Abstract
There are large socioeconomic disparities in adult mortality in Russia, although the biological mechanisms are not well understood. With data from the study of Stress, Aging, and Health in Russia (SAHR), we use Gompertz hazard models to assess the relationship between educational attainment and mortality among older adults in Moscow and to evaluate biomarkers associated with inflammation, neuroendocrine function, heart rate variability, and clinical cardiovascular and metabolic risk as potential mediators of that relationship. We do this by assessing the extent to which the addition of biomarker variables into hazard models of mortality attenuates the association between educational attainment and mortality. We find that an additional year of education is associated with about 5% lower risk of age-specific all-cause and cardiovascular mortality. Inflammation biomarkers are best able to account for this relationship, explaining 25% of the education-all-cause mortality association, and 35% of the education-cardiovascular mortality association. Clinical markers perform next best, accounting for 13% and 23% of the relationship between education and all-cause and cardiovascular mortality, respectively. Although heart rate biomarkers are strongly associated with subsequent mortality, they explain very little of the education-mortality link. Neuroendocrine biomarkers fail to account for any portion of the link. These findings suggest that inflammation may be important for understanding mortality disparities by socioeconomic status.
Keywords: Russia, socioeconomic status, biomarkers, mortality, aging, inflammation, education
Background
Mortality in Russia
Russia faces a heavy burden of premature mortality. Adult mortality increased between the 1960s and 1980s, then fluctuated significantly with further increases until the mid-2000s (Shkolnikov et al., 2004a). The worst of what we now call the mortality crisis occurred in 1994, when life expectancy at birth fell to just 57 for men and 71 for women (Brainerd and Cutler, 2005). Russia’s mortality trend was a dramatic departure from the improvements seen in much of the rest of the world, leading to a deepening of the “mortality divide” between Eastern and Western Europe (Andreev et al., 2003; McMichael et al., 2004; Moser et al., 2005). Compared to Western Europe, life expectancy in Russia in 1994 was 16 years lower for men and 10 years lower for women (European Health for All Database, 2015). After 2004, Russia experienced substantial progress, with life expectancy reaching 65 for men and 75 for women in 2013 (Rosstat, 2015; Shkolnikov et al., 2013). Despite these recent improvements, life expectancies for Russian men and women remain 11–12 years and 8–9 years lower respectively than in Western Europe.
Adult mortality in Russia is characterized by extremely high mortality rates at working ages (15–60 years) and high mortality at older ages, particularly among men. Excess mortality at working ages is largely attributable to accidents and violence, alcohol-related causes, and premature cardiovascular mortality (Leon et al., 2010), while cardiovascular disease is largely responsible for excess mortality at older ages (Meslé, 2004; Powles et al., 2005; Shkolnikov et al., 2013). A reduction in cardiovascular disease mortality played a major role in the overall mortality decline in many countries, but cardiovascular mortality in Russia has remained high, with reductions occurring only very recently (Grigoriev et al., 2014; Levi et al., 2009).
Socioeconomic differentials in health have not been studied extensively in Russia, due in part to a prohibition against examining health disparities during the Soviet era (Shkolnikov et al., 1998). Nevertheless, there are some studies showing that socioeconomic disparities in mortality within Russia are at least as large as—and probably larger than—within western countries (Malyutina et al., 2004; Shkolnikov et al., 2004b, 1998). Increasing mortality between the 1970s and the 1990s was concentrated in manual workers and those with low levels of education (Andreev et al., 2009; Shkolnikov et al., 2004b).
Both macro- and micro-level evidence suggest that alcohol, particularly binge and hazardous drinking, is responsible for a large part of excess Russian mortality among young and middle-aged adults, especially men, and may partly explain the socioeconomic differentials (Leon et al., 1997; Shkolnikov et al., 2004a; Zaridze et al., 2014; Tomkins et al., 2012). Alcohol consumption increases mortality from a range of causes, including external causes (e.g., accidents, homicide, suicide), acute internal causes (e.g., alcohol poisoning, sudden cardiac arrest), and chronic internal causes (e.g., hypertension, stroke, cirrhosis) (Leon et al., 2010).
It is well established that in Russia as in other countries excess mortality and socioeconomic differences in mortality continue into old age (Hoffmann, 2008; Huisman et al., 2004; Shkolnikov et al., 2008; Wolfson et al., 1993), though it is not clear if the causes implicated for younger adults are equally important in this age group. Among older adults, heavy drinking is rare and unexpected deaths from external and acute conditions are much less frequent; instead, most deaths are related to chronic cardiovascular disease. This fact led researchers in the past few decades to collect clinical measures of cardiovascular disease risk, such as blood pressure, cholesterol, obesity and smoking. However, these measures were unable to fully explain excess Russian mortality or the mortality disparities by educational attainment (Averina et al., 2003; Dennis et al., 1993; Ginter, 1995; Kuulasmaa et al., 2000; Sidorenkov et al., 2010).
Nevertheless, if socioeconomic status influences physical disease, it must act on some physiological process (Steptoe and Marmot, 2002), and evidence of this dysfunction may be detectable before death. Can preexisting morbidity or risk factors explain socioeconomic disparities in mortality at older ages? We address this question by studying a broad set of biomarkers that may be driving mortality disparities among older Russians.
Mortality prediction from biomarkers
In recent years, a small but growing number of population-based studies have begun collecting a wide range of biomarkers with the intention of improving mortality prediction and our understanding of the biological underpinnings of socioeconomic disparities in health (Crimmins et al., 2010). The study on Stress, Aging and Health in Russia (SAHR) is one such study. The extensive biological data collected in SAHR provide an opportunity to compare biomarkers of health and disease not typically collected in population-based studies of health and aging. In addition to standard clinical cardiovascular and metabolic risk factors, SAHR collects measures of heart rate variability, an indicator of cardiovascular function; inflammation, a key aspect of the immune system; and neuroendocrine function, which directs the body’s stress response. In this study, we examine nine clinical measures of cardiovascular and metabolic function, four measures of heart rate function, three biomarkers of inflammation, and four markers of neuroendocrine function. A brief description of each biomarker is shown in Table 1.
Table 1.
Biomarker index components
| Biomarker | Description | High-risk definition |
|---|---|---|
| Clinical cardiovascular and metabolic markers | ||
| Systolic blood pressure | The maximum arterial blood pressure during each heartbeat. |
> 140 mmHG |
| Diastolic blood pressure | The minimum arterial blood pressure during each heartbeat. |
> 90 mmHG |
| Total cholesterol | A measure of cholesterol that includes low- density lipoprotein (LDL, or “bad” cholesterol) and high-density lipoprotein HDL, or “good” cholesterol). Excess cholesterol contributes to plaques in blood vessels. |
>= 240 mg/dL |
| High-density lipoprotein (HDL) cholesterol | “Good” cholesterol; helps remove cholesterol from arteries. |
< 40 mg/dL |
| Triglycerides | A lipid widespread in adipose tissue, circulates in the blood as lipoproteins. |
>= 200 mg/dL |
| Glycosylated hemoglobin | A protein created when hemoglobin binds to glucose. A measure of blood glucose levels over the past several months. |
> 6.5% |
| Insulin resistance | Estimated from the homeostatic model assessment (HOMA-IR). Derived from fasting blood glucose levels. |
Highest sex-specific quintile |
| Body mass index (BMI) | Weight in kilograms / height in meters squared. A measure of body fat. |
> 30 or <18.5 |
| Waist circumference | Waist circumference in centimeters. A marker of abdominal fat. |
> 102 cm for men > 88 cm for women |
| Heart rate variability markers | ||
| Mean heart rate | Mean heart rate over 24 hours (beats per minute). |
Highest sex-specific quintile |
| Ratio of daytime to nighttime heart rate | Ratio of mean daytime heart rate (8:00 am – 8:00 pm) to mean nighttime heart rate (12:00 am - 4:00 am). |
Lowest sex-specific quintile |
| Standard deviation of normal beat-to-beat intervals (SDNN) |
Standard deviation of all normal intervals over 24 hours. Measure of longer term variability. |
< 100 ms |
| Root mean square of successive differences in normal beat-to-beat intervals (RMSSD) |
The square root of the mean of the squares of successive differences between adjacent normal heart beats. Measure of shorter term variability. |
Lowest sex-specific decile |
| Inflammation markers | ||
| C-reactive protein | Protein that is part of the inflammatory response. Increases in response to interleukin-6. |
> 3 mg/L |
| Interleukin-6 | Inflammatory cytokine, promotes production of inflammatory proteins. |
Highest sex-specific quintile |
| Fibrinogen | Protein that assists in blood clot formation. Increases as part of the inflammatory response. |
Highest sex-specific quintile |
| Neuroendocrine markers | ||
| Cortisol | Stress hormone produced by the adrenal gland. | Highest sex-specific quintile |
| Dehydroepiandrosterone sulfate (DHEAS) | Steroid hormone produced by the adrenal gland. May counterbalance the effect of cortisol. |
Lowest sex-specific quintile |
| Epinephrine | Hormone produced by the adrenal gland. Part of the fight-or-flight response. |
Highest or lowest sex-specific decile |
| Norepinephrine | Hormone produced by the adrenal gland. Precursor to epinephrine. |
Highest sex-specific quintile |
Note: Each biomarker risk index represents a count of the number of biomarkers for which a participant falls in the high-risk category.
Elevated heart rate has been shown to predict all-cause and cardiovascular mortality in population-based studies in Japan (Fujiura et al., 2001), Finland (Reunanen et al., 2000), and the United States (Kannel et al., 1987). Low heart rate variability is associated with increased cardiovascular risk (Dekker et al., 2000) and all-cause mortality in population-based samples (Dekker et al., 1997; Stein and Kleiger, 1999; Tsuji et al., 1994). However, another population-based study found mixed results, depending on the measure of heart rate variability and outcome examined (Hansen et al., 2008).
Heart rate variability is easy to collect in a hospital setting, but reproducibility and prognostic value are improved with 24 hours of monitoring in ordinary circumstances (Palatini et al., 2000). Six-lead Holter monitors worn by SAHR participants collected heart rate and detailed electrocardiogram data over 24 hours as they went about their daily life (Shkolnikova et al., 2009). Such data on heart rate variability are rarely collected in population-based studies.
There is only limited evidence on the social determinants of heart rate variability. Marmot and Steptoe (2002) found that job strain, characterized by high demand-low control work environments, is associated with reduced heart rate variability; Amelsvoort et al.(2000) found lower variability among shift workers as compared to day workers; and Shishehbor et al. (2006) found that worse neighborhood socioeconomic conditions (measured as average income and educational attainment) are associated with reduced variability.
Although the acute inflammatory response is an essential defense against injury and illness, chronic, low-grade inflammation is believed to be maladaptive and to contribute to a number of chronic diseases of aging, including cardiovascular disease (Bruunsgaard et al., 2003; De Martinis et al., 2006; Franceschi and Campisi, 2014; Pawelec et al., 2014). Suggestive evidence has led researchers to argue that atherosclerosis is best understood as an inflammatory disease. For example, some genetic studies have linked polymorphisms related to inflammation with cardiovascular disease risk (Hansson et al., 2006), and elevated levels of the inflammatory molecule C-reactive protein have been used in studies since the 1990s as an indicator of cardiovascular risk (Ridker et al., 2000; von Haehling et al., 2009). These studies, together with accumulating evidence of an association between SES and inflammation (see, e.g., Steptoe, 2012 for a review), suggest a mediating role for inflammation in the association between SES and cardiovascular health (Aiello and Kaplan, 2009).
However, it remains unclear whether inflammation represents an independent causal pathway to cardiovascular disease (Danesh and Pepys, 2009; Jialal et al., 2004; Lagrand et al., 1999; von Haehling et al., 2009). This uncertainty is underscored by findings linking C-reactive protein—the most-studied inflammatory biomarker—to other cardiovascular risk factors, suggesting the possibility of confounding or reverse causality. For example, a randomized trial found that statin drugs used on patients with high C-reactive protein and normal cholesterol levels lowered the risk of negative cardiovascular outcomes (Ridker et al., 2008), but because statins lower both C-reactive protein and cholesterol, it is not clear that C-reactive protein was responsible (Libby et al., 2011). Adipose tissue promotes inflammation, so high levels of C-reactive protein may reflect metabolic risk (Hansson, 2005; Hansson et al., 2006). C-reactive protein has been found to be associated with elevated heart rate and reduced heart-rate variability, leading authors to speculate a common etiology (Sajadieh et al., 2004).
The neuroendocrine system has been hypothesized to propagate mortality disparities via psychosocial stress. The underlying idea is that excess stress causes chronic wear and tear on the neuroendocrine system; neuroendocrine dysregulation, in turn, can disrupt numerous physiological systems, including the immune and cardiovascular systems (McEwen, 2012). Evidence supports the link between neuroendocrine dysfunction and mortality: high levels of cortisol (Gruenewald et al., 2006; Turra et al., 2005) and norepinephrine (Katayama et al., 2004; Reuben et al., 2000) have been found to be associated with all-cause mortality, as have low levels of dehydroepiandrosterone sulfate (DHEAS; Mazat et al., 2001; Roth et al., 2002) and extreme values (both high and low) of epinephrine (Goldman et al., 2009; Turra et al., 2005). To the extent that psychosocial stress is correlated with socioeconomic status, neuroendocrine dysfunction could represent a biological pathway to health disparities (Adler and Snibbe, 2003).
In this study, we compare standard clinical markers of cardiovascular and metabolic risk with biomarkers of heart rate variability, inflammation, and neuroendocrine function to determine the extent to which these markers account for disparities in mortality by socioeconomic status.
Data and method
Data
The study on Stress, Aging, and Health in Russia (SAHR) is a prospective population-based study of Moscow residents aged 55 and older (Shkolnikova et al., 2009). SAHR was conducted jointly by the State Research Center for Preventive Medicine (Moscow, Russia), the Max Planck Institute for Demographic Research (Rostock, Germany) and Duke University (Durham, United States). Study participants were randomly selected from seven previous epidemiological cohorts (the Lipid Research Clinics (LRC) and the multinational MONItoring of trends and determinants in CArdiovascular disease (MONICA) cohorts) constructed between the 1970s and 1990s. Since these cohorts included only residents of Moscow before the mid-1980s, a small number of additional participants representing those who moved to Moscow after 1985 were identified from the Moscow Outpatient Clinics’ registry.
The baseline survey was fielded between 2006 and 2009. It included 1,800 participants who were interviewed and participated in a medical examination (response rate = 66%). Interviews and examinations were administered at the hospital; only participants unable or unwilling to come to the hospital were interviewed at home.
The biomedical data collected at baseline include a rich array of biomarkers: anthropometry, blood pressure measurements, routine blood tests and blood biochemistry from a venous blood sample, concentrations of stress hormones in urine samples, and characteristics of heart function from a 24-hour electrocardiogram (EKG). Anthropometry was measured using a calibrated set of measurement tools. Blood pressure was the average of three manual measures. Venous blood was taken after an overnight fast. Participants collected a twelve-hour overnight urine sample before performing the medical examination. The 24-hour EKG was measured via a small, wearable three-channel Schiller Holter monitor, which allowed for dynamic measurement as a respondent went about his or her day.
Mortality follow-up is ongoing; the most recent mortality update includes deaths through January 31, 2014. Deaths were verified in residential registers.
SAHR was approved by the Ethics committee of the State Research Centre of Preventative Medicine and by the institutional review board at Duke University. All participants provided informed consent prior to data collection. See Shkolnikova et al. (2009) for more details on SAHR.
Measures
We use years of educational attainment as a measure of SES, for reasons related to both SES measurement in general, and SES measurement in Russia in particular. First, because educational attainment is determined relatively early in adulthood, it may better represent a stable measure of lifetime SES, and may be more accurately reported than income or wealth. Given the age of the study sample, with many retired respondents, income and occupation may be less informative than for a working age population.
Second, education is preferable to income and occupation as an indicator of SES in Russia specifically; this preference is reflected in most epidemiological and public health studies linking mortality and health with socioeconomic status in Russia (Dennis et al., 1993; Glei et al., 2013a; Metelskaya et al., 2012; Shkolnikov et al., 2004b, 1998). Income measurement in Russia has been more challenging than in other developed countries. Income differentials have fluctuated in Russia, with smaller differentials in the Soviet era and much larger differentials post-transition (Gerber and Hout, 1998; Shkolnikov et al., 1998). The hyperinflation and economic instability that characterized the post-Soviet era encouraged substantial cash-based non-declared payments, making income figures unreliable (Perlman and Bobak, 2008). Additionally, while the effect of education is typically believed to act partly via income and/or occupation, this is less likely to be the case in Russia. Income returns to education are lower and the link between a prestigious occupation and high income is weaker in Russia versus the West (Bessudnov et al., 2011; Gerber, 2000; Gerber and Hout, 1998; Shkolnikov et al., 1998).
The biomarkers used in this study are briefly described in Table 1. Urinary cortisol, epinephrine, and norepinephrine are measured per gram of creatinine to adjust for urine concentration. Laboratory methods are outlined in Appendix section A1; for more information, see Shkolnikova et al. (2009) and Glei et al. (2013a).
We combine the biomarkers in each category (clinical, heart rate, inflammation, neuroendocrine) into a high-risk index representing a count of biomarkers for which a respondent falls into the high-risk range (Seeman et al., 2004; Seplaki et al., 2005). Following Glei et al. (2013a), we define high-risk with established cut-points when possible. Because many of the biomarkers do not have clinical high-risk cut-points, we use being in the sex-specific highest risk 20% (upper or lower quintile depending on the particular marker) to define high risk for these markers; for markers where both extremes are believed to be high risk, we use the highest and lowest sex-specific deciles. (Results are robust to an alternative index calculation based on z-scores; see Appendix Table A.2.) Table 1 lists the high-risk definition for each biomarker.
Analytic strategy
Limiting the sample to respondents with non-missing information on demographic characteristics and biomarkers yields a sample of 1,604 (Table 2). On average, those with missing biomarkers are older (71 vs. 68 years old, p < 0.001), slightly less educated (13 vs. 14 years of schooling, p < 0.001), and more likely to have died by follow-up (31% vs. 16%, p < 0.001). Results are robust to using multiple imputation instead of casewise deletion (see Appendix Table A.3).
Table 2.
Creating the analytic sample
| Total number of respondents | 1,800 |
|---|---|
| Respondents with information missing on: | |
| Sex | 0 |
| Age | 0 |
| Education (degree) | 0 |
| Education (years) | 8 |
| Mortality status | 0 |
| Inflammation index | 34 |
| Cardiovascular index | 42 |
| Heart rate variability index | 70 |
| Neuroendocrine index | 72 |
| Any biomarker index | 191 |
| Respondents in analytic sample | 1,604 |
We estimate four models to assess the biomarker indexes as mediators of the education-mortality relationship. First, we examine whether education predicts age-specific mortality. Second, we test whether biomarker indexes are associated with education in ordinary least squares (OLS) regression models. Third, we study the relationship between the indexes and mortality. Finally, we assess whether the indexes account for the relationship between education and mortality by comparing the coefficient on education in hazard models that do and do not include each index as a predictor. We use Gompertz hazard models, which assume the natural log of the hazard increases linearly with age; this is testable by examining the models’ gamma parameters, which describe the shape of the baseline hazard function. The Gompertz functional form is a well-known approximation of mortality after age 40 (Horiuchi and Coale, 1982). Our findings are robust to using a Cox specification (results not shown).
Because cardiovascular disease is most clearly implicated in pathways associated with the biomarkers examined, we additionally analyze cause-specific models of cardiovascular disease-related death. Furthermore, cardiovascular mortality excludes causes of death (such as accidents and violence) unlikely to be powerfully influenced by biomarkers. For these reasons, we hypothesize that the relationship between education and cardiovascular mortality will be stronger than the relationship between education and all-cause mortality. In these models, respondents who died from causes other than cardiovascular disease are treated as censored as of the date of death. Cause of death is based on the International Classification of Disease (eighth revision) group codes. Deaths resulting from ischemic heart disease, cerebrovascular causes, and other circulatory diseases are considered cardiovascular deaths. All analyses are conducted in Stata (version 12.1; StataCorp., College Station, TX) .
Results
Table 3 shows descriptive statistics of the analytic sample. By January 31, 2014, 252 respondents had died (about 16% of the total sample; 23% of males, and 9% of females). As expected, cardiovascular disease is the most common cause of death, accounting for 147 of the 252 deaths (not shown). Neoplasms account for another 66 deaths. Eight individuals died from external causes, 20 from other causes, and 11 from unknown causes.
Table 3.
Demographic and biological characteristics of the analytic sample
| Full sample | Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean/ Prop. | SD | Median | Mean/ Prop. |
SD | Median | Mean/ Prop. |
SD | Median | |
| Demographic characteristics | |||||||||
| Male (%) | 45.9% | -- | -- | 100.0% | -- | -- | 0.0% | -- | -- |
| Age (years) | 67.95 | 7.56 | 68.00 | 68.62 | 8.06 | 68.00 | 67.39 | 7.07 | 68.00 |
| 55–59 | 15.3% | -- | -- | 15.4% | -- | -- | 15.3% | -- | -- |
| 60–64 | 20.9% | -- | -- | 20.1% | -- | -- | 21.5% | -- | -- |
| 65–69 | 21.9% | -- | -- | 18.8% | -- | -- | 24.7% | -- | -- |
| 70–74 | 21.8% | -- | -- | 20.2% | -- | -- | 23.2% | -- | -- |
| 75–79 | 11.2% | -- | -- | 14.9% | -- | -- | 7.9% | -- | -- |
| 80 + | 8.9% | -- | -- | 10.6% | -- | -- | 7.4% | -- | -- |
| Education, completed years | 14.02 | 3.53 | 15.00 | 13.90 | 3.83 | 15.00 | 14.13 | 3.26 | 15.00 |
| Mortality | |||||||||
| Died by January 31, 2014 (%) | 15.7% | -- | -- | 23.1% | -- | -- | 9.4% | -- | -- |
| Biomarker risk indexes | |||||||||
| Clinical index (possible range: 0–9) | 2.52 | 1.81 | 2.00 | 2.36 | 1.83 | 2.00 | 2.64 | 1.78 | 2.00 |
| Heart rate index (possible range: 0–4) | 0.69 | 0.95 | 0.00 | 0.71 | 0.97 | 0.00 | 0.67 | 0.94 | 0.00 |
| Inflammation index (possible range: 0–3) | 0.71 | 0.85 | 0.00 | 0.71 | 0.84 | 0.00 | 0.72 | 0.85 | 1.00 |
| Neuroendocrine index (possible range: 0–4) | 0.79 | 0.85 | 1.00 | 0.77 | 0.86 | 1.00 | 0.80 | 0.85 | 1.00 |
| Inflammation biomarkers | |||||||||
| C-reactive protein (mg/L) | 3.31 | 5.26 | 1.70 | 3.49 | 5.92 | 1.60 | 3.16 | 4.63 | 1.75 |
| Interleukin-6 (pg/L) | 1.53 | 5.30 | 0.05 | 1.74 | 5.29 | 0.05 | 1.35 | 5.31 | 0.05 |
| Fibrinogen (g/L) | 3.84 | 0.82 | 3.75 | 3.84 | 0.90 | 3.70 | 3.84 | 0.75 | 3.78 |
| Neuroendocrine biomarkers | |||||||||
| Dehydroepiandrosterone sulfate (micromol/L) | 2.48 | 1.90 | 1.90 | 3.05 | 2.21 | 2.51 | 2.00 | 1.43 | 1.60 |
| Cortisol (microgram/ g creatinine) | 30.74 | 24.35 | 24.27 | 31.03 | 23.14 | 25.96 | 30.49 | 25.35 | 22.68 |
| Epinephrine (microgram/ g creatinine) | 8.24 | 7.43 | 6.38 | 7.08 | 7.18 | 5.27 | 9.22 | 7.51 | 7.18 |
| Norepinephrine (microgram/ g creatinine) | 37.98 | 33.85 | 29.17 | 32.91 | 29.83 | 25.19 | 42.29 | 36.38 | 33.10 |
| Heart rate biomarkers | |||||||||
| Mean heart rate (bpm) | 74.28 | 9.47 | 74.00 | 73.52 | 10.30 | 72.00 | 74.92 | 8.67 | 75.00 |
| Ratio of mean daytime heart rate to mean nighttime heart rate | 1.32 | 0.13 | 1.31 | 1.31 | 0.13 | 1.31 | 1.32 | 0.12 | 1.32 |
| Standard deviation of normal beat-to-beat intervals (SDNN) (ms) | 142.23 | 36.15 | 141.36 | 142.31 | 37.81 | 142.09 | 142.17 | 34.71 | 139.74 |
| Root-mean-square of successive diffs in beat-to-beat intervals (RMSSD) | 26.24 | 21.37 | 21.10 | 25.91 | 20.25 | 20.47 | 26.51 | 22.29 | 21.67 |
| Cardiovascular biomarkers | |||||||||
| Systolic blood pressure (mmHG) | 142.08 | 23.39 | 140.00 | 144.96 | 23.75 | 142.00 | 139.64 | 22.80 | 138.00 |
| Diastolic blood pressure (mmHG) | 81.28 | 12.70 | 80.00 | 83.42 | 12.76 | 82.00 | 79.46 | 12.38 | 79.25 |
| Total cholesterol (mg/dL) | 5.96 | 1.18 | 5.90 | 5.60 | 1.11 | 5.57 | 6.26 | 1.15 | 6.20 |
| HDL cholesterol (mg/dL) | 1.28 | 0.32 | 1.24 | 1.21 | 0.31 | 1.16 | 1.34 | 0.32 | 1.32 |
| Triglycerides (mg/dL) | 1.35 | 0.73 | 1.20 | 1.32 | 0.70 | 1.16 | 1.37 | 0.75 | 1.25 |
| Glycosylated hemoglobin (%) | 6.02 | 0.90 | 5.90 | 5.99 | 0.88 | 5.80 | 6.05 | 0.91 | 5.90 |
| Insulin resistance (HOMA-IR) | 2.83 | 2.45 | 2.21 | 2.70 | 2.51 | 2.05 | 2.95 | 2.39 | 2.34 |
| Body mass index | 28.58 | 4.88 | 28.34 | 27.55 | 4.47 | 27.28 | 29.45 | 5.05 | 29.32 |
| Waist circumference (cm) | 93.06 | 12.51 | 93.00 | 96.44 | 12.57 | 96.00 | 90.18 | 11.72 | 90.00 |
| N | 1,604 | 736 | 868 | ||||||
Figure 1 shows Kaplan-Meier survival curves by three categories of educational attainment, based on the type of degree obtained. Those with the most education have greater age-specific survival probabilities than those with less education; a log rank test rejects the equality of the three survival curves (p≈0.004). Median remaining lifetime at age 55 is estimated as 23.1 years for those with no degree, 26.8 for those with a secondary degree, and 29.7 for those with a post-secondary degree. These estimates are conditional on surviving to at least age 55 (in order to be eligible for SAHR); thus, lifetime mortality disparities are almost certainly underestimated, since those with less education are less likely to survive to age 55.
Figure 1. Kaplan-Meier survival by educational attainment, all-cause mortality by January 31, 2014.
Note: Survival probabilities are conditional on living to age 55 or older. The observed survival period begins at baseline survey (between 2006 and 2009) and ends January 31, 2014.
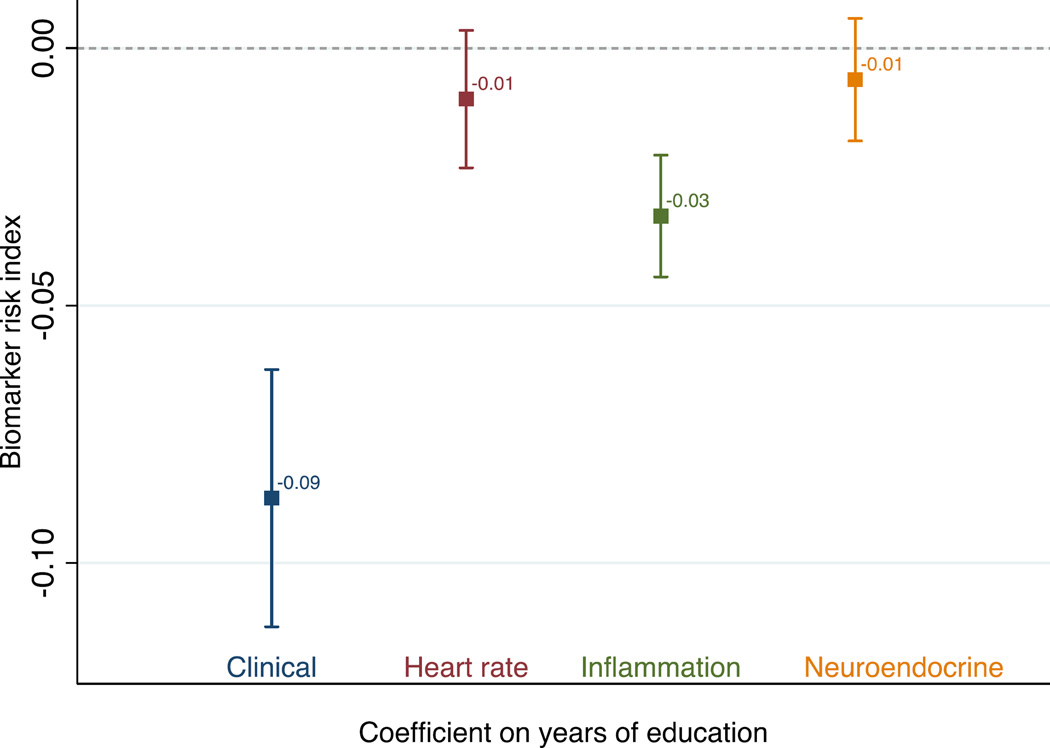
Figure 2 shows results from OLS regression models of the biomarker indexes. The figure presents the coefficients for years of education from separate OLS models of each biomarker index regressed on years of education, controlling for sex and age. The clinical and inflammation markers show a small but significant inverse relationship with education: an additional year of education is associated with having 0.09 (clinical) or 0.03 (inflammation) fewer biomarkers in the high-risk category. The associations between education and biomarkers of neuroendocrine function and heart rate variability are even smaller and not statistically significant.
Figure 2. Coefficient on years of education in four ordinary least squares regressions of each biomarker risk index on education.
Note: Shown are coefficients and 95% confidence intervals on education from four separate ordinary least squares regressions of the high-risk biomarker indexes. Models control for age and sex. See Table 1 for index definitions. N=1,604.
The results of survival models with biomarkers as the predictors are shown in Figure 3. Hazard ratios associated with the four biomarker indexes, from separate models, are shown. Having one additional heart rate or inflammation biomarker in the high-risk category is associated with a nearly 50% increase in the risk of mortality at any age. The neuroendocrine and clinical markers are also significantly associated with mortality, though less strongly. An additional high-risk neuroendocrine or clinical biomarker is associated with a 21% or 11%, respectively, increased risk of mortality.
Figure 3. Hazard ratios on biomarker indexes from proportional hazards Gompertz survival models.
Note: Shown are hazard ratios and 95% confidence intervals on each biomarker index from four separate Gompertz models. Models control for sex and age (implicitly). See Table 1 for index definitions. N=1,604.
Table 4 presents a series of survival models. Model 1 estimates age-specific mortality hazard ratios associated with years of education, controlling for sex. The four high-risk indexes are added to the model one at a time (Models 2–5); finally all indexes are considered in the same model (Model 6).
Table 4.
Survival models of age-specific all-cause mortality by January 31, 2014
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Years of education | 0.947 | (0.917, 0.977) | 0.954 | (0.923, 0.985) | 0.953 | (0.923, 0.983) | 0.960 | (0.930, 0.991) | 0.947 | (0.918, 0.978) | 0.968 | (0.936, 1.000) |
| Clinical index | 1.088 | (1.015, 1.167) | 1.036 | (0.965, 1.113) | ||||||||
| Heart rate index | 1.355 | (1.209, 1.518) | 1.321 | (1.178, 1.482) | ||||||||
| Inflammation index | 1.454 | (1.279, 1.653) | 1.404 | (1.232, 1.600) | ||||||||
| Neuroendocrine index | 1.202 | (1.057, 1.368) | 1.182 | (1.037, 1.347) | ||||||||
| Male | 2.411 | (1.851, 3.141) | 2.494 | (1.912, 3.253) | 2.429 | (1.865, 3.164) | 2.525 | (1.938, 3.291) | 2.443 | (1.875, 3.182) | 2.607 | (1.998, 3.402) |
| Gamma | 0.082 | (0.067, 0.098) | 0.085 | (0.069, 0.101) | 0.083 | (0.067, 0.099) | 0.078 | (0.062, 0.094) | 0.078 | (0.062, 0.094) | 0.077 | (0.061, 0.093) |
| Number of observations | 1604 | 1604 | 1604 | 1604 | 1604 | 1604 | ||||||
| Attenuation in education coefficient from Model 1 |
-- | 13.2% | 11.3% | 24.5% | 0.0% | 39.6% | ||||||
Note: Shown are Gompertz models of all-cause mortality. In Model 1, education is the only explanatory variable. In Models 2–5, each biomarker index is individually considered. In Model 6, all biomarker indexes are included. The bottom row shows attenuation (from Model 1) in the hazard ratio associated with education.
In Model 1, with no biomarkers, an additional year of education is associated with about 5% lower risk of mortality at any age. The gamma parameter is greater than zero (0.082, 95% confidence interval: (0.067, 0.098)), indicating that the baseline hazard function increases with age; that is, the risk of mortality increases exponentially as respondents get older, as expected. When biomarkers are added in Models 2–6, the hazard ratio associated with education attenuates toward one; the extent of this attenuation is shown in the bottom row of Table 4. In Model 2, including the clinical index attenuates the hazard ratio associated with years of education by 13%. The inflammation index, in Model 4, has the largest effect on the education hazard ratio, resulting in an attenuation of 25%. When the heart rate index is included in Model 3, the education hazard ratio attenuates by 11%; it does not attenuate at all when the neuroendocrine index is added in Model 5. In Model 6, all four biomarker indexes are considered in the same model; the hazard ratio on education attenuates by 40%, and is marginally significant (at the 5% level) in predicting mortality.
Next, we consider models of cause-specific mortality. Table 5 shows the results of cause-specific survival models. As predicted, the high-risk biomarker indexes are able to account for a larger share of the education-cardiovascular mortality relationship than the education-all-cause mortality relationship. Just as for all-cause mortality, the hazard ratio associated with education attenuates the most when inflammation is added to the model.
Table 5.
Survival models of age-specific cardiovascular mortality by January 31, 2014
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Years of education | 0.949 | (0.912, 0.989) | 0.961 | (0.922, 1.003) | 0.960 | (0.921, 1.000) | 0.967 | (0.927, 1.008) | 0.950 | (0.912, 0.989) | 0.984 | (0.942, 1.027) |
| Clinical index | 1.147 | (1.048, 1.256) | 1.074 | (0.979, 1.178) | ||||||||
| Heart rate index | 1.574 | (1.367, 1.812) | 1.516 | (1.315, 1.748) | ||||||||
| Inflammation index | 1.592 | (1.350, 1.876) | 1.526 | (1.290, 1.806) | ||||||||
| Neuroendocrine index | 1.159 | (0.979, 1.372) | 1.138 | (0.958, 1.352) | ||||||||
| Male | 2.707 | (1.895, 3.868) | 2.860 | (1.998, 4.094) | 2.768 | (1.937, 3.953) | 2.896 | (2.026, 4.139) | 2.737 | (1.915, 3.911) | 3.084 | (2.154, 4.416) |
| Gamma | 0.107 | (0.086, 0.128) | 0.112 | (0.091, 0.133) | 0.108 | (0.088, 0.129) | 0.101 | (0.080, 0.121) | 0.104 | (0.083, 0.125) | 0.103 | (0.082, 0.124) |
| Number of observations | 1604 | 1604 | 1604 | 1604 | 1604 | 1604 | ||||||
| Attenuation in education coefficient from Model 1 |
-- | 23.5% | 21.6% | 35.3% | 2.0% | 68.6% | ||||||
Note: Shown are Gompertz models of cardiovascular mortality. In Model 1, education is the only explanatory variable. In Models 2–5, each biomarker index is individually considered. In Model 6, all biomarker indexes are included. The bottom row shows attenuation (from Model 1) in the hazard ratio associated with education.
Discussion
The purpose of this study is to assess the relationship between educational attainment and mortality among older adults in Moscow, and to evaluate biomarkers associated with inflammation, neuroendocrine function, heart rate variability, and clinical cardiovascular and metabolic risk as potential mediators of that relationship. We find that lower educational attainment is associated with higher all-cause and cardiovascular mortality risk. In sex-adjusted models, a year of education is associated with about a 5% reduction in age-specific all-cause mortality and in cardiovascular mortality. This is similar in magnitude to findings from other Russian studies that examine educational differences in life expectancy at age 20 (Murphy et al., 2006) and in life expectancy between 40 and 75 (Shkolnikov et al., 2004b).
When we add biomarker indexes individually to the hazard models, we find that inflammation markers are best able to account for health disparities: inflammation biomarkers are both predictive of mortality and associated with educational attainment to a greater extent than any other set of biomarkers. While heart rate markers are predictive of mortality, they are not significantly associated with education. Clinical markers are associated with education and subsequent mortality, but the relationship with mortality is not as strong as that between inflammation and mortality.
Inflammatory markers account for 25% of the education–all-cause mortality relationship and 35% of the education–cardiovascular mortality relationship. Standard clinical markers perform next best, accounting for 13% and 24% of these two relationships, respectively. This last estimate is in line with a previous study of Russian adults from the Lipid Research Clinics, which found that clinical markers were able to account for about 22% of the excess coronary heart disease mortality experienced by the least educated (Dennis et al., 1993). Our results regarding clinical markers and all-cause mortality are also similar in magnitude to findings from the MacArthur Studies of Successful Aging, which followed a group of healthy, highly functioning older Americans. In the MacArthur cohort, clinical markers accounted for about 13% of the education– mortality relationship (Seeman et al., 2004). The MacArthur study found that neuroendocrine markers accounted for about 4% of the education–mortality link, consistent with our estimate that neuroendocrine biomarkers have no effect. However, the MacArthur study’s conclusions regarding inflammation differ from our results. In the MacArthur study, inflammation markers accounted for only 10% of the education–mortality relationship, lower than our estimate of 25%. These differences could be due to the study populations (e.g., the MacArthur study included only participants with high scores for health and function measures; there may also be substantial differences in lifestyle, infectious exposure, and health care access between the two countries) or to differences in measurement and methods between the two studies.
Our results suggest that inflammation may be an important biological pathway underlying mortality disparities in this sample of older adult Muscovites. How might SES influence inflammation? Three main hypotheses have been proposed: infectious exposure, health behaviors, and stress. Finch and Crimmins (2004) argue that early exposure to pathogens upregulates the lifelong inflammatory response, leading to increased risk of chronic disease in later life, such as heart disease and stroke. Supporting this theory, studies have found that children of low SES face a higher infectious burden compared to children of high SES (Dowd et al., 2009b), that adults of low SES are more likely to be infected with pathogens typically acquired at young ages (Steptoe et al., 2007), and that infant mortality, a proxy for early infectious environment, is associated with later life disease, including cardiovascular disease and stroke (Leon, 2001).
Health behaviors, such as alcohol consumption, smoking, and especially obesity, may also link SES and inflammation (Steptoe, 2012). Problematic drinking, smoking, and obesity are inversely associated with education in SAHR (results not shown; see also Metelskaya et al., 2012), and these health behaviors have been shown to be associated with inflammation (Ferrante, 2007; Gonçalves et al., 2011; Szabo and Mandrekar, 2009). Obesity and inflammation are consistently linked to the extent that many consider obesity a state of chronic inflammation (de Heredia et al., 2012). However, the role of obesity in linking SES and mortality is questionable. Recent studies have highlighted inconsistencies in the BMI-mortality relationship (Ahima and Lazar, 2013), and in the present sample, obesity (BMI>30) is not significantly associated with subsequent mortality (results not shown). Sensitivity analyses in the present study show that the importance of inflammation in attenuating the education-mortality relationship persists after health behaviors are taken into account (see Appendix Table A.4). This suggests that health behaviors are not the primary link between SES and inflammation.
Finally, psychosocial stress could mediate the SES-inflammation relationship, either via indirect means, such as the health behaviors described above, or via direct psychobiological processes. Low SES has been linked to greater exposure to acute and chronic stressors and to greater perceived stress (Cohen and Janicki-Deverts, 2012), and stress has been found to be related to inflammation in numerous studies (Black and Garbutt, 2002; Hänsel et al., 2010). Perceived stress has been found to be associated with biological dysregulation in this sample (Glei et al., 2013b), and the strength of this association appears to be higher than in other countries; future work on stress and mortality is planned by the SAHR team.
This study provides evidence for the hypothesis laid out in the previous literature that inflammation plays a key role in health disparities (Aiello and Kaplan, 2009; e.g., Steptoe, 2012). Replication in other population health studies – including both longitudinal observational studies and experiments – would further build the case, as would additional biomedical research into the (potentially causal) role of inflammation in cardiovascular disease and other chronic diseases. With significant further research, one or more inflammation biomarkers could become standard clinical measures of mortality risk, possibly serving as a metric for screenings, interventions, and preventative health care.
This study has several limitations. The sample size and follow-up period are relatively modest (mean follow-up: 5.9 years); a larger sample and longer follow-up would provide additional power. Biomarkers are collected only at baseline; variation across years, weeks, or even hours could be important for accurately measuring biological systems. For inflammation, high levels may reflect acute illness rather than the chronic inflammation that leads to cardiovascular disease. We performed a crude test for this problem by excluding respondents with the highest levels of C-reactive protein and found no substantive change in the results (see Appendix Table A.5). For neuroendocrine markers especially, missed shorter-term variation may be important; e.g., diurnal variation in cortisol is believed to be a better measure of neuroendocrine function than a one-time summary measure (Dowd et al., 2009a). The one-time biomarker collection further limits our ability to address causality. Inflammation may be causally related to mortality, or it may instead be a response to the underlying disease process, resulting from reverse causality or confounding (Danesh and Pepys, 2009; Jialal et al., 2004; von Haehling et al., 2009). We are unable in this study to adjudicate between causal and non-causal explanations.
These findings may not be generalizable to a younger age group, nor to Russia as a whole, since Muscovites are richer, better educated, and generally better off than other Russians (“All-Russia Population Census,” 2002). The differences between Moscow and Russia may make the sample somewhat more comparable to the low mortality populations of other developed countries, however.
Still, this analysis has the considerable benefit of rich data: this is the first study to assess the relationship between SES, extensive biomarker measures, and mortality in Russia. We find that measures of inflammation are consistently associated with lower educational attainment, are predictive of all-cause and cardiovascular mortality, and attenuate the relationship between education and mortality when added to survival models. These findings suggest that inflammation is important for understanding mortality disparities by socioeconomic status.
Supplementary Material
Acknowledgments
We are grateful to Evgeny Andreev at the New Economic School in Moscow, and Alexander Deev at the National Research Center for Preventive Medicine (NRCPM) in Moscow for considerable effort on data cleaning and processing, to Svetlana Shalnova at the NRCPM for major contributions to the collection, handling and processing of various biological markers and for her help in ensuring the quality of these data, and to Viktoria Metelskaya at the NRCPM for providing consultation on the biochemical measurements.
We would also like to thank Jennifer Dowd, Scott Lynch, and Germán Rodríguez for helpful comments on earlier drafts.
Funding: This work was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (grant P2CHD047879). Data collection was funded by the National Institute on Aging (grant R01AG026786). Vladimir Shkolnikov was partly funded by the Dynasty Foundation (Russia). The funding agencies had no role in the design, execution, analysis, interpretation, or writing of the study.
Footnotes
Ethics approval: SAHR was approved by the Ethics committee of the State Research Centre of Preventative Medicine (Moscow, Russia) and by the institutional review board at Duke University (Durham, NC). All participants provided informed consent prior to data collection.
Disclosure statement: No author has an actual or potential conflict of interest.
References
- Adler NE, Snibbe AC. The Role of Psychosocial Processes in Explaining the Gradient Between Socioeconomic Status and Health. Current Directions in Psychological Science. 2003;12:119–123. [Google Scholar]
- Ahima RS, Lazar MA. The Health Risk of Obesity—Better Metrics Imperative. Science. 2013;341:856–858. doi: 10.1126/science.1241244. [DOI] [PubMed] [Google Scholar]
- Aiello AE, Kaplan GA. Socioeconomic Position and Inflammatory and Immune Biomarkers of Cardiovascular Disease: Applications to the Panel Study of Income Dynamics. Biodemography and Social Biology. 2009;55:178–205. doi: 10.1080/19485560903382304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- All-Russia Population Census. 2002 [Google Scholar]
- Amelsvoort LGPM, van Schouten EG, Maan AC, Swenne CA, Kok FJ. Occupational determinants of heart rate variability. Int Arch Occup Environ Health. 2000;73:255–262. doi: 10.1007/s004200050425. [DOI] [PubMed] [Google Scholar]
- Andreev EM, Hoffmann R, Carlson E, Shkolnikov V, Kharkova TL. Concentration of Working-Age Male Mortality Among Manual Workers in Urban Latvia and Russia, 1970–1989. European Societies. 2009;11:161–185. [Google Scholar]
- Andreev EM, McKee M, Shkolnikov VM. Health expectancy in the Russian Federation: a new perspective on the health divide in Europe. Bulletin of the World Health Organization. 2003;81:778–787. [PMC free article] [PubMed] [Google Scholar]
- Averina M, Nilssen O, Brenn T, Brox J, Kalinin AG, Arkhipovsky VL. High cardiovascular mortality in Russia cannot be explained by the classical risk factors. The Arkhangelsk Study 2000. Eur. J. Epidemiol. 2003;18:871–878. doi: 10.1023/a:1025626202235. [DOI] [PubMed] [Google Scholar]
- Bessudnov A, McKee M, Stuckler D. Inequalities in male mortality by occupational class, perceived status and education in Russia, 1994–2006. The European Journal of Public Health ckr130. 2011 doi: 10.1093/eurpub/ckr130. [DOI] [PubMed] [Google Scholar]
- Black PH, Garbutt LD. Stress, inflammation and cardiovascular disease. Journal of Psychosomatic Research. 2002;52:1–23. doi: 10.1016/s0022-3999(01)00302-6. [DOI] [PubMed] [Google Scholar]
- Brainerd E, Cutler DM. Autopsy on an Empire: Understanding Mortality in Russia and the Former Soviet Union. The Journal of Economic Perspectives. 2005;19:107–130. [Google Scholar]
- Bruunsgaard H, Ladelund S, Pedersen AN, Schroll M, Jørgensen T, Pedersen BK. Predicting death from tumour necrosis factor-alpha and interleukin-6 in 80-year-old people. Clinical & Experimental Immunology. 2003;132:24–31. doi: 10.1046/j.1365-2249.2003.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D. Who’s stressed? Distributions of psychological stress in the United States in probability samples from 1983, 2006, and 20091. Journal of Applied Social Psychology. 2012;42:1320–1334. [Google Scholar]
- Crimmins EM, Kim JK, Vasunilashorn S. Biodemography: New approaches to understanding trends and differences in population health and mortality. Demography. 2010;47:S41–S64. doi: 10.1353/dem.2010.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh J, Pepys MB. C-Reactive Protein and Coronary Disease Is There a Causal Link? Circulation. 2009;120:2036–2039. doi: 10.1161/CIRCULATIONAHA.109.907212. [DOI] [PubMed] [Google Scholar]
- de Heredia FP, Gómez-Martínez S, Marcos A. Obesity, inflammation and the immune system. Proceedings of the Nutrition Society. 2012;71:332–338. doi: 10.1017/S0029665112000092. [DOI] [PubMed] [Google Scholar]
- Dekker JM, Crow RS, Folsom AR, Hannan PJ, Liao D, Swenne CA, Schouten EG. Low Heart Rate Variability in a 2-Minute Rhythm Strip Predicts Risk of Coronary Heart Disease and Mortality From Several Causes The ARIC Study. Circulation. 2000;102:1239–1244. doi: 10.1161/01.cir.102.11.1239. [DOI] [PubMed] [Google Scholar]
- Dekker JM, Schouten EG, Klootwijk P, Pool J, Swenne CA, Kromhout D. Heart Rate Variability from Short Electrocardiographic Recordings Predicts Mortality from All Causes in Middle-aged and Elderly Men The Zutphen Study. Am. J. Epidemiol. 1997;145:899–908. doi: 10.1093/oxfordjournals.aje.a009049. [DOI] [PubMed] [Google Scholar]
- De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflammation markers predicting frailty and mortality in the elderly. Experimental and Molecular Pathology. 2006;80:219–227. doi: 10.1016/j.yexmp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Dennis BH, Zhukovsky GS, Shestov DB, Davis CE, Deev AD, Kim H, Tyroler HA. The Association of Education with Coronary Heart Disease Mortality in the USSR Lipid Research Clinics Study. Int. J. Epidemiol. 1993;22:420–427. doi: 10.1093/ije/22.3.420. [DOI] [PubMed] [Google Scholar]
- Dowd JB, Simanek AM, Aiello AE. Socio-economic status, cortisol and allostatic load: a review of the literature. Int J Epidemiol. 2009a;38:1297–1309. doi: 10.1093/ije/dyp277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd JB, Zajacova A, Aiello A. Early origins of health disparities: burden of infection, health, and socioeconomic status in U.S. children. Soc Sci Med. 2009b;68:699–707. doi: 10.1016/j.socscimed.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Health for All Database. 2015 United Nations. [Google Scholar]
- Ferrante AW. Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. Journal of Internal Medicine. 2007;262:408–414. doi: 10.1111/j.1365-2796.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- Finch CE, Crimmins EM. Inflammatory Exposure and Historical Changes in Human Life-Spans. Science. 2004;305:1736–1739. doi: 10.1126/science.1092556. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Campisi J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J Gerontol A Biol Sci Med Sci. 2014;69:S4–S9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- Fujiura Y, Adachi H, Tsuruta M, Jacobs DR, Jr, Hirai Y, Imaizumi T. Heart rate and mortality in a Japanese general population: An 18-year follow-up study. Journal of Clinical Epidemiology. 2001;54:495–500. doi: 10.1016/s0895-4356(00)00323-1. [DOI] [PubMed] [Google Scholar]
- Gerber TP. Educational Stratification in Contemporary Russia: Stability and Change in the Face of Economic and Institutional Crisis. Sociology of Education. 2000;73:219–246. [Google Scholar]
- Gerber TP, Hout M. More Shock than Therapy: Market Transition, Employment, and Income in Russia, 1991–1995. American Journal of Sociology. 1998;104 AJSv104p1-50. [Google Scholar]
- Ginter DE. Cardiovascular risk factors in the former communist countries. Eur J Epidemiol. 1995;11:199–205. doi: 10.1007/BF01719488. [DOI] [PubMed] [Google Scholar]
- Glei DA, Goldman N, Shkolnikov VM, Jdanov D, Shalnova S, Shkolnikova M, Weinstein M. To what extent do biomarkers account for the large social disparities in health in Moscow? Social Science & Medicine. 2013a;77:164–172. doi: 10.1016/j.socscimed.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glei DA, Goldman N, Shkolnikov VM, Jdanov D, Shkolnikova M, Vaupel JW, Weinstein M. Perceived stress and biological risk: is the link stronger in Russians than in Taiwanese and Americans? Stress. 2013b:1–10. doi: 10.3109/10253890.2013.789015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman N, Glei DA, Lin Y-H, Weinstein M. Improving Mortality Prediction Using Biosocial Surveys. Am. J. Epidemiol. 2009;169:769–779. doi: 10.1093/aje/kwn389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves RB, Coletta RD, Silvério KG, Benevides L, Casati MZ, Silva da JSFHN., Jr Impact of smoking on inflammation: overview of molecular mechanisms. Inflamm. Res. 2011;60:409–424. doi: 10.1007/s00011-011-0308-7. [DOI] [PubMed] [Google Scholar]
- Grigoriev P, Meslé F, Shkolnikov VM, Andreev EM, Fihel A, Pechholdova M, Vallin J. The Recent Mortality Decline in Russia: Beginning of the Cardiovascular Revolution? Population and Development Review. 2014;40:107–129. [Google Scholar]
- Gruenewald TL, Seeman TE, Ryff CD, Karlamangla AS, Singer BH. Combinations of biomarkers predictive of later life mortality. PNAS. 2006;103:14158–14163. doi: 10.1073/pnas.0606215103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänsel A, Hong S, Cámara RJA, von Känel R. Inflammation as a psychophysiological biomarker in chronic psychosocial stress. Neuroscience & Biobehavioral Reviews, Psychophysiological Biomarkers of Health. 2010;35:115–121. doi: 10.1016/j.neubiorev.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Hansen TW, Thijs L, Boggia J, Li Y, Kikuya M, Björklund-Bodegård K, Richart T, Ohkubo T, Jeppesen J, Torp-Pedersen C, Lind L, Sandoya E, Imai Y, Wang J, Ibsen H, O’Brien E, Staessen JA. Prognostic Value of Ambulatory Heart Rate Revisited in 6928 Subjects From 6 Populations. Hypertension. 2008;52:229–235. doi: 10.1161/HYPERTENSIONAHA.108.113191. [DOI] [PubMed] [Google Scholar]
- Hansson GK. Inflammation, Atherosclerosis, and Coronary Artery Disease. New England Journal of Medicine. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Hansson GK, Robertson A-KL, Söderberg-Nauclér C. Inflammation and Atherosclerosis. Annual Review of Pathology: Mechanisms of Disease. 2006;1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- Hoffmann R. Socioeconomic Differences in Old Age Mortality. 2008 [Google Scholar]
- Horiuchi S, Coale AJ. A Simple Equation for Estimating the Expectation of Life at Old Ages. Population Studies. 1982;36:317–326. doi: 10.1080/00324728.1982.10409034. [DOI] [PubMed] [Google Scholar]
- Huisman M, Kunst AE, Andersen O, Bopp M, Borgan J-K, Borrell C, Costa G, Deboosere P, Desplanques G, Donkin A, Gadeyne S, Minder C, Regidor E, Spadea T, Valkonen T, Mackenbach JP. Socioeconomic inequalities in mortality among elderly people in 11 European populations. J Epidemiol Community Health. 2004;58:468–475. doi: 10.1136/jech.2003.010496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jialal I, Devaraj S, Venugopal SK. C-Reactive Protein: Risk Marker or Mediator in Atherothrombosis? Hypertension. 2004;44:6–11. doi: 10.1161/01.HYP.0000130484.20501.df. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Kannel C, Paffenbarger RS, Jr, Cupples LA. Heart rate and cardiovascular mortality: The Framingham study. American Heart Journal. 1987;113:1489–1494. doi: 10.1016/0002-8703(87)90666-1. [DOI] [PubMed] [Google Scholar]
- Katayama T, Nakashima H, Furudono S, Honda Y, Suzuki S, Yano K. Evaluation of Neurohumoral Activation (Adrenomedullin, BNP, Catecholamines, etc.) in Patients with Acute Myocardial Infarction. Internal Medicine. 2004;43:1015–1022. doi: 10.2169/internalmedicine.43.1015. [DOI] [PubMed] [Google Scholar]
- Kuulasmaa K, Tunstall-Pedoe H, Dobson A, Fortmann S, Sans S, Tolonen H, Evans A, Ferrario M. Estimation of contribution of changes in classic risk factors to trends in coronary-event rates across the WHO MONICA Project populations. The Lancet. 2000;355:675–687. doi: 10.1016/s0140-6736(99)11180-2. [DOI] [PubMed] [Google Scholar]
- Lagrand WK, Visser CA, Hermens WT, Niessen HWM, Verheugt FWA, Wolbink G-J, Hack CE. C-Reactive Protein as a Cardiovascular Risk Factor More Than an Epiphenomenon? Circulation. 1999;100:96–102. doi: 10.1161/01.cir.100.1.96. [DOI] [PubMed] [Google Scholar]
- Leon DA. Common threads: underlying components of inequalities in mortality between and within countries. In: Leon DA, Walt G, editors. Poverty, Inequality, and Health: An International Perspective. Oxford University Press; 2001. pp. 58–87. [Google Scholar]
- Leon DA, Chenet L, Shkolnikov VM, Zakharov S, Shapiro J, Rakhmanova G, Vassin S, McKee M. Huge variation in Russian mortality rates 1984–94: artefact, alcohol, or what? The Lancet. 1997;350:383–388. doi: 10.1016/S0140-6736(97)03360-6. [DOI] [PubMed] [Google Scholar]
- Leon DA, Shkolnikov VM, McKee M, Kiryanov N, Andreev EM. Alcohol increases circulatory disease mortality in Russia: acute and chronic effects or misattribution of cause? Int. J. Epidemiol. 2010;39:1279–1290. doi: 10.1093/ije/dyq102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi F, Chatenoud L, Bertuccio P, Lucchini F, Negri E, Vecchia CL. Mortality from cardiovascular and cerebrovascular diseases in Europe and other areas of the world: an update. European Journal of Cardiovascular Prevention & Rehabilitation. 2009;16:333–350. doi: 10.1097/HJR.0b013e328325d67d. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- Malyutina S, Bobak M, Simonova G, Gafarov V, Nikitin Y, Marmot M. Education, marital status, and total and cardiovascular mortality in Novosibirsk, Russia: A prospective cohort study. Annals of Epidemiology. 2004;14:244–249. doi: 10.1016/S1047-2797(03)00133-9. [DOI] [PubMed] [Google Scholar]
- Mazat L, Lafont S, Berr C, Debuire B, Tessier J-F, Dartigues J-F, Baulieu E-E. Prospective measurements of dehydroepiandrosterone sulfate in a cohort of elderly subjects: Relationship to gender, subjective health, smoking habits, and 10-year mortality. PNAS. 2001;98:8145–8150. doi: 10.1073/pnas.121177998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Brain on stress: How the social environment gets under the skin. Proceedings of the National Academy of Sciences. 2012;109:17180–17185. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael AJ, McKee M, Shkolnikov VM, Valkonen T. Mortality trends and setbacks: global convergence or divergence? The Lancet. 2004;363:1155–1159. doi: 10.1016/S0140-6736(04)15902-3. [DOI] [PubMed] [Google Scholar]
- Meslé F. Mortality in central and eastern Europe: Long-term trends and recent upturns. Demographic Research. 2004:10. [Google Scholar]
- Metelskaya VA, Shkolnikova MA, Shalnova SA, Andreev EM, Deev AD, Jdanov DA, Shkolnikov VM, Vaupel JW. Prevalence, components, and correlates of metabolic syndrome (MetS) among elderly Muscovites. Archives of Gerontology and Geriatrics. 2012;55:231–237. doi: 10.1016/j.archger.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser K, Shkolnikov V, Leon DA. World mortality 1950–2000: divergence replaces convergence from the late 1980s. Bulletin of the World Health Organization. 2005;83:202–209. [PMC free article] [PubMed] [Google Scholar]
- Murphy M, Bobak M, Nicholson A, Rose R, Marmot M. The Widening Gap in Mortality by Educational Level in the Russian Federation, 1980–2001. Am J Public Health. 2006;96:1293–1299. doi: 10.2105/AJPH.2004.056929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palatini P, Winnicki M, Santonastaso M, De Venuto G, Zanata G, Bertolo O, Frigo G, Pessina AC. Reproducibility of heart rate measured in the clinic and with 24-hour intermittent recorders. Am. J. Hypertens. 2000;13:92–98. doi: 10.1016/s0895-7061(99)00170-3. [DOI] [PubMed] [Google Scholar]
- Pawelec G, Goldeck D, Derhovanessian E. Inflammation, ageing and chronic disease. Current Opinion in Immunology, Host pathogens * Immune senescence. 2014;29:23–28. doi: 10.1016/j.coi.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Perlman F, Bobak M. Socioeconomic and Behavioral Determinants of Mortality in Posttransition Russia: A Prospective Population Study. Annals of Epidemiology. 2008;18:92–100. doi: 10.1016/j.annepidem.2007.07.093. [DOI] [PubMed] [Google Scholar]
- Powles JW, Zatonski W, Hoorn SV, Ezzati M. The contribution of leading diseases and risk factors to excess losses of healthy life in eastern Europe: burden of disease study. BMC Public Health. 2005;5:116. doi: 10.1186/1471-2458-5-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben DB, Talvi SLA, Rowe JW, Seeman TE. High Urinary Catecholamine Excretion Predicts Mortality and Functional Decline in High-Functioning, Community-Dwelling Older Persons MacArthur Studies of Successful Aging. J Gerontol A Biol Sci Med Sci. 2000;55:M618–M624. doi: 10.1093/gerona/55.10.m618. [DOI] [PubMed] [Google Scholar]
- Reunanen A, Karjalainen J, Ristola P, Heliövaara M, Knekt P, Aromaa A. Heart rate and mortality. Journal of Internal Medicine. 2000;247:231–239. doi: 10.1046/j.1365-2796.2000.00602.x. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AM, Kastelein JJP, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to Prevent Vascular Events in Men and Women with Elevated C-Reactive Protein. New England Journal of Medicine. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Buring JE, Rifai N. C-Reactive Protein and Other Markers of Inflammation in the Prediction of Cardiovascular Disease in Women. New England Journal of Medicine. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- Rosstat. Population. Natural Reproduction. Expected length of life. 2015 [Google Scholar]
- Roth GS, Lane MA, Ingram DK, Mattison JA, Elahi D, Tobin JD, Muller D, Metter EJ. Biomarkers of Caloric Restriction May Predict Longevity in Humans. Science. 2002;297:811–811. doi: 10.1126/science.1071851. [DOI] [PubMed] [Google Scholar]
- Sajadieh A, Nielsen OW, Rasmussen V, Hein HO, Abedini S, Hansen JF. Increased heart rate and reduced heart-rate variability are associated with subclinical inflammation in middle-aged and elderly subjects with no apparent heart disease. European Heart Journal. 2004;25:363–370. doi: 10.1016/j.ehj.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Crimmins E, Huang M-H, Singer B, Bucur A, Gruenewald T, Berkman LF, Reuben DB. Cumulative biological risk and socio-economic differences in mortality: MacArthur Studies of Successful Aging. Social Science & Medicine. 2004;58:1985–1997. doi: 10.1016/S0277-9536(03)00402-7. [DOI] [PubMed] [Google Scholar]
- Seplaki CL, Goldman N, Glei D, Weinstein M. A comparative analysis of measurement approaches for physiological dysregulation in an older population. Experimental Gerontology. 2005;40:438–449. doi: 10.1016/j.exger.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Shishehbor MH. Association of Socioeconomic Status With Functional Capacity, Heart Rate Recovery, and All-Cause Mortality. JAMA. 2006;295:784. doi: 10.1001/jama.295.7.784. [DOI] [PubMed] [Google Scholar]
- Shkolnikova M, Shalnova S, Shkolnikov VM, Metelskaya V, Deev A, Andreev E, Jdanov D, Vaupel JW. Biological mechanisms of disease and death in Moscow: rationale and design of the survey on Stress Aging and Health in Russia (SAHR) BMC Public Health. 2009;9:293. doi: 10.1186/1471-2458-9-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shkolnikov VM, Andreev EM, Leon DA, McKee M, Mesle F, Vallin J. Mortality reversal in Russia: the story so far. Hygiea Internationalis. 2004a;4:29–80. [Google Scholar]
- Shkolnikov VM, Andreev EM, McKee M, Leon DA. Components and possible determinants of decrease in Russian mortality in 2004–2010. Demographic Research. 2013;28:917–950. [Google Scholar]
- Shkolnikov VM, Deev AD, Kravdal Ø, Valkonen T. Educational differentials in male mortality in Russia and northern Europe. Demographic Research. 2004b;10:1–26. [Google Scholar]
- Shkolnikov VM, Leon DA, Adamets S, Andreev EM, Deev A. Educational level and adult mortality in Russia: An analysis of routine data 1979 to 1994. Social Science & Medicine. 1998;47:357–369. doi: 10.1016/s0277-9536(98)00096-3. [DOI] [PubMed] [Google Scholar]
- Shkolnikov VM, Scholz R, Jdanov DA, Stegmann M, Gaudecker H-M. von. Length of life and the pensions of five million retired German men. The European Journal of Public Health. 2008;18:264–269. doi: 10.1093/eurpub/ckm102. [DOI] [PubMed] [Google Scholar]
- Sidorenkov O, Nilssen O, Grjibovski AM. Metabolic syndrome in Russian adults: associated factors and mortality from cardiovascular diseases and all causes. BMC Public Health. 2010;10:582. doi: 10.1186/1471-2458-10-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein PKPhD, Kleiger REMD. Insights from the Study of Heart Rate Variability. Annual Review of Medicine. 1999;50:249–261. doi: 10.1146/annurev.med.50.1.249. [DOI] [PubMed] [Google Scholar]
- Steptoe A. Socioeconomic Status, Inflammation, and Immune Function. In: Segerstrom S, editor. The Oxford Handbook of Psychoneuroimmunology. Oxford University Press; 2012. [Google Scholar]
- Steptoe A, Marmot M. The role of psychobiological pathways in socio-economic inequalities in cardiovascular disease risk. Eur. Heart J. 2002;23:13–25. doi: 10.1053/euhj.2001.2611. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Shamaei-Tousi A, Gylfe Å, Henderson B, Bergström S, Marmot M. Socioeconomic status, pathogen burden and cardiovascular disease risk. Heart. 2007;93:1567–1570. doi: 10.1136/hrt.2006.113993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G, Mandrekar P. A Recent Perspective on Alcohol, Immunity, and Host Defense. Alcoholism: Clinical and Experimental Research. 2009;33:220–232. doi: 10.1111/j.1530-0277.2008.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkins S, Collier T, Oralov A, Saburova L, McKee M, Shkolnikov V, Kiryanov N, Leon DA. Hazardous Alcohol Consumption Is a Major Factor in Male Premature Mortality in a Typical Russian City: Prospective Cohort Study 2003–2009. PLoS ONE. 2012;7:e30274. doi: 10.1371/journal.pone.0030274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji H, Venditti FJ, Manders ES, Evans JC, Larson MG, Feldman CL, Levy D. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation. 1994;90:878–883. doi: 10.1161/01.cir.90.2.878. [DOI] [PubMed] [Google Scholar]
- Turra CM, Goldman N, Seplaki CL, Glei DA, Lin Y-H, Weinstein M. Determinants of Mortality at Older Ages: The Role of Biological Markers of Chronic Disease. Population and Development Review. 2005;31:675–698. [Google Scholar]
- von Haehling S, Schefold JC, Lainscak M, Doehner W, Anker SD. Inflammatory Biomarkers in Heart Failure Revisited: Much More than Innocent Bystanders. Heart Failure Clinics, Biomarkers in Heart Failure. 2009;5:549–560. doi: 10.1016/j.hfc.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Wolfson M, Rowe G, Gentleman JF, Tomiak M. Career Earnings and Death: A Longitudinal Analysis of Older Canadian Men. J Gerontol. 1993;48:S167–S179. doi: 10.1093/geronj/48.4.s167. [DOI] [PubMed] [Google Scholar]
- Zaridze D, Lewington S, Boroda A, Scélo G, Karpov R, Lazarev A, Konobeevskaya I, Igitov V, Terechova T, Boffetta P, Sherliker P, Kong X, Whitlock G, Boreham J, Brennan P, Peto R. Alcohol and mortality in Russia: prospective observational study of 151 000 adults. The Lancet. 2014;383:1465–1473. doi: 10.1016/S0140-6736(13)62247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.