Abstract
Marital status have been found as an independent prognostic factor for survival and spousal support could provide a survival advantage in various cancer types. However, the specific effect of marital status on survival in hepatocellular carcinoma (HCC) has not been explored in detail. In this study, we used the Surveillance, Epidemiology and End Results program to identify iagnosed with HCC between 1988 and 2007. Kaplan-Meier methods and multivariable Cox regression models were used to analyze long-term cancer-specific survival (CSS) outcomes and risk factors stratified by marital status. There were significant differences among these different marital status subgroups with regard to 5-year CSS rates (P < 0.001). Married HCC patients had a better 5 year CSS rate than those unmarried patients, and widowed patients were more likely to die of their cancer. A stratified analysis showed that widowed patients always had the lowest CSS rate across different cancer stage, age and gender subgroups. Even after adjusting for known confounders, unmarried patients were at greater risk of cancer-specific mortality. Social support aimed at this population could improve the likelihood of achieving cure.
It has been shown that married individuals have longer overall and cancer-specific survival (CSS) than those people who are single, widowed or divorced1,2. People who are married receive better social support, which subsequently promote health and survival3. Spouses can not only provide basic emotional support, but also facilitate the patients to receive more critical health care services4. Aizer et al. used the Surveillance, Epidemiology and End Results (SEER) database to study nearly 1 million contemporary cancer patients in the United States and found that unmarried patients, compared with married patients, are at higher risk of presentation with metastatic cancer, under-treatment, and death resulting from their corresponding cancer5. Thus, marital status is considered as an independent prognostic factor of survival in many cancers5,6,7,8. Prior investigations have also demonstrated that marital status plays a mixed or nonsignificant effect on disease-specific survival9,10,11. However, the role of marital status in affecting survival of patients with hepatocellular carcinoma (HCC) has not yet been assessed.
Liver cancer (LC) ranks the fifth most common malignancy and the third leading cause of cancer-related deaths globally12. HCC is the most common type of LC accounting for approximately 80 percent of all liver cancers13. We noticed that most studies only compare prognosis between married and unmarried individuals, and those separated, divorced and widowed patients were ignored without differentiating5. Given that 51 percent of Americans are married and HCC is one of the most common malignancies, targeted social support interventions could significantly prolong survival5,14. In this study, we searched the SEER population-based database of individuals diagnosed between 1988 and 2007 to evaluate discrepancies in survival trends among different marital status. Our primary objectives were to make generalizable conclusions regarding the survival discrepancies that might exist in these groups.
Materials and Methods
Patients
The SEER Cancer Statistics Review (http://seer.cancer.gov/data/citation.html), a report on the most recent cancer incidence, mortality, survival, prevalence, and lifetime risk statistics, is published annually by the Data Analysis and Interpretation Branch of the National Cancer Institute (Bethesda, MD). The current SEER database consists of 18 population-based cancer registries that represent approximately 26% of the population in the United States. SEER data contain no identifiers and are publicly available for studies of cancer-based epidemiology and survival analysis.
Cases of invasive HCC diagnosed between January 1, 1988, and December 31, 2007, were extracted from the SEER database (SEER*Stat 8.2.1) according to the Site Recode Classifications. Only those patients who underwent surgery at an age of between 18 and 85 years at diagnosis were included. Patients were excluded if they had incomplete staging, distant metastasis (M1), no evaluation of histological type, or follow-up. Age, sex, race, histologic type, stage, tumor grade, tumor size, and cancer-specific survival (CSS) rates were assessed. Adjuvant chemotherapy was not evaluated because the SEER registry does not include this information. The primary end point of the study is 5-year CSS rate, which was calculated from the date of diagnosis to the date of cancer-specific death. Cancer-specific deaths were treated as events, and deaths from other causes were treated as censored observations. The median follow-up period of patients was calculated from the date of diagnosis to the date of cancer-specific death. Marital status is coded as married, divorced, widowed, separated, and never married. Individuals in the separated and divorced group were clustered together as the divorced/separated group in this study.
This study was based on public data from the SEER database; we obtained permission to access research data files with the reference number 10504-Nov 2014. The data did not include the use of human subjects or personal identifying information. Thus, no informed consent was required for this part of the study.
Statistical Analyses
Categorical variables were presented as frequency (%), and continuous variables were presented as median (interquartile range) or mean ± SD. The association between marital status categories and clinicopathological parameters was assessed using the chi-square (χ2) test. Continuous variables were compared using the Student t test. Survival curves were generated using the Kaplan-Meier method; differences between the curves were analyzed by using the log-rank test. Multivariable Cox proportional hazards regression models were used to assess potential risk factors for survival outcomes. All statistical analyses were performed using the statistical software package SPSS for Windows, version 17 (SPSS, Inc). The results were considered statistically significant when a 2-tailed test provided a P value of less than 0.05.
Results
Patient Characteristics
We identified 8621 eligible patients with HCC in the SEER database during the 20-year study period (between 1988 and 2007). A total of 6341 (73.6%) were men, and 2280 (26.4%) were women. Of these, 5457 (63.5%) were married, 1399 (16.2%) had never married, 940 (10.9%) were divorced/separated, and 825 (9.6%) were widowed. Patients who were widowed were less likely to be younger than 45 (0.5%), have less <3 cm tumor (8.5%) (P < 0.001). The rate of surgery performed was comparable between the married and widowed groups (84.4% vs 83.9%). Married patients were also less likely to present with advanced tumor and stage than widowed patients (P < 0.001). Patient demographics and pathologic features are summarized in Table 1.
Table 1. Characteristics of Patients from SEER Database by marital status.
| Characteristic | No. (%) of patients | |||||
|---|---|---|---|---|---|---|
| Total | Married | Never married | Divorced/ Separated | Widowed | P value | |
| n = 8621 | n = 5457 | n = 1399 | n = 940 | n = 825 | ||
| Media follow up (mo) | 37 | 40 | 36 | 36 | 25 | |
| (IQR) | 5–63 | 6–67 | 5–60 | 5–62 | 4–31 | |
| Years of diagnosis | P < 0.001 | |||||
| 1988–1994 | 557(6.5) | 404(7.4) | 51(3.6) | 39(4.1) | 63(7.6) | |
| 1995–2001 | 2212(25.6) | 1450(26.6) | 319(22.8) | 192(20.4) | 251(30.4) | |
| 2002–2007 | 5852(67.9) | 3603(66.0) | 1029(73.6) | 709(75.5) | 511(62.0) | |
| Sex | P < 0.001 | |||||
| Male | 6341(73.6) | 4306(78.9) | 1058(75.6) | 688(73.2) | 289(35.0) | |
| Female | 2280(26.4) | 1151(21.1) | 341(24.4) | 252(26.8) | 536(65.0) | |
| Age | P < 0.001 | |||||
| <45 | 659(7.6) | 322(5.9) | 291(20,8) | 42(4.5) | 4(0.5) | |
| 45–60 | 3520(40.8) | 2199(40.3) | 701(50.1) | 520(55.3) | 100(12.1) | |
| 61–75 | 3283(38.1) | 2276(41.7) | 323(23.1) | 323(34.4) | 361(43.8) | |
| >75 | 1159(13.5) | 660(12.1) | 84(6.0) | 55(5.9) | 360(43.6) | |
| Race | P < 0.001 | |||||
| Caucasian | 5425(63.7) | 3361(61.6) | 878(62.8) | 667(71.0) | 519(62.9) | |
| African American | 914(11.4) | 384(7.0) | 312(22.3) | 137(14.6) | 81(9.8) | |
| Others* | 2282(24.9) | 1712(31.4) | 209(14.9) | 136(14.4) | 225(27.3) | |
| Pathological grading | 0.677 | |||||
| High/Moderate | 6564(28.3) | 4147(76.0) | 1055(75.4) | 724(77.0) | 638(77.3) | |
| Poor/UD | 2057(10.0) | 1310(24.0) | 344(24.6) | 216(23.0) | 187(22.7) | |
| Stage | 0.015 | |||||
| Localized | 5216(42.7) | 3310(60.7) | 800(57.2) | 584(62.2) | 522(63.2) | |
| Regional | 2471(27.2) | 1584(29.0) | 424(30.3) | 257(27.3) | 206(25.0) | |
| Distant | 934(15.9) | 563(10.3) | 175(12.5) | 99(10.5) | 97(11.8) | |
| Tumor size | P < 0.001 | |||||
| <3 cm | 1747(12.8) | 1125(20.6) | 267(19.1) | 220(23.4) | 135(16.4) | |
| 3–5 cm | 2434(19.8) | 1513(27.7) | 383(27.4) | 319(33.9) | 219(26.5) | |
| >5 cm | 4440(34.0) | 2819(51.7) | 749(53.5) | 401(42.7) | 471(57.1) | |
*Including other (American Indian/AK Native, Asian/Pacific Islander) and unknowns.
Clinicopathological Differences Between the Groups
As illustrated in Table 1, there were significant differences observed between the 4 groups, including the calendar years of diagnosis (more frequent in 2002–2007, 67.9%; P < 0.001), sex (more frequent in men, 73.6%; P < 0.001), age (more frequent in 45–60 and 61–75 years, 78.9%; P < 0.001), race (more frequent in Caucasian, 63.7%; P < 0.001), pathologic grade (less poor/undifferentiated in grade, 10.0%; P < 0.001), stage (more localized, 42.7%; P < 0.001), and tumor size (more >5 cm, 34.0%; P < 0.001).
Impact of Marital Status on Survival Outcomes
The univariate log-rank test showed that the 3-year and 5-year CSS were 44.5% and 36.9% in the married group, 40.6% and 33.4% in the never married group, 40.2% and 33.5% in the divorced/separated group, 20.2% and 21.8% in the widowed group, respectively (P < 0.001) (Fig. 1). Moreover, an early year of diagnosis (1988–1994), men, age more than 75 years, African American race, poor/undifferentiated tumor grade, higher stage, and larger tumor size (P < 0.001) were regarded as significant risk factors by univariate analysis (Table 2). Multivariate analysis with Cox regression was performed, and the following 7 factors were found to be independent prognostic factors (Table 3), including year of diagnosis (1995–2001: HR, 0.974; 95% CI, 0.875–1.084; 2002–2007: HR, 0.803; 95% CI, 0.725–0.889), age (45–60 year: HR, 1.617; 95% CI, 1.441–1.814; 45–60 year: HR, 1.617; 95% CI, 1.441–1.814; 61–75 year: HR, 2.098; 95% CI, 1.869–2.355;>75 year: HR, 2.410; 95% CI, 2.115–2.747), race (African American: HR, 1.189; 95% CI, 1.092–1.295), pathological grading (poor/undifferentiated: HR, 1.396; 95% CI, 1.315–1.483), stage (regional: HR, 1.797; 95% CI, 1.694–1.907; distant: HR, 2.924; 95% CI, 2.692–3.177), tumor size (3–5 cm: HR, 1.736, 95% CI, 1.586–1.901; >5 cm: HR 2.529, 95% CI, 2.322–2.754), and marital status(never married: HR, 1.109, 95% CI, 1.025–1.200; divorced/separated: HR, 1.181, 95% CI, 1.082–1.288;. widowed: HR, 1.198, 95% CI, 1.093–1.312).
Figure 1. Survival curves in hepatocellular carcinoma patients according to marital status, χ2 = 77.744, P < 0.001.
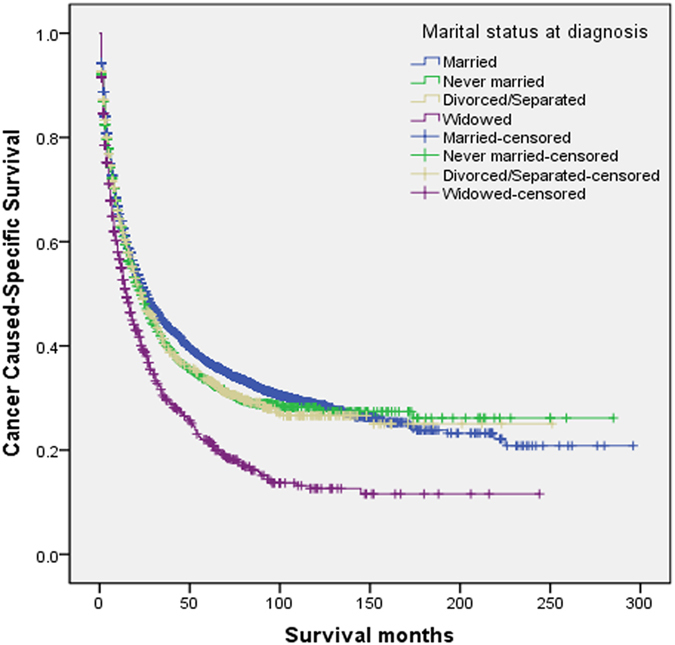
Table 2. Univariate survival analyses of HCC patients according to various clinicopathological variables.
| Variable | n | 3-year CSS (%) | 5-year CSS (%) | Log rank χ2 test | P |
|---|---|---|---|---|---|
| Years of diagnosis | 124.997 | P < 0.001 | |||
| 1988–1994 | 557 | 29.7% | 23.0% | ||
| 1995–2001 | 2212 | 35.4% | 27.8% | ||
| 2002–2007 | 5852 | 45.8% | 38.3% | ||
| Sex | 1.991 | 0.158 | |||
| Male | 6341 | 41.8% | 34.3% | ||
| Female | 2280 | 42.9% | 35.7% | ||
| Age | 309.794 | P < 0.001 | |||
| <45 | 659 | 50.2% | 43.9% | ||
| 45–60 | 3520 | 49.1% | 42.5% | ||
| 61–75 | 3283 | 37.9% | 29.5% | ||
| >75 | 1159 | 26.6% | 17.7% | ||
| Race | 38.560 | P < 0.001 | |||
| Caucasian | 5425 | 42.1% | 35.1% | ||
| African American | 914 | 34.0% | 26.0% | ||
| Others* | 2282 | 45.3% | 36.9% | ||
| Pathological grading | 327.616 | P < 0.001 | |||
| High/Moderate | 6564 | 45.6% | 38.4% | ||
| Poor/undifferentiation | 2057 | 28.0% | 22.4% | ||
| Stage | 1440.866 | P < 0.001 | |||
| Localized | 5216 | 54.6% | 45.9% | ||
| Regional | 2471 | 27.2% | 20.7% | ||
| Distant | 934 | 10.1% | 6.7% | ||
| Tumor size (mm) | 1019.417 | P < 0.001 | |||
| <3 cm | 1747 | 69.9% | 62.2% | ||
| 3–5 cm | 2434 | 46.7% | 38.5% | ||
| >5 cm | 4440 | 28.3% | 21.3% | ||
| Marital Status | 77.744 | P < 0.001 | |||
| Married | 5457 | 44.5% | 36.9% | ||
| Never married | 1399 | 40.6% | 33.4% | ||
| Divorced/Separated | 940 | 40.2% | 33.5% | ||
| Widowed | 825 | 20.2% | 21.8% |
*Including other (American Indian/AK Native, Asian/Pacific Islander) and unknowns.
Table 3. Multivariate Cox model analyses of prognostic factors of HCC.
| Variable | Hazard Ratio | 95% CI | P |
|---|---|---|---|
| Years of diagnosis | P < 0.001 | ||
| 1988–1994 | 1 | Reference | |
| 1995–2001 | 0.974 | 0.875–1.084 | |
| 2002–2007 | 0.803 | 0.725–0.889 | |
| Age | P < 0.001 | ||
| <45 | 1 | Reference | |
| 45–60 | 1.617 | 1.441–1.814 | |
| 61–75 | 2.098 | 1.869–2.355 | |
| >75 | 2.410 | 2.115–2.747 | |
| Race | P < 0.001 | ||
| Caucasian | 1 | Reference | |
| African American | 1.189 | 1.092–1.295 | |
| Others* | 0.866 | 0.813–0.921 | |
| Pathological grading | P < 0.001 | ||
| High/Moderate | 1 | Reference | |
| Poor/undifferentiation | 1.396 | 1.315–1.483 | |
| Stage | P < 0.001 | ||
| Localized | 1 | Reference | |
| Regional | 1.797 | 1.694–1.907 | |
| Distant | 2.924 | 2.692–3.177 | |
| Tumor size (mm) | P < 0.001 | ||
| <3 cm | 1 | Reference | |
| 3–5 cm | 1.736 | 1.586–1.901 | |
| >5 cm | 2.529 | 2.322–2.754 | |
| Marital Status | P < 0.001 | ||
| Married | 1 | Reference | |
| Never married | 1.109 | 1.025–1.200 | |
| Divorced/Separated | 1.181 | 1.082–1.288 | |
| Widowed | 1.198 | 1.093–1.312 |
*Including other (American Indian/AK Native, Asian/Pacific Islander) and unknowns.
Stratified Analysis of Marital Status Effect on CSS Rates
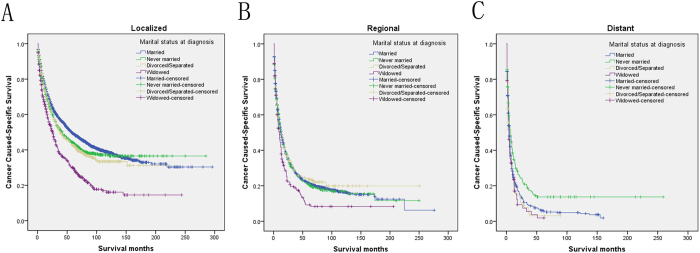
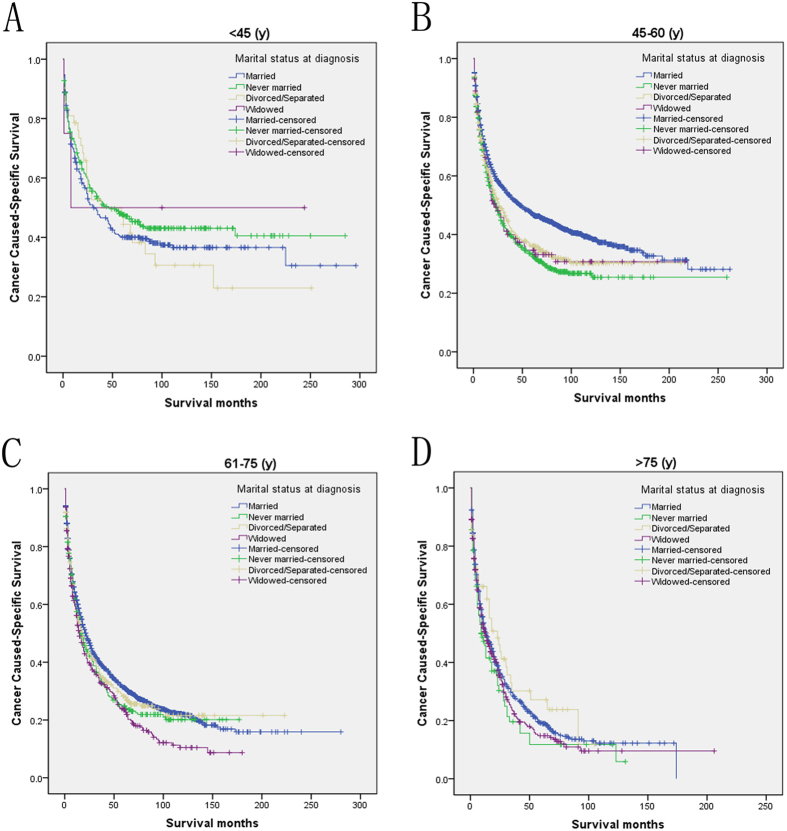
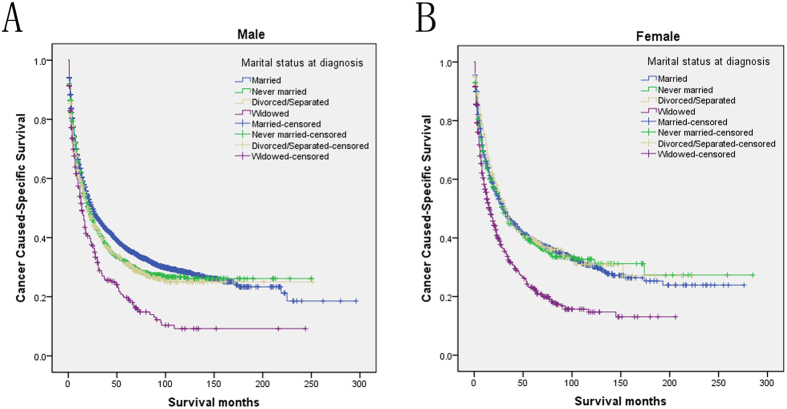
We then further analyzed the effect of marital status on CSS rates in each stage (Fig. 2). Both univariate and multivariate analysis showed that marital status was an independent prognostic factor in each tumor stage (P < 0.001). In addition to this, we also observed two interesting findings. First, the widowed group, compared with the other groups, always had the lowest CSS rate in the localized and regional stage. Widowed patients had 19.4% reduction in 5-year CSS compared with married patients in the localized stage (49.2% versus 29.8%, P < 0.001), 13.2% reduction in the regional stage (21.5% versus 8.3%, P < 0.001), 4.2% reduction in the distant stage (6.1% versus 1.9%, P = 0.166). Second, the divorced/separated group also had decreased 5-year CSS across several subgroups compared with patients in the never married group (Table 4). Furthermore, we made further stratified analysis of survival rates and hazard by gender and age (Figs 3 and 4). Unmarried patients always had the lowest CSS rate, which were consistent with aboved results (Table 5 and 6).
Figure 2. Subgroup analysis for evaluating the effect of marital status for hepatocellular carcinoma patients according different cancer stage.
(A) The localized stage group: χ2 = 88.888, P < 0.001; (B) The regional stage group: χ2 = 12.846, P = 0.005; (C) The distant stage group: χ2 = 18.761, P < 0.001.
Table 4. Univariate and multivariate analyses for evaluating marital status influencing CSS in HCC based on different cancer stage.
| Variable | 5-year CSS (%) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| Log rank χ2 test | P | HR(95% CI) | P | ||
| Localized | |||||
| Marital status | 88.888 | P < 0.001 | P < 0.001 | ||
| Married | 49.2% | Reference | |||
| Never married | 44.1% | 1.259(1.128–1.404) | P < 0.001 | ||
| Divorced/Separated | 42.4% | 1.265(1.123–1.424) | P < 0.001 | ||
| Widowed | 29.8% | 1.264(1.121–1.425) | P < 0.001 | ||
| Regional | |||||
| Marital status | 12.846 | 0.005 | 0.045 | ||
| Married | 21.5% | Reference | |||
| Never married | 20.8% | 1.089(0.953–1.243) | 0.251 | ||
| Divorced/Separated | 23.5% | 1.096(0.937–1.281) | 0.010 | ||
| Widowed | 8.3% | 1.265(1.057–1.514) | P < 0.001 | ||
| Distant | |||||
| Marital status | 18.761 | P < 0.001 | 0.039 | ||
| Married | 6.1% | Reference | |||
| Never married | 13.7% | 0.827(0.670–1.022) | 0.079 | ||
| Divorced/Separated | 3.1% | 1.192(0.942–1.509) | 0.143 | ||
| Widowed | 1.9% | 1.197(0.928–1.543) | 0.166 | ||
Figure 3. Subgroup analysis for evaluating the effect of marital status for hepatocellular carcinoma patients according different age.
(A) <45 year: χ2 = 2.097, P = 0.553; (B) 45–60 year: χ2 = 46.729, P < 0.001; (C) 61–75 year: χ2 = 14.877, P = 0.002; (D) >75 year: χ2 = 5.327, P = 0.149.
Figure 4. Subgroup analysis for evaluating the effect of marital status for hepatocellular carcinoma patients according different gender.
(A) Male: χ2 = 43.265, P < 0.001; (B). Female: χ2 = 53.101, P < 0.001.
Table 5. Univariate and multivariate analyses for evaluating marital status influencing CSS in HCC based on different age.
| Variable | 5-year CSS (%) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| Log rank χ2 test | P | HR(95%CI) | P | ||
| <45 | |||||
| Marital status | 2.097 | 0.553 | NI | ||
| Married | 40.0% | ||||
| Never married | 47.6% | ||||
| Divorced/Separated | 44.4% | ||||
| Widowed | NI | ||||
| 45–60 | |||||
| Marital status | 46.729 | P < 0.001 | P < 0.001 | ||
| Married | 47.1% | Reference | |||
| Never married | 33.2% | 1.377(1.231–1.541) | P < 0.001 | ||
| Divorced/Separated | 35.8% | 1.347(1.189–1.525) | P < 0.001 | ||
| Widowed | 33.1% | 1.852(1.430–2.397) | P < 0.001 | ||
| 61–75 | |||||
| Marital status | 14.877 | 0.002 | 0.001 | ||
| Married | 31.2% | Reference | |||
| Never married | 24.8% | 1.057(0.916–1.219) | 0.449 | ||
| Divorced/Separated | 27.8% | 1.112(0.964–1.282) | 0.146 | ||
| Widowed | 23.5% | 1.320(1.154–1.510) | P < 0.001 | ||
| >75 | |||||
| Marital status | 5.327 | 0.149 | NI | ||
| Married | 19.2% | ||||
| Never married | 11.8% | ||||
| Divorced/Separated | 23.8% | ||||
| Widowed | 14.8% | ||||
Table 6. Univariate and multivariate analyses for evaluating marital status influencing CSS in HCC based on different gender.
| Variable | 5-year CSS (%) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| Log rank χ2 test | P | HR(95%CI) | P | ||
| Male | |||||
| Marital status | 43.265 | P < 0.001 | P < 0.001 | ||
| Married | 36.3% | Reference | |||
| Never married | 31.6% | 1.141(1.043–1.247) | 0.004 | ||
| Divorced/Separated | 31.0% | 1.256(1.136–1.390) | P < 0.001 | ||
| Widowed | 19.2% | 1.318(1.145–1.517) | P < 0.001 | ||
| Female | |||||
| Marital status | 53.101 | P < 0.001 | 0.074 | ||
| Married | 39.3% | Reference | |||
| Never married | 38.2% | 1.048(0.887–1.238) | 0.580 | ||
| Divorced/Separated | 39.5% | 1.040(0.872–1.240) | 0.666 | ||
| Widowed | 22.9% | 1.199(1.048–1.373) | 0.008 | ||
NI: not included in multivariate survival analysis.
P values were adjusted for years of diagnosis, sex, age, race, pathological grading, stage and tumor size as covariates between the two groups.
Discussion
Despite the impact of marriage on cancer survival has been performed in some studies15,16,17, no research has been focused on the heterogeneity of unmarried patients in HCC or performed on stage by stage comparisons of the impact of marital status on survival. Our study showed that unmarried patients, including the widowed ones, are at significantly greater risk of death resulting from their cancer when compared with married patients. This survival discrepancy existed in each stage, age and gender. In addition, after adjusting for sex, pathological grading, stage, etc., marital status remained to serve as an independent prognostic predictor. Meanwhile, we also obeserved that more cancer cases were diagnosed in later years (more frequent in 2002–2007) which could be atrributed to the inclusion of more cancer registries in the SEER database over the years.
Being married has been shown to possess a survival disadvantage for patients with many types of cancers18,19. Delayed diagnosis and under-treatment are the mainly reported reasons of poor survival in unmarried patients5,20. In our study, we found that the percentage of patients with HCC in the widowed group (63.2%) was the highest in the localized stage compare with married (60.7%), never married (57.2%), and divorced/separated group (62.2%). Apparently, delayed diagnosis could not explain the result because the widowed group had the highest percentage. Another reason can be explain the unfavorable prognosis of unmarried individuals was under-treatment. However, surgery, rather than adjunctive therapy, is recommended for those resectable HCC patients. Interestingly, we found that the widowed patients, compared with those in the married group, still had a disadvantage of 19.4% in the localized stage, 13.2% in the regional stage and 4.2% in the distant stage regarding the 5-year CSS. Unmarried patients were at an increased risk of cancer mortality in contrast to married patients with different gender and age subgroups after adjusted for confounding factors. When comparing with married patients, widowed patients always had the worse CSS in all subgroups. Besides, no significantly difference of surgical resection rates was observed between the married and widowed groups. Thus, the hypothesis of under-treatment could not be supported by these findings.
Married patients have better adherence with prescribed treatments than unmarried patients. Delayed radiation treatments in head/neck cancer patients due to impaired adherence can result in increased rates of recurrence and poorer survival21. Similar results are also observed in other cancers22,23. Support systems, ranging from financial to emotional, are always lacking in unmarried patients. Spouses can provide adequate financial support to cover the costs of cancer treatment. Contrarily, unmarried patients might be reluctant to receive the treatment they needed due to economic reasons. Other than financial support, patients also have an emotion pillar to lean on provided by spouses during some of the more difficult times of their lives. Schlegel et al, also demonstrated that single patients had higher rates of depression24.
Psychologically, unmarried patients display more stress and depression when they are diagnosed with cancer, which can alter immune function and result in tumor progression25,26. DiMatteo et al. reported that married patients displayed lower risk of depression27. Moreover, Goodwin et al. found that women with depression were at greater risk for undergoing non-definitive treatment and display worse survival after a diagnosis of breast cancer28. A perceived lack of social support was associated with higher cortisol levels in patients with cancer, and chronic stress might promote cortisol secretion29,30. Lower natural-killer cell count and survival was also observed in those patients whom lack of social support31. Increased cortisol levels may downregulate the cortisol receptors, thus reduce anti-inflammatory response and promote inflammation32. In addition, a five year observational cohort study demonstrated that depression and anxiety were correlated with breast cancer recurrence33. Stress mediators produced in chronic stress could result in tumor metastasis through activation of specific signaling pathways and the tumor microenvironment25.
Although this study is based on a large population and partly answer the questions about marital status and prognosis in HCC, potential limitations should also be considered. First, the SEER database only collects the marital status at diagnosis, which could serve as a time dependent variable and may be changed after diagnosis. The changed marital status could also affect survival. Second, the information on smoking and alcohol use may not be available in SEER, and some studies have reported that unmarried patients may be at greater risk of such habits34. Furthermore, the SEER database also lacks important information regarding therapy options, income/insurance status, education and quality of marriage, which could not be adjusted by our analyses. Importantly, due to the retrospective nature, psychological tests could not be used to validate our hypothesis that psychosocial factors may be the main reasons for poor survival in unmarried patients.
Despite these limitations, our study indicates that unmarried patients are at greater risk of delayed diagnosis and cancer-specific mortality. Our study also reveals that unmarried patients groups form essentially a heterogeneous group, and widowed patients are always at the highest risk of mortality. Physicians caring for unmarried patients with HCC, especially in widowed ones, should realize the poorer outcomes in this population. It raises the possibility that investments in targeted social support services and interventions aimed at this population could significantly improve the likelihood of achieving cure.
Additional Information
How to cite this article: Zhang, W. et al. Prognostic value of marital status on stage at diagnosis in hepatocellular carcinoma. Sci. Rep. 7, 41695; doi: 10.1038/srep41695 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
This work was supported by grants from National Natural Science Foundation for Distinguished Young Scholars (81225017 to B.S.); The State Key Program of National Natural Science of China (81430062 to B.S.); B.S. is Yangtze River Scholars Distinguished Professor.
Footnotes
The authors declare no competing financial interests.
Author Contributions W.J.Z. and B.C.S.: Conceptualization, methodology, validation, investigation, writing–original draft, writing–review and editing, and visualization. W.J.Z., Z.K.X. and B.C.S.: Data curation and writing–review and editing. W.J.Z., X.C.W. and R.Y.H.: Formal analysis, investigation, and writing–review and editing. X.C.W.: Investigation, resources, writing–original draft, and writing–review and editing. W.J.Z. and B.C.S.: Formal analysis, writing–original draft, writing–review and editing, visualization, and supervision. K.P.J. and G.Y.Z.Y.: Investigation, resources, writing–original draft, and writing–review and editing. W.W.Y. and Y.Y.: Formal analysis, investigation, resources, and writing–review and editing. H.W., Z.K.X. and B.C.S.: Conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing–original draft, writing–review and editing, visualization, and supervision.
References
- Kaplan R. M. & Kronick R. G. Marital status and longevity in the United States population. J Epidemiol Community Health 60, 760–765, doi: 10.1136/jech.2005.037606 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda A. et al. Marital status and mortality among Japanese men and women: the Japan Collaborative Cohort Study. BMC Public Health 7, 73, doi: 10.1186/1471-2458-7-73 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino B. N. Social support and health: a review of physiological processes potentially underlying links to disease outcomes. J Behav Med 29, 377–387, doi: 10.1007/s10865-006-9056-5 (2006). [DOI] [PubMed] [Google Scholar]
- Molloy G. J., Stamatakis E., Randall G. & Hamer M. Marital status, gender and cardiovascular mortality: behavioural, psychological distress and metabolic explanations. Soc Sci Med 69, 223–228, doi: 10.1016/j.socscimed.2009.05.010 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizer A. A. et al. Marital status and survival in patients with cancer. J Clin Oncol 31, 3869–3876, doi: 10.1200/JCO.2013.49.6489 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denberg T. D., Beaty B. L., Kim F. J. & Steiner J. F. Marriage and ethnicity predict treatment in localized prostate carcinoma. Cancer 103, 1819–1825, doi: 10.1002/cncr.20982 (2005). [DOI] [PubMed] [Google Scholar]
- Li Q., Gan L., Liang L., Li X. & Cai S. The influence of marital status on stage at diagnosis and survival of patients with colorectal cancer. Oncotarget 6, 7339–7347, doi: 3129 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Wilson S. E., Stewart D. B. & Hollenbeak C. S. Marital status and colon cancer outcomes in US Surveillance, Epidemiology and End Results registries: does marriage affect cancer survival by gender and stage? Cancer Epidemiol 35, 417–422, doi: 10.1016/j.canep.2011.02.004 (2011). [DOI] [PubMed] [Google Scholar]
- Reyes Ortiz C. A., Freeman J. L., Kuo Y. F. & Goodwin J. S. The influence of marital status on stage at diagnosis and survival of older persons with melanoma. J Gerontol A Biol Sci Med Sci 62, 892–898, doi: 62/8/892 (2007). [DOI] [PubMed] [Google Scholar]
- Jatoi A. et al. Does marital status impact survival and quality of life in patients with non-small cell lung cancer?Observations from the mayo clinic lung cancer cohort. Oncologist 12, 1456–1463, doi: 10.1634/theoncologist.12-12-1456 (2007). [DOI] [PubMed] [Google Scholar]
- Greenberg E. R. et al. Social and economic factors in the choice of lung cancer treatment. A population-based study in two rural states. N Engl J Med 318, 612–617, doi: 10.1056/NEJM198803103181006 (1988). [DOI] [PubMed] [Google Scholar]
- Shen Q. et al. Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large-scale, multicentre study. Lancet Oncol 13, 817–826, doi: 10.1016/S1470-2045(12)70233-4 (2012). [DOI] [PubMed] [Google Scholar]
- Zhang W. & Sun B. Impact of age on the survival of patients with liver cancer: an analysis of 27,255 patients in the SEER database. Oncotarget 6, 633–641, doi: 2719 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passel J. S. Demography of immigrant youth: past, present, and future. Future Child 21, 19–41 (2011). [DOI] [PubMed] [Google Scholar]
- Alamanda V. K., Song Y. & Holt G. E. Effect of marital status on treatment and survival of extremity soft tissue sarcoma. Ann Oncol 25, 725–729, doi: 10.1093/annonc/mdt583 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravdal O. The impact of marital status on cancer survival. Soc Sci Med 52, 357–368, doi: S0277953600001398 (2001). [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Hunt W. C., Key C. R. & Samet J. M. The effect of marital status on stage, treatment, and survival of cancer patients. JAMA 258, 3125–3130 (1987). [PubMed] [Google Scholar]
- Holm K. E. et al. The impact of age on outcomes in chronic obstructive pulmonary disease differs by relationship status. J Behav Med 37, 654–663, doi: 10.1007/s10865-013-9516-7 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelles J. L., Joseph S. A. & Konety B. R. The impact of marriage on bladder cancer mortality. Urol Oncol 27, 263–267, doi: 10.1016/j.urolonc.2008.04.016 (2009). [DOI] [PubMed] [Google Scholar]
- Osborne C., Ostir G. V., Du X., Peek M. K. & Goodwin J. S. The influence of marital status on the stage at diagnosis, treatment, and survival of older women with breast cancer. Breast Cancer Res Treat 93, 41–47, doi: 10.1007/s10549-005-3702-4 (2005). [DOI] [PubMed] [Google Scholar]
- Pajak T. F. et al. Elapsed treatment days–a critical item for radiotherapy quality control review in head and neck trials: RTOG report. Int J Radiat Oncol Biol Phys 20, 13–20, doi: 0360-3016(91)90132-N (1991). [DOI] [PubMed] [Google Scholar]
- Li B. D. et al. Patient compliance is critical for equivalent clinical outcomes for breast cancer treated by breast-conservation therapy. Ann Surg 231, 883–889 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. L., Shelton D. R., Krailo M. & Levine A. M. The effect of compliance with treatment on survival among patients with hematologic malignancies. J Clin Oncol 8, 356–364 (1990). [DOI] [PubMed] [Google Scholar]
- Schlegel R. J., Manning M. A., Molix L. A., Talley A. E. & Bettencourt B. A. Predictors of depressive symptoms among breast cancer patients during the first year post diagnosis. Psychol Health 27, 277–293, doi: 10.1080/08870446.2011.559232 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Smith M., Lutgendorf S. K. & Sood A. K. Impact of stress on cancer metastasis. Future Oncol 6, 1863–1881, doi: 10.2217/fon.10.142 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar L. S. & Anisman H. Stress and coping factors influence tumor growth. Science 205, 513–515 (1979). [DOI] [PubMed] [Google Scholar]
- DiMatteo M. R., Lepper H. S. & Croghan T. W. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med 160, 2101–2107, doi: ioi90679 (2000). [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Zhang D. D. & Ostir G. V. Effect of depression on diagnosis, treatment, and survival of older women with breast cancer. J Am Geriatr Soc 52, 106–111, doi: 52018 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. S. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 87, 873–904, doi: 10.1152/physrev.00041.2006 (2007). [DOI] [PubMed] [Google Scholar]
- Sephton S. E., Sapolsky R. M., Kraemer H. C. & Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst 92, 994–1000 (2000). [DOI] [PubMed] [Google Scholar]
- Levy S. M. et al. Perceived social support and tumor estrogen/progesterone receptor status as predictors of natural killer cell activity in breast cancer patients. Psychosom Med 52, 73–85 (1990). [DOI] [PubMed] [Google Scholar]
- Cruces J., Venero C., Pereda-Peeez I. & De la Fuente M. The effect of psychological stress and social isolation on neuroimmunoendocrine communication. Curr Pharm Des 20, 4608–4628, doi: CPD-EPUB-58922 (2014). [DOI] [PubMed] [Google Scholar]
- Burgess C. et al. Depression and anxiety in women with early breast cancer: five year observational cohort study. BMJ 330, 702, doi: 10.1136/bmj.38343.670868.D3 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom M. Social capital, economic conditions, marital status and daily smoking: a population-based study. Public Health 124, 71–77, doi: 10.1016/j.puhe.2010.01.003 (2010). [DOI] [PubMed] [Google Scholar]