Abstract
Background:
Elderly multiple myeloma (MM) patients often tend to suffer a variety of diseases, so the treatment of choice is very difficult for the elderly myeloma patients. The overall survival (OS) time and side effects with elderly patients are unclear in China. The study tried to find out the role of geriatric assessment in the Chinese elderly MM.
Methods:
We retrospectively analyzed the data of 628 newly diagnosed patients from six hospitals from June 2011 to June 2013. A geriatric assessment had been performed to assess comorbidities, cognitive, and physical status for these patients. The primary endpoint was to evaluate different physical states of elderly patients with OS time and treatment-related side effects.
Results:
An additive scoring system (range: 0–5), based on age, Katz's Activity of Daily Living (ADL) and Lawton's Instrumental Activity of Daily Living (IADL) ≤5 and Charlson Comorbidity Index (CCI) was developed to identify three groups: fit (score = 0); intermediate-fitness (score = 1); and frail (score ≥2). The 3-year OS was 63% in fit patients, 63% in intermediate-fitness patients, and 49% in frail patients ≥3 hematologic adverse events (AEs) were documented in 45 (35.4%) fit, 34 (34%) intermediate-fitness, and 121 (30.2%) frail patients. The risk of a grade ≥3 hematologic AEs was not significantly increase in intermediate-fitness (hazard ratios [HR]: 0.99, 95% confidence interval [CI]: 0.54–1.47, P = 1.000) and in frail patients (HR: 1.16, 95% CI: 0.70–1.93, P = 0.558) compared with fit ones.
Conclusions:
MM occurs earlier in life and being advanced when the diagnosis is made in the mainland of China. The overall survival in frailty with International Staging System (ISS) II/III was the worst in all patients.
Keywords: Geriatric Assessment, Multicenter Study, Multiple Myeloma
Introduction
Multiple myeloma (MM) is a clonal B-cell malignancy of terminally differentiated plasma cells. MM is the second most common cancer of blood system. As the aging of the population in recent years, the incidence of this disease increased gradually.[1] Although the treatment method was improved in the recent 10 years, the outcomes of MM has improved,[2,3,4,5] patients older than seventy benefits less from these new treatments than younger patients.[6] At present, many biological and genetic prognostic factors and the International Staging System (ISS) are available to predict outcome; however, these factors are not sufficient to explain the aforementioned age-based difference in the outcome.[7,8,9] Specific assessment strategies are needed to define the frailty profiles of older patients.[10] Frailty and comprehensive geriatric assessment (CGA) are being incorporated to guide treatment decisions for cancer patients.[11,12] However, the value of a geriatric assessment (GA) for MM patients in China has not yet been prospectively evaluated. We assessed the predictive role of a baseline GA in 628 newly diagnosed MM patients to define a frailty score and assess the influence of this score on clinical outcomes and toxicity.
Methods
The study was a multicenter retrospective study that enrolled newly diagnosed inpatients from six hospitals from June 2011 to June 2013. Six hundred and twenty-eight patients were enrolled. The patients’ demographic features, clinical features, treatments, and outcome data were collected and analyzed [Table 1]. MM was defined according to the criteria of the International Myeloma Working Group (IMWG).[1] This study was retrospective, and all patients’ data remained anonymous. Therefore, the requirement to obtain informed consent was waived. This study was complied with the Declaration of Helsinki and was approved by Beijing Peoples’ Hospital Ethics Committees.
Table 1.
The basic characteristics of the patients
| Characteristics | Number of patients (n = 628), n (%) | Median (IQR) |
|---|---|---|
| Gender | ||
| Female | 276 (43.94) | – |
| Male | 352 (56.05) | |
| Age (years) | ||
| <65 | 432 (68.79) | 58 (52–66) |
| 65–75 | 159 (25.30) | |
| >75 | 37 (5.89) | |
| ISS | ||
| I | 139 (22.13) | – |
| II | 169 (26.91) | |
| III | 320 (50.95) | |
| ADL | ||
| >4 | 206 (32.80) | 4 (3–5) |
| ≤4 | 422 (67.20) | |
| IADL | ||
| >5 | 281 (44.75) | 5 (4–7) |
| ≤5 | 347 (55.25) | |
| Charlson Comorbidity Index | ||
| ≤1 | 476 (75.80) | 0 (0–1) |
| ≥2 | 152 (24.20) | |
| Therapy | ||
| Bortezomibbased regimens | 287 (45.70) | – |
| Other | 341 (54.30) |
IQR: Interquartile range; ISS: International Staging System; ADL: Activity of Daily Living; IADL: Instrumental Activity Of Daily Living; –: Not applicable.
The primary objective of this analysis was to identify a simple scoring system based on geriatric parameters to predict overall survival (OS). The secondary objectives included an evaluation of the impact of the frailty scoring system on treatment-related toxicity and progression-free survival (PFS).
The GA consisted of the following three tools: Katz's Activities of Daily Living (ADL) index, Lawton's Instrumental Activities of Daily Living (IADL) index, and the Charlson Comorbidity Index (CCI). The ADL and the IADL scales were adopted to assess the self-care activities, tasks of household management and independence status. The patients’ general conditions, ISS scores, beta-2-microglobulin and albumin levels, and some chromosomal abnormalities were assessed and collected. We also assessed adverse events (AEs) in these patients, and we graded these events according to the National Cancer Institute Common Terminology Criteria (NCI-CTC) for Adverse Events version 3.0.
Statistical analysis
We used the Kaplan-Meier method to estimate survival and compared between risk groups using log-rank testing. Estimates of the predictive effect of GA for OS were expressed as hazard ratios (HRs). A value of P ≤ 0.05 was considered to be significant differences. The statistical analysis was performed using Statistical Product and Service Solutions (SPSS) version 22.0 statistical software (SPSS Inc., Chicago, IL, USA).
Results
This retrospective study included 628 newly diagnosed MM patients. The median age was 58 years, and the majority of patients (68.79%) were <65 years [Table 1]. Among the 628 patients, 50.95% were Stage III, 26.91% were Stage II, and 22.13% were Stage I MM according to the ISS. Four hundred and twenty-two patients (67.2%) had ADL scores ≤4, 347 (55.25%) had IADL scores ≤5, and 152 (24.2%) had CCIs ≥2. Nearly, half of the patients received bortezomib-based treatment.
In this real practice study of MM, a multivariate Cox regression model showed that geriatric components influenced OS. In the frail patients, including those >75 years old, functional declines indicated by the ADL and IADL scores and the presence of comorbidities were associated with worse OS. Reduced OS was observed for patients aged >75 years (HR: 1.25), for patients with ADL scores ≤4 (HR: 1.36) and IADL scores ≤5 (HR: 1.31), and for patients with CCI scores ≥2 (HR: 1.11) [Table 2]. The OS did not differ between the fit and the intermediately fit patients. An ISS-adjusted multivariate analysis revealed that the risk of death was higher for patients older than 75 years. The proportions of the frail patients in the ISS I, II, and III risk groups and the OSs of these groups are illustrated [Table 3]. Grade 3 or higher hematologic AEs were documented in 45 (35.4%) fit, 34 (34%) intermediately fit, and 121 (30.2%) frail patients. The risks of developing a grade ≥3 hematologic AEs were not significantly higher in the intermediately fit (HR: 0.99, 95% confidence interval [CI]: 0.54–1.47, P = 1.000) or frail patients (HR: 1.16, 95% CI: 0.70–1.93, P = 0.558) compared with the fit patients. Grade 3 or higher nonhematologic AEs were examined. The risk of developing a grade 3 or higher nonhematologic AE was slightly increased in the intermediately fit group (HR: 1.57, 95% CI: 0.89–2.77, P = 0.121) and significantly increased in the frail patients (HR: 1.79, 95% CI: 1.11–2.90, P = 0.018) compared with the fit patients [Table 4]. Drug discontinuation for any cause other than progression and death was reported in 25 (19.7%) fit, 22 (22%) intermediately fit, and 140 (34.9%) frail patients. The risk of drug discontinuation was higher in the intermediately fit patients (HR: 1.16, 95% CI: 0.16–8.26, P = 0.882) and significantly higher in the frail patients (HR: 3.41, 95% CI: 0.78–14.99, P < 0.105) than that in the fit patients. The combination of the ISS with the frailty score identified six groups. The median follow-up time was 13.4 months (8–33 months). The survival curves for these six groups are shown in Figure 1. The frail, ISS II/III patients were considered to be very high-risk patients and exhibited a 3-year OS of 49%. The fit, ISS I patients were deemed to be low-risk patients and exhibited the best prognoses and a 3-year OS of 90%.
Table 2.
The final Cox regression model* of the patients
| Variables | HR (95% CI) | P | Score |
|---|---|---|---|
| Age (years) | |||
| <65 | 1 | – | 0 |
| 65–75 | 1.74 (0.98–3.09) | 0.058 | 1 |
| >75 | 1.25 (0.44–3.51) | 0.677 | 2 |
| ADL | |||
| >4 | 1 | – | 0 |
| ≤4 | 1.36 (0.78–2.37) | 0.282 | 1 |
| IADL | |||
| >5 | 1 | – | 0 |
| ≤5 | 1.31 (0.78–2.20) | 0.309 | 1 |
| Charlson Comorbidity Index | |||
| ≤1 | 1 | – | 0 |
| ≥2 | 1.11 (0.61–2.01) | 0.736 | 1 |
| International Staging System | |||
| I | 1 | – | – |
| II | 1.26 (0.54–2.94) | 0.598 | – |
| III | 2.19 (1.06–4.53) | 0.034 | – |
| Therapy | |||
| Other | 1 | – | – |
| Contain bortezomib | 0.59 (0.35–0.99) | 0.046 | – |
*HRs and relative risks are for overall survival in patients with the factors as compared with those without the factors. The model was adjusted for International Staging System and therapy. Harrell’s C index=0.75 (95% CI: 0.51–0.94), Akaike information criterion=585.90. HR: Hazard ratio; CI: Confidence interval; ADL: Activity of Daily Living; IADL: Instrumental Activity of Daily Living; ISS: International Staging System; –: Not applicable.
Table 3.
Additive total score and related rate of overall survival at 3 years
| Additive total score | Patient status | Patients, n (%) | Overall survival (%, 95% CI) |
|---|---|---|---|
| 0 | Fit | 127 (20.22) | 63 (53.67–72.25) |
| 1 | Intermediate-fitness | 100 (15.92) | 63 (53.38–73.90) |
| ≥2 | Frail | 401 (63.85) | 49 (44.14–54.74) |
In univariate Cox model the Harrell’s C index = 0.87 (95% CI: 0.66–1.01) and the Akaike information criterion = 628.54. In multivariate Cox model the Harrell’s C index = 0.72 (95% CI: 0.47–0.92) and the Akaike information criterion = 586.36. CI: Confidence interval.
Table 4.
Univariate and multivariate analysis of the impact of the frailty profile for patients on overall survival, progression-free survival, discontinuation rate, and incidence of Grade 3 or higher toxicity
| Variables | Overall survival | Discontinuation | Grade >3 nonhematologic toxicity | Grade >3 hematologic toxicity | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Crude | ||||||||
| Fit | 1 | – | 1 | – | 1 | – | 1 | – |
| Intermediate-fitness | 0.97 (0.39–2.40) | 0.939 | 1.16 (0.16–8.26) | 0.882 | 1.57 (0.89–2.77) | 0.121 | 0.99 (0.54–1.47) | 1.000 |
| Frail | 1.61 (0.83–3.12) | 0.159 | 3.41 (0.78–14.99) | 0.105 | 1.79 (1.11–2.90) | 0.018 | 1.16 (0.70–1.93) | 0.558 |
| Adjusted* | ||||||||
| Fit | 1 | – | 1 | – | 1 | – | 1 | – |
| Intermediate-fitness | 0.80 (0.31–2.06) | 0.641 | 1.10 (0.15–7.91) | 0.923 | 1.48 (0.84–2.62) | 0.177 | 1.06 (0.65–1.53) | 1.000 |
| Frail | 1.50 (0.77–2.93) | 0.231 | 3.26 (0.74–14.43) | 0.120 | 1.80 (1.11–2.91) | 0.018 | 1.12 (0.67–1.86) | 0.671 |
*Adjusted for ISS and therapy. HR: Hazard ratio; CI: Confidence interval; ISS: International Staging System; –: Not applicable.
Figure 1.
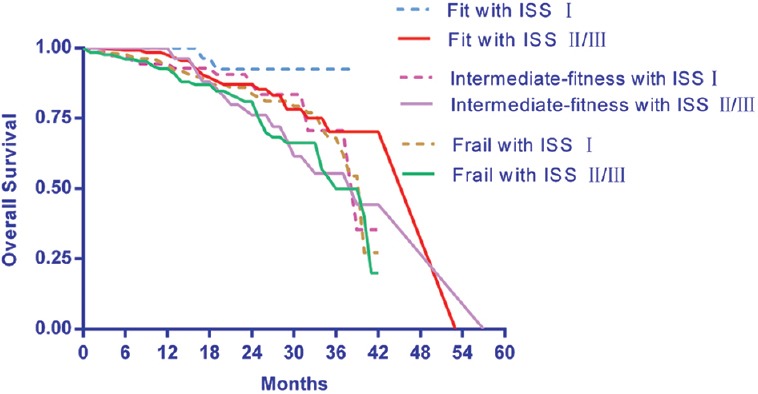
OSs of the patients classified into six categories according to recursive partitioning analysis following the combination of two independent prognostic scores, i.e., the frailty score and the ISS score. OSs: Overall survivals; ISS: International Staging System.
Discussion
The median age observed in the study was similar to the age reported by Dr. Lu et al.[13] This retrospective analysis revealed that the frailty score, which combined age, functional status and comorbidities, was associated with survival and toxicity and could be useful to guide dosage adjustments for the treatment of Chinese MM patients. The frailty profile was relevant to progression, nonhematologic AEs and treatment discontinuation irrespective of the ISS stage and type. The study validated the recent IMWG publication of Dr. Palumbo et al.[14]
In our analysis, the 3-year OS rates were 63% in the fit, 63% in the intermediately fit and 50% in the frail patients. In our results, OS did not significantly differ between groups, primarily because the doses were reduced during the treatment of the fit patients at some centers. The reduced survival of the frail patients was due to reductions in doses and age. A significantly higher cumulative incidence of nonhematologic toxicities and a significantly increased rate of drug discontinuation were observed in the frail patients compared with the fit patients, and the nonhematologic AEs and drug discontinuations might have reduced survival. The findings suggest that dose reductions reduced the occurrence of hematological toxicity but did not affect the toxicity of hematology.
Following the combination of the frailty score with the established ISS, the 3-year OS rates were found to be 49% in the frail, ISS II/III group and 90% in the fit, ISS I group. Maybe people with more advanced stage might have worse physical activity and tolerance with regimen. We hope the combination of the two independent parameters will produce a single index that significantly improved the prognostic value and will consequently serve as an important prediction strategy in the future.
The chronological order of the patient's age, the patient's performance status, and the doctor's clinical judgment are insufficient characteristics to define vulnerable populations. The GA could be used in routine clinical practice and in research to ensure that appropriate elderly patients were selected to facilitate more accurate cross-trial comparisons. Frail patients might benefit from mild methods or even palliative/less supportive treatment.[15,16,17]
The power of this analysis was great due to the widely representative and fairly homogeneous group of real-world data provided by the six Chinese centers. The GAs were prospectively obtained prior to the initiation of chemotherapy and reflected the patients’ baseline health statuses rather than the toxicities induced by the therapies. Older people required special care in terms of treatment decisions, and efficacy and side effects must be carefully balanced.
The study is subject to some limitations. This study was retrospective, the value of the information was not very high, and the ADL and IADL scores from the different centers were not uniform. The treatments differed, and these differences biased the survival time data. Dose adjustments for the frail patients resulted in weak doses, and significant differences were consequently not observed between the fit and frail patients.
In conclusion, this study of real-world MM treatment in China revealed that in the mainland of China, the majority of patients were young, in the later ISS stages, and in poor physical condition. The fit, ISS I patients survived the longest. The specific disease risk factors of a patient need to be established prior to selecting a treatment.
Financial support and sponsorship
This work was partly supported by a grant from the National Natural Science Foundation of China (No.81670192).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank all the patients who participated in the source studies and Stefanie K for providing editorial assistance.
Footnotes
Edited by: Peng Lyu
References
- 1.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–60. doi: 10.1056/NEJMra1011442. doi:10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 2.San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–17. doi: 10.1056/NEJMoa0801479. doi:10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 3.Fayers PM, Palumbo A, Hulin C, Waage A, Wijermans P, Beksaç M, et al. Thalidomide for previously untreated elderly patients with multiple myeloma:Meta-analysis of 1685 individual patient data from 6 randomized clinical trials. Blood. 2011;118:1239–47. doi: 10.1182/blood-2011-03-341669. doi:10.1182/blood-2011-03-341669. [DOI] [PubMed] [Google Scholar]
- 4.Palumbo A, Hajek R, Delforge M, Kropff M, Petrucci MT, Catalano J, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med. 2012;366:1759–69. doi: 10.1056/NEJMoa1112704. doi:10.1056/NEJMoa1112704. [DOI] [PubMed] [Google Scholar]
- 5.San Miguel JF, Weisel KC, Song KW, Delforge M, Karlin L, Goldschmidt H, et al. Impact of prior treatment and depth of response on survival in MM-003, a randomized phase 3 study comparing pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone in relapsed/refractory multiple myeloma. Haematologica. 2015;100:1334–9. doi: 10.3324/haematol.2015.125864. doi:10.3324/haematol.2015.125864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mols F, Oerlemans S, Vos AH, Koster A, Verelst S, Sonneveld P, et al. Health-related quality of life and disease-specific complaints among multiple myeloma patients up to 10 yr after diagnosis: Results from a population-based study using the PROFILES registry. Eur J Haematol. 2012;89:311–9. doi: 10.1111/j.1600-0609.2012.01831.x. doi:10.1111/j.1600-0609.2012.01831.x. [DOI] [PubMed] [Google Scholar]
- 7.Chng WJ, Dispenzieri A, Chim CS, Fonseca R, Goldschmidt H, Lentzsch S, et al. IMWG consensus on risk stratification in multiple myeloma. Leukemia. 2014;28:269–77. doi: 10.1038/leu.2013.247. doi:10.1038/leu.2013.247. [DOI] [PubMed] [Google Scholar]
- 8.Kumar SK, Dispenzieri A, Gertz MA, Lacy JA, Lust SR, Hayman FK, et al. Continued improvement in survival in multiple myeloma and the impact of novel agents. Blood. 2012;120:3972. doi:10.1038/leu.2013.313. [Google Scholar]
- 9.Siegel DS, Desikan KR, Mehta J, Singhal S, Fassas A, Munshi N, et al. Age is not a prognostic variable with autotransplants for multiple myeloma. Blood. 1999;93:51–4. [PubMed] [Google Scholar]
- 10.Palumbo A, Waage A, Hulin C, Beksac M, Zweegman S, Gay F, et al. Safety of thalidomide in newly diagnosed elderly myeloma patients: A meta-analysis of data from individual patients in six randomized trials. Haematologica. 2013;98:87–94. doi: 10.3324/haematol.2012.067058. doi:10.3324/haematol.2012.067058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamaker ME, Jonker JM, de Rooij SE, Vos AG, Smorenburg CH, van Munster BC. Frailty screening methods for predicting outcome of a comprehensive geriatric assessment in elderly patients with cancer: A systematic review. Lancet Oncol. 2012;13:e437–44. doi: 10.1016/S1470-2045(12)70259-0. doi:10.1016/S1470-2045(12)70259-0. [DOI] [PubMed] [Google Scholar]
- 12.Larocca A, Bringhen S, Petrucci MT, Oliva S, Falcone AP, Caravita T, et al. A phase 2 study of three low-dose intensity subcutaneous bortezomib regimens in elderly frail patients with untreated multiple myeloma. Leukemia. 2016;30:1320–6. doi: 10.1038/leu.2016.36. doi:10.1038/leu.2016.36. [DOI] [PubMed] [Google Scholar]
- 13.Lu J, Lu J, Chen W, Huo Y, Huang X, Hou J Chinese Medical Doctor Association Hematology Branch. Clinical features and treatment outcome in newly diagnosed Chinese patients with multiple myeloma: Results of a multicenter analysis. Blood Cancer J. 2014;4:e239. doi: 10.1038/bcj.2014.55. doi:10.1038/bcj.2014.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palumbo A, Bringhen S, Mateos MV, Larocca A, Facon T, Kumar SK, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: An International Myeloma Working Group report. Blood. 2015;125:2068–74. doi: 10.1182/blood-2014-12-615187. doi:10.1182/blood-2014-12-615187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larocca A, Palumbo A. How I treat fragile myeloma patients. Blood. 2015;126:2179–85. doi: 10.1182/blood-2015-05-612960. doi:10.1182/blood-2015-05-612960. [DOI] [PubMed] [Google Scholar]
- 16.Maes H, Delforge M. Optimizing quality of life in multiple myeloma patients: Current options, challenges and recommendations. Expert Rev Hematol. 2015;8:355–66. doi: 10.1586/17474086.2015.1021772. doi:10.1586/17474086.2015.1021772. [DOI] [PubMed] [Google Scholar]
- 17.Lyu WW, Zhao QC, Song DH, Zhang JJ, Ding ZX, Li BY, et al. Thalidomide-based regimens for elderly and/or transplant ineligible patients with multiple myeloma: A meta-analysis. Chin Med J. 2016;129:320–5. doi: 10.4103/0366-6999.174497. doi:10.4103/0366-6999.174497. [DOI] [PMC free article] [PubMed] [Google Scholar]


