 | |||
| normal range*5→ clinical range*7 | 7 | 10.3 | |||
*1 Mental Development Index (MDI) of Bayley Scales of Infant Development II (Bayley II)
*2 Intelligence quotient (IQ) of the Kaufman Assessment Battery for Children (K-ABC) or Wechsler Intelligence Scales for Children IV (WISC IV)
*3 abnormal according to set of criteria (see eBox)
*4 Developmental Coordination Disorder (DCD) or abnormal DCD screening or abnormal according to set of criteria (see eBox)
*5 T value according to standardization Child Behavior Checklist 4–18 (CBCL/4–18) = 59
*6 T value according to standardization Child Behavior Checklist 4–18 (CBCL/4–18) 60–63
*7 T value according to standardization Child Behavior Checklist 4–18 (CBCL/4–18) = 64
In contrast, with motor development assessment the proportion of deteriorations (negative category changes) was high with 24.7% (n = 96) in the age between 2 and 5 years. Among the 10-year-olds, however, a positive category change was observed in 22.4% (n = 19), i.e. these children were assessed as having better motor function. The assessment of behavior was very instable. A category change was observed in 42.6% (n = 29) of the children. The proportion of children with behavioral problems was 32.3% ([22.4; 44.2], [n = 22]) at age 5 years and increased to 38.2% ([27.6; 50.1], [n = 26]) at age 10.
Discussion
These data from a German non-city federal state reveal a mortality rate of 25% among extremely premature infants which was constant during the observation period. Mortality rates decreased with increasing gestational age. The results also confirmed the high risk of developmental disorders in this group. Disabilities and impairments in the areas of cognition, language, behavior, and motor function were very common among the 5-year-olds.
The proportion of children with cognitive impairment (IQ <70) was very high (14.1%) among the premature infants; this finding was in line with data from other studies (3, 8). The expected percentage rate in the normal population is 2.3% (Kaufman Assessment Battery for Children [K-ABC]) (14). At age 5 years, the proportion of children with abnormalities in language development was conspicuously high with 40.4%. In the control population of term births, 20.2% of children had language difficulties (12). Behavioral abnormalities were identified in 33.1% of the 5-year-olds, while the expected rate was 15% based on normalized data (Child Behavior Checklist [CBCL]/4–18) (15).
With 17.4%, the prevalence of CP was higher in our study population than the 11% reported in comparable studies (16, 17). One reason for this finding could be that parents of premature infants with significant impairments regularly visit Sociopediatric Centers and consequently attend to follow-up appointments more frequently. Furthermore, it appears that very centralized health care systems, such as the one in Sweden, achieve exceptionally good outcomes with their health care programs for premature infants (18). The high proportion of children with abnormalities explains why at age 5 years 72.5% of the preterm-born children required treatment. In the comparison sample of term births (12), only 15% of the children received treatment at age 5. This result is in line with data from a study conducted in Berlin (19), showing that at the time of primary school enrollment children born as extremely small premature infants had special educational needs significantly more frequently than term-born children.
Analyzing the impact of peri- and neonatal as well as social factors on the long-term outcome achieved among these children, the following risk factors, which have previously been described elsewhere (10, 20), were also described in the Lower Saxony project: birth at <26 weeks’ gestation, severe brain injury and mechanical ventilation for >2 weeks increased the risk of developmental disorders significantly. The key risk factor for CP, the most severe form of motor disability, was severe brain injury (table 3). With regard to cognitive development, lower maternal educational attainment as a social risk factor (11) also had a significant negative impact on cognitive development, besides severe brain injury. Data from fathers were not included in the analysis as the rate of single mothers was high in the study population. Because in most cases mothers spend more time with their children, their impact on the children’s upbringing was considered to be of greater importance than that of the fathers.
Of special interest was the question whether developmental disorders can be reliably predicted at age 2 years which could only be addressed by longitudinal data analysis. To this end, data from the 10-year follow-up were used to evaluate the long-term development of the children until school age. It was found that between ages 2 and 5 years still major changes in the assessment of both cognition and motor development were observed (table 4). However, the results of the evaluation of the cognitive development at age 5 years were already largely identical with those at age 10, as evidenced by the fact that the evaluation outcome (category change) changed only in 8.8% each, in both directions. Taking into account that, besides statistical uncertainty, the measurement tool used at age 5 years was different from that used at age 10 years, this can be considered a low value. In contrast, motor assessment revealed significantly better results at age 10 years compared to age 5 years. These may be due to natural ripening processes of the nervous system and/or can be regarded as treatment effects.
The development of behavior is the most difficult to predict, at least based on parental assessment data. At age 10 years, 38.2% of the children were assessed as having behavioral problems, more than at age 5 years. Interestingly, the severity of the behavioral problems at age 5 was independent of the gestational age (table 2). The high rate (23.5%) of negative behavior category changes indicates that many parents regard the behavior of preterm-born children at age 5 years more positive compared to age 10 years. One explantation could be that at school the children are confronted with higher expectations and are supposed to follow more rules compared to the time at kindergarten. Consequently, at preschool age certain behavioral problems do not occur or are not perceived by parents as such. However, assessor-performed evaluations based on observations of the children in situations with higher behavioral expectations already differed considerably from parent-reported evaluations at the 5-year follow-up, indicating potentially more serious problems (12). Thus, Sociopediatric Centers should provide relevant information to pediatric and family physicians and parents to enable early preventative interventions to control behavioral problems.
Limitations
The critical limitation to this study is the high rate of children lost to follow-up in this patient population. The follow-up examination rate decreased with increasing patient age. At age 5, 63.0% of the survivors could be examined at follow-up, while at age 10 it were only 38.8% of the first one and a half year groups. In addition, the number of examined 10-year-olds is even smaller as the examination of two and a half year groups had not yet been completed at the time of publication. One reason for the increasing drop-out rate is the increasing mobility of the population (21) with many children lost to follow-up due to their parents moving out of the territory of the state of Lower Saxony. Apart from that, the interest in medical examinations decreases with increasing age, as known from the attendance rates in pediatric and adolescent screening programs (22, 23).
When the examination of all potential participants cannot be achieved, it is necessary to try to identify potentially underlying systematic causes. However, the comparison of the 5-year-olds included in the analysis with the total study population of survivors did not indicate the presence of a selection factor with regard to the relevant medical and biological risk factors (Table 1).
There still is the possibility of differences in sociodemographic or cultural status between the examined and non-examined children which cannot be excluded. In addition, the as yet low number of 10-year-olds increases the risk of selection bias. It seems possible that children with severe motor impairment have participated slightly more frequently due to their regular treatment needs at a Sociopediatric Center compared to children of parents with no consultation needs.
Another limitation is related to the way the motor status of the children was assessed: In the 10-year-olds, a standardized method, the Movement Assessment Battery for Children-2 (M-ABC-2) (24), was used, while among the 2-to-5-year-olds the assessment was carried out by experienced developmental neurologists using a defined set of criteria. The project was not conducted under study conditions, but embedded in a multicenter routine examination setting in the Sociopediatric Centers.
Strengths
In this study, disability and impairment prevalence data of several year groups obtained at several follow-up time points in an entire German federal state (Lower Saxony) are presented. With this, state-wide information about the development of German premature infants until age 10 years has become available for the first time. Since the study population comprised not only children treated in level-1 centers, but also those receiving care in hospitals of a lower level of care, this analysis provides a more real-world view compared to studies undertaken in distinct highly qualified centers. It provides physicians involved in the follow-up care of these children with important information about premature infants at particularly high risk and helps them to identify children with a greater need for treatment and support. By combining the study data with peri- and neonatal data, it is possible to derive information about the quality of care which is used in the project “Benchmarking in preterm infant care“ (25), a project initiated in the State of Lower Saxony in 2012 to advance quality development in neonatology.
Conclusion
The results of this state-wide, multicenter follow-up study in the German State of Lower Saxony confirmed the known high risk of developmental disorders in extremely premature infants <28 weeks’ gestation. Disabilities such as cerebral palsy or mental retardation, but also speech developmental disorders, motor deficits and behavioral problems are common. Consequently, almost three quarters of the children are still receiving treatment and support measures at age 5.
The presented data help in clinical practice to already identify children with an unfavorable developmental prognosis (<26 weeks’ gestation, severe brain injury, mechanical ventilation for >2 weeks, lower maternal educational attainment) when taking the medical history and to address their special developmental diagnostic and treatment needs, particularly in patients with risk factor combinations.
At age 2 years, even experienced specialists can only predict the long-term motor and cognitive development with limited accuracy. From age 2 to age 5 years, the analysis of longitudinal data shows significant shifts. Thus, it may be asked whether the age of two years set by the Federal Joint Committee (G-BA) as the time to measure the outcome quality of neonatal care is in fact appropriate (26).
Key Messages.
At the age of 5 years, 64% of the extremely premature infants have an at least average cognitive development; 60% show no language abnormalities and 45% no motor disorders.
Disabilities (cerebral palsy in 17%; cognitive impairment in 14%) and developmental disorders are common among former extremely immature premature infants and require treatment in 73% of cases.
Key variables affecting the long-term outcome include severe brain injury (grade 3 intraventricular hemorrhage, periventricular hemorrhage, periventricular leukomalacia), prematurity <26 weeks’ gestation, and lower maternal educational attainment.
In more than 25% of cases, predictions made at the age of 2 years regarding the children’s future mental and motor development turn out to be inaccurate.
Preliminary data from the 10-year follow-up indicate a high prevalence of behavioral disorders. This problem should be addressed early.
Acknowledgments
Acknowledgement
This project has been financially supported by the Kaufmännische Krankenkasse (KKH), the Techniker Krankenkasse and the Qualitätsinitiative e. V., Lower Saxony Society for the Promotion of Quality in Healthcare.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
Translated from the original German by Ralf Thoene, MD.
References
- 1.AQUA-Institut für angewandte Qualitätsförderung und Forschung im Gesundheitswesen GmbH. Bundesauswertung 2014: Geburtshilfe. www.sqg.de/ergebnisse/leistungsbereiche/geburtshilfe/(last ac-cessed on 23 March 2016) [Google Scholar]
- 2.Wilson-Castello D, Friedman H, Minich N, et al. Improved neurodevelopmental outcomes for extremely low birth weight infants in 2000-2002. Pediatrics. 2007;119:37–45. doi: 10.1542/peds.2006-1416. [DOI] [PubMed] [Google Scholar]
- 3.Doyle LW, Anderson PJ. Victorian Infant Collaborative Study Group: Improved neurosensory outcome at 8 years of age of extremely low birthweight children born in Victoria over three distinct eras. Arch Dis Child Fetal Neonatal. 2005;90:484–488. doi: 10.1136/adc.2004.063362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.AQUA-Institut für angewandte Qualitätsförderung und Forschung im Gesundheitswesen GmbH. Bundesauswertung 2014: Neonatologie. www.sqg.de/ergebnisse/leistungsbereiche/neonatologie/(last accessed on 23 March 2016) [Google Scholar]
- 5.Robertson CM, Hrynchyshyn GJ, Etches PC, Pain KS. Population-based study of the incidence, complexity, and severity of neurologic disability among survivors weighing 500 through 1250 grams at birth: a comparison of two birth cohorts. Pediatrics. 1992;90:750–755. [PubMed] [Google Scholar]
- 6.Marlow N, Wolke D, Bracewell MA, Samara M. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352:9–19. doi: 10.1056/NEJMoa041367. [DOI] [PubMed] [Google Scholar]
- 7.Larroque B, Ancel PY, Marret S, et al. Neurodevelopmental disabilities and special care of 5-year-old children born before 33 weeks of gestation (the EPIPAGE study): a longitudinal cohort study. Lancet. 2008;371:813–820. doi: 10.1016/S0140-6736(08)60380-3. [DOI] [PubMed] [Google Scholar]
- 8.Hutchinson EA, De Luca CR, Doyle LW, Roberts G, Anderson PJ. School-age outcomes of extremely preterm or extremely low birth weight children. Pediatrics. 2013;131:1053–1061. doi: 10.1542/peds.2012-2311. [DOI] [PubMed] [Google Scholar]
- 9.Wolke D, Meyer R. Cognitive status, language attainment, and prereading skills of 6-year-old very preterm children and their peers: the Bavarian longitudinal study. Dev Med Child Neurol. 1999;41:94–109. doi: 10.1017/s0012162299000201. [DOI] [PubMed] [Google Scholar]
- 10.Neubauer AP, Voss W, Kattner E. Outcome of extremely low birth weight survivors at school age: the influence of perinatal parameters on neurodevelopment. Eur J Pediatr. 2007;167:87–95. doi: 10.1007/s00431-007-0435-x. [DOI] [PubMed] [Google Scholar]
- 11.Voss W, Jungmann T, Wachtendorf M, Neubauer AP. Long-term cognitive outcomes of extremely low-birth-weight infants: the influence of the maternal educational background. Acta Paediatr. 2012;101:569–573. doi: 10.1111/j.1651-2227.2012.02601.x. [DOI] [PubMed] [Google Scholar]
- 12.Damm G, Macha T, Petermann F, Voss W, Sens B. Qualitätsanalysen zur Entwicklung Frühgeborener: Ergebnisse des Niedersächsischen Frühgeborenen-Nachuntersuchungsprojekts und eines Vergleichs-kollektivs reif geborener Kinder. Z Evid Fortbild Qual Gesundhwesen. 2015;109:6–17. doi: 10.1016/j.zefq.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Zentrum für Qualität und Management im Gesundheitswesen. Geburtshilfe Jahresstatistiken 2005-2008. www.aekn.de/zq/projekte/perinatalerhebung/download-statistiken (last accessed on 23 March 2016) [Google Scholar]
- 14.Melchers P, Preuß U. Pearson Assessment. Frankfurt/M.: 2009. Kaufman Assessment Battery for Children (deutsche Version) [Google Scholar]
- 15.Döpfner M, Plück J, Bölte S, et al. Arbeitsgruppe Kinder-, Jugendlichen- und Familiendiagnostik (KJFD) Köln: 1998. Elternfragebogen über das Verhalten von Kindern und Jugendlichen Deutsche Bearbeitung der Child Behaviour Checklist (CBCL/4-18) [Google Scholar]
- 16.Serenius F, Källén K, Blennow M, et al. Neurodevelopmental out-come in extremely preterm infants at 25 years after active perinatal care in Sweden. JAMA. 2013;309:1810–1820. doi: 10.1001/jama.2013.3786. [DOI] [PubMed] [Google Scholar]
- 17.Oskoui M, Coutinho F, Dykeman J, Jetté N, Pringsheim T. An update on the prevalence of cerebral palsy: a systematic review and meta-analysis. Dev Med Child Neurol. 2013;55:501–519. doi: 10.1111/dmcn.12080. [DOI] [PubMed] [Google Scholar]
- 18.Rossi R, Poets C, Jorch G. Perinatalmedizinische Versorgung: Maximale Sicherheit für Mutter und Kind anstreben. Dtsch Arztebl. 2015;112:18–20. [Google Scholar]
- 19.Bettge S, Oberwöhrmann S, Brockstedt M, Bührer C. Birth weight and special educational needs—results of a population-based study in Berlin. Dtsch Arztebl Int. 2014;111:337–344. doi: 10.3238/arztebl.2014.0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voss W, Neubauer AP. Entwicklung von Frühgeborenen mit einem Geburtsgewicht @@<@@ 1000 g - Langzeitentwicklung bis zum Schulalter Welche Risikofaktoren sind bei der Entlassung definierbar? Pädiatrische Praxis. 2009;73:373–382. [Google Scholar]
- 21.Umzug AG. 11 Prozent Umzugsquote - ist Deutschland Umzugsweltmeister? www.pressebox.de/pressemitteilung/umzug-ag/11-Prozent-Umzugsquote-ist-Deutschland-UmzugsweltmeisterOE/boxid/434875 (last accessed on 23 March 2016) [Google Scholar]
- 22.Riens B, Mangiapane S. Zentralinstitut für die kassenärztliche Versorgung in Deutschland. Berlin: 2013. Teilnahme an der Jugendgesundheitsuntersuchung J1 - Eine retrospektive Kohortenstudie. [Google Scholar]
- 23.Robert Koch-Institut Erkennen - Bewerten - Handeln. Robert Koch-Institut. Berlin: 2008. Zur Gesundheit von Kindern und Jugendlichen in Deutschland; pp. 127–133. [Google Scholar]
- 24.Petermann F. Pearson Assessment & Information. Frankfurt/M.: 2011. Movement assessment battery for children-2 (movement ABC-2) [Google Scholar]
- 25.Damm G, Cloppenburg E, Küster H, Mayer C, Kattner E. Benchmarking in der Frühgeborenenversorgung: Ein Projekt zur Qualitätsentwicklung in der Neonatologie. Niedersachs Arztebl. 2016;6:17–19. [Google Scholar]
- 26.Gemeinsamer Bundesausschuss. Qualitätssicherungs-Richtlinie Früh- und Reifgeborene - QFR-RL. www.g-ba.de/informationen/richtlinien/41 (last accessed on 23 March 2016) [Google Scholar]
- E1.Reuner G, Rosenkranz J, Pietz J, Horn R. Pearson Clinical & Talent Assessment. Frankfurt/M.: 2008. Bayley scales of infant development (Bayley II) - Deutsche Fassung. [Google Scholar]
- E2.Grimm H. Sprachentwicklungstest für drei- bis fünfjährige Kinder (SETK 3-5) Göttingen: Hogrefe. 2010 [Google Scholar]
- E3.Petermann U. Pearson Assessment & Information. Frankfurt/M.: 2011. Wechsler Intelligence Scale for Children—fourth edition (WISC-IV) [Google Scholar]
- E4.Petermann F. Pearson Assessment & Information. Frankfurt/M.: 2011. Movement assessment battery for children-2 (movement ABC-2) [Google Scholar]
- E5.Kennedy-Behr A, Wilson BN, Rodger S, Mickan S. Georg Thieme Verlag. New York: 2013. Cross-cultural adaptation of the developmental coordination disorder questionnaire 2007 for German-speaking countries: DCDQ-G Stuttgart, [DOI] [PubMed] [Google Scholar]
- E6.Surveillance of cerebral palsy in Europe a collaboration of cerebral palsy surveys and registers. Surveillance of cerebral palsy in Europe (SCPE) Dev Med Child Neurol. 2000;42:816–824. doi: 10.1017/s0012162200001511. [DOI] [PubMed] [Google Scholar]
- E7.UNESCO Institute for Statistics. International standard classification of education. www.uis.unesco.org/Education/Pages/international-standard-classification-of-education.aspx (last accessed on 23 March 2016) [Google Scholar]