Abstract
Background:
The 13C urea breath test (13C-UBT) is the gold standard for detecting Helicobacter pylori infection. H. pylori pathogenesis in patients with hepatitis B virus (HBV) and related diseases remains obscure. We used 13C-UBT to detect H. pylori infection in patients with chronic HBV infection, HBV-related cirrhosis, HBV-related hepatic carcinoma, and other chronic hepatic diseases.
Methods:
A total of 131 patients with chronic hepatitis B (HB), 179 with HBV-related cirrhosis, 103 with HBV-related hepatic carcinoma, 45 with HBV-negative hepatic carcinoma, and 150 controls were tested for H. pylori infection using 13C-UBT. We compared H. pylori infection rate, liver function, complications of chronic hepatic disease, serum HBV-DNA, serum alpha-fetoprotein (AFP), and portal hypertensive gastropathy (PHG) incidence among groups.
Results:
HBV-related cirrhosis was associated with the highest H. pylori infection rate (79.3%). H. pylori infection rate in chronic HB was significantly higher than in the HBV-negative hepatic carcinoma and control groups (P < 0.001). H. pylori infection rate in patients with HBV-DNA ≥103 copies/ml was significantly higher than in those with HBV-DNA <103 copies/ml (76.8% vs. 52.4%, P < 0.001). Prothrombin time (21.3 ± 3.5 s vs. 18.8 ± 4.3 s), total bilirubin (47.3±12.3 μmol/L vs. 26.6 ±7.9 μmol/L), aspartate aminotransferase (184.5 ± 37.6 U/L vs. 98.4 ± 23.5 U/L), blood ammonia (93.4 ± 43.6 μmol/L vs. 35.5 ± 11.7 μmol/L), and AFP (203.4 ± 62.6 μg/L vs. 113.2 ± 45.8 μg/L) in the 13C-UBT-positive group were significantly higher than in the 13C-UBT-negative group (P < 0.01). The incidence rates of esophageal fundus variceal bleeding (25.4% vs. 16.0%), ascites (28.9% vs. 17.8%), and hepatic encephalopathy (24.8% vs. 13.4%) in the 13C-UBT-positive group were significantly higher than in the 13C-UBT-negative group (P < 0.01). The percentages of patients with liver function in Child-Pugh Grade C (29.6% vs. 8.1%) and PHG (43.0% vs. 24.3%) in the 13C-UBT-positive group were significantly higher than in the 13C-UBT-negative group (P < 0.05).
Conclusions:
It is possible that H. pylori infection could increase liver damage caused by HBV. H. pylori eradication should be performed in patients with complicating H. pylori infection to delay hepatic disease progression.
Keywords: Helicobacter Pylori Infection, Hepatitis B Virus, Hepatitis B Virus-related Cirrhosis, Hepatitis B Virus-related Hepatic Carcinoma, Urea Breath Test
Introduction
The pathogenesis of hepatitis B virus (HBV) in the progression of chronic hepatic disease is generally accepted. Helicobacter pylori mainly causes disease in the stomach and duodenum, where it can induce chronic infection and ulcers.[1,2] In recent years, investigators have found that H. pylori is associated with the progression of diseases other than gastrointestinal disease, such as chronic bronchitis and coronary sclerosis.[3,4] H. pylori DNA could be detected in hepatic tissue specimens of patients with chronic hepatic disease, suggesting that coinfection with H. pylori could aggravate a patient's condition.[5] The 13C-urea breath test (13C-UBT) is the internationally accepted gold standard for the detection of H. pylori infection and for monitoring the curative effect of H. pylori elimination treatment.[6] The pathogenesis of H. pylori infection in patients with HBV-related disease remains obscure. This study explored the H. pylori infection state in patients with chronic hepatic disease and the relationship of H. pylori infection with liver function, serum alpha-fetoprotein (AFP), complications of hepatic disease, and portal hypertensive gastropathy (PHG).
Methods
Patients
From January 2008 to December 2015, we performed a prospective study on the relationship of H. pylori infection with hepatic disease. We designed a table before the study, set a test end point, if patients fit the enrollment standard, and they were enrolled in the corresponding group. Sample size was estimated using Microsoft Excel 2007 (Microsoft Corporation, USA); the sample size in this study was larger than the estimated value. Patients who were treated in the department of gastroenterology at our hospital were randomly enrolled: 131 patients with chronic hepatitis B (HB) (Group A); 179 patients with HBV-related cirrhosis (Group B); 103 patients with HBV-related hepatic carcinoma (Group C); 45 patients with HBV-negative hepatic carcinoma (Group D); and 150 healthy volunteers in the same period were enrolled as controls (Group E). Enrollment standard: the diagnosis fit the guidelines of prevention and treatment for chronic HB produced by the Chinese Society of Hepatology and the Chinese Society of Infectious Diseases, Chinese Medical Association in 2015.[7] The diagnosis was confirmed by the presence of HB surface antigen, HB surface antibody, HB envelope antigen, HB envelope antibody, HB core antibody, HBV-DNA, and analysis of liver function, blood clotting function, liver computed tomography, and Doppler color ultrasonography. Among the five groups, the age, sex, and other general information were not significantly different [P > 0.05, Table 1]. The clinical profile of patients was noted from their medical records, and informed consent was obtained from all patients. Patients with intake of antibiotics (up to 1 month) or prior therapy for eradication of H. pylori were excluded from the study. The Research Ethics Committee of the Affiliated Yantai Yuhuangding Hospital of Qingdao University approved this study. Informed consent was obtained from all the enrolled patients.
Table 1.
Information of patients and volunteers
| Characteristics | Chronic hepatitis B (n = 131) | HBV-related cirrhosis (n = 179) | HBV-related hepatic carcinoma (n = 103) | HBV-negative hepatic carcinoma (n = 45) | Healthy volunteers (n = 150) | P |
|---|---|---|---|---|---|---|
| Sex (male/female) | 85/46 | 104/75 | 68/35 | 25/20 | 82/68 | 0.261 |
| Age (years), mean ± SD | 49.3 ± 13.1 | 53.5 ± 11.2 | 52.4 ± 11.6 | 58.3 ± 10.9 | 52.3 ± 11.7 | 0.347 |
HBV: Hepatitis B virus.
Sample collection and 13C urea breath test testing method
Four weeks before the test, antibiotics, bismuth, and proton pump inhibitors were discontinued. The patients fasted for at least 6 h before the 13C-UBT. First, a baseline breath specimen was collected and marked as δ‰ (0 min). Then, 30 min after ingestion of 13C urea, a second breath specimen was collected and marked as δ‰ (30 min).[8,9] The equipment used was a HY-IREXB 13C breath detector (Guangzhou Huayou photoelectricity Co. Ltd. China). Test threshold: for detected value = δ‰(30 min) −δ‰ (0 min), a positive value was >4.0 and a negative value was <4.0. In our hospital, somatostatin and octreotide were only used for patients with complicated esophageal fundus variceal bleeding; for such patients, 13C-UBT was performed during a period when neither somatostatin nor octreotide was used.
Hepatitis B virus-DNA specimen collection and testing
Blood specimens were collected from all patients and centrifuged as soon as possible. Serum was collected and, if not tested promptly, was stored at −18°C. HBV-DNA quantity was tested by polymerase chain reaction. Samples with <103 copies/ml HBV-DNA were judged to be negative, and samples with >103 copies/ml were judged to be positive.[10]
Serum specimen examination
Serum prothrombin time (PT), total bilirubin (TBIL), aspartate aminotransferase (AST), blood ammonia (NH3), and AFP were tested using standard methods.[11]
Gastroscopy examination
The purpose of gastroscopy was to evaluate the relationship of H. pylori infection with PHG. As PHG could only occur in patients with cirrhosis, they underwent gastroscopy to assess the grade of varices and the severity of PHG. The severity of PHG was classified according to McCormack's classification into two classes: mild and severe. Mild PHG comprises a snake-skin or mosaic pattern or fine pink speckling, and severe PHG comprises cherry-red spots with or without spontaneous bleeding.[12] Patients with diseases such as peptic ulcer, cardio and cerebro-vascular disease, or acute gastric mucosal lesions induced by nonsteroidal anti-inflammatory drugs were excluded from the study.
Statistical analysis
The data were represented as mean ± standard deviation (SD). Measurement data were analyzed using t-test; numeration data were analyzed using Chi-square test; A value of P < 0.05 was considered statistically significant. The statistical package SPSS software version 17.0 for Windows (SPSS Inc., USA) was used. H. pylori infection rate, liver function, complications of chronic hepatic disease, serum HBV-DNA, AFP, and PHG incidence among the groups were compared.
Results
13C urea breath test positive rate
The HBV-related cirrhosis patient group had the highest 13C-UBT-positive rate (79.3%). The rate was significantly higher than that of the chronic HB, HBV-negative hepatic carcinoma, and healthy volunteer groups (P < 0.001). The 13C-UBT positive rate of the chronic HB group was significantly higher than that of the HBV-negative hepatic carcinoma and healthy volunteer groups [P < 0.001, Table 2].
Table 2.
Results of 13C-UBT
| Group | Case number | 13C-UBT (+), n | Helicobacter pylori infection rate (%) |
|---|---|---|---|
| Chronic hepatitis B (A) | 131 | 76 | 58.0 |
| HBV-related cirrhosis (B) | 179 | 142 | 79.3 |
| HBV-related hepatic carcinoma (C) | 103 | 71 | 68.9 |
| HBV-negative hepatic carcinoma (D) | 45 | 15 | 33.3 |
| Healthy volunteers (E) | 150 | 35 | 23.3 |
χ2 = 120.817; P<0.001. 13C-UBT: 13C-urea breath test; HBV: Hepatitis B virus.
Relationship of hepatitis B virus-DNA with Helicobacter pylori infection
The percentage of patients with a positive 13C-UBT in the group with ≥103 copies/ml HBV-DNA was significantly higher than that in the group with HBV-DNA <103 copies/ml [P < 0.001, Table 3]. There was no significant difference between the group of patients with 103–106 copies/ml HBV-DNA and the group with >106 copies/ml HBV-DNA (P > 0.05).
Table 3.
Relationship between HBV-DNA level and Helicobacter pylori infection
| Group | Case number | 13C-UBT positive | Helicobacter pylori infection rate (%) |
|---|---|---|---|
| HBV-DNA (copies/ml) | |||
| <103 | 103 | 54 | 52.4 |
| ≥103 | 310 | 238 | 76.8 |
| 103–106 | 181 | 135 | 74.6 |
| >106 | 129 | 103 | 79.8 |
χ2 = 20.965; P<0.001. 13C-UBT: 13C-urea breath test; HBV: Hepatitis B virus.
Relationship between 13C urea breath test and liver function
PT, TBIL, AST, NH3, and AFP levels in the 13C-UBT-positive group were significantly higher than those in the 13C-UBT-negative group [P < 0.01, Table 4].
Table 4.
Relationship between 13C-UBT positivity and liver function
| Characteristics | 13C-UBT-positive (n = 339) | 13C-UBT-negative (n = 269) | P |
|---|---|---|---|
| PT (s) | 21.3 ± 3.5 | 18.8 ± 4.3 | 0.006 |
| TBIL (μmol/L) | 47.3 ± 12.3 | 26.6 ± 7.9 | 0.007 |
| AST (U/L) | 184.5 ± 37.6 | 98.4 ± 23.5 | 0.004 |
| NH3 (μmol/L) | 93.4 ± 43.6 | 35.5 ± 11.7 | 0.004 |
| AFP (µg/L) | 203.4 ± 62.6 | 113.2 ± 45.8 | 0.009 |
Data are presented as mean ± SD. 13C-UBT: 13C-urea breath test; AFP: Alpha-fetoprotein; NH3: Blood ammonia; AST: Aspartate aminotransferase; TBIL: Total bilirubin; PT: Prothrombin time.
Of the 179 patients with HBV-related cirrhosis, 142 exhibited a positive 13C-UBT, and 37 were 13C-UBT negative. This showed that in the 13C-UBT-positive group, the percentage of patients whose liver function is in Child-Pugh Grade C was significantly higher than that in the 13C-UBT-negative group [P < 0.01, Table 5].
Table 5.
Relationship between 13C-UBT positivity and liver pathology (Child-Pugh classification) in patients with HBV-related cirrhosis (n = 179)
| Group | Number | Grade A, n (%) | Grade B, n (%) | Grade C, n (%) |
|---|---|---|---|---|
| 13C-UBT positive | 142 | 43 (30.3) | 57 (40.1) | 42 (29.6) |
| 13C-UBT negative | 37 | 22 (59.5) | 12 (32.4) | 3 (8.1) |
χ2 = 12.716; P<0.01. HBV: Hepatitis B virus; 13C-UBT: 13C-urea breath test.
Relationship of 13C urea breath test with complications of liver disease
The incidence of esophageal fundus variceal bleeding, ascites, and hepatic encephalopathy in the 13C-UBT-positive group was significantly higher than in the 13C-UBT-negative group [P < 0.01, Table 6].
Table 6.
Relationship between 13C-UBT and complications of liver disease
| Characteristics | 13C-UBT positive (n = 339) | 13C-UBT negative (n = 269) | P |
|---|---|---|---|
| Esophageal fundus variceal bleeding, n (%) | 86 (25.4) | 43 (16.0) | 0.007 |
| Ascites, n (%) | 98 (28.9) | 48 (17.8) | 0.002 |
| Hepatic encephalopathy, n (%) | 84 (24.8) | 36 (13.4) | 0.001 |
13C-UBT: 13C-urea breath test.
Relationship of 13C urea breath test with portal hypertensive gastropathy in patients with hepatitis B virus-related cirrhosis
In patients with HBV-related cirrhosis, the incidence of PHG in the 13C-UBT-positive group (43.0%, 61/142) was significantly higher than that in the 13C-UBT-negative group (24.3%, 9/37) (P < 0.05). Of the 61 patients with PHG who were 13C-UBT positive, 40 had severe PHG and 21 had mild PHG; two patients had severe PHG and seven had mild PHG in the group of 13C-UBT-negative patients. The difference was statistically significant (P < 0.05, odds ratio = 0.150, 95% confidence interval = 0.029–0.787) [Figure 1].
Figure 1.
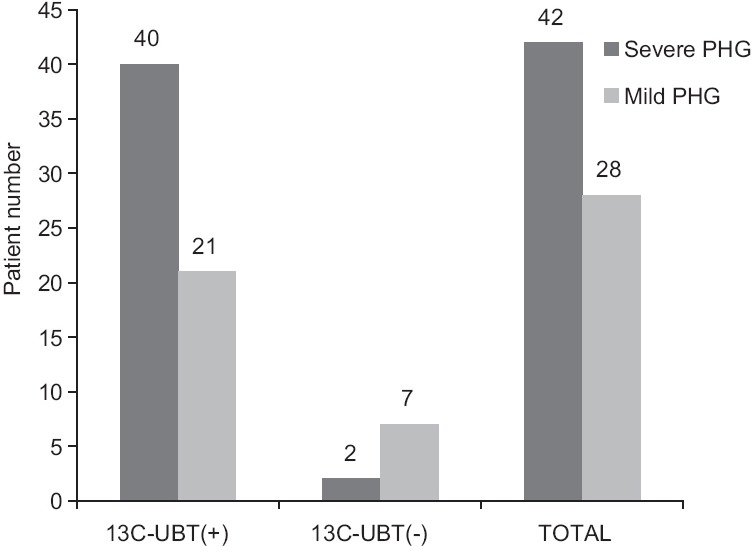
Association of 13C-UBT with severity of PHG (P = 0.035, OR = 0.150, 95% CI = 0.029–0.787). 13C-UBT: 13C urea breath test; PHG: Portal hypertensive gastropathy; OR: Odds ratio; CI: Confidence interval.
Discussion
H. pylori infections can be diagnosed by a variety of invasive and noninvasive methods. Originally, endoscopic biopsy was the gold standard, but it is invasive and prone to sampling error, as H. pylori tends to be heterogeneously distributed in the stomach. Serologic examinations are noninvasive and convenient but do not accurately reflect infection status. The 13C-UBT is based on the potent urease activity of H. pylori in the gastric mucosa. It uses 13C-labeled urea for the test, is noninvasive, and was developed to overcome the shortcomings of serologic testing. 13C-UBT is widely used for the detection of H. pylori infection because it is reported to have a sensitivity and specificity of more than 90%, and it is more convenient to use and safer for patients. For these reasons, the 13C-UBT is now routinely used for the diagnosis of H. pylori infection.[13,14,15] This study used 13C-UBT for the detection of H. pylori infection and was easily accepted by patients and volunteers.
In recent years, investigators have paid close attention to the relationship between H. pylori infection and liver disease. It was reported that the H. pylori infection rate in patients with HBV was significantly higher than in volunteers. Moreover, HB patients with H. pylori coinfection exhibited high levels of HBV-DNA.[16] Studies indicated that H. pylori infection rates in patients with HBV-related cirrhosis and HBV-related hepatic carcinoma were significantly higher than in the control group.[17] H. pylori could be detected not only in human gastric mucosa, but also in human hepatic tissue.[18]
The present study demonstrates that the HBV-related cirrhosis patient group had the highest H. pylori infection rate (79.3%). It was significantly higher than that in the chronic HB, HBV-negative hepatic carcinoma, and control groups, which indicated that H. pylori infection rate increased with progression of disease in patients with chronic HB. This result suggests that H. pylori contributes to pathogenesis in coordination with HBV. The H. pylori infection rate in patients with ≥103 copies/ml HBV-DNA was significantly higher than in those with <103 copies/ml HBV-DNA (P < 0.05), suggesting that HBV-DNA replication could increase the H. pylori infection rate. However, there was no significant difference between the group with 103–106 copies/ml HBV-DNA and the group with >106 copies/ml HBV-DNA, indicating that the H. pylori infection rate was not correlated with viral load.
This study shows that PT, TBIL, AST, blood NH3, and AFP levels in the 13C-UBT positive group were significantly higher than those in the 13C-UBT-negative group (P < 0.05). In addition, patients with HBV-related cirrhosis whose 13C-UBT was positive had worse liver function, suggesting that H. pylori infection may aggravate liver pathology. The percentage of patients with liver function of Child-Pugh Grade C in the 13C-UBT-positive group was significantly higher than in the 13C-UBT-negative group. Meanwhile, we found that serum AFP level in the 13C-UBT-positive group was significantly higher than that in the 13C-UBT-negative group, indicating that H. pylori infection was perhaps a risk factor for the occurrence of hepatic carcinoma. It is possible that H. pylori infection is associated with immunopathogenic damage and immunological tolerance in patients with hepatic disease. Interestingly, with the progression of the disease, the H. pylori infection rate was increased. As patients with chronic HB are often immunocompromised, they exhibit signs of immunopathogenic damage as well as immunological tolerance. This results in a reduction in the ability of the gastrointestinal mucosa to defend against infection, leading to an alteration in the flora of the gastrointestinal tract. The gastric mucosa then reaches a state of hyperemia and anoxia, which makes eradication of H. pylori difficult, leading to an increase in the H. pylori infection rate.[19] The incidence of esophageal fundus variceal bleeding, ascites, hepatic encephalopathy, and PHG in the 13C-UBT-positive group was significantly higher than for the 13C-UBT-negative group (P < 0.05). This suggests that H. pylori eradication should be performed for patients with hepatic disease with complicating H. pylori coinfection to prevent the deterioration of liver function.
High ammonia levels in the blood is the main cause of hepatic encephalopathy in patients with cirrhosis, with the main source of NH3 in the blood coming from the intestinal tract. It was found that urease produced by H. pylori could decompose urea that diffused into the digestive tract, producing large amounts of NH3. This results in an increase in the NH3 concentration in the stomach, potentially causing damage to the gastric mucosa. NH3 is then absorbed through the stomach into the blood, making H. pylori infection a likely source of high-blood NH3 in patients with cirrhosis.[20] In this study, we demonstrated that blood NH3 levels in the 13C-UBT-positive group were significantly higher than those in the 13C-UBT-negative group, with a concomitant increase in the incidence of hepatic encephalopathy. This supports the observations that H. pylori in the stomach contributes to high-blood NH3, and that H. pylori infection can lead to hepatic encephalopathy. When gastric mucosa is in a state of congestion and edema, local tissue becomes ischemic, anoxic, and prone to circulatory disorders, providing conditions prone to H. pylori infection and gastric mucosal erosion and bleeding. Moreover, changes in intestinal flora make H. pylori eradication difficult. In addition, the cytotoxic action of H. pylori could aggravate hepatic injury and induce hyperammonemia and hepatic encephalopathy.[21,22]
The pathogenesis of PHG is complex. Many factors, including splanchnic blood flow, local disturbances in the regulation of vascular tone, and portal pressure, have been examined to determine the underlying mechanisms. It is postulated that PHG develops as a result of vascular congestion induced by blockade of gastric blood drainage rather than by hyperemia. H. pylori infection is well documented to be associated with many gastric mucosal lesions and peptic ulcers; however, its role in the development of PHG is unclear. This study confirms that H. pylori infection is significantly correlated with the severity of PHG, as previously reported.[12] Our results show that patients with H. pylori infection are nearly twice as likely to develop PHG as patients without H. pylori infection. Our study also showed that severity of PHG was associated with H. pylori infection (P < 0.05). Thus, we demonstrated a significant association of H. pylori with PHG in cirrhosis and with the severity of PHG. The results suggest that the eradication of H. pylori may delay the progression of PHG.
In conclusion, our analysis showed a positive association between H. pylori infection and the risk of chronic HB, HBV-related cirrhosis, and HBV-related hepatic carcinoma. This finding suggests that H. pylori infection may be associated with clinical manifestation and disease progression. We wish to highlight the importance of H. pylori screening of patients with chronic HB, particularly those with HBV-related cirrhosis and HBV-related hepatic carcinoma. However, given the limitations of the included studies, our study itself had some limitations. These findings must, therefore, be confirmed by larger prospective trials.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yi Cui
References
- 1.Choi YJ, Kim N, Paik JH, Kim JM, Lee SH, Park YS, et al. Characteristics of Helicobacter pylori-positive and Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphoma and their influence on clinical outcome. Helicobacter. 2013;18:197–205. doi: 10.1111/hel.12033. doi:10.1111/hel.12033. [DOI] [PubMed] [Google Scholar]
- 2.Song ZQ, Zhou LY. Helicobacter pylori and gastric cancer: Clinical aspects. Chin Med J. 2015;128:3101–5. doi: 10.4103/0366-6999.169107. doi:10.4103/0366-6999.169107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matusiak A, Chalubinski M, Broncel M, Rechcinski T, Rudnicka K, Miszczyk E, et al. Putative consequences of exposure to Helicobacter pylori infection in patients with coronary heart disease in terms of humoral immune response and inflammation. Arch Med Sci. 2016;12:45–54. doi: 10.5114/aoms.2015.50772. doi:10.5114/aoms.2015.50772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang DZ, Chen W, Yang S, Wang J, Li Q, Fu Q, et al. Helicobacter pylori infection in Chinese patients with atrial fibrillation. Clin Interv Aging. 2015;10:813–9. doi: 10.2147/CIA.S72724. doi:10.2147/CIA.S72724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabelo-Gonçalves EM, Sgardioli IC, Lopes-Cendes I, Escanhoela CA, Almeida JR, Zeitune JM. Improved detection of Helicobacter pylori DNA in formalin-fixed paraffin-embedded (FFPE) tissue of patients with hepatocellular carcinoma using laser capture microdissection (LCM) Helicobacter. 2013;18:244–5. doi: 10.1111/hel.12040. doi:10.1111/hel.12040. [DOI] [PubMed] [Google Scholar]
- 6.Çinar A, Sadiç M, Atilgan HI, Baskin A, Koca G, Demirel K, et al. Prevalence of Helicobacter pylori infection in school and pre-school aged children with C-14 urea breath test and the association with familial and environmental factors. Mol Imaging Radionucl Ther. 2015;24:66–70. doi: 10.4274/mirt.53215. doi:10.4274/mirt.53215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chinese Society of Hepatology and Chinese Society of Infectious Diseases, Chinese Medical Association. The guideline of prevention and treatment for chronic hepatitis B. Chin J Liver Dis (Electron Version) 2015;7:1–18. doi:10.3969/j.issn.1674-7380.2015.03.001. [Google Scholar]
- 8.Honar N, Minazadeh A, Shakibazad N, Haghighat M, Saki F, Javaherizadeh H. Diagnostic accuracy of urea breath test for Helicobacter pylori infection in children with dyspepsia in comparison to histopathology. Arq Gastroenterol. 2016;53:108–12. doi: 10.1590/S0004-28032016000200011. doi:10.1590/S0004-28032016000200011. [DOI] [PubMed] [Google Scholar]
- 9.Somily AM, Morshed MG. An update of laboratory diagnosis of Helicobacter pylori in the Kingdom of Saudi Arabia. J Infect Dev Ctries. 2015;9:806–14. doi: 10.3855/jidc.5842. doi:10.3855/jidc.5842. [DOI] [PubMed] [Google Scholar]
- 10.Mu D, Yan L, Tang H, Liao Y. A sensitive and accurate quantification method for the detection of hepatitis B virus covalently closed circular DNA by the application of a droplet digital polymerase chain reaction amplification system. Biotechnol Lett. 2015;37:2063–73. doi: 10.1007/s10529-015-1890-5. doi:10.1007/s10529-015-1890-5. [DOI] [PubMed] [Google Scholar]
- 11.Qu B, Wang H, Liu Y, Jia Y. Effects of H. pylori infection on carotid intima-media thickness, serum glucose, serum uric acid, liver and kidney function in subjects with chronic alcohol ingestion. Int J Cardiol. 2015;187:470–1. doi: 10.1016/j.ijcard.2015.03.338. doi:10.1016/j.ijcard.2015.03.338. [DOI] [PubMed] [Google Scholar]
- 12.Gjeorgjievski M, Cappell MS. Portal hypertensive gastropathy: A systematic review of the pathophysiology, clinical presentation, natural history and therapy. World J Hepatol. 2016;8:231–62. doi: 10.4254/wjh.v8.i4.231. doi:10.4254/wjh.v8.i4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon YH, Kim N, Lee JY, Choi YJ, Yoon K, Yoon H, et al. The diagnostic validity of the (13) c-urea breath test in the gastrectomized patients: Single tertiary center retrospective cohort study. J Cancer Prev. 2014;19:309–17. doi: 10.15430/JCP.2014.19.4.309. doi:10.15430/JCP.2014.19.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boklage SH, Mangel AW, Ramamohan V, Mladsi D, Wang T. Cost-effectiveness analysis of universal noninvasive testing for post-treatment confirmation of Helicobacter pylori eradication and the impact of patient adherence. Patient Prefer Adherence. 2016;10:1025–35. doi: 10.2147/PPA.S102760. doi:10.2147/PPA.S102760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirata Y, Serizawa T, Shichijo S, Suzuki N, Sakitani K, Hayakawa Y, et al. Efficacy of triple therapy with esomeprazole, amoxicillin, and sitafloxacin as a third-line Helicobacter pylori eradication regimen. Int J Infect Dis. 2016;51:66–9. doi: 10.1016/j.ijid.2016.08.019. doi:10.1016/j.ijid.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 16.Waluga M, Kukla M, Zorniak M, Bacik A, Kotulski R. From the stomach to other organs: Helicobacter pylori and the liver. World J Hepatol. 2015;7:2136–46. doi: 10.4254/wjh.v7.i18.2136. doi:10.4254/wjh.v7.i18.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Segura-López FK, Güitrón-Cantú A, Torres J. Association between Helicobacter spp. infections and hepatobiliary malignancies: A review. World J Gastroenterol. 2015;21:1414–23. doi: 10.3748/wjg.v21.i5.1414. doi:10.3748/wjg.v21.i5.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng H, Zhou X, Zhang G. Association between cirrhosis and Helicobacter pylori infection: A meta-analysis. Eur J Gastroenterol Hepatol. 2014;26:1309–19. doi: 10.1097/MEG.0000000000000220. doi:10.1097/MEG.0000000000000220. [DOI] [PubMed] [Google Scholar]
- 19.Rabelo-Gonçalves E, Roesler B, Guardia AC, Milan A, Hara N, Escanhoela C, et al. Evaluation of five DNA extraction methods for detection of H. pylori in formalin-fixed paraffin-embedded (FFPE) liver tissue from patients with hepatocellular carcinoma. Pathol Res Pract. 2014;210:142–6. doi: 10.1016/j.prp.2013.11.003. doi:10.1016/j.prp.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Schulz C, Schütte K, Reisener N, Voss J, Malfertheiner P. Prevalence of Helicobacter pylori infection in patients with minimal hepatic encephalopathy. J Gastrointestin Liver Dis. 2016;25:191–5. doi: 10.15403/jgld.2014.1121.252.hpy. doi:10.15403/jgld.2014.1121.252.hpy. [DOI] [PubMed] [Google Scholar]
- 21.Sakamoto Y, Oho K, Toyonaga A, Kumamoto M, Haruta T, Inoue H, et al. Effect of Helicobacter pylori infection on esophagogastric variceal bleeding in patients with liver cirrhosis and portal hypertension. J Gastroenterol Hepatol. 2013;28:1444–9. doi: 10.1111/jgh.12221. doi:10.1111/jgh.12221. [DOI] [PubMed] [Google Scholar]
- 22.Xu XS, Chen W, Miao RC, Zhou YY, Wang ZX, Zhang LQ, et al. Survival analysis of hepatocellular carcinoma: A comparison between young patients and aged patients. Chin Med J. 2015;128:1793–800. doi: 10.4103/0366-6999.159356. doi:10.4103/0366-6999.159356. [DOI] [PMC free article] [PubMed] [Google Scholar]


