Abstract
The extent of our knowledge on the number of chemical compounds related to anthropogenic activities that can cause damage to the environment and to organisms is increasing. Endocrine disrupting chemicals (EDCs) are one group of potentially hazardous substances that include natural and synthetic chemicals and have the ability to mimic endogenous hormones, interfering with their biosynthesis, metabolism, and normal functions. Adverse effects associated with EDC exposure have been documented in aquatic biota and there is widespread interest in the characterization and understanding of their modes of action. Fish are considered one of the primary risk organisms for EDCs. Zebrafish (Danio rerio) are increasingly used as an animal model to study the effects of endocrine disruptors, due to their advantages compared to other model organisms. One approach to assess the toxicity of a compound is to identify those patterns of gene expression found in a tissue or organ exposed to particular classes of chemicals, through new technologies in genomics (toxicogenomics), such as microarrays or whole-genome sequencing. Application of these technologies permit the quantitative analysis of thousands of gene expression changes simultaneously in a single experiment and offer the opportunity to use transcript profiling as a tool to predict toxic outcomes of exposure to particular compounds. The application of toxicogenomic tools for identification of chemicals with endocrine disrupting capacity using the zebrafish model system is reviewed.
Keywords: Danio rerio, Endocrine disruptors, Environmental pollutants, Fish, Omics, Toxicity, Toxicogenomics, Zebrafish.
INTRODUCTION
Water is essential to sustain life. An adequate, safe, and accessible water supply must be available to all living organisms, communities, and economies. Water suppliers around the world have the responsibility to provide drinking water of good quality in the necessary quantity. However, one of the issues concerning the quality of drinking water is the presence of several environmental pollutants including endocrine disrupting chemicals (EDCs), pharmaceuticals and personal care products (PPCPs), and other substances [1]. Some of these environmental pollutants are ubiquitous and may not be removed from water treatment plants during water potabilization for human consumption [2]. In fact, these pollutants can bioaccumulate in aquatic organisms, especially fish, and are an important criterion in risk assessment. Bioconcentration from water must be considered in context with toxicity, biotic and abiotic degradation, and other physical-chemical factors in order to protect freshwater and marine environments and their organisms. Furthermore, it is necessary to prevent human consumption of contaminated aquatic food, thereby decreasing the risk of exposure to EDCs.
The major adverse effects of EDCs include their ability to interfere with the hormonal system altering endocrine regulation of essential physiological functions [3]. The EDCs most commonly found to be of concern for aquatic wildlife are those resulting from the discharge of either treated or untreated municipal and industrial wastewaters [4, 5]. The most potent EDCs contained in these effluents are the natural and synthetic steroid estrogens, such as 17β-estradiol (E2), estrone (E1), and 17 α-ethinylestradiol (EE2). Other common industrial chemicals such as bisphenol A (BPA) and nonylphenol (NP) are also suspected to disrupt the endocrine system in animals [6]. Several studies provide evidence that exposure to these compounds can lead to abnormal modulation or disruption of development and reproduction in aquatic wildlife, especially fish. For example, exposure to the pharmaceutical estrogen EE2 and the anabolic androgen 17β-trenbolone (Tb) in zebrafish (Danio rerio) produces an increase in vitellogenin (vtg) concentration (a biomarker response for estrogen exposure) and feminization of fish after exposure to 10 ng/L EE2, as well as a decrease in vtg production and masculinization after exposure to 50 ng/L of Tb [7]. Furthermore, a decrease in gonadal development [8], reduced fecundity and/or fertility [8, 9], and alterations to gonadal differentiation [10], are among the most commonly observed effects.
Currently, omic technologies provide valuable tools to increase our understanding of changes in genomes (genomics, epigenomics), global gene expression (transcriptomics), protein levels (proteomics), and biochemical molecules involved in metabolism (metabolomics). Evaluation of the entire transcriptome of a cell, tissue, or organism at a particular time [11] is widely used to elucidate changes in gene expression profiles after exposure to EDCs [12-14]. Proteomics aims to understand changes in the entire set of proteins [15], and metabolomics studies the identification and quantification of small biomolecules (metabolites) in biological samples under various environmental and genetic conditions [16].
Toxicogenomics is a field that emerged from the combination of conventional toxicology and functional genomics. In recent years, the application of these tools contributed to defining adverse molecular effects resulting from environmental stressors, toxins, drugs, and chemicals. This has been possible through microarray and whole-genome sequencing technologies, permitting large-scale detection and quantification of mRNA transcripts and of microRNAs, allowing the detection of alterations in mRNA stability or gene regulation [17].
An inherent assumption of toxicology studies is that the biological effects of a chemical in a laboratory animal model system are predictive of similar effects in humans. An essential step for the development of omic applications for endocrine disruption research was refining their use in model species applied in understanding both the highly conserved and species-specific differences of the endocrine system. The potential risk posed by EDCs to humans and wildlife has instigated efforts to establish regulatory programs for EDC hazard assessment. Since the aquatic environment is a major sink for EDCs, particular attention is given to the development and validation of testing approaches using fish, in particular the very well characterized model, the zebrafish. The zebrafish develops from a fertilized egg to a mature adult within a few months and, therefore, allows performing full life-cycle tests with a variety of developmental and reproductive endpoints that might be affected by EDCs [18]. The zebrafish genome matches approximately 70% to human orthologs, further supporting their already recognized value as a model species for biomedical research [19].
The present review summarizes available toxicogenomic data on EDCs using zebrafish as a model system. Online databases, reference lists of key publications, and journals were searched for potentially eligible studies. A literature review on toxicogenomic approaches with regard to effects of EDCs was searched using PubMed, Scopus, Science Direct, and Google Scholar through February 2015. The search included the following keywords: EDCs, toxicogenomics, transcriptomics, toxicity, genes, DNA sequencing, and microarrays.
ENDOCRINE DISRUPTING CHEMICALS (EDCS)
EDCs are defined as exogenous agents that interfere with the production, release, transport, metabolism, binding, action, or elimination of hormones responsible for the maintenance of homeostasis and the regulation of developmental processes [20]. EDCs are a structurally diverse group of chemicals that may adversely affect the health of humans, wildlife and fisheries, or their progenies, by interaction with the endocrine system. Endocrine disruptors affect many aspects of transcription and transcriptional regulation that influence gene expression [21]. EDCs comprise a broad-class of exogenous substances including many man-made chemicals that are widely dispersed in the environment. Some molecules identified as endocrine disruptors include plastic additives [e.g., BPA used to make plastics harder, clearer, and more resistant to heat stress], plasticizers (e.g., phthalates), synthetic chemicals used as industrial solvents/lubricants and their byproducts [e.g., polychlorinated biphenyls (PCBs), polybrominated biphenyls (PBBs), dioxins], alkyl phenols (present in detergents and surfactants), pesticides [e.g., methoxychlor, chlorpyrifos, dichlorodiphenyltrichloroethane (DDT), vinclozolin], pharmaceutical agents [e.g., diethylstilbestrol (DES)], metals (e.g., arsenic compounds), drugs, anabolic agents, fungi (e.g., mycoestrogenzearalenone) and some phytoestrogens, including isoflavones and lignans, which are present in some food items such as soy and in cosmetics with active ingredients of vegetable origin, among many other xenobiotics.
Sources of EDCs in the Environment
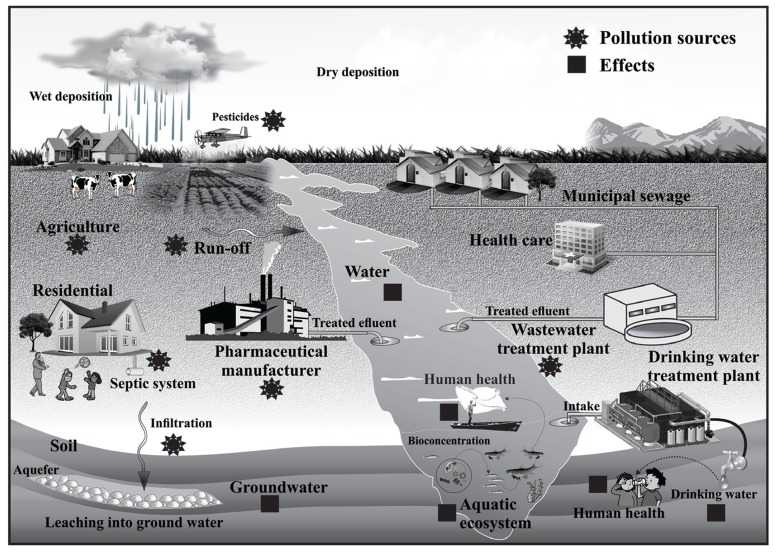
EDCs can originate from numerous sources and enter the environment by many routes. From the air, soil, and water, EDCs enter the aquatic ecosystem via disposed wastewater, agricultural run-off, and groundwater discharge, and can accumulate both in sediments and in biota including fish [22]. Since some of these compounds are lipophilic and persistent, they have the potential to bioaccumulate and become part of a plant’s or animal’s body burden and biomagnify in higher trophic levels. This is of concern to public health as a primary route of human exposure to EDCs is through eating contaminated fish. Discharges from municipal wastewater treatment plants (WWTPs) are identified as significant contributors of EDCs to surface waters. The actual sources are upstream discharges to the treatment facilities, which include natural hormones and pharmaceutical estrogens excreted by humans and flushed down home toilets, PPCPs excreted or washed from the body, plant material, items treated with fire retardants, other household cleaning products, and pesticides. WWTPs might also receive effluents from industrial processes that use cleaners containing NPs and plastics containing BPA, or health care and storm water run-off that contain EDCs. However, run-off from agricultural fields and livestock feeding operations, and land amended with biosolids or manure also contribute as nonpoint sources for EDCs in the aquatic environment. In addition, the potential exists for agricultural run-off containing pesticides and fertilizers to contain the estrogenic surfactants that make up the chemical formulation. Other potential sources include private septic systems, untreated storm water flows and urban run-off, industrial effluents, landfill leachate, and atmospheric deposition. Human exposure can occur via ingestion of food, dust, and water, inhalation of gases and particles in the air, and skin contact. A diagram showing the environmental fate and human exposure of these contaminants is summarized in (Fig. 1).
Fig. (1).
Sources of EDCs in environmental waters and human exposure (adapted from Bradley and Kolpin [105]). (Star: Pollution sources, Square: Effects).
Some EDCs are reported to bioaccumulate in fish and to biomagnify through the food chain. In fact, EDCs can affect the developing embryo due to its transfer during egg development. Fish are particularly vulnerable to exposure to EDCs such as BPA [23], NP [24], polybrominateddiphenyl ethers (PBDEs) [25], and phthalates [26], among other pollutants that can reach bodies of water. Alterations due to EDCs have been studied for several aspects of fish physiology, most commonly in features relating to growth, development, and reproduction [27-29]. Several studies report associations between fish consumption and human risk attributable to EDCs such as triclosan [30], organochlorine pesticides (dieldrin, heptachlor epoxide, and hexachlorobenzene) [31], among others. Populations that are particularly at risk for EDC exposure include people with high fish consumption, the elderly, pregnant women and their fetuses, nursing infants of mothers who consume contaminated fish, and people living near hazardous waste sites.
EDCs in Freshwater and Effects on Fish
The adverse effects of EDCs have become an important issue drawing public attention, especially because of the link between a synthetic steroidal estrogen used in birth control pills (e.g., ethinylestradiol) and the toxicological impact on fish [32]. In wildlife, these chemical agents affect aquatic life and their proliferation and can eventually cause reduced population density and species biodiversity [33], change of sex in fish [34], eggshell thinning in birds [35], and other problems. Since hormone receptor systems function similarly in humans and animals, these observations have raised concern about potential human health effects.
Occurrence of EDCs in aquatic organisms has been extensively monitored, with spot sampling followed by laboratory analysis being the most widely used monitoring approach. However, combined chemical analysis and bioassays can fully reveal the water pollution level. Exposure to EDCs in water is associated with a range of reproductive impacts, particularly in fish, including the induction of intersex (presence of both male and female sex organs) [36], lowered hormone levels, and reduced gamete production and fertilization capability. In most cases researchers are unable to pinpoint the specific chemicals responsible for effects indicating endocrine disruption in exposed fish. Estradiol, estrone, ethinylestradiol, NP, octylphenol, alkylphenolethoxylates, and BPA are identified as likely causes based on their concentrations in water (see Table S1 (323KB, pdf) and Fig. 2). In accordance with compounds, the most frequently (100%) found in freshwater are metals [e.g., Cadmium (Cd), Nickel (Ni), Chromium (Cr), Zinc (Zn), Copper (Cu), Cobalt (Co) and lead (Pb)], caffeine, PCBs, hexa-chlorocyclohexanes (HCHs), phenolic compounds [e.g., 4-n-nonylphenol (4-n-NP), 4-cumylphenol (4-CP), nonylphenolmonoethoxylate (NP1EO), and nonylphenoldiethoxylate (NP2EO)], and phthalates [e.g., dimethyl phthalate (DMP)]. Cadmium presented the highest concentrations (135 ppb). Cadmium is a non-essential trace metal that is extremely toxic to aquatic biota. It is commonly found in surface waters contaminated with industrial effluents. When dissolved in water, Cd can rapidly cause physiological changes in the gills and kidneys of freshwater fish. da Silva and Martinez [37] reported that acute exposure to Cd decreases the activity of enzymes, which culminated in the loss of the fish’s ability to regulate the levels of calcium in the blood, leading to hypocalcemia. Meanwhile, HCHs and 4-CP were detected at very low concentrations (0.01 ppb).
Fig. (2).
Means of detection frequencies (weighted by the number of samples analyzed in each study) in freshwater with their standard error of the mean (taken from the data in Table S1 (323KB, pdf) ). Values shown above each bar are the average concentrations in ppb (μg/L). EDC detection frequency <100% (A) and equal to 100% (B). [Abbreviations: 4-CP: 4-cumylphenol; 4-n-NP: 4-n-nonylphenol; BBP: butyl benzyl phthalate; BPA: Bisphenol A; Cd: Cadmium; Co: Cobalt; Cr: Chromium; Cu: Copper; DDT: dichlorodiphenyltrichloroethane; DEHP: Di(2-ethylhexyl)phthalate; DEP: Diethyl phthalate; DES: Diethylstilbestrol; DMP: Dimethyl phthalate; DnBP: Di-n-butyl phthalate; DnOP: Di-n-octylphthalate; E1: Estrone; E2: Estradiol; E3: Estriol; EE2: 17α-ethynylestradiol; HCHs: Hexachlorocyclohexanes; Ni: Nickel; NP: Nonylphenol; NP1EO: Nonylphenol monoethoxylate; NP2EO: Nonylphenol diethoxylate; OP: Organophosphates; PAHs: Polycyclic aromatic hydrocarbons; Pb: Lead; PCBs: Polychlorinated biphenyls; Zn: Zinc].
Apart from these compounds, BPA and triclosan are found in significant levels in the aquatic environment and may contribute to the presence of effects on aquatic organisms through unexpected modes of action. Both showed frequency detection above 80% with a mean of 0.06 and 0.2 ppb, respectively. Triclosan is a commonly used antimicrobial that is incorporated into dish soap, detergent, toothpaste, mouthwash, hand soap, fabric, deodorant, and shampoo, in addition to innumerable other personal care and household products. Triclosan is a polychlorophenoxy phenol used as an antibacterial and antifungal agent in household cleaning products and its bioaccumulation has been reported in freshwater fish from India [30]. BPA has also been found to accumulate in fish with concentrations of 10 ppm [38] and may also pose risks to ecosystems and human health.
Recent studies showed multiple stressors on fish populations, including estrogens, heavy metals, and alkylphenols (Table 1). High concentrations of metals such as Zn (3418-6580 ppb) and Arsenic (As; 3135-11024 ppb) were observed in fish muscle. Arsenic receives special attention due to its potential general health hazard to the aquatic ecosystem and human life. However, Pb and Cd had the lowest concentrations in fish muscle (0.4-1 ppb and 3-21 ppb, respectively), as well as Mercury (Hg). Nevárez et al. [39] investigated contamination levels and safety for consumers in fish from Northern Mexico. They found that the provisional tolerable weekly intake (PTWI) suggested by the World Health Organization for methyl mercury (1.6 µg/kg body weight (b.w.) per week) was exceeded in the spring season in the San Marcos dam (1.94 µg/kg b.w. per week) and concluded that this concentration might put consumers at risk for mercury poisoning. Alkylphenolpolyethoxylate compounds (APEs) are widely used as non-ionic surfactants in detergents, pesticides, herbicides, emulsifiers, paints, cosmetics, plastic wares, and even in jet fuel. APEs are commonly found in wastewater discharges and effluents from sewage treatment plants. Nonylphenol is the most abundant derivative of APEs demonstrated to stay biologically active for a longer period of time in the body than an endogenous estrogen. NP competes with estrogen and binds to the estrogen receptor, affecting reproduction [40] and development in fish [41]. This compound was observed in species such as Carassius carassius [42] and Cyprinus carpio [43] in concentrations above 1000 ppb, showing that fish are at risk from these pollutants found in bodies of water.
Table 1. Concentrations of EDCs in different fish species.
| Compounds | Species | Body Part | Concentration (ng/g) | References |
|---|---|---|---|---|
| Nonylphenols | Crucian carp (Carassius carassius) Common carp (Cyprinus carpio) |
Muscle Muscle Liver |
1132-3111 1920 3210 |
[42] [43] |
| Octylphenols | Crucian carp (Carassius carassius) Common carp (Cyprinus carpio) |
Muscle Muscle |
6-46 3240 |
[42] [43] |
| Bisphenol A (BPA) | Crucian carp (Carassius carassius) Striped bass (Morone saxatilis) Common carp (Cyprinus carpio) |
Muscle Muscle Blood Bile Muscle Liver |
4-59 5.06-8.94 1.18-6.21 0.61 1580 2150 |
[42] [99] [43] |
| Arsenic (As) |
Engrauli sencrasicolus Trachurus trachurus Scomber scombrus Mullus barbatus Arnoglossus laterna |
Muscle | 5275 5409 3669 11024 3135 |
[100] |
| Cadmium (Cd) |
Engrauli sencrasicolus Trachurus trachurus Scomber scombrus Mullus barbatus Arnoglossus laterna |
Muscle | 1 0.9 1.3 0.4 1.3 |
[100] |
| Lead (Pb) |
Engrauli sencrasicolus Trachurus trachurus Scomber scombrus Mullus barbatus Arnoglossus laterna |
Muscle | 5 4 3 5 21 |
[100] |
| Manganese (Mn) |
Engrauli sencrasicolus Trachurus trachurus Scomber scombrus Mullus barbatus Arnoglossus laterna |
Muscle | 257 196 122 219 2454 |
[100] |
| Zinc (Zn) |
Engrauli sencrasicolus Trachurus trachurus Scomber scombrus Mullus barbatus Arnoglossus laterna |
Muscle | 6580 5677 4875 3418 4952 |
[100] |
| Mercury (Hg) |
Mojarra amarilla (Caquetaia kraussii) Bocachico (Prochilodus magdalenae) Moncholo (Hoplias malabaricus) Blanquillo (Sorubim cuspicaudus) |
Muscle |
450 20 90 120 |
[101] [101] [102] [102] |
TOXICOGENOMICS AND EDCS
Biological effects of EDCs in fish can be detected at various levels: molecular, cellular, organ, organism, population, and ecosystem [44]. Early alterations will be found at the molecular level since this is one of the first targets in the cell. Thus, one approach to detect effects at an early stage is to look at the effects at the gene expression level (toxicogenomics), at the protein level (proteomics), or at the level of metabolites (metabolomics) [44].
Toxicogenomics is defined as the application of genomic technology in toxicology research [45]. More simply put, it is the application of omic technologies to assess endpoints of toxicology. Under this paradigm, transcription profiles are generated from as many genes as possible, preferably the entire genome of the organism, when exposed to a given model toxicant. This transcriptome data can then serve as a training set to develop a more predictive and robust assay based on the expression profiles of a smaller number of genes. Transcriptomics measures and analyzes the gene expression profiles in a cell, organ, or organism under the influence of chemical stressors or toxic compounds or in disease states, and may provide information on the molecular basis of the observed adverse effects [46]. Toxicogenomics has been applied successfully within aquatic toxicology to assist in chemical testing, determination of mechanisms, and environmental monitoring [47]. Furthermore, it is a thorough mean for high-throughput discovery of biomarkers using the latest technologies (genomics, transcriptomics, proteomics, and metabolomics) [48]. Examples of toxicogenomic applications include prediction of genotoxicity or carcinogenicity [49], target organ toxicity [50], and endocrine disruption [51]. Toxicologists have long used information from gene sequences, polymorphisms, and changes in transcript and protein levels to study mechanisms of toxicity and as biomarkers of susceptibility and toxicity. What is offered by the expansive nature of the omics technologies is the ability to probe more deeply the complexities of gene-environment interactions and the responses of biological pathways and networks to chemical perturbations in a higher throughput fashion.
ZEBRAFISH (Danio rerio)
The zebrafish has emerged as an important tool for studying the biological effects of hormones and EDCs [28]. The zebrafish is a small tropical freshwater fish which lives in rivers of northern India, northern Pakistan, Nepal, and Bhutan in South Asia [52]. This organism is frequently employed in toxicological studies [53], because of its multiple advantages such as small size, short generation time (approximately three to five months), high fecundity, and rapid ex utero development of embryos which are optically transparent. In contrast to many other fish species, adult zebrafish are about 3-5 cm in length, and they can be easily managed in large numbers in the laboratory [54]. Embryonic development of the zebrafish is very rapid in the first 72 hours [55]. After three to four months organisms are sexually mature and can generate new offspring. A single female on average can lay up to 200 eggs per week [56].
The zebrafish has become a popular model for new technologies, including toxicogenomics [47]. In the past, this model was primarily used in developmental and genetic research [57], but today this model is gaining importance in other fields. The availability of the complete genome sequence of zebrafish allowed the development of commercially available microarrays that offer a standardized tool set for zebrafish transcriptional profiling studies. The zebrafish is genetically closely related to humans and shares high similarity in developmental processes, physiology, and behavior [19]. In addition, the above-mentioned advantages offered by this model organism are a valuable resource for toxicologists not only to assess toxicity of EDCs, but also to dissect their mechanisms of toxicity.
Endocrine System of Zebrafish
For fish to be a relevant model for the study of endocrine systems of other vertebrates (including humans), they must be significantly similar in function. The endocrine system in fish consists of various glands located throughout the body which synthesize and secrete hormones to regulate an array of biological processes (Fig. 3). For example, the thyroid gland secretes the hormones thyroxine (T4) and triiodothyronine (T3), which aid fish in adapting to changes in temperature and osmotic stress. The pituitary and thyroid glands may also be targets for EDCs. One of the most studied pathways that can be affected by EDCs is the hypothalamic–pituitary–gonadal (HPG) axis, which has been employed to illustrate how EDCs can affect the endocrine system in fish. In addition, Shi et al. [58] reported that exposure of zebrafish embryos to perfluorooctanesulfonate (PFOS), a persistent compound widely distributed in the aquatic environment and wildlife, could alter gene expression in the hypothalamic-pituitary-thyroid (HPT) axis, and also disrupt the thyroid status at several steps in the synthesis, regulation, and action of thyroid hormones. Teleost hypothalamic hormones appear to have similar activities to mammalian equivalents. However, the additional roles found in some studies may point to a more complex system in the fish.
Fig. (3).
Schematic of the human and zebrafish endocrine system. [Abbreviations: ACTH: Adrenocorticotropin; ADH: Antidiuretic Hormone; FSH: Follicle-Stimulating Hormone; GH: Growth Hormone; LH: Luteinizing Hormone; PRL: Prolactin; T3: Triiodothyronine; T4: Thyroxine].
In vertebrates, gonadotropin-releasing hormone (GnRH) is the key factor controlling the activity of the reproductive axis by finely tuning the synthesis and release of the pituitary gonadotropins luteinizing hormone (lh) and follicle-stimulating hormone (fsh). In turn, these two hormones regulate gametogenesis and steroidogenesis in the gonads. The development and functioning of GnRH neurons are finely tuned by a series of factors, notably sex steroids, making these neurons potential targets for EDCs in aquatic species [59]. The gonadotropins released into systemic circulation elicit increased androgen and estrogen production by the gonads. Vorges et al. [60] reported that exposure of zebrafish to 17α-Ethinylestradiol, a potent synthetic estrogen, disrupted the ontogeny of the GnRH system by inducing an increase in the number of GnRH-ir neurons and GnRH fibers based on their immunoreactivity, as well as a decrease in the size of the GnRH-ir soma and a modification of the migration profile of GnRH-ir neurons. The major estrogen in female fish, E2, is produced primarily in the ovary by the follicular cells. Production of E2 in the zebrafish ovary follows similar synthesis pathways as other vertebrates. Exposure of an organism to levels of natural hormones or EDCs, which alter or interfere with the proper functioning of the endocrine system, has the potential to seriously affect the health of an organism and its progeny.
Toxic Effects of EDCs on Zebrafish
The use of fish as a model to reveal endocrine disrupters is justified because of the striking similarity of the hormonal system among vertebrates. Compared to other vertebrates, fish are easier and less expensive when involved in genetic and embryological manipulation. Zebrafish are widely used since they are very robust and spawn continuously. Zebrafish are one of a group of small fish species that can be kept in the laboratory, are easily exposed to EDCs in tank water at different stages in the life cycle, and exhibit measurable sensitivity to EDCs. Several studies have shown toxic effects of EDCs on zebrafish. Some of these have been related to organochlorine insecticides such as methoxychlor, endosulfan, and heptachlor. The use of methoxychlor increased following the ban of DDT in the USA in 1972. Methoxychlor is less environmentally persistent than DDT. In the case of endosulfan, fish are highly sensitive to low concentrations, with median lethal concentration (LC50) values reported in parts per billion (ppb) [61]. Heptachlor is also very toxic and highly persistent in the environment. Exposure of zebrafish larvae to these compounds showed that at 96 hours post fertilization (hpf) LC50 values were 0.24, 1.74, and 1.59 mg/L to endosulfan, heptachlor and methoxychlor, respectively. These data found the larvae to be most sensitive to endosulfan. Also, impacts following exposure to bromophenols are observed. Bromophenols are formed by the biodegradation of other pollutants such as brominated benzenes and some PBDEs; they may also be generated as byproducts of the photochemical degradation of tetrabromobisphenol A (TBBPA) in water or the decomposition of plastics. Among bromophenols, 2,4,6-tribromophenol (TBP) is the most widely produced brominated phenol. The effect of TBP was assessed on zebrafish embryos and it altered the sex ratio to a male-dominant state. The F1-generation larvae exhibited increased malformations, reduced survival, and retarded growth, suggesting that TBP in the aquatic environment has significant adverse effects on the fish population [62].
Embryonic exposure to butachlor (a chloroacetanilide herbicide widely employed in weed control of important crops) in zebrafish showed that butachlor was highly toxic, hindering the hatching process, resulting in a series of malformations, followed by mortality. The malformations observed included pericardial edema (PE) and yolk sac edema (YSE), which showed concentration-dependent responses. The mortalities were 93.3% and 100% for 16 μM and 20 μM exposure, respectively, and the LC50 value of butachlor at 84 hpf was 14 μM [63]. Triadimefon is another pesticide which disrupts zebrafish development [64]. The weight and length of female fish exposed to 0.5 μg/mL triadimefon were significantly lower than those of the control at 120 days. Meanwhile, male fish exposed to triadimefon (0.25 and 0.5 μg/mL) had decreased length and weight compared to the control fish.
Santos et al. [65] investigated whether exposure to estrogenic compounds can block the masculinizing effect of tributyltin (TBT). Five days post-fertilization zebrafish larvae were exposed for a four month period to TBT and to the synthetic estrogen EE2. These authors found that fish exposed to TBT showed a male sex bias (62.5% males in control tanks and 86% and 82% in TBT 25 ng/g and TBT 100 ng/g, respectively). Co-exposure to EE2 completely blocked the masculinizing effect of TBT, which suggests that in the aquatic environment the presence of estrogens may neutralize the fish masculinizing effect of TBT. However, zebrafish exposed to environmentally relevant concentrations of the androgenic steroid trenbolone acetate (TbA) metabolite Tb showed a strong and irreversible masculinization that raises concern about the effects of androgenic discharges in the aquatic environment [66].
EDC Toxicogenomic Studies Using Zebrafish
Fish populations constitute an important part of aquatic ecosystems. The accumulation of substances that can alter the endocrine system, may pose risks to the environment and human health. With regard to toxicogenomic approaches, several studies are reported for different EDCs using zebrafish as a model (Table S2 (323KB, pdf) ). One of the most studied compounds is BPA, which is commonly found in consumer plastic goods [67]. BPA was identified as a high priority for assessment of human health risk because it was considered to present the greatest potential for human exposure. Most studies of the health effects of BPA are focused on endocrine disruption leading to reproductive toxicity, but BPA also displays additional side effects including liver damage, disrupted pancreatic β-cell function, thyroid hormone disruption, and obesity-promoting effects [68]. BPA’s effects have been reported in wild freshwater fish, farmed freshwater fish, wild marine fish, and farmed marine fish collected in Taiwan (China) [38], and also in water, sediments, fish, clam, and river snail from Taihu Lake in China [69]. These results show that BPA is highly toxic to aquatic organisms. Recently Chow et al. [70] reported a study of the effects on zebrafish showing vtg1 expression after exposure of embryos at a concentration of 3.93 mg/L. Ji et al. [71] reported an exposure to 50 μg/L of Bisphenol AF (BAF) in livers. Vitellogenin is reported as a sensitive biomarker of estrogenic contamination in aquatic environments. In fish, vitellogenins are a class of phosphoglycolipoprotein that are precursors to yolk-proteins, and act to transport nutrients such as amino acids, lipids, and sugars into oocytes for the developing embryo. Hepatic vitellogenin synthesis is regulated through E2 activation of estrogen receptors (ERs) (zfERα, zfERβ1, and zfERβ2 in zebrafish), which dimerize and translocate into the nucleus to induce transcription [72]. In zebrafish, seven vitellogenin genes (vtg1-7) are expressed exclusively in the female liver and can be induced in the male liver by E2 with vtg1 suspected to be the dominant isoform [69]. Several studies in zebrafish suggest that vtg is expressed also in extrahepatic tissue due to exposure to different EDCs. Expression of vtg is also reported in the testis following exposure to EE2 [73].
Many pesticides in aquatic organisms are identified as EDCs, which are capable of impacting the reproduction, development, and survival of various fish species. The effects of these chemicals on zebrafish are reported for fungicides [64, 74-76], chloroacetanilide herbicides [63], phosphomethyl amino acids herbicides [77], and organochlorine insecticides [70, 78]. Roundup and its active ingredient glyphosate are among the most widely used herbicide worldwide and may contaminate surface waters. Uren Webster et al. [77] reported that exposure of zebrafish at a relatively high concentration of 10 mg/L Roundup or glyphosate induced changes in the transcript profiling of the gonads of cyp19a1 and esr1 in the ovary and hsd3b2, cat, and sod1 in the testis. These results showed that likely mechanisms of toxicity include disruption of the steroidogenic biosynthesis pathway and oxidative stress.
Phytoestrogens are plant-derived estrogens that are structurally similar to 17-β estradiol, the major endogenous estrogen present in females (also present in males), with the capability of exerting various biological effects comparable to those of estrogens. Isoflavones, coumestans, and lignans are three major categories of phytoestrogens. Genistein is an isoflavone that occurs naturally in soy and other legumes. Schiller et al. [79] reported that 48 hpf zebrafish embryos exposed to genistein (2200 μg/L) resulted in edema, head and tail malformations, and spontaneous movement, as well as a reduced blood circulation. In the same study, zebrafish microarrays resulted in the detection of 881 differentially expressed genes, which interfered significantly with multiple molecular pathways in 2 day old zebrafish. Corresponding to the microarray results, genes such as vtg1 (response to estradiol stimulus), cyp19a1b (response to estradiol stimulus), and sc4mol (steroid biosynthesis) were upregulated, whereas pax2a (thyroid gland development), nkx2.1 (thyroid gland development), hoxa9a, hoxa10b, and hoxa11b were downregulated at a concentration of 2400 μg/L. Meanwhile, Santos et al. [80] found downregulation in hormone receptor genes (esr2a, esr2b, and ar) at concentrations of 0.2 and 2 μg/L.
Of all the groups of EDCs, alkylphenols are considered to be the most prevalent, with special attention paid to octylphenol and NP. These chemicals are produced from the degradation of some alkylphenolethoxylates, non-ionic surfactants widely used in pesticides, spermicides, paints, wetting agents, textiles, plastics, and paper products. Octylphenol and NP are more stable, persistent, and toxic than their parent compounds and they can easily accumulate in biological tissues because of their lipophilicity [81]. Kazeto et al. [82] reported that para-nonylphenol, strongly enhanced the expression of the cyp19a2 gene in a dose-dependent manner. Transcript abundance of cyp19a2 in the para-nonylphenol-exposed group at the highest concentration (1 μM) was approximately 60 times higher than that of the control group. Puy-Azurmendi et al. [41] observed upregulation in cyp19a1b after exposure to commercial NP and alkyl-phenol isomers (33-octylphenol), which suggests that cyp19 genes would make excellent transcriptional targets for endocrine disruption. Aromatase is an enzyme that catalyzes the synthesis of estrogens, which are important in reproduction, gonad development, bone mineralization, glucose metabolism, behavior, and other functions. Aromatase is expressed mainly in gonads and, to a lesser extent, also in the brain, placenta, adipose tissue, skin, and endometrium. Cytochrome P450 aromatase is a crucial steroidogenic enzyme catalyzing the final, rate-limiting step in the conversion of androgens to estrogens. The zebrafish, and many other teleosts, have two aromatase genes (cyp19a1 and cyp19a2) that are expressed predominantly in the gonads and brain, respectively [83] cyp19a1b is the brain form of aromatase in zebrafish that is highly responsive to estrogens [62].
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous organic contaminants arising from incomplete combustion or pyrolysis of organic material. Benzo(a)pyrene (BaP) is a representative PAH, which is ubiquitous in the environment. Ren et al. [84] demonstrated that BaP (≥1 μM) can cause significant malformation and mortality in developing zebrafish embryos. Also, Huang et al. [85], with the help of microarray analysis, demonstrated that BaP was capable of inducing visual system developmental defects and dysfunction by perturbing photoreceptor development correlated genes. Other authors, including Kazeto et al. [82] found upregulation in cyp19a2 gene after exposure of zebrafish to BaP (10 μM).
Tributyltin is one of the organotin compounds which are used worldwide in agriculture and industry as a biocide, heat stabilizer, marine antifouling paint, and chemical catalytic converter. This compound gained notoriety as the chemical that induced masculinization of female gastropod mollusks, a condition known as imposex [86]; fish as well are reported as exhibiting masculinization [87]. Meanwhile, McGinnis and Crivello [88] examined the actions of TBT as an endocrine disruptor in zebrafish. They observed overexpression of genes such asdax1, sox9a, sf1 (brain), cyp19a, dax1, zfERβ1 (liver), and cyp19a (gonads), as well as decreased expression of genes such as sox9a and fst in the liver.
Phthalates are ubiquitous in the aquatic environment and are extensively used as plasticizers in many mass-produced products including food packaging, toys, electrical equipment, medical devices, paints, and cosmetics. Phthalates also enter surface waters via waste-water treatment effluents and from the atmosphere via plastic manufacturing and burning. The effects of mono-(2-ethylhexyl)phthalate (MEHP) on zebrafish larvae were reported by Zhai et al. [26]. These authors found that at a 200 μg/L concentration of MEHP, genes involved in the HPT system such as tshβ (thyroid hormone synthesis), pax8 (thyroid development), nkx2.1 (thyroid development), nis (thyroid hormone synthesis), tg (thyroid hormone synthesis), dio1 (thyronine deiodinase), dio2 (thyroid hormone metabolism), and ugt1ab (thyroid hormone metabolism) exhibited upregulation, while ttr (thyroid hormones binding) showed downregulation.
Finally, other compounds which have been identified in toxicogenomic studies are: brominated flame retardants (BD-209, TBBA), alkaloid in the tobacco plant (nicotine), estrogens (EE2), phenolic compounds (DE-71, TBP), aromatase inhibitor (fadrozole), synthetic progestin (MTA), chlorinated organophosphate (TDCPP), and organic perfluorinated compounds (PFOA). Zebrafish exposed to compounds such as EE2 [73], TBP (male) [62], and TBBA [70] exhibit an overexpression of vtg, while DE-71 [89], TBP (female) [62], and nicotine [90] produce decreased expression. Also, cytochrome cyp19 genes showed overexpression (cyp19a2, cyp19b in males, cyp19a in testis) and decreased expression (cyp19a in gonads, cyp19b in brain, cyp19a1b, cyp19a1a) in zebrafish exposed to EE2 [82], DE-71 [89], TBP [62], and nicotine [90]. EDCs such as EE2 [73] and DE-71[89] showed changes in expression of estrogen receptors (er-β), as well as PFOA [91], TDCPP [92], and BDE-209 [93] which presented changes in expression of pax8a gene involved in thyroid development.
Zebrafish As a Model of Human Endocrine Diseases
Traditional animal models and assays are currently being used to assess the potential human and ecological hazards and risks posed by tens of thousands of chemicals including those that can disrupt the endocrine system. The zebrafish presents as a complementary vertebrate model to assess endocrine disruption. The endocrine systems of zebrafish and humans are shown in (Fig. 3). Many of the same hormones and expression sites are shared between the zebrafish and humans. Hypothalamic hormones in fish have similar activities to mammalian equivalents, although the system in fish can be more complex. In addition, pituitary hormones in the zebrafish perform the same or similar functions as the mammalian pituitary hormones [94]. The thyroid gland in the adult zebrafish is not a compact structure encapsulated in connective tissue, but rather a loose aggregation of follicles, close to the ventral aorta, distributed between the first gill and the heart [95], but the thyroid gland develops from the same tissue as the thyroid in mammals, indicating that they are homologous structures [94]. Zebrafish do not possess an adrenal gland, but rather an intermingled group of cells located in the anterior kidney that produce steroid hormones [96]. The cells producing steroid hormones in this region are referred to as the interrenals. The interrenal cells produce cortisol, which regulates both metabolism and electrolyte balance. Pancreatic development in the zebrafish and mammals consists of both an endocrine component (the principal islet) and exocrine (parenchyma) component [97]. In short, endocrine function is a well-conserved process between zebrafish and mammals with the majority of hormones, hormone receptors, and activities being conserved [94].
The zebrafish model has been suggested as a model species to identify targets as well as modes of EDC action. In fact, the zebrafish has been found useful in EDC screening, in EDC effects assessment, and in studying targets and mechanisms of EDC action [44]. Since many of the environmental EDCs interfere with the sex steroid system of vertebrates, most EDC studies with zebrafish addressed disruption of sexual differentiation [73] and reproduction [98]. However, other targets of EDCs action must not be overlooked. For application of a species as a toxicological model, a good understanding of the biological traits of this species is a pre-requisite for the proper design of test protocols and endpoints as well as for the interpretation and extrapolation of the toxicological findings. Due to the genomic resources available for zebrafish and the long experience with zebrafish in toxicity testing, it is easily possible to establish molecular endpoints for EDC effects assessment. Additionally, genes expressed by different disruptors in zebrafish are orthologous to human genes (Table 2).
Table 2. Genes identified to be altered by endocrine disruptors using toxicogenomic tools in zebrafish.
| Zebrafish Gene | Human Gene | EDC(s) That Alter Expression in Zebrafish | References |
|---|---|---|---|
| amh | AMH | Estrogen | [103] |
| ar | AR | Phytoestrogens and aromatase inhibitor | [80] |
| bax | BAX | Insecticide | [78] |
| bcl2 | BCL2 | Pesticide and insecticide | [74, 78] |
| c3 | C3 | Phthalates | [104] |
| cat | CAT | Pesticides and insecticides | [77, 78] |
| cpt | CPT2 | Insecticide | [78] |
| Cu/Zn-sod | SOD1 | Pesticides and insecticides | [77, 78] |
| cxcl-clc | CXCL12 | Pesticides and phthalates | [74, 104] |
| cyp19a1a | CYP19A1 | Alkaloid in the tobacco plant Nicotiana tabacum | [90] |
| cyp19a1b | CYP19A1 | Alkaloid in the tobacco plant Nicotiana tabacum | [90] |
| esr1 | ESR1 | Pesticides, estrogen, aromatase inhibitor, and organic perfluorinated compounds | [77, 80, 91] |
| fshr | FSHR | Organic chemical | [71] |
| fshβ | FSHβ | Organic chemical and phenolic compounds | [71, 89] |
| hoxa11b | HOXA11 | Phytoestrogens | [79] |
| ifnγ | IFNY | Phthalates | [104] |
| IL-1β | IL-1β | Pesticide and phthalates | [63, 104] |
| lyz | LYZ | Phthalates | [104, 105] |
| Mn-sod | SOD3 | Insecticide | [78] |
| nkx2.1 | NKX2.1 | Phthalates, phytoestrogens, chlorinated organophosphate, and brominated flame retardants | [26, 79, 92, 93] |
| nr5a1b | NR5A1 | Estrogen | [73] |
| ogg1 | OGG1 | Pesticide | [74] |
| p53 | P53 | Pesticide, insecticide, and aromatase inhibitor | [74, 78, 80] |
| pax2a | PAX2 | Phytoestrogens | [79] |
| pax8 | PAX8 | Phthalates, organic perfluorinated compounds, chlorinated organophosphate, and brominated flame retardants | [26, 91-93] |
| pparα | PPARα | Insecticide | [78] |
| slc5a5 | SLC5A5 | Chlorinated organophosphate | [92] |
| sox9a | SOX9 | Estrogen and organotins | [73, 88] |
| tg | TG | Phthalates, chlorinated organophosphate, and brominated flame retardants | [26, 92, 93] |
| tnfα | TNF | Pesticide | [74] |
| tshβ | TSHβ | Phthalates, pesticide, Chlorinated organophosphate, and brominated flame retardants | [26, 75, 76, 92, 93] |
| ttr | TTR | Phthalates, pesticide, and brominated flame retardants | [26, 75, 93] |
Abbreviations: Anti-Mullerian hormone (amh), Androgen receptor (ar), Bcl2-associated X protein (bax), B-cell lymphoma/leukaemia-2gene (bcl2), complement factor C3B (c3), catalase (cat), arnitine palmitoyl transferase (cpt), Cu/Zn-superoxide dismutase (Cu/Zn-Sod), CC-chemokine (cxcl-clc), Cytochrome P450 19A1A (cyp19a1a), Cytochrome P450 19A1B (cyp19a1b), Estrogen receptor 1 (esr1), Follicle stimulating hormone receptor (fshr), Follicle stimulating hormone β (fshβ), homeobox a11b (hoxa11b), interferón γ (ifnγ), interleukin 1 β (IL-1β), lysozyme (lyz), manganese superoxide dismutase (Mn-sod), NK2 homeobox 1 (nkx2.1), nuclear receptor subfamily 5, group A, member 1b (nr5a1b), 8-oxoguanine DNA glycosylase 1 (ogg1), paired box 2a (pax2a), paired box 8 (pax8), peroxisome proliferator-activated receptors α (pparα), sodium/iodide symporter (slc5a5), SRY (sex determining region Y)-box 9a (sox9a), thyroglobulin (tg), tumor necrosis factor α (tnfα), thyroid-stimulating hormone (tshβ), Transthyretin (ttr).
CONCLUSION
Degradation of water quality is an emerging problem in many countries. Bioassays are an effective approach to monitor quality of water in aquatic environments. The pollutants capable of interfering with hormonal feedback regulations and metabolic pathways by mimicking endogenous hormones are termed EDCs. EDCs are a ubiquitous global problem with exposures occurring at home, in the office, on the farm, in the air we breathe, in food, and in water. The fate of EDCs depends on the environmental media where they exist, their liability to physicochemical reactions, and their tendency to bioaccumulate. The mechanism of endocrine disruption in a cell involves both genomic and non-genomic pathways. The ultimate effects of exposure are decreased fertility, increased birth defects, and altered sexual expression, among others. The use of toxicogenomic tools to assess pollutant responses in aquatic organisms has become widespread. The development of microarrays and the use of next generation sequencing for fish models has served to advance our understanding of the action of multiple chemicals including both those that are well-studied and those that are emerging. Zebrafish represent a cost-effective model for testing potential EDCs and determining mechanisms of toxicity. Overall considering the strengths, genetic similarities, and parallels of the zebrafish endocrine system with humans and other mammals, the zebrafish is an excellent complementary model for assessing and defining mechanisms of EDCs.
ACKNOWLEDGEMENTS
The authors thank the National Program for Doctoral Formation (COLCIENCIAS, 567-2012); COLCIENCIAS (Bogota, Colombia) and the University of Cartagena (Cartagena, Colombia), Grant RC 589-2013 (1107-569-33952); and the School of Health Sciences, Purdue University (West Lafayette, USA).
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s web site along with the published article.
REFERENCES
- 1.Padhye L.P., Yao H., Kung'u F.T., Huang C-H. Year-long evaluation on the occurrence and fate of pharmaceuticals, personal care products, and endocrine disrupting chemicals in an urban drinking water treatment plant. Water Res. 2014;51:266–276. doi: 10.1016/j.watres.2013.10.070. [DOI] [PubMed] [Google Scholar]
- 2.Schaider L.A., Rudel R.A., Ackerman J.M., Dunagan S.C., Brody J.G. Pharmaceuticals, perfluorosurfactants, and other organic wastewater compounds in public drinking water wells in a shallow sand and gravel aquifer. Sci. Total Environ. 2014;468:384–393. doi: 10.1016/j.scitotenv.2013.08.067. [DOI] [PubMed] [Google Scholar]
- 3.Morales M., Martínez-Paz P., Martín R., Planelló R., Urien J., Martínez-Guitarte J.L., Morcillo G. Transcriptional changes induced by in vivo exposure to pentachlorophenol (PCP) in Chironomus riparius (Diptera) aquatic larvae. Aquat. Toxicol. 2014;157:1–9. doi: 10.1016/j.aquatox.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Kolpin D.W., Furlong E.T., Meyer M.T., Thurman E.M., Zaugg S.D., Barber L.B., Buxton H.T. Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999-2000: a national reconnaissance. Sci. Total Environ. 2002;36(6):1202–1211. doi: 10.1021/es011055j. [DOI] [PubMed] [Google Scholar]
- 5.Petrovic M., Solé M., López De Alda M.J., Barceló D. Endocrine disruptors in sewage treatment plants, receiving river waters, and sediments: integration of chemical analysis and biological effects on feral carp. Environ. Toxicol. Chem. 2002;21(10):2146–2156. [PubMed] [Google Scholar]
- 6.Xu W., Yan W., Huang W., Miao L., Zhong L. Endocrine-disrupting chemicals in the Pearl River Delta and coastal environment: sources, transfer, and implications. Environ. Geochem. Health. 2014;36(6):1095–1104. doi: 10.1007/s10653-014-9618-3. a. [DOI] [PubMed] [Google Scholar]
- 7.Örn S., Yamani S., Norrgren L. Comparison of vitellogenin induction, sex ratio, and gonad morphology between zebrafish and Japanese medaka after exposure to 17α-ethinylestradiol and 17β-trenbolone. Arch. Environ. Contam. Toxicol. 2006;51(2):237–243. doi: 10.1007/s00244-005-0103-y. [DOI] [PubMed] [Google Scholar]
- 8.Xu H., Yang J., Wang Y., Jiang Q., Chen H., Song H. Exposure to 17α-ethynylestradiol impairs reproductive functions of both male and female zebrafish (Danio rerio). Aquat. Toxicol. 2008;88(1):1–8. doi: 10.1016/j.aquatox.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 9.Van den Belt K., Wester P.W., van der Ven L., Verheyen R., Witters H. Effects of ethynylestradiol on the reproductive physiology in zebrafish (Danio rerio): time dependency and reversibility. Environ. Toxicol. Chem. 2002;21(4):767–775. [PubMed] [Google Scholar]
- 10.Fenske M., Segner H. Aromatase modulation alters gonadal differentiation in developing zebrafish (Danio rerio). Aquat. Toxicol. 2004;67(2):105–126. doi: 10.1016/j.aquatox.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Brandenburg A. 2014. [Google Scholar]
- 12.Miracle A.L., Ankley G.T. Ecotoxicogenomics: linkages between exposure and effects in assessing risks of aquatic contaminants to fish. Reprod. Toxicol. 2005;19(3):321–326. doi: 10.1016/j.reprotox.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Reyero N., Denslow N.D. Applications of genomic technologies to the study of organochlorine pesticide-induced reproductive toxicity in fish. J. Pestic. Sci. 2006;31(3):252–262. [Google Scholar]
- 14.Lam S.H., Hlaing M.M., Zhang X., Yan C., Duan Z., Zhu L., Ung C.Y., Mathavan S., Ong C.N., Gong Z. Toxicogenomic and phenotypic analyses of bisphenol-A early-life exposure toxicity in zebrafish. PLoS One. 2011;6(12):e28273. doi: 10.1371/journal.pone.0028273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derks K.W., Hoeijmakers J.H., Pothof J. The DNA damage response: The omics era and its impact. DNA Repair (Amst.) 2014;19:214–220. doi: 10.1016/j.dnarep.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahman A.M., Pawling J., Ryczko M., Caudy A.A., Dennis J.W. Targeted metabolomics in cultured cells and tissues by mass spectrometry: Method development and validation. Anal. Chim. Acta. 2014;845:53–61. doi: 10.1016/j.aca.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Reamon-Buettner S.M., Mutschler V., Borlak J. The next innovation cycle in toxicogenomics: environmental epigenetics. Mutat. Res. Rev. Mutat. Res. 2008;659(1):158–165. doi: 10.1016/j.mrrev.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Ankley G.T., Johnson R.D. Small fish models for identifying and assessing the effects of endocrine-disrupting chemicals. ILAR J. 2004;45(4):469–483. doi: 10.1093/ilar.45.4.469. [DOI] [PubMed] [Google Scholar]
- 19.Howe K., Clark M.D., Torroja C.F., Torrance J., Berthelot C., Muffato M., Collins J.E., Humphray S., McLaren K., Matthews L. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496(7446):498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.EPA, Environmental Protection Agency (EPA) Endocrine Disruptor Research. 2014.
- 21.Dominguez G.A., Bisesi J.H., Kroll K.J., Denslow N.D., Sabo-Attwood T. Control of Transcriptional Repression of the Vitellogenin Receptor Gene in Largemouth Bass (Micropterus Salmoides) by Select Estrogen Receptors Isotypes. Toxicol. Sci. 2014;141(2):423–431. doi: 10.1093/toxsci/kfu145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Söffker M., Tyler C.R. Endocrine disrupting chemicals and sexual behaviors in fish-a critical review on effects and possible consequences. Crit. Rev. Toxicol. 2012;42(8):653–668. doi: 10.3109/10408444.2012.692114. [DOI] [PubMed] [Google Scholar]
- 23.Chung E., Genco M.C., Megrelis L., Ruderman J.V. Effects of bisphenol A and triclocarban on brain-specific expression of aromatase in early zebrafish embryos. Proc. Natl. Acad. Sci. USA. 2011;108(43):17732–17737. doi: 10.1073/pnas.1115187108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu M., Xu H., Shen Y., Qiu W., Yang M. Oxidative stress in zebrafish embryos induced by short‐term exposure to bisphenol A, nonylphenol, and their mixture. Environ. Toxicol. Chem. 2011;30(10):2335–2341. doi: 10.1002/etc.634. [DOI] [PubMed] [Google Scholar]
- 25.Yu L., Lam J.C., Guo Y., Wu R.S., Lam P.K., Zhou B. Parental transfer of polybrominated diphenyl ethers (PBDEs) and thyroid endocrine disruption in zebrafish. Environ. Toxicol. Chem. 2011;45(24):10652–10659. doi: 10.1021/es2026592. [DOI] [PubMed] [Google Scholar]
- 26.Zhai W., Huang Z., Chen L., Feng C., Li B., Li T. Thyroid endocrine disruption in zebrafish larvae after exposure to mono-(2-ethylhexyl) phthalate (MEHP). PLoS One. 2014;9(3):e92465. doi: 10.1371/journal.pone.0092465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arukwe A., Olufsen M., Cicero N., Hansen M.D. Effects on Development, Growth Responses and Thyroid-Hormone Systems in Eyed-Eggs and Yolk-Sac Larvae of Atlantic Salmon (Salmo salar) Continuously Exposed to 3, 3′, 4, 4′-Tetrachlorobiphenyl (PCB-77). J. Toxicol. Environ. Health A. 2014;77(9-11):574–586. doi: 10.1080/15287394.2014.887422. [DOI] [PubMed] [Google Scholar]
- 28.Naderi M., Wong M.Y., Gholami F. Developmental exposure of zebrafish (Danio rerio) to bisphenol-S impairs subsequent reproduction potential and hormonal balance in adults. Aquat. Toxicol. 2014;148:195–203. doi: 10.1016/j.aquatox.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Xu N., Chen P., Liu L., Zeng Y., Zhou H., Li S. Effects of combined exposure to 17α-ethynylestradiol and dibutyl phthalate on the growth and reproduction of adult male zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2014;107:61–70. doi: 10.1016/j.ecoenv.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Shanmugam G., Ramasamy K., Selvaraj K.K., Sampath S., Ramaswamy B.R. Triclosan in Fresh Water Fish Gibelion Catla from the Kaveri River, India, and Its Consumption Risk Assessment. Environ. Forensics. 2014;15(3):207–212. [Google Scholar]
- 31.Lee S-H., Ra J-S., Choi J-W., Yim B-J., Jung M-S., Kim S-D. Human health risks associated with dietary exposure to persistent organic pollutants (POPs) in river water in Korea. Sci. Total Environ. 2014;470:1362–1369. doi: 10.1016/j.scitotenv.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 32.Roggio M., Guyón N., Hued A., Amé M., Valdés M., Giojalas L., Wunderlin D., Bistoni M. Effects of the synthetic estrogen 17α-ethinylestradiol on aromatase expression, reproductive behavior and sperm quality in the fish Jenynsia multidentata. Bull. Environ. Contam. Toxicol. 2014;92(5):579–584. doi: 10.1007/s00128-013-1185-2. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y., Zhou J. Endocrine disrupting chemicals in aquatic environments: A potential reason for organism extinction? Aquat. Ecosyst. Health Manage. 2013;16(1):88–93. a. [Google Scholar]
- 34.Baumann L., Knörr S., Keiter S., Nagel T., Segner H., Braunbeck T. 2014. [DOI] [PubMed]
- 35.Olivero-Verbel J., Agudelo-Frias D., Caballero-Gallardo K. Morphometric parameters and total mercury in eggs of snowy egret (Egretta thula) from Cartagena Bay and Totumo Marsh, north of Colombia. Mar. Pollut. Bull. 2013;69(1):105–109. doi: 10.1016/j.marpolbul.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 36.Barnhoorn I., Bornman M., Pieterse G., Van Vuren J. Histological evidence of intersex in feral sharptooth catfish (Clarias gariepinus) from an estrogen‐polluted water source in Gauteng, South Africa. Environ. Toxicol. 2004;19(6):603–608. doi: 10.1002/tox.20068. [DOI] [PubMed] [Google Scholar]
- 37.da Silva A.O., Martinez C.B. Acute effects of cadmium on osmoregulation of the freshwater teleost Prochilodus lineatus: Enzymes activity and plasma ions. Aquat. Toxicol. 2014;156:161–168. doi: 10.1016/j.aquatox.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Lee C-C., Jiang L-Y., Kuo Y-L., Chen C-Y., Hsieh C-Y., Hung C-F., Tien C-J. Characteristics of nonylphenol and bisphenol A accumulation by fish and implications for ecological and human health. Sci. Total Environ. 2015;502:417–425. doi: 10.1016/j.scitotenv.2014.09.042. [DOI] [PubMed] [Google Scholar]
- 39.Nevárez M., Leal L.O., Moreno M. Estimation of Seasonal Risk. Caused by the Intake of Lead, Mercury and Cadmium through Freshwater Fish Consumption from Urban Water Reservoirs in Arid Areas of Northern Mexico. Int. J. Environ. Res. Public Health. 2015;12(2):1803–1816. doi: 10.3390/ijerph120201803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaube R., Gautam G.J., Joy K.P. Teratogenic effects of 4-nonylphenol on early embryonic and larval development of the catfish Heteropneustes fossilis. Arch. Environ. Contam. Toxicol. 2013;64(4):554–561. doi: 10.1007/s00244-012-9851-7. [DOI] [PubMed] [Google Scholar]
- 41.Puy-Azurmendi E., Olivares A., Vallejo A., Ortiz-Zarragoitia M., Piña B., Zuloaga O., Cajaraville M. Estrogenic effects of nonylphenol and octylphenol isomers in vitro by recombinant yeast assay (RYA) and in vivo with early life stages of zebrafish. Sci. Total Environ. 2014;466:1–10. doi: 10.1016/j.scitotenv.2013.06.060. [DOI] [PubMed] [Google Scholar]
- 42.Zheng B., Liu R., Liu Y., Jin F., An L. Phenolic endocrine-disrupting chemicals and intersex in wild crucian carp from Hun River, China. Chemosphere. 2015;120:743–749. doi: 10.1016/j.chemosphere.2014.10.049. [DOI] [PubMed] [Google Scholar]
- 43.Mortazavi S., Bakhtiari A.R., Sari A.E., Bahramifar N., Rahbarizadeh F. Occurrence of Endocrine Disruption Chemicals (Bisphenol A, 4-Nonylphenol, and Octylphenol) in Muscle and Liver of, Cyprinus Carpino Common, from Anzali Wetland, Iran. Bull. Environ. Contam. Toxicol. 2013;90(5):578–584. doi: 10.1007/s00128-013-0964-0. [DOI] [PubMed] [Google Scholar]
- 44.Ankley G.T., Bencic D.C., Breen M.S., Collette T.W., Conolly R.B., Denslow N.D., Edwards S.W., Ekman D.R., Garcia-Reyero N., Jensen K.M. Endocrine disrupting chemicals in fish: developing exposure indicators and predictive models of effects based on mechanism of action. Aquat. Toxicol. 2009;92(3):168–178. doi: 10.1016/j.aquatox.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 45.Frawley R., Germolec D., Loveren H. V. 2014.
- 46.Ellinger-Ziegelbauer H., Ahr H-J. Omics in Toxicology. Regulatory Toxicol; 2014. pp. 173–179. [Google Scholar]
- 47.Williams T.D., Mirbahai L., Chipman J.K. The toxicological application of transcriptomics and epigenomics in zebrafish and other teleosts. Brief. Funct. Genomics. 2014;13(2):157–171. doi: 10.1093/bfgp/elt053. [DOI] [PubMed] [Google Scholar]
- 48.Collings F.B., Vaidya V.S. Novel technologies for the discovery and quantitation of biomarkers of toxicity. Toxicology. 2008;245(3):167–174. doi: 10.1016/j.tox.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ellinger-Ziegelbauer H., Aubrecht J., Kleinjans J.C., Ahr H-J. Application of toxicogenomics to study mechanisms of genotoxicity and carcinogenicity. Toxicol. Lett. 2009;186(1):36–44. doi: 10.1016/j.toxlet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 50.Umbright C., Sellamuthu R., Li S., Kashon M., Luster M., Joseph P. Blood gene expression markers to detect and distinguish target organ toxicity. Mol. Cell. Biochem. 2010;335(1-2):223–234. doi: 10.1007/s11010-009-0272-5. [DOI] [PubMed] [Google Scholar]
- 51.Larkin P., Knoebl I., Denslow N. Differential gene expression analysis in fish exposed to endocrine disrupting compounds. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2003;136(2):149–161. doi: 10.1016/s1096-4959(03)00228-8. [DOI] [PubMed] [Google Scholar]
- 52.Sharma P., Sharma S., Patial V., Singh D., Padwad Y.S. Zebrafish (Danio rerio): A potential model for nephroprotective drug screening. Clinical Queries: Nephrol. 2014;3(2):97–105. [Google Scholar]
- 53.Dai Y.J., Jia Y.F., Chen N., Bian W.P., Li Q.K., Ma Y.B., Chen Y.L., Pei D.S. Zebrafish as a model system to study toxicology. Environ. Toxicol. Chem. 2014;33(1):11–17. doi: 10.1002/etc.2406. [DOI] [PubMed] [Google Scholar]
- 54.ZFIN 2014.
- 55.Kimmel C.B., Ballard W.W., Kimmel S.R., Ullmann B., Schilling T.F. Stages of embryonic development of the zebrafish. . Dev. Dyn. 1995;203(3):253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 56.Vliegenthart A., Tucker C.S., Del Pozo J., Dear J.W. Zebrafish as model organisms for studying drug-induced liver injury. Br. J. Clin. Pharmacol. 2014;78(6):1217–1227. doi: 10.1111/bcp.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hill A.J., Teraoka H., Heideman W., Peterson R.E. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005;86(1):6–19. doi: 10.1093/toxsci/kfi110. [DOI] [PubMed] [Google Scholar]
- 58.Shi X., Liu C., Wu G., Zhou B. Waterborne exposure to PFOS causes disruption of the hypothalamus–pituitary–thyroid axis in zebrafish larvae. Chemosphere. 2009;77(7):1010–1018. doi: 10.1016/j.chemosphere.2009.07.074. [DOI] [PubMed] [Google Scholar]
- 59.Clelland E., Peng C. Endocrine/paracrine control of zebrafish ovarian development. Mol. Cell. Endocrinol. 2009;312(1):42–52. doi: 10.1016/j.mce.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 60.Vosges M., Le Page Y., Chung B-C., Combarnous Y., Porcher J-M., Kah O., Brion F. 17α-Ethinylestradiol disrupts the ontogeny of the forebrain GnRH system and the expression of brain aromatase during early development of zebrafish. Aquat. Toxicol. 2010;99(4):479–491. doi: 10.1016/j.aquatox.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 61.Jonsson C.M., Toledo M.C. Bioaccumulation and elimination of endosulfan in the fish yellow tetra (Hyphessobrycon bifasciatus). Bull. Environ. Contam. Toxicol. 1993;50(4):572–577. doi: 10.1007/BF00191248. [DOI] [PubMed] [Google Scholar]
- 62.Deng J., Liu C., Yu L., Zhou B. Chronic exposure to environmental levels of tribromophenol impairs zebrafish reproduction. Toxicol. Appl. Pharmacol. 2010;243(1):87–95. doi: 10.1016/j.taap.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 63.Tu W., Niu L., Liu W., Xu C. Embryonic exposure to butachlor in zebrafish (Danio rerio): endocrine disruption, developmental toxicity and immunotoxicity. Ecotoxicol. Environ. Saf. 2013;89:189–195. doi: 10.1016/j.ecoenv.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 64.Liu S-Y., Jin Q., Huang X-H., Zhu G-N. Disruption of zebrafish (Danio rerio) sexual development after full life-cycle exposure to environmental levels of triadimefon. Environ. Toxicol. Pharmacol. 2014;37(1):468–475. doi: 10.1016/j.etap.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 65.Santos M., Micael J., Carvalho A., Morabito R., Booy P., Massanisso P., Lamoree M., Reis-Henriques M. Estrogens counteract the masculinizing effect of tributyltin in zebrafish. Comp. Biochem. Physiol. C Pharmacol. 2006;142(1):151–155. doi: 10.1016/j.cbpc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 66.Morthorst J.E., Holbech H., Bjerregaard P. Trenbolone causes irreversible masculinization of zebrafish at environmentally relevant concentrations. Aquat. Toxicol. 2010;98(4):336–343. doi: 10.1016/j.aquatox.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 67.Alenazi N.A., Manthorpe J.M., Lai E.P. Selective extraction of BPA in milk analysis by capillary electrophoresis using a chemically modified molecularly imprinted polymer. Food Contr. 2015;50:778–783. [Google Scholar]
- 68.Le Corre L., Besnard P., Chagnon M-C. BPA, an energy balance disruptor. Crit. Rev. Food Sci. Nutr. 2015;55(6):769–777. doi: 10.1080/10408398.2012.678421. [DOI] [PubMed] [Google Scholar]
- 69.Wang Y., Wang Q., Hu L., Lu G., Li Y. Occurrence of estrogens in water, sediment and biota and their ecological risk in Northern Taihu Lake in China. Environ. Geochem. Health. 2015;37(1):147–156. doi: 10.1007/s10653-014-9637-0. [DOI] [PubMed] [Google Scholar]
- 70.Chow W.S., Chan W.K., Chan K.M. Toxicity assessment and vitellogenin expression in zebrafish (Danio rerio) embryos and larvae acutely exposed to bisphenol A, endosulfan, heptachlor, methoxychlor and tetrabromobisphenol A. J. Appl. Toxicol. 2013;33(7):670–678. doi: 10.1002/jat.2723. [DOI] [PubMed] [Google Scholar]
- 71.Ji K., Hong S., Kho Y., Choi K. Effects of bisphenol S exposure on endocrine functions and reproduction of zebrafish. Environ. Sci. Technol. 2013;47(15):8793–8800. doi: 10.1021/es400329t. [DOI] [PubMed] [Google Scholar]
- 72.Menuet A., Pellegrini E., Anglade I., Blaise O., Laudet V., Kah O., Pakdel F. Molecular characterization of three estrogen receptor forms in zebrafish: binding characteristics, transactivation properties, and tissue distributions. Biol. Reprod. 2002;66(6):1881–1892. doi: 10.1095/biolreprod66.6.1881. [DOI] [PubMed] [Google Scholar]
- 73.Reyhanian Caspillo N.V. K.; Hallgren, S.; Olsson, P.E.; Porsch-Hällström, I. Short-term treatment of adult male zebrafish (Danio rerio) with 17α-ethinyl estradiol affects the transcription of genes involved in development and male sex differentiation. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2014;164:35–42. doi: 10.1016/j.cbpc.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 74.Jiang J., Wu S., Wu C., An X., Cai L., Zhao X. Embryonic exposure to carbendazim induces the transcription of genes related to apoptosis, immunotoxicity and endocrine disruption in zebrafish (Danio rerio). Fish Shellfish Immunol. 2014;41(2):493–500. doi: 10.1016/j.fsi.2014.09.037. [DOI] [PubMed] [Google Scholar]
- 75.Yu L., Chen M., Liu Y., Gui W., Zhu G. Thyroid endocrine disruption in zebrafish larvae following exposure to hexaconazole and tebuconazole. Aquat. Toxicol. 2013;138:35–42. doi: 10.1016/j.aquatox.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 76.Yu L.Q., Zhao G.F., Feng M., Wen W., Li K., Zhang P.W., Peng X., Huo W.J., Zhou H.D. Chronic exposure to pentachlorophenol alters thyroid hormones and thyroid hormone pathway mRNAs in zebrafish. Environ. Toxicol. Chem. 2014;33(1):170–176. doi: 10.1002/etc.2408. [DOI] [PubMed] [Google Scholar]
- 77.Uren Webster T.M., Laing L.V., Florance H., Santos E.M. Effects of glyphosate and its formulation, Roundup, on reproduction in Zebrafish (Danio rerio). Environ. Sci. Technol. 2014;48(2):1271–1279. doi: 10.1021/es404258h. [DOI] [PubMed] [Google Scholar]
- 78.Jin Y., Zheng S., Pu Y., Shu L., Sun L., Liu W., Fu Z. Cypermethrin has the potential to induce hepatic oxidative stress, DNA damage and apoptosis in adult zebrafish (Danio rerio). Chemosphere. 2011;82(3):398–404. doi: 10.1016/j.chemosphere.2010.09.072. [DOI] [PubMed] [Google Scholar]
- 79.Schiller V., Wichmann A., Kriehuber R., Muth-Köhne E., Giesy J.P., Hecker M., Fenske M. Studying the effects of genistein on gene expression of fish embryos as an alternative testing approach for endocrine disruption. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2013;157(1):41–53. doi: 10.1016/j.cbpc.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 80.Santos D., Matos M., Coimbra A.M. Developmental toxicity of endocrine disruptors in early life stages of zebrafish, a genetic and embryogenesis study. Neurotoxicol. Teratol. 2014;46:18–25. doi: 10.1016/j.ntt.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 81.USEPA 2005.
- 82.Kazeto Y., Place A.R., Trant J.M. Effects of endocrine disrupting chemicals on the expression of CYP19 genes in zebrafish (Danio rerio) juveniles. Aquat. Toxicol. 2004;69(1):25–34. doi: 10.1016/j.aquatox.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 83.Goto-Kazeto R., Kight K.E., Zohar Y., Place A.R., Trant J.M. Localization and expression of aromatase mRNA in adult zebrafish. Gen. Comp. Endocrinol. 2004;139(1):72–84. doi: 10.1016/j.ygcen.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 84.Ren X., Lu F., Cui Y., Wang X., Bai C., Chen J., Huang C., Yang D. Protective effects of genistein and estradiol on PAHs-induced developmental toxicity in zebrafish embryos. Hum. Exp. Toxicol. 2012;31(11):1161–1169. doi: 10.1177/0960327112450900. [DOI] [PubMed] [Google Scholar]
- 85.Huang L., Zuo Z., Zhang Y., Wu M., Lin J.J., Wang C. Use of toxicogenomics to predict the potential toxic effect of benzo (a) pyrene on zebrafish embryos: ocular developmental toxicity. Chemosphere. 2014;108:55–61. doi: 10.1016/j.chemosphere.2014.02.078. [DOI] [PubMed] [Google Scholar]
- 86.Abidli S., Santos M.M., Lahbib Y., Castro L.F., Reis-Henriques M.A., El Menif N.T. Tributyltin (TBT) effects on Hexaplex trunculus and Bolinus brandaris (Gastropoda: Muricidae): imposex induction and sex hormone levels insights. Ecol. Indic. 2012;13(1):13–21. [Google Scholar]
- 87.Shimasaki Y., Kitano T., Oshima Y., Inoue S., Imada N., Honjo T. Tributyltin causes masculinization in fish. Environ. Toxicol. Chem. 2003;22(1):141–144. [PubMed] [Google Scholar]
- 88.McGinnis C.L., Crivello J.F. Elucidating the mechanism of action of tributyltin (TBT) in zebrafish. Aquat. Toxicol. 2011;103(1):25–31. doi: 10.1016/j.aquatox.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 89.Yu L., Liu C., Chen Q., Zhou B. Endocrine disruption and reproduction impairment in zebrafish after long-term exposure to DE-71. Environ. Toxicol. Chem. 2014;33(6):1354–1362. doi: 10.1002/etc.2562. b. [DOI] [PubMed] [Google Scholar]
- 90.Kanungo J., Cuevas E., Guo X., Lopez A.G., Ramirez-Lee M.A., Trickler W., Paule M.G., Ali S.F. Nicotine alters the expression of molecular markers of endocrine disruption in zebrafish. Neurosci. Lett. 2012;526(2):133–137. doi: 10.1016/j.neulet.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 91.Du G., Huang H., Hu J., Qin Y., Wu D., Song L., Xia Y., Wang X. Endocrine-related effects of perfluorooctanoic acid (PFOA) in zebrafish, H295R steroidogenesis and receptor reporter gene assays. Chemosphere. 2013;91(8):1099–1106. doi: 10.1016/j.chemosphere.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 92.Wang Q., Liang K., Liu J., Yang L., Guo Y., Liu C., Zhou B. Exposure of zebrafish embryos/larvae to TDCPP alters concentrations of thyroid hormones and transcriptions of genes involved in the hypothalamic–pituitary–thyroid axis. Aquat. Toxicol. 2013;126:207–213. doi: 10.1016/j.aquatox.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 93.Chen Q., Yu L., Yang L., Zhou B. Bioconcentration and metabolism of decabromodiphenyl ether (BDE-209) result in thyroid endocrine disruption in zebrafish larvae. Aquat. Toxicol. 2012;110:141–148. doi: 10.1016/j.aquatox.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 94.McGonnell I., Fowkes R. Fishing for gene function–endocrine modelling in the zebrafish. J. Endocrinol. 2006;189(3):425–439. doi: 10.1677/joe.1.06683. [DOI] [PubMed] [Google Scholar]
- 95.Wendl T., Lun K., Mione M., Favor J., Brand M., Wilson S.W., Rohr K.B. Pax2. 1 is required for the development of thyroid follicles in zebrafish. Development. 2002;129(15):3751–3760. doi: 10.1242/dev.129.15.3751. [DOI] [PubMed] [Google Scholar]
- 96.Gallo V.P., Civinini A. Survey of the adrenal homolog in teleosts. Int. Rev. Cytol. 2003;230:89–187. doi: 10.1016/s0074-7696(03)30003-8. [DOI] [PubMed] [Google Scholar]
- 97.Argenton F., Zecchin E., Bortolussi M. Early appearance of pancreatic hormone-expressing cells in the zebrafish embryo. Mech. Dev. 1999;87(1):217–221. doi: 10.1016/s0925-4773(99)00151-3. [DOI] [PubMed] [Google Scholar]
- 98.Han J., Wang Q., Wang X., Li Y., Wen S., Liu S., Ying G., Guo Y., Zhou B. The synthetic progestin megestrol acetate adversely affects zebrafish reproduction. Aquat. Toxicol. 2014;150:66–72. doi: 10.1016/j.aquatox.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 99.Yu Y., Wu L. 2014.
- 100.Copat C., Arena G., Fiore M., Ledda C., Fallico R., Sciacca S., Ferrante M. Heavy metals concentrations in fish and shellfish from eastern Mediterranean Sea: consumption advisories. Food Chem. Toxicol. 2013;53:33–37. doi: 10.1016/j.fct.2012.11.038. [DOI] [PubMed] [Google Scholar]
- 101.Olivero-Verbel J., Caballero-Gallardo K., Turizo-Tapia A. 2014. [DOI] [PubMed]
- 102.Olivero-Verbel J., Caballero-Gallardo K. Nematode and mercury content in freshwater fish belonging to different trophic levels. Parasitol. Res. 2013;112(6):2187–2195. doi: 10.1007/s00436-013-3378-3. [DOI] [PubMed] [Google Scholar]
- 103.Caspillo N.R., Volkova K., Hallgren S., Olsson P-E., Porsch-Hällström I. Short-term treatment of adult male zebrafish (Danio rerio) with 17α-ethinyl estradiol affects the transcription of genes involved in development and male sex differentiation. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2014;164:35–42. doi: 10.1016/j.cbpc.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 104.Xu H., Shao X., Zhang Z., Zou Y., Wu X., Yang L. Oxidative stress and immune related gene expression following exposure to di-n-butyl phthalate and diethyl phthalate in zebrafish embryos. Ecotoxicol. Environ. Saf. 2013;93:39–44. doi: 10.1016/j.ecoenv.2013.03.038. [DOI] [PubMed] [Google Scholar]
- 105.Bradley P.M., Kolpin D.W. Managing the effects of endocrine disrupting chemicals in wastewater-impacted streams. INTECH Open Access Publisher; 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher’s web site along with the published article.