Abstract
The complexity and diversity of a neural network requires regulated elongation and branching of axons, as well as the formation of synapses between neurons. In the present study we explore the role of AP-2, a key endocytic adaptor protein complex, in the development of rat hippocampal neurons. We found that the loss of AP-2 during the early stage of development resulted in impaired axon extension and failed maturation of the axon initial segment (AIS). Normally the AIS performs two tasks in concert, stabilizing neural polarity and generating action potentials. In AP-2 silenced axons polarity is established, however there is a failure to establish action potential firing. Consequently, this impairs activity-driven Ca2+ influx and exocytosis at nerve terminals. In contrast, removal of AP-2 from older neurons does not impair axonal growth or signaling and synaptic function. Our data reveal that AP-2 has important roles in functional axogenesis by proper extension of axon as well as the formation of AIS during the early step of neurodevelopment.
In order to properly integrate into functional circuits, neurons must establish cellular polarity including the elaboration of dendritic branches and elongation of a single axon that navigates through guidance cues to establish synaptic terminals. Some of the critical molecular signaling pathways have been identified in axogenesis1, path finding2, and synaptogenesis3. However, less is known about how these systems rely on basic endocytic machinery to function. One would expect endocytosis to be important for a number of stages in neuronal development as precise sorting of cargoes or membrane proteins is essential during development of hippocampal neurons4. Forward extension of growth cones in neurites involves continuous addition and retrieval of membrane to drive the leading edge. Furthermore, endocytosis of signaling receptors such as netrin-DCC5,6, Slit-Robo7,8, semaphorin-neuropilin9, and ephrin-eph receptor10 is critical for correct axon guidance and outgrowth11,12,13. Molecules such as AP-2 and clathrin are usually involved in clearing such receptors from the cell surface, similar to the role they play in the delivery of iron through endocytosis of the transferrin receptor.
Another critical aspect of axogenesis, aside from signaling and guidance is establishing subcellular signaling domains. These domains are created by the precise sorting of axonal and dendritic proteins including voltage-gated ion channels14,15,16,17. A key stage of this process is the formation of the axon initial segment (AIS). The AIS serves as a barrier to maintain polarity as well as a functional role in signaling by initiating action potentials. The AIS is composed of an array of structural and cytoskeletal proteins which localize to the proximal region of axon helping to establish a barrier for selective transport of cargo to the axon18. The signaling features of the AIS are enabled because this region of axon is studded with a precise array of voltage-gated ion channels including a high concentration of voltage-gated sodium (NaV) channels to initiate action potentials. At present, the molecular mechanisms enabling the delivery of these ion channels are unknown19,20. One possibility in neurons is that the enrichment of ion channels along the axon and at the AIS are selectively retrieved from other areas of the cell (proximal dendrites and soma) for subsequent delivery early in polarization similar to signaling receptors. Clathrin-mediated endocytosis has been identified as an important mechanism for enriching Na+ channels in epithelial tissue (a classic polarized cell)21,22. Endocytosis was also identified as a critical process for modulating ligand-gated channels at the postsynaptic density of dendritic spines23. Taken together, these observations warrant a closer study of endocytic proteins in neuronal axogenesis and signaling.
Previously, using shRNA-mediated ablation of the μ2-subunit to deplete overall AP-2 complex levels in mature polarized hippocampal neurons, we demonstrated that this clathrin-associated adaptor protein complex is critical for efficient synaptic vesicle endocytosis24,25. Here we show that removal of AP-2 at early stages of neuronal development impedes axogenesis, prevents accumulation of NaV channels at the AIS, and impairs synaptic transmission. In contrast, removal of AP-2 once the axon has formed does not alter signal propagation and synaptic transmission. Together, these data demonstrate a critical window during which AP-2 is needed in the process of establishing axogenesis including axonal branching and establishing the repertoire of ion channels critical for signal propagation to synapses.
Results
Early depletion of AP-2 impairs proper axon extension in hippocampal neurons
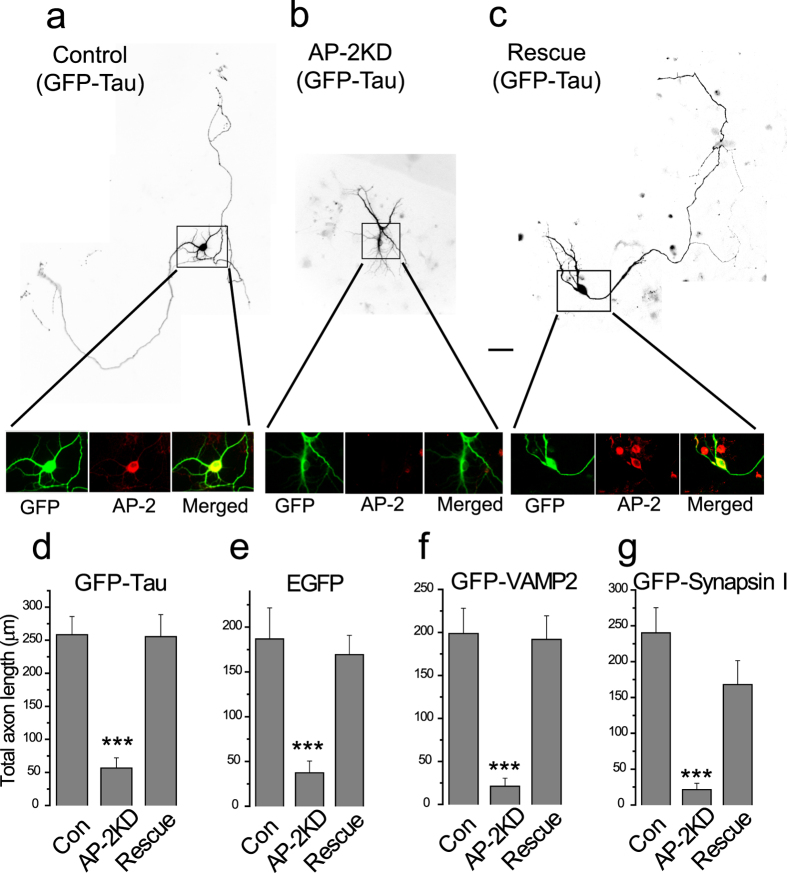
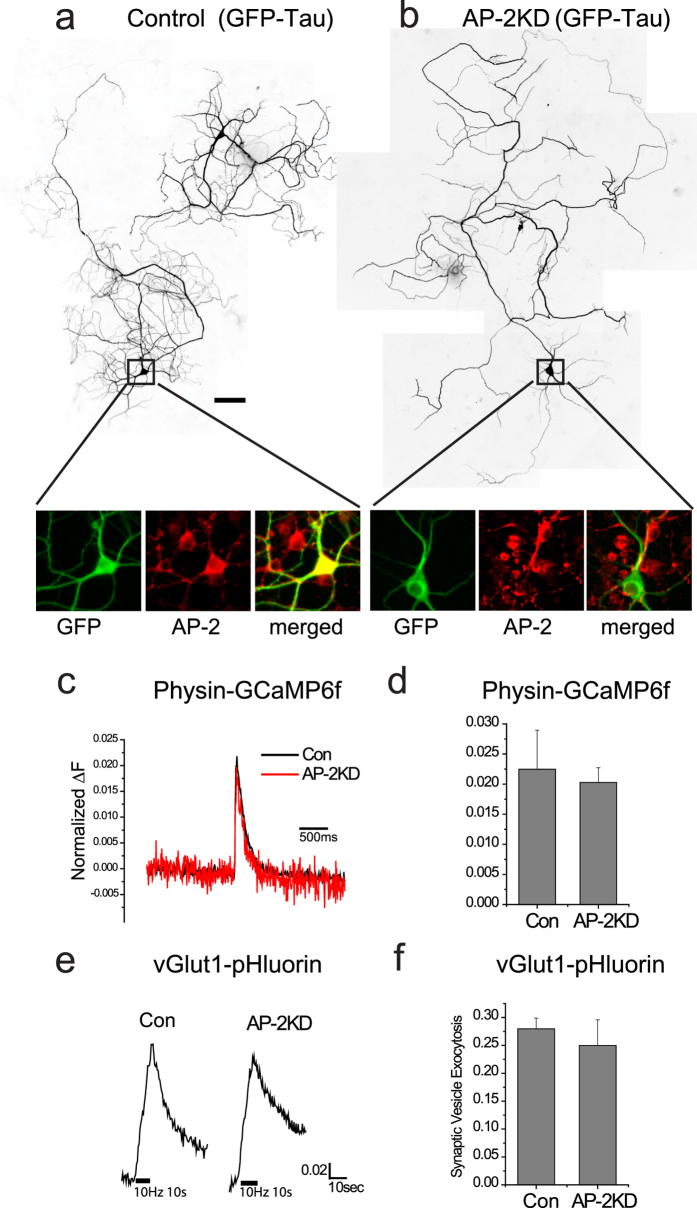
Dissociated hippocampal neurons are known to go through a series of morphological changes when plated in vitro26. This includes the formation of lamellipodia, extension and retraction of short neurites and the emergence of a single rapidly growing axon. To determine if AP-2 complex used in synaptic endocytosis plays an important role in axogenesis we delivered shRNA targeting the μ2 subunit of the AP-2 protein complex to neurons ~36 hours after plating. AP-2 consists of an obligate hetero-tetramer consisting of α, β2, μ2 and σ2. Removal of a single one of these subunits in mammalian cells results in loss of the AP-2 complex27. The efficiency of the knockdown in individual neurons was determined by retrospective immunocytochemistry against the AP-2 α subunit as a proxy for the AP-2 complex as a whole, which showed that shRNA treatment targeting the μ2 subunit resulted in >90% loss of the AP-2 complex (Fig. 1a–c inset and supplementary Fig. 1). In order to examine the morphology of the transfected cell, we co-transfected neurons receiving shRNA with either a cytosolic marker (soluble GFP) or GFP-tagged proteins that eventually become polarized to the axon (GFP-Tau, GFP-VAMP2, GFP-synapsin I). Five days after transfection, neurons were fixed and imaged. Under control conditions (cells receiving GFP alone or a GFP-tagged protein) we observed the expected morphology for this age in vitro: a cell body with a number of shorter thick neuritic extensions as well as a single, often branched narrow process that extends over a large distance (Fig. 1a also see supplementary Fig. 2), which we take to be the nascent axon. However, in cells in which AP-2 had been ablated the cell body had less developed neuritic extensions (Fig. 1b) with the longest neurite on average only 25% of the axonal length in control neurons (Fig. 1a). These results were invariant with respect to the type of marker used to examine the morphology (Fig. 1d–g, see also supplementary Fig. 2). Although the axon extension was impaired, there was still a strong development of polarity for axon specific markers only in one neurite extension including, synapsin, VAMP2 and Tau proteins (supplementary Fig. 2). Proper polarization was also confirmed by sorting of endogenous microtubule associated protein 2 (MAP2), which was properly confined to the dendrites of both control and AP-2KD neurons (supplementary Fig. 2). We confirmed the specificity of the shRNA by demonstrating that these effects were fully reversed when a plasmid encoding an shRNA-resistant μ2 was co-transfected with the shRNA and the morphological marker (Fig. 1c,d). Equivalent results were obtained with all 4 of the different markers used in rescue-transfected neurons, which were indistinguishable from WT (Fig. 1d–g). Thus the defects in axonal extension resulted from AP-2 ablation through μ2 subunit deletion during early development.
Figure 1. Early AP-2 depletion has defect in axon formation in hippocampal neurons.
(a–c) Representative images of axon extension in hippocampal neurons (cultured for 7 days) expressing GFP-Tau taken from: (a) Control cell (expressing GFP-Tau alone), (b) AP-2KD cell (expressing GFP-Tau + shRNA targeting μ2 subunit of AP-2), and (c) Rescue cell (Expressing GFP-Tau, shRNA targeting μ2 subunit of AP-2, and shRNA-μ2 resistant μ2 plasmid). Knockdown shRNA was added at 36–48 hours after plating. (a–c) (Inset) magnified cell body of neurons that were immunolabeled with left to right: anti-GFP (green) and anti-α-adaptin AP-2 subunit antibody (α subunit of AP-2 complex), (red) and merged (overlay of red and green imaged). (d) Total axon length of neurons from all three conditions (Control, AP-2KD, and Rescue). Total length of axon including primary and all sub-branched axons were measured: Total length of axon; GFP-Tau con = 258.3 ± 27.5 μm (n = 12), GFP-Tau AP-2KD = 56.8 ± 15.2 μm (n = 18), GFP-Tau Rescue = 255.39 ± 33.4 μm (n = 12), (e–g) Total –axon length of neurons from all three conditions (Control, AP-2KD, and Rescue) using exogenously expressed cytosolic marker (e; EGFP only) or axonal markers (f; GFP-VAMP2, (g) GFP-Synapsin I respectively) with the same method as (d). (e) EGFP con = 186.9 ± 34.5 μm (n = 12), EGFPAP-2KD = 37.4 ± 13.0 μm (n = 14), EGFP Rescue = 169.3 ± 21.6 μm (n = 8). (f) GFP-VAMP2 con = 198.8 ± 29.3 μm (n = 16), GFP-VAMP2 AP-2KD = 21.1 ± 9.5 μm (n = 9), GFP-VAMP2Rescue = 191.9 ± 27.4 μm (n = 10). (g) GFP-Synapsin I con = 240.1 ± 35.2 μm (n = 10), GFP-Synapsin I AP-2KD = 21.4 ± 9.0 μm (n = 13), GFP-Synapsin I Rescue = 168.1 ± 33.4 μm (n = 5). Scale bar represents 10 μm. ***p < 0.001. One-way ANOVA.
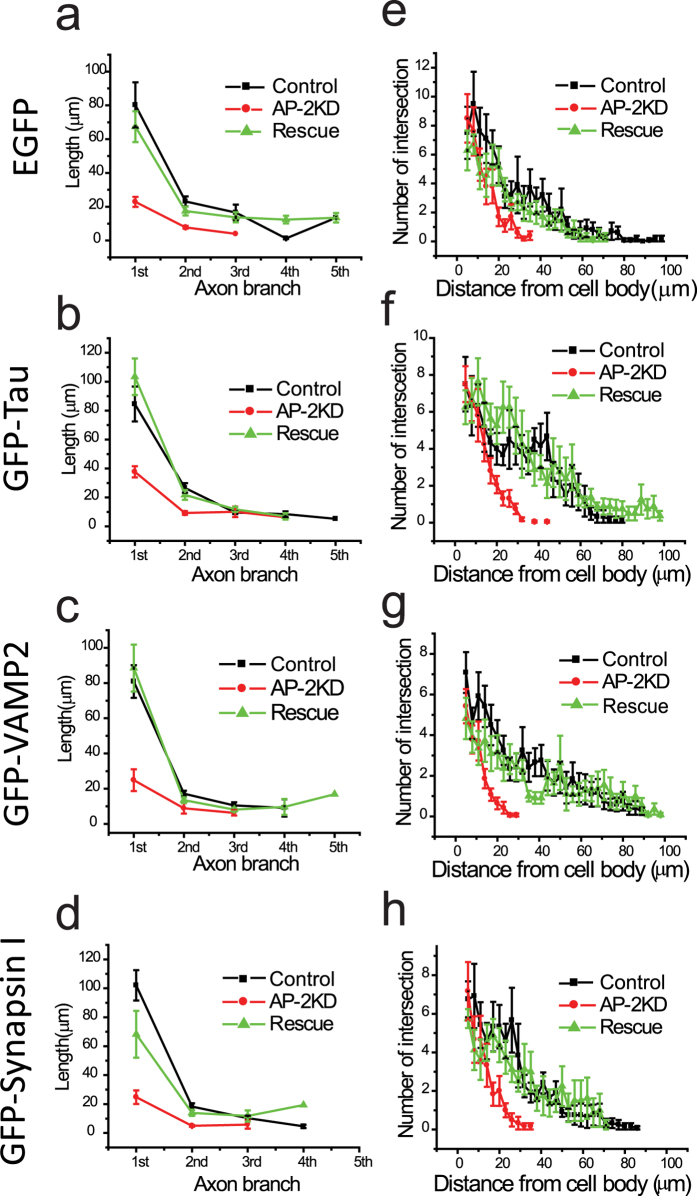
In order to examine the morphological variations in a more systematic manner, we measured branching frequency and branch length using the aforementioned markers (Fig. 2a–d) and carried out a Sholl analysis (Fig. 2e–h) in the AP-2KD, WT and rescue neurons (AP-2 KD + shRNA-resistant μ2 plasmid) (Fig. 2). These data all showed that in the absence of AP-2, axons become severely blunted and form fewer branches using multiple axonal markers. We also attempted to examine neurons that had been depleted of AP-2 at early stages but kept in culture for longer periods (>14 days in vitro) but were unable to find transfected neurons as assayed by expression of our GFP reporters. Presumably, those neurons were not able to sustain viability, reminiscent of the of AP-2 knockout mouse phenotype28.
Figure 2. Impairment of AP-2 expression leads to a reduction in neuronal process.
(a–d) The lengths of individual branch of axon were measured using four markers (EGFP. GFP-Tau, GFP-VAMP2 and GFP-Synapsin I). AP-2 depleted neurons have significantly deficient axon extension in each axon branch. (e–h) Sholl analysis was applied in WT, AP-2KD, and rescued neurons transfected four different markers. AP-2KD neuron has significantly decreased neuronal process (<40 μm) as measured by all four different markers compare to that of WT or rescued neurons (~100 μm).
Early AP-2 ablated neurons still undergo synaptogenesis
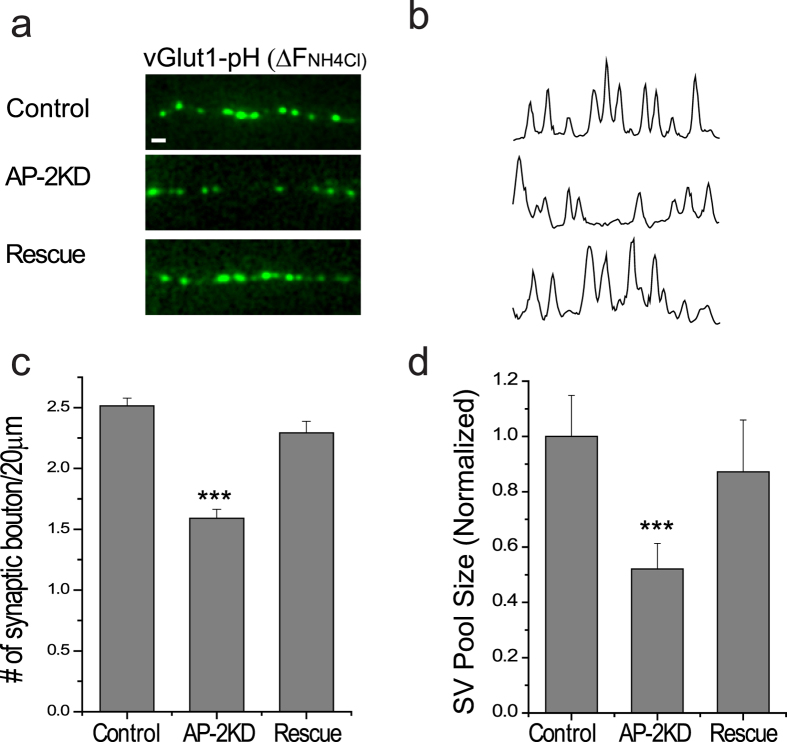
Following axon elongation and polarization, synaptogenesis of en passant boutons occurs in hippocampal neurons during development. Although we quantitatively observed less axogenesis in AP-2KD neurons, synaptogenesis still persisted. We identified synapses using the expression of a vesicle specific glutamate transporter (vGlut1) with an attached lumenal pH-sensitive GFP molecule (pHluorin). Intracellular vesicles can be visualized by a brief application of NH4Cl (pH7.4) that neutralized the alkaline lumen of the vesicle29 (Fig. 3a,b). The density of synaptic boutons (vG-pH positive puncta) measured at 7–8 days in vitro (DIV) was decreased by ~30% in AP-2KD axons compared to control (2.51 ± 0.06/20 μm and 1.58 ± 0.07/20 μm for control and AP-2KD neurons respectively) (Fig. 3c). In addition to having fewer boutons, the size of the synaptic vesicle pool (defined by the average maximal NH4Cl vG-pH response) at each bouton in AP-2KD neurons was also decreased by 50% compared to control synapses (Fig. 3d). In each case, we confirmed that these defects from early AP-2 depletion were fully restored by expressing shRNA-insensitive μ2. These results indicate that the loss of AP-2 does not preclude synaptogenesis in the truncated axon, although the density and size of boutons are decreased (Fig. 3a–d).
Figure 3. Synaptogenesis is maintained in early AP-2 depleted neurons.
(a) Representative images of axons expressing vGlut1-pHluorin after exposure to 50 mM NH4Cl (pH 7.4) to reveal intracellular pool of vesicles for control, AP-2KD and rescue conditions. Scale bar represent 5 μm. (b) Line scans of intensity from images in (a). (c) Mean values of the number of synaptic boutons per 20 μm of axon in Control, AP-2KD, and Rescue neurons. Control = 2.51 ± 0.06/20 μm (n = 6 cells), AP-2KD = 1.58 ± 0.07/20 μm (n = 5 cells), Rescue = 2.29216 ± 0.0956/20 μm (n = 6 cells). (d) Quantification of the total synaptic vesicle pool as measured by NH4Cl application in Control, AP-2KD, and Rescue neurons expressing vGlut1-pHlourin normalized to control cells. Control = 100.0 ± 14.8% (n = 8 cells), AP-2KD = 52.1 ± 9.2% (n = 5 cells), Rescue = 87.2 ± 1.8% (n = 5 cells). ***p < 0.001. One-way ANOVA.
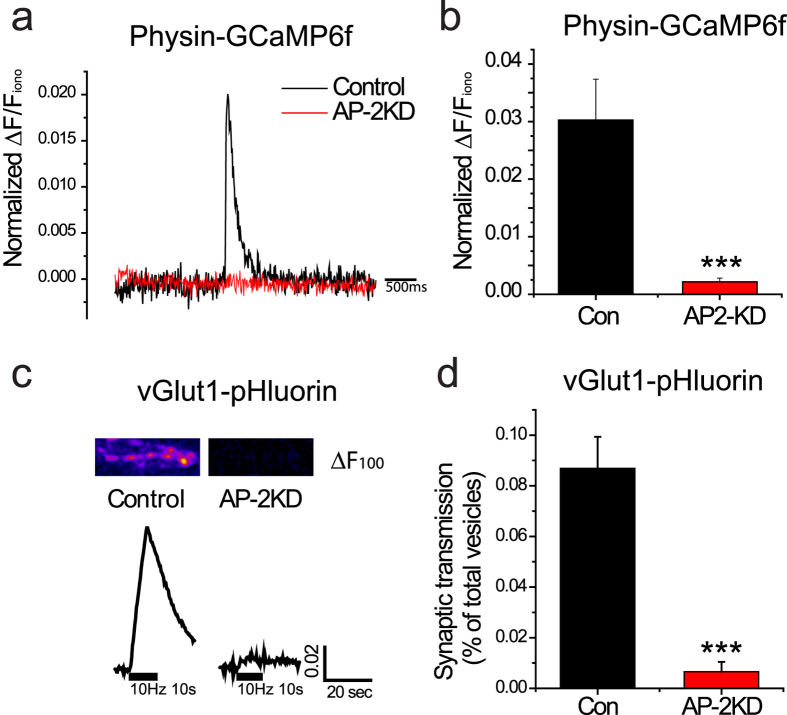
Activity-driven Ca2+ influx and synaptic vesicle release at nerve terminal is disrupted in early AP-2 depleted neurons
When an action potential invades a presynaptic terminal it activates voltage-gated Ca2+ (CaV) channels, which catalyzes vesicle fusion and release of neurotransmitter. Next, we undertook measurements of synaptic function at the nerve terminals of AP-2KD neurons. We made use of a sensitive genetically-encoded Ca2+ indicator, GCaMP6f 30, fused to synaptophysin to specifically measure Ca2+ influx at presynaptic nerve terminals. We compared activity-driven responses to the value obtained following perfusion of ionomycin, which should saturate the probe31. As shown in Fig. 4a,b, activity-driven Ca2+ influx is absent from early AP-2KD neurons while control neurons show a robust increase even from stimulation with a single AP. Synaptic transmission is tightly regulated by Ca2+ influx at the nerve terminal32. As such, we also utilized vGlut1-pHlourin (vG-pH) system, to measure synaptic vesicle release during trains of stimulation as a sensitive assay29 for activation of CaV channels at nerve terminals31,33. We delivered 100 stimuli at 10 Hz. Under these conditions, control neurons released 8.69 ± 1.2% of their synaptic vesicles, whereas AP-2KD neurons showed a response just above baseline even under prolonged stimulation (0.65 ± 0.3%) (Fig. 4c,d).
Figure 4. Activity-driven Ca2+ influx and vesicle fusion are impaired in early AP-2 depleted synapses.
(a) Representative single action potential stimulated GCaMP6f traces in control (black) and early AP-2KD neurons (red). (b) Mean value of GCaMP6f 1AP response amplitudes in control and AP-2KD neuron. Con = 0.0302 ± 0.71 (n = 7 cells), AP-2KD = 0.0021 ± 0.06 (n = 8 cells). (c) Representative vG-pH trace of neuron in control and early AP-2KD neurons. Neurons expressing vG-pH only, vG-pH with shRNA against AP-2 were stimulated with 100 action potential at 10 Hz. (Inset) ΔF image of synaptic bouton after 100 action potential at 10 Hz. (d) Mean values of the amount of synaptic transmission by 100 AP. Con = 8.69 ± 1.24% (n = 8 cells), AP-2KD = 0.65 ± 0.38% (n = 7 cells). ***p < 0.001.
AP-2 in not essential at later stages of synaptogenesis
The retardation of axogenesis by early AP-2KD suggests that AP-2 is critical during the initiation period of polarization for establishing functional synaptic terminals capable of neurotransmission. In order to determine whether the dependence on AP-2 integrity is maintained at later stages of axon development we examined the consequences of AP-2KD applied to cells at DIV 7–8 and examined at >DIV 14. We previously showed that KD at this stage results in a >96% KD of AP-224. Between DIV 7–8 and DIV14 the axon continues significant growth, including additional branching and elaboration preventing a full quantitative analysis as applied to the earlier developmental stage. However, neurons transfected with shRNA targeting μ2 and GFP-Tau (Fig. 5a,b) or GFP-VAMP2 (data not shown) at 7–8 DIV showed no obvious impairment in axon growth. Similar to early AP-2KD there was no impairment of polarization of tau protein in later stage AP-2KD (Fig. 5b). Analysis of Ca2+ influx and vesicle exocytosis did not show any impairment in late AP-2KD neurons (Fig. 5c–f), though there was an impairment in synaptic vesicle endocytosis as previously reported24.
Figure 5. Late-stage AP-2 depletion does not impair synaptic function.
(a,b) Representative images of axon extension in hippocampal neurons (cultured for 14–16 days) expressing GFP-Tau with as an axonal marker taken from: (a) Control cell (expressing GFP-Tau alone), (b) Late AP-2KD cell (expressing GFP-Tau + shRNA targeting μ2 subunit of AP-2). Knockdown shRNA was added at 8 DIV after plating. A-B (Inset), magnified cell body of neurons that were immunolabeled with left to right: anti-GFP (green) and anti-α-adaptin antibody (red) and merged (overlay of red and green imaged). (c) Representative 1 action potential stimulated GCaMP6f traces in control (black) and late AP-2KD neurons (red). (d) Mean value of Physin-GCaMP6f 1 action potential response amplitudes in control and AP-2KD neuron. Con = 2.24 ± 0.64% (n = 4 cells), AP-2KD = 2.02 ± 0.24% (n = 4 cells). (e) Representative vG-pH traces of control and late AP-2KD neurons 100 action potential pulses at 10 Hz. (f) Mean values of peak vG-pH in control and AP-2KD cells normalized to NH4Cl response. Con = 27.98 ± 0.01% (n = 11 cells), AP-2KD = 24.98 ± 4.58% (n = 8 cells), p = 0.51.
Early AP-2 depletion impairs action potential generation
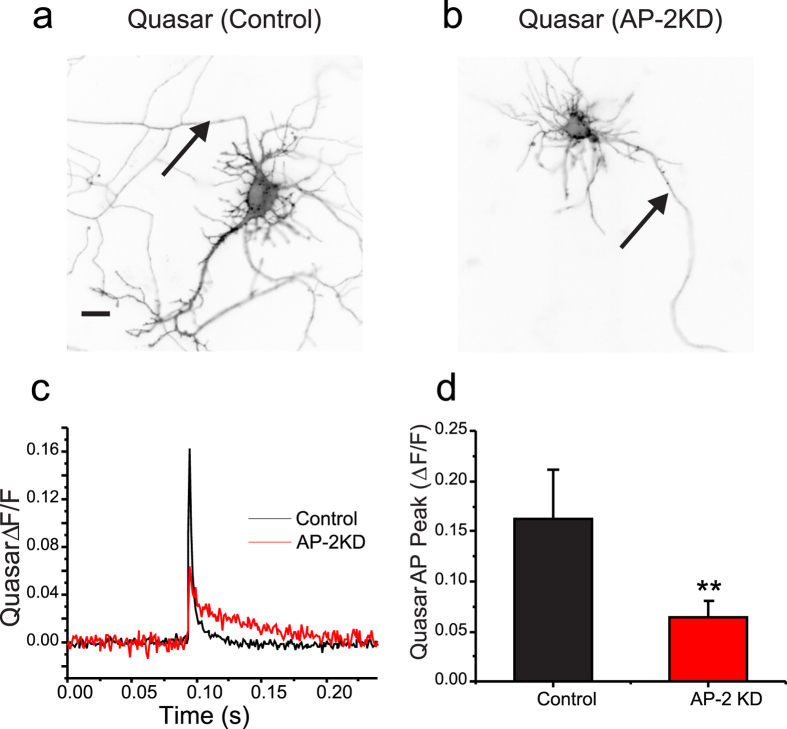
The failure of field-stimulation to elicit vesicle exocytosis or Ca2+ -influx at presynaptic terminals in early AP-2KD neurons raised the question of functional electrical activity and signal propagation to synaptic terminal. Recently using a genetically-encoded voltage indicator in combination with high speed imaging, it has been possible to make subcellular measurements of electrical activity in hippocampal neurons34. In the present study, we took advantage of the latest version of archaerhodopsin-based voltage indicator QuasAr235 to examine the action potential waveform at the somas of control and early AP-2KD neurons (Fig. 6a,b). As shown in Figs 6c,d, the peak amplitude of the action potential waveform in AP-2KD neurons was severely decreased (0.05 ± 0.02 ΔF/F, n = 13) compared to control neurons (0.17 ± 0.04 ΔF/F, n = 11). Indeed, the waveform itself did not resemble an action potential with its low amplitude and lack of rapid polarization (Fig. 6c,d). Measurements at the presynaptic terminals in AP-2KD neurons did not yield any measurable signal making comparison to control cells impossible (supplementary Fig. 3). Thus, although AP-2 KD neurons establish polarity and synaptic terminals, they are unable to generate or propagate action potentials. This was not the case in mature neurons, when depleting AP-2 complex levels at later stages of development. In these cells, action potential generation and propagation was still quite robust within the distal axon (>200 μm from soma; supplementary Fig. 4) as expected from prior measurements of synaptic function when AP-KD occurs at DIV 7–8 neurons.
Figure 6. The waveform of action potentials is disrupted in AP-2 depleted neurons.
(a,b) Representative images of Quasar transfected neurons ± AP-2KD with arrows indicating AIS. Scale bar = 5 μm. (c) Average traces of optically recorded action potentials from control (Black; n = 11) and AP-2KD (red; n = 13) neurons after subtraction of field stimulus, isolated by inclusion of TTX (300 nM) extracellularly, see methods. (d) Amplitude of AP waveforms recorded from soma of control (black; 0.177 ± 0.044) and AP-2KD (red; 0.0596 ± 0.016). **p < 0.01.
Early AP-2 depletion impairs the maturation of the Axon Initial Segment (AIS) in hippocampal neuron
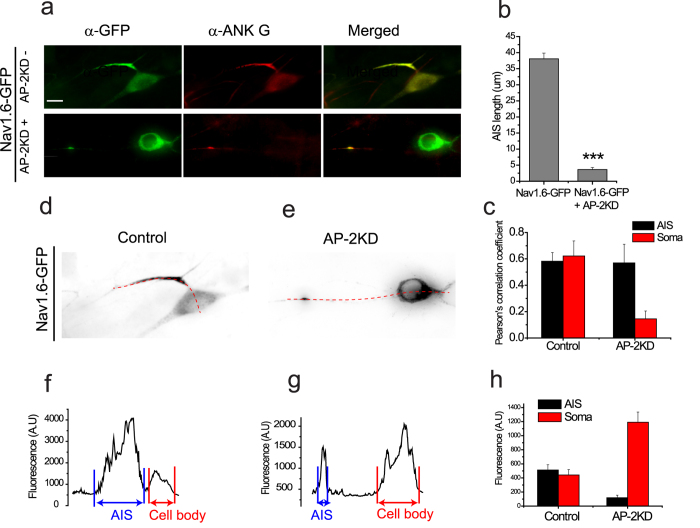
In addition to axonal elongation and synaptogenesis, another important aspect of axogenesis is the generation of specialized signaling domains with voltage-gated ion channels. One such domain is the AIS, a structural and functional hallmark of the axon that both maintains polarity and it the site for action potential initiation. Structurally the AIS is composed of several ion channels (e.g. NaV channels) and structural proteins such as ankyrin G (AnkG)36. It has previously been demonstrated that the silencing AnkG dismantles the AIS and causes axons to acquire the molecular characteristics of dendrites37. Although we didn’t observe changes in polarity with early AP-2KD, we reasoned that the maturation of the AIS where other components with AnkG binding motifs including sodium channels interact with AnkG might be impaired38. In dissociated hippocampal neurons the AIS appears four days after plating in vitro (supplementary Fig. 5)39. We monitored AIS formation for control and AP-2KD neurons using AIS markers. Given the importance of NaV channels for signaling in AIS, we measured their trafficking by expressing a functional exogenous NaV1.6 channel tagged with GFP40. In control neurons expressing NaV1.6-GFP only, we measured a distinct segment of sodium channels colocalized with AnkG measuring 38.09 ± 1.75 μm (Fig. 7a,b) in close agreement with previous reports41. However, in AP-2KD neurons it was hard to detect any significant enrichment in AP-2 depleted neurons with enrichment less than 10% of control neurons, NaV1.6-GFP + AP-2KD = 3.61 ± 0.65 μm (Fig. 7a,b). Intriguingly, Nav1.6 is easily visible in the soma of AP-2KD suggesting a defect in trafficking of the channel not its stability. As shown in Fig. 7c–h, Nav1.6-GFP is enriched at the cell body of AP-2KD neurons (2-fold higher than control), whereas Nav1.6-GFP is distributed more at the AIS than at cell body of control neurons (Fig. 7c). Although knockdown of AP-2 doesn’t alter polarization in a similar manner to AnkG silencing (supplementary Fig. 6), there is a total impairment of action potential initiation. These results suggest that AIS maturation and Nav1.6 trafficking to the AIS are mediated by AP-2.
Figure 7. AP-2 ablation impairs the formation of axon initial segment.
(a) Representative images of cells transfected at 2 DIV with Nav1.6-GFP ± shRNA against AP-2 (AP-2KD). Neurons were fixed at 7 DIV and incubated with anti-GFP (green) and anti-ankyrin G (red). Arrow indicates AIS. (Inset) magnified images of AIS region to clearly visualize. (b) Mean value of the length of the AIS in each condition. Nav1.6-GFP only = 38.09 ± 1.75 μm (n = 24 cells), Nav1.6-GFP + AP-2KD = 3.61 ± 0.65 μm (n = 17 cells). ***p < 0.001. (c) Mean value of Pearson’s co-localization coefficient of Nav1.6-GFP and AnkyrinG in AIS and Soma. Control AIS = 0.58 ± 0.07, Control soma = 0.62 ± 0.11. AP-2KDAIS = 0.57 ± 0.14, AP-2KDsoma = 0.15 ± 0.59. (d–h) Measurement of Nav1.6-GFP at AIS and soma in control and AP-2KD neuron. (d,e) Dashed lines indicate line scan of Nav1.6-GFP distribution from AIS to Soma. (f and g) Profiles of line scan of Nav1.6-GFP in control (f) and AP-2KD (g) neurons. (h) Mean values of density of Nav1.6-GFP at AIS and soma in control and AP-2KD neurons. Control AIS = 513.63 ± 72.69 a.u., Control soma = 442.51 ± 76.19 a.u. (n = 21 cells). AP-2KDAIS = 119.43 ± 35.23 a.u., AP-2KDsoma = 1190.86 ± 145.63 a.u. (n = 15 cells).
Discussion
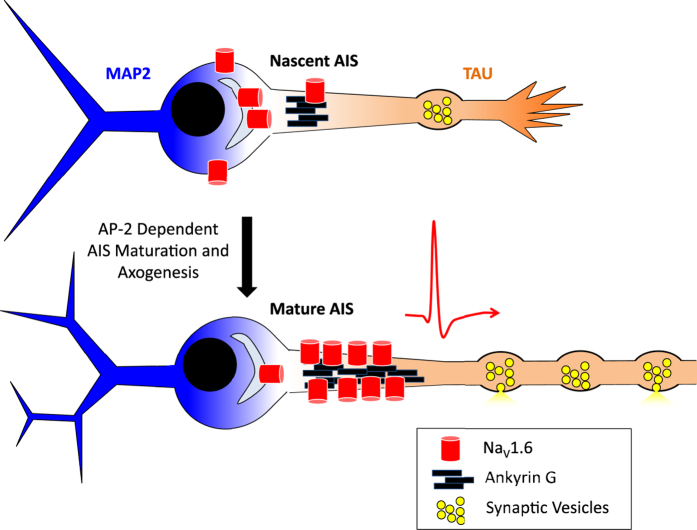
In the present study, we demonstrate that AP-2 has a unique role in the early development of the neuron that is critical for establishing a functional axon. We characterize three distinct phenotypes of neurons that lack AP-2 at the early stage of development: First, impaired extension and arborization of the axon; Second, failure of the axon initial segment to elongate and mature with functional enrichment of NaV channels; and third, the defects in action potential generation and propagation (Fig. 8). The well-studied development of the AIS has always displayed a tightly aligned temporal sequence of protein enrichment anchored by AnkG that has always maintained two important but unique roles that have previously seemed intertwined, maintaining polarity and transforming synaptic inputs into action potentials. Interestingly, it seems that AP-2 is necessary for maturation of the nascent AIS and accumulation of proteins important for electrical signaling in young neurons. This role for AP-2 in the development of the AIS is independent of establishing polarity as axonal proteins still exclusively clustered into axons (Fig. 1 and supplementary Fig. 2) even with a truncated AIS (Fig. 7). Here, we show a unique role for AP-2 in establishing electrical signaling and clustering NaV channels at the axon initial segment that is independent of polarization at the AIS. To our knowledge this is the first time that these two roles have been independently observed in development.
Figure 8. Model of axogenesis in an early AP-2 depleted neuron.
Suggested model of axogenesis with and without AP-2 present. AP-2 mediated endocytosis is essential for establishing and maintaining axon elongation. Furthermore, AP-2 plays a critical role for establishing a functional AIS, the loss of which leads to impaired synaptogenesis and signal transduction.
The adaptor complex AP-2 is the major clathrin-associated adaptor for cargo recognition at the plasma membrane. There is accumulating evidence that endocytosis contributes to the formation of cellular polarity by proper sorting and localizing key molecules in a number of tissue types20,21,22. In our results, neurons display a deficit in axon extension when AP-2 complex levels are depleted at the early period of cellular development, but these axons still go on to form synaptic boutons. Indeed, tau, VAMP2 and synapsin still segregate from the other neurites of the young neuron (Fig. 1). This likely indicates that the role of the AIS for modulating trafficking of synaptic components is maintained in its depleted state, but not its role for integrating synaptic activity to fire action potentials. The observed loss of physiological responses at presynaptic terminal to electrical stimulation are dramatically impaired without any appreciable Ca2+ influx or vesicle fusion when stimulated in AP-2KD neurons. We attribute these impairments to the lack of propagating action potentials that are likely caused by the truncated AIS as measured by AnkG and a failure to establish significant concentrations of NaV1.6 at the AIS (Fig. 7).
The AIS is composed of a host of proteins that subsequently enrich at the AIS including cytoskeletal proteins, cell adhesion molecules (CAMs) and ion channels. The first two proteins to localize to the AIS are βIV-spectrin and AnkG. The establishment of AnkG is followed by the localization of NaV 1.6 channels and the L1-CAMS (neurofascin and NrCAM) as the AIS matures with varying dynamics38,42. These varied modes of enrichment and maturation suggest different modes of trafficking of AIS components. Although it has been well established that AnkG is necessary for clustering sodium channels36,40, it has also been demonstrated that knocking down NaV channels in early stages of neural development destabilized the AIS in motor neurons43. Since the loss of AP-2 proteins mimics the loss of NaV channels we think that AP-2 may contribute to an endocytosis pathway that plays a critical role in the formation of the AIS. Furthermore, the loss of NaV channel enrichment halts the subsequent addition of other proteins that make up the functional AIS.
Due to technical limitations, we were not able to trace NaV or AnkG protein translocation and detect a role for AP-2 mediated endocytosis and targeted delivery. Recent work suggests that NaV channels are delivered by exocytosis to the AIS44. There are a number of receptors and other proteins whose endocytosis is required for axogenesis. One recently described pathway that initiates axogenesis involves the retrieval of activated type II TGFβ receptor1. This receptor is known to directly associate with AP-2 through the β2 subunit45. One possible explanation of the acute requirement for endocytic function at early developmental stages is that successful signaling to initiate and maintain a preliminary axogenesis program requires internalization of the TGFβ ligand-receptor complex, perhaps to generate a signaling endosome. Another example of AP-2 modulated signaling during development involves the establishment of planar cell polarity46,47, and neurite outgrowth by Wnt/Frizzled2. AP-2 with Dishevelled, a downstream hub molecule of Wnt/Frizzled signaling, appears to regulate endocytosis of the Frizzled receptor and contribute to planer cell polarity47,48. Our results indicate that AP-2 is also involved in separate sorting for establishing an essential component of neuronal polarity and excitability, the AIS. It is well known that a number of voltage-gated ion channels are properly sorted by interacting with AP-2 complex20,49. It is likely that early depletion of AP-2 disrupts the correct sorting or targeting of voltage-gated ion channels to the AIS, and eventually destabilizes the other structural and transynaptic proteins that accumulate at the AIS. Specific antibodies that could recognize endogenous NaV1.6 channels with high specificity were not available for immunostaining. We used a low expression plasmid to express a functional NaV1.6-eGFP as a proxy for endogenous channel trafficking40. Although our result using this exogenous proteins shows that NaV1.6 clearly fails to accumulate to the AIS, it is still unclear if Nav1.6 itself is directly retrieved by AP-2, and if this may also be the case for other voltage-gated ion channels. The fact that NaV1.6 accumulates in the somata of AP-2KD neurons does indicate that retrieval and delivery rather than simple diffusion likely plays a critical role for establishing a concentration of channels at the AIS (Fig. 7c–h). We cannot exclude the possibility that CaV channel targeting to presynaptic terminals as well as other channels are also modulated by AP-2. However, the propagation of action potentials is upstream of Ca2+ entry and K+ channel activation masking other deficits at terminals.
Although cargo-internalization during endocytosis is a principal function of AP-2, we cannot rule out that other mechanisms for this protein or the μ2-subunit at the AIS. Indeed, studies in C. elegans have demonstrated a role for hemicomplexes of AP-250. Additionally, the mechanisms for AnkG delivery to the AIS is unknown, as such our results also cannot rule out an important role for AP-2 to maintain or enrich AnkG at the AIS, which could also account for the observed deficiencies in the accumulation of NaV channels. AnkG plays an important role as an adaptor for motor protein entrance and delivery of cargo to the AIS, so a specific disruption to this process by AP-2 KD could account for the observed deficits in AIS maturation and axogenesis51.
The lack of phenotype in later stages of development with AP-2KD suggests the existence of alternate endocytic or other trafficking pathways that only arise in later developmental stages. Alternatively the lack of phenotype suggests that endocytosis is only needed a low rate for maintenance that we are insensitive to in our experiments as we only observe a short 2-week window that may not fit with the turnover of these proteins. We attempted to explore this is late stage neurons DIV18-21, but still found action potential generation and propagation to be present (supplementary Fig. 4). There were small changes in the shape of the action potential during repolarization, but initiation at the AIS was clearly unperturbed.
Despite the fact that all of our results are from an in vitro system we believe that it provides a good conceptual framework for axogenesis and formation of the AIS. Because in vitro cultured hippocampal neurons have originally already formed neuronal polarity before dissected, it might still retain information for axogenesis and in a sense, axon regeneration. Our molecular manipulation demonstrates that AP-2 has a critical role in the early developmental window of axogenesis and maturation of the AIS.
Methods
Cell culture and immunofluorescence
Hippocampal CA3-CA1 regions were dissected from 0–1 day old Sprague Dawley rats, dissociated, and plated onto poly-ornithine-coated glass for 7–9 or 14–20 days as previously described24. Animal treatment was carried out in accordance with the Animal Care and Use Guidelines by Kyung Hee University. All experiments were approved by the Animal Care Committee of the Kyung Hee University. To make early stage knock down of AP-2, shRNA-μ2 with other plasmids were transfected 36–48 hours after plating. Neurons were fixed 7 days in vitro (DIV) after plating (5 days after transfection). To make late stage knock down of AP-2, constructs were transfected 7–8 days after plating and were fixed 14–16 DIV after plating (6–7 days after transfection). Neurons were fixed with 4% paraformaldehyde and permeabilized with 0.2% Triton X-100, blocked with 5% BSA, and subsequently incubated with appropriated primary antibodies [anti-GFP (invitrogen), anti-α-adaptin (ABR) (subunit of AP-2), anti-MAP2 (Millipore), anti-ankyrin G (Neuromab)]. Alexa 488 or Alexa 546 conjugated secondary antibodies (Invitrogen) were applied in primary antibody incubated samples with different color combinations as needed.
Optical Setup and imaging
For immunofluorescence, images of fixed cells were acquired with using an epi-fluorescence microscope through a 20x (0.75 NA) or 40x (1.3 NA) Fluar Zeiss objective a recorded on an EMCCD camera (Andor iXon +, Model # DU-897E-BV) or PL APO 63X (1.32 NA) Leica objective (Leica DMRBE) with CoolSNAP HQ camera (Photometrics) driven by Metamorph software. Images of entire neurons were obtained by tiling images of several different fields of view with partial overlap and stitched together using Image J plugin (Stitching 2D, http://pacific.mpi-cbg.de/wiki/index.php/Stitching_2D/3D).
For action potential waveform imaging, specimens of Quasar transfected neurons were illuminated by a 637 nm laser 140 mW (Coherent OBIS Laser) with HQ620/60x and 660LP dichroic (Chroma) through a 40 × 1.3NA Zeiss Fluar Objective and a custom beam-expander to provide a ~25 μm diameter spot with a final power density of ~1850 W/cm2. Archaerhodopsin fluorescent emission was collected through a HQ700/75 m filter (Chroma) and captured with an IXON3 897 Ultra camera (Andor) in a cropped sensor mode (10 MHz readout, 500 ns pixel shift speed) to achieve 1 kHz frame rate imaging (exposure time of 985 μs) over an 85 × 45 pixel area with the use of an intermediate image plane mask (Optomask, Cairn Research distributed by Andor) to prevent light exposure of non-relevant pixels. Light was collected through an HQ700/75 m filter (Chroma). All voltage imaging experiments were carried out at 34 °C.
For presynaptic terminal live imaging for synaptic vesicle release and Ca2+ entry, constructs (vG-pH with/without shRNA-μ2 or synaptophysin-GCaMP6f with/without shRNA-μ2) were transfected 1.5~2 days or 8 days after plating. Experiments were carried out either 7–8 DIV for young neurons or 14–20DIV for mature neurons after plating. Coverslips were mounted in a stimulation chamber with laminar-flow perfusion on the stage of a custom-built laser-illuminated epi-fluorescence microscope. Live images were acquired with an Andor iXon Ultra 897 (Model # DU-897U-CS0-#BV) back-illuminated EM CCD camera. A diode pumped OBIS 488 laser (Coherent) shuttering by synchronizing TTL on/off signal from EMCCD camera during acquisition was utilized as a light source. Fluorescence excitation/emission and collection was achieved using a 40x (1.3 NA) Fluar objective lens using 500–550 nm emission and 498 nm dichroic filters (Chroma) for pHluorin and GCaMP6f. Action potentials were evoked by passing 1 ms current pulse through platinum-iridium electrodes form an isolated current stimulator (World Precision Instruments). Neurons were perfused in a saline based buffer containing (in mM) 119 NaCl, 2.5 KCl, 2 CaCl2, 2 MgCl2, 25 HEPES, 30 glucose, 10 μM 6-cyano-7nitroquinoxaline-2,3-dione (CNQX), and 50 μM D,L-2-amino-5-phosphonovaleric acid (AP5) (adjusted to pH 7.4). Temperature was clamped at 30 °C to remove effect from temperature fluctuation. All chemicals were purchased from Sigma, unless noted. NH4Cl solution applications were done with 50 mM NH4Cl in substitution of 50 mM of NaCl (pH7.4). Imaging for vG-pH, vG-pH with/without shRNA-μ2 transfected neurons were stimulated for 10 s at 10 Hz. Subsequently, NH4Cl was applied to measure total synaptic vesicle pool. Images were acquired at 2 Hz by exposing 50 ms. Imaging for Physin-GCaMP6f, Physin-GCaMP6f with/without shRNA-μ2 transfected neurons were stimulated with 1 AP with 2 mM external Ca2+. Imaging of Ca2+ influx by 1AP was recorded at 100 Hz by exposing 9.73 ms in frame transfer mode. Ionomycin 200 μM (Alomone Labs) was applied to record maximum fluorescence of Physin-GCaMP6f at the end of each experiment.
Image analysis
Image J was utilized for all image analysis (http://rsb.info.nih.gov/ij). Counting and measurement of axonal length were performed using Image J plugin Neuron J.
(http://www.imagescience.org/meijering/software/neuronj), and sholl analysis was carried out using a Sholl analysis plugin (http://www-biology.ucsd.edu/labs/ghosh/software/). The longest axon directly originating from cell body was defined as the primary axon. Subsequent branches were designated in their order of appearance. Some images were displayed as inverted grey or pseudo-color to show neuronal morphology. To analyze the length of the AIS, axon containing an ankyrin G positive area was selected by line scan. The AIS was defined as the region over which the line-scanned signal was 5 times higher than standard deviation of the baseline of axon signal.
For analysis Quasar, Quasar images were also analyzed in Image J by using custom-written plugins (http://rsb.info.nih.gov/ij/plugins/time-series.html) as previously described34. Briefly, stimulation with 300 nM TTX present was used to isolate the field stimulation from AP signal, and was subtracted from our AP recordings at the soma and bouton. ΔF/F values of the AP were calculated after background subtraction. Measurements of background were measured using a square 10 × 10 pixel ROI. Automated peak and FWHM analyses were carried out using Origin Pro 8′s (Northampton, MA) peak fitting algorithm.
For analysis pHluorin and GCaMP6f, we followed previously described methods31 with minor modifications. Images were analyzed using Image J with the plugin Time-series analyzer. Fluorescence traces were analyzed with Origin Pro 8.0. For synaptic vesicle release, vG-pH positive boutons are selected as a region of interest. The peak amplitude at 100AP (ΔF values of each 100AP responses) was taken and normalized by maximum value of NH4Cl (total synaptic vesicle pool). For Ca2+ entry, Physin-GCaMP6f positive boutons were used to mark ROIs. Amplitudes of 1AP response were taken by averaging 5–6 trials. The peak values are normalized by peak of ionomycin fluorescence. Silent boutons were defined as previously described that boutons where the vG-pH ΔF 100 or Physin-GCaMP6f ΔF 1AP was smaller than one standard deviation of the baseline before stimulation.
For statistical analysis, either one-way ANOVA (over three samples) or student t-test (two samples) was applied. Data are presented as means ± S.E.M (standard error of mean).
Molecular biology and others
shRNA against μ2 and HA-μ2 plasmids resistant to shRNA μ2 were constructed as previously described24. 60-mers oligonucleotides containing rat μ2 subunit cDNA target sequences (GTGGATGCCTTTCGCGTCA) of AP-2 were synthesized, annealed, and ligated into pSUPER vector according to manufacturer’s instruction. HA-μ2 construct was generated using Quick Change Site-Directed mutagenesis (Stratagene). The target sequence for shRNA μ2 was mutated to GTGGACGCTTTCCGGGTCA without the amino acid sequence change. EGFP-C2 is from clontech, GFP-Tau, VMAP2-GFP, GFP-synapsin-1, Nav1.6-GFP was cloned as previously described52. GCaMP6f from Addgene30 is conjugated to synaptophysin.
Additional Information
How to cite this article: Kyung, J. W. et al. Adaptor Protein 2 (AP-2) complex is essential for functional axogenesis in hippocampal neurons. Sci. Rep. 7, 41620; doi: 10.1038/srep41620 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We would like to thank Dr. Pablo Ariel for valuable discussions. We also thank members of the Hoppa lab and The Kim lab for comments. This work is supported by Basic Science Research Program through the Nation Research Foundation of Korea (NRF) funded by the Ministry of science, ICT & Future Planning (NRF- 2016M3C7A1905072), and a grant from Kyung Hee Univeristy in 2016 (KHU-20160702) for S.H.K and The Brain Research Foundation (BRFSG-2015-05) for I.H.C and M.B.H (Chicago Illinois).
Footnotes
The authors declare no competing financial interests.
Author Contributions J.W.K. and I.H.C designed and performed experiments and analyzed data. S.L. performed experiments and analyzed data. W.K.S. and T.A.R designed and analyzed data. M.B.H. and S.H.K. analyzed data and co-wrote the paper.
References
- Yi J. J., Barnes A. P., Hand R., Polleux F. & Ehlers M. D. TGF-beta signaling specifies axons during brain development. Cell 142, 144–157 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S. et al. A Wnt-Frz/Ror-Dsh pathway regulates neurite outgrowth in Caenorhabditis elegans. PLoS Genet 6, doi: 10.1371/journal.pgen.1001056 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colon-Ramos D. A. Synapse formation in developing neural circuits. Curr Top Dev Biol 87, 53–79 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M. A. et al. Sorting and directed transport of membrane proteins during development of hippocampal neurons in culture. Proc Natl Acad Sci USA 98, 7051–7057 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini T. et al. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell 78, 409–424 (1994). [DOI] [PubMed] [Google Scholar]
- Adler C. E., Fetter R. D. & Bargmann C. I. UNC-6/Netrin induces neuronal asymmetry and defines the site of axon formation. Nat Neurosci 9, 511–518 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd T. et al. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell 92, 205–215 (1998). [DOI] [PubMed] [Google Scholar]
- Pappu K. S. & Zipursky S. L. Axon guidance: repulsion and attraction in roundabout ways. Curr Biol 20, R400–402 (2010). [DOI] [PubMed] [Google Scholar]
- Huber A. B. et al. Distinct roles for secreted semaphorin signaling in spinal motor axon guidance. Neuron 48, 949–964 (2005). [DOI] [PubMed] [Google Scholar]
- Hornberger M. R. et al. Modulation of EphA receptor function by coexpressed ephrinA ligands on retinal ganglion cell axons. Neuron 22, 731–742 (1999). [DOI] [PubMed] [Google Scholar]
- Kamiguchi H. The mechanism of axon growth: what we have learned from the cell adhesion molecule L1. Mol Neurobiol 28, 219–228 (2003). [DOI] [PubMed] [Google Scholar]
- Kamiguchi H. & Yoshihara F. The role of endocytic l1 trafficking in polarized adhesion and migration of nerve growth cones. J Neurosci 21, 9194–9203 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan C. W. et al. Vav family GEFs link activated Ephs to endocytosis and axon guidance. Neuron 46, 205–217 (2005). [DOI] [PubMed] [Google Scholar]
- Bean B. P. The action potential in mammalian central neurons. Nat Rev Neurosci 8, 451–465 (2007). [DOI] [PubMed] [Google Scholar]
- Lai H. C. & Jan L. Y. The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci 7, 548–562 (2006). [DOI] [PubMed] [Google Scholar]
- Vacher H. & Trimmer J. S. Trafficking mechanisms underlying neuronal voltage-gated ion channel localization at the axon initial segment. Epilepsia 53 Suppl 9, 21–31 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido J. J. et al. Identification of an axonal determinant in the C-terminus of the sodium channel Na(v)1.2. EMBO J 20, 5950–5961, doi: 10.1093/emboj/20.21.5950 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband M. N. The axon initial segment and the maintenance of neuronal polarity. Nat Rev Neurosci 11, 552–562 (2010). [DOI] [PubMed] [Google Scholar]
- Fache M. P. et al. Endocytotic elimination and domain-selective tethering constitute a potential mechanism of protein segregation at the axonal initial segment. J Cell Biol 166, 571–578 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier C., Brachet A., Fache M. P. & Dargent B. Voltage-gated sodium channel organization in neurons: protein interactions and trafficking pathways. Neurosci Lett 486, 92–100 (2010). [DOI] [PubMed] [Google Scholar]
- Staruschenko A., Pochynyuk O. & Stockand J. D. Regulation of epithelial Na+ channel activity by conserved serine/threonine switches within sorting signals. J Biol Chem 280, 39161–39167 (2005). [DOI] [PubMed] [Google Scholar]
- Wang H. et al. Clathrin-mediated endocytosis of the epithelial sodium channel. Role of epsin. J Biol Chem 281, 14129–14135 (2006). [DOI] [PubMed] [Google Scholar]
- Lu J. et al. Postsynaptic positioning of endocytic zones and AMPA receptor cycling by physical coupling of dynamin-3 to Homer. Neuron 55, 874–889 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H. & Ryan T. A. Synaptic vesicle recycling at CNS snapses without AP-2. J Neurosci 29, 3865–3874 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H. & Ryan T. A. A distributed set of interactions controls mu2 functionality in the role of AP-2 as a sorting adaptor in synaptic vesicle endocytosis. J Biol Chem 284, 32803–32812 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A. M. & Banker G. Neuronal polarity. Annu Rev Neurosci 17, 267–310 (1994). [DOI] [PubMed] [Google Scholar]
- Deborde S. et al. Clathrin is a key regulator of basolateral polarity. Nature 452, 719–723, doi: 10.1038/nature06828 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsunari T. et al. Clathrin adaptor AP-2 is essential for early embryonal development. Mol Cell Biol 25, 9318–9323 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan S. & Ryan T. A. Real-time measurements of vesicle-SNARE recycling in synapses of the central nervous system. Nat Cell Biol 2, 197–204 (2000). [DOI] [PubMed] [Google Scholar]
- Chen T. W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H. & Ryan T. A. Balance of Calcineurin Aalpha and CDK5 Activities Sets Release Probability at Nerve Terminals. J Neurosci 33, 8937–8950 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R. & Neher E. Presynaptic calcium and control of vesicle fusion. Curr Opin Neurobiol 15, 266–274 (2005). [DOI] [PubMed] [Google Scholar]
- Kim S. H. & Ryan T. A. CDK5 serves as a major control point in neurotransmitter release. Neuron 67, 797–809 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppa M. B., Gouzer G., Armbruster M. & Ryan T. A. Control and plasticity of the presynaptic action potential waveform at small CNS nerve terminals. Neuron 84, 778–789 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochbaum D. R. et al. All-optical electrophysiology in mammalian neurons using engineered microbial rhodopsins. Nat Methods 11, 825–833 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D. et al. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J Cell Biol 143, 1295–1304 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedstrom K. L., Ogawa Y. & Rasband M. N. AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. J Cell Biol 183, 635–640 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. L., Korobova F. & Svitkina T. Axon initial segment cytoskeleton comprises a multiprotein submembranous coat containing sparse actin filaments. J Cell Biol 205, 67–81 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes A. P. & Polleux F. Establishment of axon-dendrite polarity in developing neurons. Annu Rev Neurosci 32, 347–381 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser A. et al. An ankyrinG-binding motif is necessary and sufficient for targeting Nav1.6 sodium channels to axon initial segments and nodes of Ranvier. J Neurosci 32, 7232–7243 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender K. J. & Trussell L. O. The physiology of the axon initial segment. Annu Rev Neurosci 35, 249–265, doi: 10.1146/annurev-neuro-062111-150339 (2012). [DOI] [PubMed] [Google Scholar]
- Jenkins S. M. & Bennett V. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J Cell Biol 155, 739–746 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X. & Shrager P. Dependence of axon initial segment formation on Na+ channel expression. J Neurosci Res 79, 428–441 (2005). [DOI] [PubMed] [Google Scholar]
- Akin E. J., Sole L., Dib-Hajj S. D., Waxman S. G. & Tamkun M. M. Preferential targeting of Nav1.6 voltage-gated Na+ Channels to the axon initial segment during development. PLoS One 10, e0124397 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao D., Ehrlich M., Henis Y. I. & Leof E. B. Transforming growth factor-beta receptors interact with AP2 by direct binding to beta2 subunit. Mol Biol Cell 13, 4001–4012 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almuedo-Castillo M., Salo E. & Adell T. Dishevelled is essential for neural connectivity and planar cell polarity in planarians. Proc Natl Acad Sci USA 108, 2813–2818 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu A. et al. Association of Dishevelled with the clathrin AP-2 adaptor is required for Frizzled endocytosis and planar cell polarity signaling. Dev Cell 12, 129–141 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu A., Xing Y., Harrison S. C. & Kirchhausen T. Structural analysis of the interaction between Dishevelled2 and clathrin AP-2 adaptor, a critical step in noncanonical Wnt signaling. Structure 18, 1311–1320 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason A. K., Jacobs B. E. & Welling P. A. AP-2-dependent internalization of potassium channel Kir2.3 is driven by a novel di-hydrophobic signal. J Biol Chem 283, 5973–5984 (2008). [DOI] [PubMed] [Google Scholar]
- Gu M. et al. AP2 hemicomplexes contribute independently to synaptic vesicle endocytosis. Elife 2, e00190 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry J. et al. Ankyrin-G directly binds to kinesin-1 to transport voltage-gated Na+ channels into axons. Dev Cell 28, 117–131 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser A. et al. Two Nedd4-binding motifs underlie modulation of sodium channel Nav1.6 by p38 MAPK. J Biol Chem 285, 26149–26161 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.