Abstract
Background & Aims
Non-alcoholic fatty liver disease (NAFLD) is highly prevalent and is associated with development of metabolic disease including atherosclerotic cardiovascular disease (CVD). Our aim is to examine the association of hepatic steatosis with prevalent clinical and subclinical CVD outcomes in a large community-based sample, the Framingham Heart Study.
Methods
Hepatic steatosis was measured in 3529 participants using multidetector computed tomography scanning. Multivariable logistic regression was used to determine whether hepatic steatosis is associated with prevalent CVD adjusted for covariates. We also tested whether associations were independent of other metabolic diseases/traits. The primary clinical outcome was composite prevalent clinical CVD defined by prior non-fatal myocardial infarction, stroke, transient ischemic attack, heart failure, or peripheral arterial disease. Subclinical cardiovascular outcomes were coronary artery calcium (CAC) and abdominal artery calcium (AAC).
Results
3014 participants were included (50.5% women). There was a non-significant association of hepatic steatosis with clinical CVD (OR 1.14 [p = 0.07]). Hepatic steatosis was associated with both CAC and AAC (OR 1.20 [p <0.001] and OR 1.16 [p <0.001], respectively). Associations persisted for CAC even when controlling for other risk factors/metabolic diseases, but for AAC, the associations became non-significant after adjustment for visceral adipose tissue. The association between hepatic steatosis and AAC was stronger in men than in women (p sex interaction = 0.022).
Conclusion
There was a significant association of hepatic steatosis with subclinical CVD outcomes independent of many metabolic diseases/traits with a trend towards association between hepatic steatosis and clinical CVD outcomes. The association with AAC was stronger in men than in women.
Keywords: Fatty liver, Abdominal aortic calcium, Coronary artery calcium, Cardiovascular disease
Introduction
As the most common liver disease in the United States, non-alcoholic fatty liver disease (NAFLD) affects approximately 30% of the population with prevalence rising to 70–90% in diabetics [1,2]. The high prevalence of NAFLD is expected to increase in the coming years due to the continued rise in diabetes and obesity [2]. Previous studies have found that NAFLD is associated with the presence of the metabolic syndrome, defined by the Adult Treatment Panel (ATP) III guidelines as the presence of at least three of the following: hypertension (HTN), hypertriglyceridemia, low high-density lipoprotein (HDL), impaired fasting glucose, and central adiposity [3–5].
In addition, NAFLD has also been associated with atherosclerotic clinical and subclinical cardiovascular disease (CVD) outcomes, though many of these studies have been performed predominantly in diabetic populations [5–9]. However, fewer large-scale population-based studies have evaluated the association of NAFLD with both clinical and subclinical cardiovascular outcomes. Subclinical CVD, in particular coronary artery calcium (CAC), is associated with increased risk of CVD events and has been shown to be both an independent predictor of CVD and to improve discrimination for risk-stratifying patients for CVD [10–12]. Though some studies have shown an association between NAFLD and CAC, it remains unclear whether and to what extent NAFLD relates to the risk of clinical and subclinical CVD outcomes in men and women in the community [9,13].
The purpose of this study is to examine the association of NAFLD, determined by multidetector CT scan, with prevalent clinical and subclinical CVD outcomes in a large, well-characterized, prospective population-based cohort, the Framingham Heart Study.
Methods
Sample
Study participants were taken from the Framingham Heart Study, a multigenerational prospective cohort study initiated in 1948 to study the incidence of coronary heart disease (CHD) as well as to identify risk factors. Selection of the initial cohort of 5209 residents of Framingham, MA has been described previously [14]. The original cohort has been followed at regular intervals with biennial history and physical examinations as well as collection of risk factor data, including imaging and lab data. In 1971, the Offspring Study began, enrolling 5124 members of the initial cohort's offspring and their spouses. These study members are also followed at regular intervals with history and physical exams every 4–8 years, as well as similar risk factor data collection like the primary cohort [15]. In 2002, 4095 third generation members and their spouses were recruited and enrolled in the study and have undergone two exams to date [16].
Multidetector CT for measurement of hepatic steatosis and vascular calcium
Between 2002 and 2005, multidetector CT scans were performed on 3529 members of both the offspring and third generation cohorts (1418 from the offspring study and 2111 from the third generation study). The inclusion criteria and overall protocol for the multidetector CT scan has been previously described [3,17]. Inclusion criteria included patients who still resided in the New England area, those older than 35 years in men and 40 in women, and negative pregnancy screening. Exclusion criteria included confirmed pregnancy and weight >160 kilograms. Additionally, subjects were excluded if CT scan results were uninterpretable for hepatic steatosis (n = 323) or if they did not attend the offspring exam (n = 23). A further 169 participants were excluded for missing a complete covariate profile. Multidetector CT scanning was conducted as reported in prior studies for the detection and quantification of CAC and abdominal aortic calcium (AAC) [3,17–19]. CAC and AAC scoring was conducted using a modified Agatston score [20]. Overall summary findings regarding the age and sex distribution of CAC and AAC have been previously reported [21,22]. For hepatic steatosis measurements, previous studies have shown that a single CT slice comparing three regions of interest in the liver as well as two from the spleen to a calibration phantom (Image Analysis, Lexington, KY) with a water-equivalent compound (CT-Water, Light Speed Ultra; General Electric, Milwaukee, WI) produced highly reliable measurements, with intraclass correlation coefficients of 0.99 [17]. Additional studies have shown that CT measures of hepatic steatosis, including liver-phantom ratios, correlate well with histology [17,23]. For the current study, a liver-phantom ratio was calculated and analyzed as a continuous variable as previously described [3,17]. The liver-phantom ratio correlates well with the liver-spleen ratio, which has previously been shown to accurately correspond to hepatic steatosis in biopsy studies (r = 0.92) [23,24]. A liver-phantom ratio of 0.33 or lower represents the presence of thirty percent or more of hepatic steatosis or fatty liver with a 98% sensitivity and 70% specificity [3]. Continuous liver-phantom ratios, rather than dichotomous liver-phantom ratios, were used in order to maximize power, as previous studies have shown that dichotomizing the liver-phantom ratio resulted in a loss of power [3]. Other causes of steatosis other than NAFLD (such as alcohol) were controlled for in regression analysis. Viral hepatitides are less likely to cause steatosis and very rare in the general population, which is reflected by our study population, so were not included in the covariate profile.
Covariate measurement
The age of the participant at time of the exam was used for analysis. Menopause was defined as cessation of menses for at least one year. Participants were considered positive for smoking if they smoked one cigarette or more per day in the year preceding entry to the study. Alcohol use was defined in drinks per week and was controlled for in multivariable regression in order to maintain adequate power to detect differences in our primary outcome. Body mass index (BMI) was defined as weight (kg)/height (m2) and was considered a continuous variable. Diabetes was defined as a fasting glucose >126 mg/dl, a random glucose >200 mg/dl or taking hypoglycemic agents. HTN was defined as a systolic blood pressure (SBP) ≥140 mmHg, a diastolic blood pressure (DBP) ≥90 mmHg, or taking an antihypertensive medication. Triglycerides and HDL were measured on fasting morning samples. Metabolic syndrome was defined according to the National Cholesterol Education Program (NCEP) ATP III guidelines [4]. Risk factors were measured at the seventh examination cycle (1998–2001) for the offspring participants or during the first examination for the third generation group. Subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) were measured by multidetector CT as previously described and were reported as cm3 [3]. Waist circumference was measured at the umbilicus and reported in mm.
Clinical and subclinical atherosclerotic CVD outcomes
The primary clinical CVD outcome was prevalent clinical CVD and was a composite of non-fatal myocardial infarction (MI), heart failure, stroke, transient ischemic attack (TIA), and peripheral arterial disease (PAD), defined by a three physician endpoint committee. The definition of clinical CVD and each of the CVD components as well as endpoint committee review have been previously described [15,16]. Subclinical CVD outcomes were determined by the CAC and AAC score. AAC and CAC outcomes were defined as dichotomous outcomes greater than or equal to the age- and sex-specific 90th percentile in healthy reference group [21,22]. Prevalent events were included if they occurred on or before the exam date.
Statistical analysis
Differences in characteristics between participants with and without CVD were determined using a t test for normally distributed variables, and chi-square test for dichotomous variables. Multivariable logistic regression analysis was performed to test the association between hepatic steatosis, defined by the liver-phantom ratio, as the continuous predictor variable, and clinical CVD. Logistic regression was also used for association analyses of the liver-phantom ratio with each of the subclinical CVD endpoints, AAC and CAC scores in the age- and sex-specific 90th percentile.
The covariates in our multivariable model included age, age2, sex, alcohol use, menopausal status, and hormone replacement therapy (HRT) [3]. To determine whether the association of hepatic steatosis was independent of related fat and metabolic disease measures, we also controlled for BMI, diabetes, HDL, HTN, metabolic syndrome, SAT, VAT, triglycerides, and waist circumference in our models. In addition, we performed analyses of two composite covariate profiles: a clinical covariate profile composed of clinically relevant and easily obtained covariates (age, sex, alcohol use, smoking, menopause, HRT use, diabetes, BMI, HDL, total cholesterol, HTN, and use of lipid-lowering medications) and a model including the new American College of Cardiology/American Heart Association (ACC/AHA) cardiovascular risk prediction model [25].
All models were tested for interactions with sex and age. Analyses were performed using SAS version 9.3 with a two-sided 0.05 alpha used to declare statistical significance. Given that the liver-phantom ratio less than or equal to 0.33 defines NAFLD, results were transformed to reflect an increased OR with higher levels of hepatic steatosis.
Results
Study sample characteristics
Baseline demographics for participants with and without prevalent CVD are shown in Table 1. In our sample of 3014 participants, 50.5% were female with an average age of 51 years. 51% of women were menopausal with 24% using HRT. 6% had diabetes and 30.9% met criteria for the metabolic syndrome. The prevalence of CVD was 5.87%. The overall prevalence of AAC and CAC levels at or above the 90th percentile were 25.7% and 19.4%, respectively. The overall prevalence of fatty liver, defined as 30% or greater levels of hepatic steatosis, was 17%, as previously reported [3]. There were statistically significant differences in age, sex, menopausal status and HRT use, glucose-related risk factors, blood pressure, lipid, and fat-related risk factors between the CVD and non-CVD groups, justifying our need to control for these in our modeling. Only SAT, current smoking, and alcohol use showed no significance between-group difference. There was a small but still statistically significant difference in liver-phantom ratio between CVD and no CVD groups.
Table 1.
Characteristics of participants.
| Category | Overall (N = 3014) | CVD (N = 177) | No CVD (N = 2837) | p value |
|---|---|---|---|---|
| Female (%) | 50.5 (1521) | 37.3 (66) | 51.3 (1455) | <0.001 |
| Age at exam (yr) | 51.1 (10.1) | 62.3 (10.2) | 50.4 (10.1) | <0.001 |
| Menopause (%)** | 51.1 (777) | 90 (60) | 49.3 (717) | <0.001 |
| Hormone replacement therapy (%)** | 24.1 (347) | 36.4 (24) | 23.6 (343) | 0.017 |
| Current cigarette use (%) | 13.0 (393) | 14.7 (26) | 12.9 (367) | 0.50 |
| Mean alcoholic drinks per week (SD) | 5.5 (7.8) | 6.2 (11.4) | 5.4 (7.5) | 0.38 |
| Glucose-related | ||||
| Fasting glucose (mg/dl) | 98.8 | 112 (42.6) | 97.9 (18.8) | <0.001 |
| Diabetes mellitus (%) | 6.1 (183) | 20.9 (37) | 5.1 (146) | <0.001 |
| Blood pressure-related | ||||
| Hypertensive drug use (%) | 18.6 (562) | 54.2 (96) | 16.4 (466) | <0.001 |
| Systolic BP (mmHg) | 121.7 (16.5) | 128.2 (18.3) | 121.3 (16.3) | <0.001 |
| Diastolic BP (mmHg) | 75.5 (9.3) | 73.3 (9.9) | 75.7 (9.3) | 0.001 |
| Lipid-related | ||||
| Triglycerides (mg/dl) | 127.2 (92.7) | 170.6 (134.3) | 124.5 (88.7) | <0.001 |
| HDL-cholesterol (mg/dl) | 53.9 (16.7) | 47.4 (15.9) | 54.3 (16.7) | <0.001 |
| Total cholesterol (mg/dl) | 196.3 (35.5) | 187.6 (40.4) | 196.9 (35.1) | <0.001 |
| Fat-related | ||||
| BMI (kg/m2) | 27.4 (4.9) | 28.6 (5.4) | 27.3 (4.9) | <0.001 |
| Waist circumference (mm) | 978.1 (128.2) | 1023 (123.1) | 975.3 (128.0) | <0.001 |
| SAT (cm3) | 2788.7 (1315.8) | 2770.6 (1158.5) | 2789.8 (1325.2) | 0.85 |
| VAT (cm3) | 1754.58 (1019.50) | 2467.5 (1250.8) | 1710.1 (986.6) | <0.001 |
| Metabolic syndrome (%) | 30.92 | 60.4 (107) | 29.1 (825) | <0.001 |
| Outcomes | ||||
| Total prevalent CVD (%) | 5.87 | -- | ||
| AAC (%)& | 25.71 | 56.5 (100) | 23.8 (675) | <0.001 |
| CAC (%)& | 19.4 | 47.5 (84) | 17.7 (502) | <0.001 |
| Predictor | ||||
| Liver-phantom ratio# | 0.36 | 0.34 (0.06) | 0.35 (0.05) | 0.03 |
BP, blood pressure; HDL, high density lipoprotein; BMI, body mass index; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue; AAC, abdominal aortic calcium; CAC, coronary artery calcium; CVD, cardiovascular disease; NAFLD, non-alcoholic fatty liver disease. Data are presented as mean (standard deviation) or % (number of individuals).
Data shown for women only: n = 1521.
Defined as 90th percentile or greater.
NAFLD defined as liver-phantom ratio of 0.33 or less.
Multivariable-adjusted correlations between hepatic steatosis and clinical and subclinical outcomes
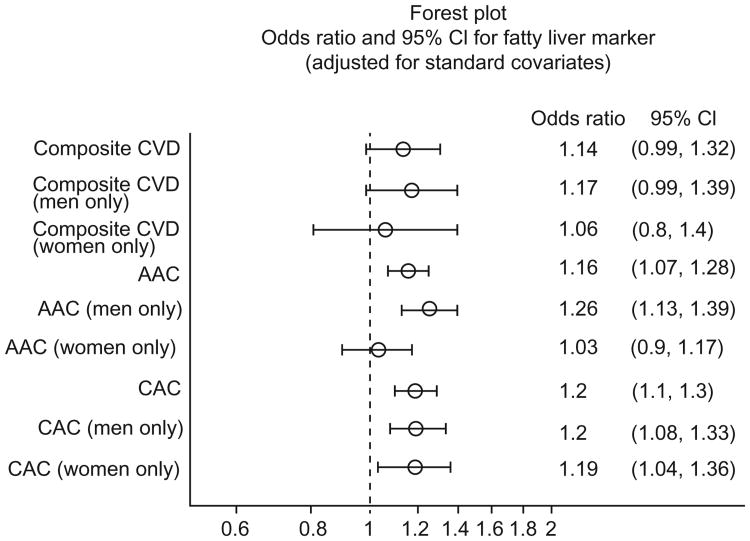
There was an association of increased levels of hepatic steatosis with prevalent clinical CVD, though this did not reach statistical significance. This was noted in age/sex-adjusted, covariates-adjusted, and covariates plus SAT-adjusted models with p values of 0.072, 0.074, and 0.074, respectively (see Table 2). With respect to subclinical outcomes, hepatic steatosis was significantly associated with CAC at or above the 90th percentile in all models. Hepatic steatosis was significantly associated with AAC at or above the 90th percentile in most models. AAC models that included additional adjustment for body fat measures such as BMI, metabolic syndrome, or VAT, showed attenuated or non-significant associations. Statistically significant interactions for sex and age were noted for AAC across all models (age interaction: range of p = 0.005–0.03; sex interaction: range of p = 0.010–0.048). No sex or age interactions were noted for any CAC models. When stratified by sex, the association between hepatic steatosis and AAC was strongest in men whereas no such sex difference was noted for CAC (see Fig. 1).
Table 2.
Multivariable analysis of clinical cardiovascular disease outcomes and hepatic steatosis.
| Overall N = 3014 Covariates | CVD | AACa,b | CACa | |||
|---|---|---|---|---|---|---|
| Odds ratio (CI) | p value | Odds ratio (CI) | p value | Odds ratio (CI) | p value | |
| Age + sex + age2 | 1.14 (0.99-1.32) | 0.072 | 1.17 (1.08-1.27) | <0.001 | 1.20 (1.10-1.30) | <0.001 |
| Covariates* | 1.14 (0.99-1.32) | 0.074 | 1.16 (1.07-1.26) | <0.001 | 1.20 (1.10-1.30) | <0.001 |
| Covariates + BMI | 1.11 (0.95-1.29) | 0.178 | 1.09 (1.00-1.19) | 0.040 | 1.13 (1.03-1.23) | 0.008 |
| Covariates + diabetes mellitus | 1.10 (0.95-1.27) | 0.218 | 1.14 (1.05-1.23) | 0.002 | 1.17 (1.08-1.28) | <0.001 |
| Covariates + HDL | 1.10 (0.95-1.27) | 0.222 | 1.12 (1.03-1.21) | 0.008 | 1.18 (1.08-1.29) | <0.001 |
| Covariates + HTN | 1.07 (0.92-1.24) | 0.388 | 1.11 (1.02-1.21) | 0.015 | 1.15 (1.05-1.25) | 0.002 |
| Covariates + metabolic syndrome | 1.05 (0.90-1.22) | 0.532 | 1.06 (0.97-1.15) | 0.207 | 1.11 (1.01-1.21) | 0.023 |
| Covariates + SAT | 1.15 (0.99-1.33) | 0.074 | 1.14 (1.05-1.24) | 0.003 | 1.16 (1.07-1.27) | <0.001 |
| Covariates + VAT | 1.04 (0.89-1.22) | 0.624 | 1.03 (0.94-1.12) | 0.573 | 1.10 (1.01-1.21) | 0.039 |
| Covariates + triglycerides | 1.08 (0.92-1.25) | 0.347 | 1.11 (1.02-1.20) | 0.020 | 1.15 (1.06-1.26) | 0.001 |
| Covariates + waist circ. | 1.10 (0.95-1.29) | 0.211 | 1.09 (1.00-1.19) | 0.050 | 1.12 (1.03-1.23) | 0.010 |
| Clinical covariate profile& | 1.06 (0.90-1.25) | 0.494 | 1.04 (0.95-1.13) | 0.450 | 1.10 (1.00-1.21) | 0.048 |
| AHA/ACC cardiovascular risk score˄ | 1.05 (0.90-1.24) | 0.516 | 1.04 (0.95-1.13) | 0.387 | 1.10 (1.01-1.21) | 0.028 |
| AHA/ACC risk score-men | 1.11 (0.92-1.34) | 0.275 | 1.17 (1.05-1.30) | 0.006 | 1.12 (1.00-1.25) | 0.051 |
| AHA/ACC risk score-women | 0.91 (0.66-1.26) | 0.582 | 0.88 (0.76-1.01) | 0.074 | 1.09 (0.94-1.26) | 0.236 |
BMI, body mass index; CI, confidence interval; HDL, high density lipoprotein; HTN, hypertension; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue; CVD, cardiovascular disease; AAC, abdominal aortic calcium; CAC, coronary artery calcium.
Covariates: age, sex, age2, alcoholic drinks, smoking, menopause, and HRT.
90th percentile values.
Statistically significant interactions for age and sex noted for AAC.
Composite of the following: age, age2, sex, alcohol use, smoking, menopause, HRT use, diabetes, BMI, HDL, total cholesterol, HTN, and presence of lipid-lowering medications.
AHA/ACC Cardiovascular Risk Score: age, presence/absence of diabetes, systolic blood pressure, hypertension treatment, smoking, total cholesterol level, and HDL level.
Fig. 1. Forest plot of odds ratios with 95% confidence intervals for association of non-alcoholic fatty liver disease with clinical and subclinical cardiovascular disease outcomes, stratified by sex.
Results for the composite clinical covariate model showed no significant association with clinical CVD (OR 1.06, p = 0.49) or AAC (OR 1.04, p = 0.45), but did show an association with CAC which just reached significance (OR 1.10, p = 0.048) (see Table 2). In the model composed of risk factors included in the new AHA/ACC risk score [25], the results were similar for CVD, AAC, and CAC outcomes (see Table 2). Results also showed that the association between hepatic steatosis and AAC in men was stronger (OR 1.17, p = 0.006) than the association in women.
Discussion
Study findings
In this cross-sectional study of clinical and subclinical CVD outcomes in the Framingham Heart Study offspring and third generation cohorts, there was no association of hepatic steatosis measured by CT scanning with prevalent clinical CVD, but there were significant multivariable-adjusted associations of hepatic steatosis with subclinical atherosclerosis, including CAC in all models but one and AAC in most models.
The most robust finding of our study was the highly significant associations between hepatic steatosis and CAC/AAC levels. Vascular calcification has long been recognized as playing a role in cardiovascular risk stratification. In particular, there are strong associations of CAC with increased risk of future CHD and other CVD outcomes independent of all traditional risk factors, and CAC is useful in risk stratification because it improves discrimination and reclassification for future CVD [10–12]. However, compared to CAC, AAC has received less attention in the literature. Prior studies in the Framingham Heart Study reported strong, independent associations of AAC, detected by lateral lumbar radiography, with future clinical CVD outcomes, including CHD, CVD and CVD mortality [26,27]. Over and above traditional risk factors, AAC provided modest, statistically significant improvement in discrimination for various CVD outcomes. A recent meta-analysis of 10 studies found an increased relative risk of coronary and cerebrovascular events, all cardiovascular events, and cardiovascular death in participants with higher AAC levels [28]. However, data are sparse regarding CVD risk using CT measures of AAC.
Only two prior studies have reported on associations between hepatic steatosis and AAC. Liu et al. found that hepatic steatosis was associated with AAC in models controlling for age and sex, but that this association was lost when controlling for other common risk factors for CVD, including alcohol use, smoking status, diabetes, HTN, and dyslipidemia [13]. McKimmie et al. did not find any significant association with either CAC or AAC on multivariable analysis [29]. A number of factors may account for the differences between our findings and those from the previous studies, including differences in ethnic composition, cohort ascertainment, and power. Participants in the Liu et al. report were drawn from the Jackson Heart Study, which is exclusively African-American. African-Americans tend to have lower amounts of both VAT and hepatic steatosis despite higher rates of other cardiovascular risk factors [30,31]. Further differences between the Framingham cohort and the Jackson Heart Study cohort selected for evaluation of NAFLD include a higher proportion of women (65% in the Jackson Heart Study compared to 51% in our study) and an older age (59 years compared to 51 years, respectively). Data from the totality of previous studies of AAC suggest that an elevated AAC in younger persons confers a stronger independent risk for future CVD risk [28].
CAC has been more widely studied and is a well-established independent risk predictor for CVD [12]. Among the prior studies evaluating associations between hepatic steatosis and CAC, results have been mixed. McKimmie et al. found no association between hepatic steatosis and CAC [29], while two large Korean population-based cohorts, which used ultrasound to diagnose fatty liver, showed that hepatic steatosis was associated with elevated CAC scores [8,9]. Kim et al. showed an association between hepatic steatosis and CAC in both sexes, an association which was independent of VAT as well as traditional cardiovascular risk factors [9]. Liu et al. found an association between CAC and hepatic steatosis, as diagnosed by CT scan, in a cohort of African-Americans in the Jackson Heart Study, though interestingly, they did not note any sex differences after testing for sex interactions [13]. A recently published analysis of the Multi-Ethnic Study of Atherosclerosis (MESA), found a positive association between hepatic steatosis and CAC, independent of other cardiovascular risk factors [32]. Furthermore, a recent systematic review and meta-analysis included 7 studies which showed an association between CAC and hepatic steatosis [33]. Our study adds to this literature by demonstrating a significant association between hepatic steatosis and CAC, independent of VAT. Given the absence of association between hepatic steatosis and AAC in the study of Liu et al. in African-Americans, further studies in larger samples are warranted to reliably examine for differences in the presence of associations or differences in mechanisms of associations in racial/ethnic populations.
Our findings provide further justification for studies to investigate the mechanistic link between hepatic steatosis and atherosclerotic CVD. Possible mechanisms by which hepatic steatosis may directly contribute to vascular disease include the production of pro-atherogenic factors. Consistent with other reports, we have previously demonstrated associations of hepatic steatosis with individual risk factors such as diabetes, HTN, impaired fasting glucose, HDL cholesterol, and triglycerides as well as the composite endpoint of metabolic syndrome, independent of other fat depots. However, in the current study, associations of hepatic steatosis with subclinical vascular disease are independent of these risk factors, suggesting that other unmeasured factors may play a role. McKimmie et al. found associations of hepatic steatosis with inflammatory markers that might mark the precursor to atherogenesis, but the study had insufficient power to detect effects on vascular disease [29]. Given that microRNAs have been recently shown to be released by the liver and promote vascular disease, further investigation is warranted to identify circulating factors that may mediate the relationship between steatosis and CVD outcomes [34].
Our study has several limitations. Given the cross-sectional nature of the study, the ability to draw causal relationships between predictors and outcomes are limited. Furthermore, prevalence of clinical CVD outcomes was low, with only 177 total cases. This is likely a consequence of the relative youth of participants, and may limit the power to detect associations between hepatic steatosis and clinical CVD outcomes. Given the existence of a gap between when risk factors are measured and when CT scans are performed, some patients may have had slightly different risk factor measurements at the time when CT scans were performed. Such difference in risk factor measurement may result in misclassification of participants which may bias our results towards non-significance. Though there may have been fatal CVD events, these were not included as this was a prevalence study.
This study has several strengths. By using the Framingham Heart Study, our study provides data on hepatic steatosis and CVD outcomes using well-measured covariates and outcomes in a large, prospective sample. Prior epidemiologic studies using this cohort have been widely validated in many other populations, increasing the generalizability of our results. Our results add to the existing literature by showing associations between hepatic steatosis and clinical and subclinical outcomes as well as demonstrating gender-dependent associations between AAC and hepatic steatosis. Though liver biopsy remains the gold-standard for NAFLD and non-alcoholic steatohepatitis (NASH) diagnosis, CT measurements of hepatic steatosis correlate well with histologic diagnosis and reflect a real-world use of diagnostic modalities for NAFLD [17,23]. Though unable to distinguish between NAFLD and NASH, use of CT for NAFLD diagnosis avoids the ethical implications of wide-spread biopsy of a whole population within the study and the attendant risks with which this would be associated. Liver attenuation as measured using CT scanning correlates well with histological steatosis (r = 0.92) [24]. Other causes of hepatic steatosis besides NAFLD, such as alcohol use, were controlled for in our models. Additional causes of secondary hepatic steatosis resulting from genotype 3 hepatitis C or certain drugs such as antiretrovirals or seizure medications, or causes of increased liver attenuation (opposite of the lower attenuation seen with steatosis), such as hemochromatosis or glycogen storage diseases, would be unlikely to substantially bias results due to their low prevalence in the general European ancestry population which constitutes the Framingham cohort. Additionally, as all members of the offspring and third generation underwent CT scanning, there was minimal loss of subjects due to exclusion criteria which limits selection bias.
In conclusion, there was a significant association of hepatic steatosis with subclinical CVD outcomes independent of many metabolic diseases/traits. Further studies with larger numbers of prospective clinical CVD outcomes are necessary to determine if hepatic steatosis is associated with incident CV outcomes as well as to determine what precise role hepatic steatosis plays in the development of clinical and subclinical CV outcomes.
Supplementary Material
Acknowledgments
This research was conducted in part using data and resources from the Framingham Heart Study of the National Heart Lung and Blood Institute of the National Institutes of Health and Boston University School of Medicine. This work was supported by the NHLBI's Framingham Heart Study (contract no. N01-HC-25195).
Financial support: JLM is supported by a T32DK062708 educational grant. EKS was supported by National Institutes of Health grant K23DK080145-01, the Doris Duke Medical Foundation, and the University of Michigan Internal Medicine Department, Division of Gastroenterology, and Biological Sciences Scholars Program. SS was supported by grants from the National Institute of Neurological Disorders and Stroke (NS17950) and the National Institute of Aging (AG08122, AG033193). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS, the NHLBI or the NIH.
Abbreviations
- AAC
abdominal aortic calcium
- ATP
Adult Treatment Panel
- BMI
body mass index
- CAC
coronary artery calcium
- CHD
coronary heart disease
- CT
computed tomography
- CVD
cardiovascular disease
- HDL
high-density lipoprotein
- HRT
hormone replacement therapy
- HTN
hypertension
- MI
myocardial infarction
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- NCEP
National Cholesterol Education Program
- PAD
peripheral arterial disease
- RNA
ribonucleic acid
- SAT
subcutaneous adipose tissue
- TIA
transient ischemic attack
- VAT
visceral adipose tissue
Footnotes
Conflict of interest: The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Authors' contribution: J.L.M- data analysis and interpretation, manuscript revision and finalization
K.M.P- data analysis and interpretation, manuscript revision and finalization
J.M.M- study design, data analysis and interpretation, manuscript revision and finalization
U.H- study design, data analysis and interpretation, manuscript revision and finalization
S.S- data analysis and interpretation, manuscript revision and finalization
C.S.F- data collection, data analysis and interpretation, manuscript revision and finalization
C.J.O- study design, data analysis and interpretation, manuscript revision and finalization
E.K.S- data collection, study design, data analysis and interpretation, manuscript revision and finalization
Supplementary data: Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jhep.2015.02. 045.
References
- 1.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 2.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 3.Speliotes EK, Massaro JM, Hoffmann U, Vasan RS, Meigs JB, Sahani DV, et al. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: The Framingham heart study. Hepatology. 2010;51:1979–1987. doi: 10.1002/hep.23593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 5.Targher G, Bertolini L, Rodella S, Tessari R, Zenari L, Lippi G, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2007;30:2119–2121. doi: 10.2337/dc07-0349. [DOI] [PubMed] [Google Scholar]
- 6.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341–1350. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 7.Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R, et al. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes. 2005;54:3541–3546. doi: 10.2337/diabetes.54.12.3541. [DOI] [PubMed] [Google Scholar]
- 8.Sung KC, Wild SH, Kwag HJ, Byrne CD. Fatty liver, insulin resistance, and features of metabolic syndrome: relationships with coronary artery calcium in 10,153 people. Diabetes Care. 2012;35:2359–2364. doi: 10.2337/dc12-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim D, Choi SY, Park EH, Lee W, Kang JH, Kim W, et al. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology. 2012;56:605–613. doi: 10.1002/hep.25593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O'Malley PG. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol. 2005;46:807–814. doi: 10.1016/j.jacc.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 11.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 12.Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O'Leary D, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788–795. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J, Musani SK, Bidulescu A, Carr JJ, Wilson JG, Taylor HA, et al. Fatty liver, abdominal adipose tissue and atherosclerotic calcification in African Americans: the Jackson Heart Study. Atherosclerosis. 2012;224:521–525. doi: 10.1016/j.atherosclerosis.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: the Framingham Study. Ann N Y Acad Sci. 1963;107:539–556. doi: 10.1111/j.1749-6632.1963.tb13299.x. [DOI] [PubMed] [Google Scholar]
- 15.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 16.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 17.Speliotes EK, Massaro JM, Hoffmann U, Foster MC, Sahani DV, Hirschhorn JN, et al. Liver fat is reproducibly measured using computed tomography in the Framingham Heart Study. J Gastroenterol Hepatol. 2008;23:894–899. doi: 10.1111/j.1440-1746.2008.05420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maurovich-Horvat P, Massaro J, Fox CS, Moselewski F, O'Donnell CJ, Hoffmann U. Comparison of anthropometric, area- and volume-based assessment of abdominal subcutaneous and visceral adipose tissue volumes using multi-detector computed tomography. Int J Obes (Lond) 2007;31:500–506. doi: 10.1038/sj.ijo.0803454. [DOI] [PubMed] [Google Scholar]
- 19.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 20.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 21.Chuang ML, Massaro JM, Levitzky YS, Fox CS, Manders ES, Hoffmann U, et al. Prevalence and distribution of abdominal aortic calcium by gender and age group in a community-based cohort (from the Framingham Heart Study) Am J Cardiol. 2012;110:891–896. doi: 10.1016/j.amjcard.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann U, Massaro JM, Fox CS, Manders E, O'Donnell CJ. Defining normal distributions of coronary artery calcium in women and men (from the Framingham Heart Study) Am J Cardiol. 2008;102:1136–1141. 1141.e1. doi: 10.1016/j.amjcard.2008.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwasaki M, Takada Y, Hayashi M, Minamiguchi S, Haga H, Maetani Y, et al. Noninvasive evaluation of graft steatosis in living donor liver transplantation. Transplantation. 2004;78:1501–1505. doi: 10.1097/01.tp.0000140499.23683.0d. [DOI] [PubMed] [Google Scholar]
- 24.Limanond P, Raman SS, Lassman C, Sayre J, Ghobrial RM, Busuttil RW, et al. Macrovesicular hepatic steatosis in living related liver donors: correlation between CT and histologic findings. Radiology. 2004;230:276–280. doi: 10.1148/radiol.2301021176. [DOI] [PubMed] [Google Scholar]
- 25.Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson PW, Kauppila LI, O'Donnell CJ, Kiel DP, Hannan M, Polak JM, et al. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. 2001;103:1529–1534. doi: 10.1161/01.cir.103.11.1529. [DOI] [PubMed] [Google Scholar]
- 27.Levitzky YS, Cupples LA, Murabito JM, Kannel WB, Kiel DP, Wilson PWF, et al. Prediction of intermittent claudication, ischemic stroke, and other cardiovascular disease by detection of abdominal aortic calcific deposits by plain lumbar radiographs. Am J Cardiol. 2008;101:326–331. doi: 10.1016/j.amjcard.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 28.Bastos Gonçalves F, Voûte MT, Hoeks SE, Chonchol MB, Boersma EE, Stolker RJ, et al. Calcification of the abdominal aorta as an independent predictor of cardiovascular events: a meta-analysis. Heart. 2012;98:988–994. doi: 10.1136/heartjnl-2011-301464. [DOI] [PubMed] [Google Scholar]
- 29.McKimmie RL, Daniel KR, Carr JJ, Bowden DW, Freedman BI, Register TC, et al. Hepatic steatosis and subclinical cardiovascular disease in a cohort enriched for type 2 diabetes: the Diabetes Heart Study. Am J Gastroenterol. 2008;103:3029–3035. doi: 10.1111/j.1572-0241.2008.02188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guerrero R, Vega GL, Grundy SM, Browning JD. Ethnic differences in hepatic steatosis: an insulin resistance paradox? Hepatology. 2009;49:791–801. doi: 10.1002/hep.22726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katzmarzyk PT, Bray GA, Greenway FL, Johnson WD, Newton RL, Ravussin E, et al. Racial differences in abdominal depot-specific adiposity in white and African American adults. Am J Clin Nutr. 2010;91:7–15. doi: 10.3945/ajcn.2009.28136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.RifaiAl M, Silverman MG, Nasir K, Budoff MJ, Blankstein R, Szklo M, et al. The association of nonalcoholic fatty liver disease, obesity, and metabolic syndrome, with systemic inflammation and subclinical atherosclerosis: The Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2015;239(2):629–633. doi: 10.1016/j.atherosclerosis.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oni ET, Agatston AS, Blaha MJ, Fialkow J, Cury R, Sposito A, et al. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care? Atherosclerosis. 2013;230:258–267. doi: 10.1016/j.atherosclerosis.2013.07.052. [DOI] [PubMed] [Google Scholar]
- 34.Speliotes EK, Yerges-Armstrong LM, Wu J, Hernaez R, Kim LJ, Palmer CD, et al. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 2011;7:e1001324. doi: 10.1371/journal.pgen.1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.