Abstract
Background:
Drug is an important cause of liver injury and accounts for up to 40% of instances of fulminant hepatic failure. Drug-induced liver injury (DILI) is increasing while the diagnosis becomes more difficult. Though many drugs may cause DILI, Chinese herbal medicines have recently emerged as a major cause due to their extensive use in China. We aimed to provide drug safety information to patients and health carers by analyzing the clinical and pathological characteristics of the DILI and the associated drug types.
Methods:
A retrospective analysis was conducted in 287 patients diagnosed with DILI enrolled in our hospital from January 2011 to December 2015. The categories of causative drugs, clinical and pathological characteristics were reviewed.
Results:
Western medicines ranked as the top cause of DILI, accounting for 163 out of the 287 DILI patients (56.79%) in our study. Among the Western medicine, antituberculosis drugs were the highest cause (18.47%, 53 patients) of DILI. Antibiotics (18 patients, 6.27%) and antithyroid (18 patients, 6.27%) drugs also ranked among the major causes of DILI. Chinese herbal medicines are another major cause of DILI, accounting for 36.59% of cases (105 patients). Most of the causative Chinese herbal medicines were those used to treat osteopathy, arthropathy, dermatosis, gastropathy, leukotrichia, alopecia, and gynecologic diseases. Hepatocellular hepatitis was prevalent in DILI, regardless of Chinese herbal medicine or Western medicine-induced DILI.
Conclusions:
Risks and the rational use of medicines should be made clear to reduce the occurrence of DILI. For patients with liver injury of unknown origin, liver tissue pathological examination is recommended for further diagnosis.
Keywords: Clinical Characteristics, Drug-induced Liver Injury, Pathology
Introduction
Liver is not only the important metabolic, energy supply, and immune regulation organ of the human body,[1] but also the main organ of drug metabolism.[2] Certain chemical or medicinal agents, when taken in overdoses and sometimes even when introduced within therapeutic dosages, may injure the organ and so cause hepatotoxicity. Drug-induced liver injury (DILI) or hepatitis is the inflammation of the liver cells caused by medication, either the drug itself or its metabolic products during the course of treatment. The former is caused by the endogenous liver toxicity and idiosyncratic reaction to the drugs. It is related to drug dosage and can be predicted. In contrast, the latter is the body allergic or idiosyncratic reaction to the drugs that induce liver damage only in a small number of sensitive individuals. It is not related to the dosage of the drugs and usually unpredictable. Due to the increase of dosage and types of newly developed drugs, there has been an increasing tendency of DILI.[3] It has been suggested that in China, DILI accounts for 1–5% of liver disease, for 10% of acute hepatitis, and for 12.2% of acute hepatitis associated with drugs.[4] However, due to the complexity of clinical manifestations of drug hepatitis and lack of specificity of laboratory examination, it remains a significant clinical challenge to timely diagnosis of DILI. To provide drug safety information to patients and health carers about the DILI, we retrospectively analyzed the clinical data of 287 patients with DILI treated in our hospital from January 2011 to December 2015. The clinical characteristics of DILI and the types of associated drugs were reviewed.
Methods
Patient characteristics
In total, 287 patients (156 males and 131 females) diagnosed with DILI in our hospital from January 2011 to December 2015 were enrolled in this retrospective study. The diagnostic standards of DILI are listed in the following section. The ages ranged from 10 to 81 years with an average of 46.95 years and a distribution as: <20 years, five patients (1.74%); 21–40 years, 99 patients (34.49%); 41–60 years, 133 patients (46.34%); and >60 years, fifty patients (17.42%). This study was approved by the Institutional Review Board of The First Affiliated Hospital of Nanchang University. Consent form was obtained from all patients in this study.
Diagnostic standards of drug-induced liver injury
DILI is divided into the hepatocellular type, cholestatic type, and mixed type.[5] Hepatocellular type: alanine transaminase (ALT) >2–3 times the upper limit of normal (ULN) or ALT/alkaline phosphatase (ALP) ≥5. Cholestatic type: ALP >2–3 times the ULN or ALT/ALP ≤2. Mixed type: ALT >2–3 times the ULN and ALP > 2 times the ULN or ALT/ALP ranged from 2 to 5. Diagnostic standards are as follows: (1) the injury all occurred 1–4 weeks after medication (not including adrenal cortical hormone and testosterone); (2) initial symptoms of allergic signs including fever, rash, and pruritus; (3) pathological changes and clinical manifestations of hepatocyte damage or intrahepatic cholestasis; (4) peripheral blood eosinophil higher than 0.06; (5) positive drug lymphocyte transformation test or macrophage migration inhibition test; (6) all serum markers of hepatitis virus negative; (7) and a history of drug-induced hepatitis and used the same drug that induced it. Patients with any two of the above-mentioned seven conditions were considered to have DILI. Patients with the following conditions were excluded from the study: (1) viral hepatitis (particularly sporadic hepatitis E); (2) nonalcoholic fatty liver disease; (3) autoimmune liver diseases (autoimmune hepatitis, primary biliary cirrhosis, and primary sclerosing cholangitis); (4) infection with acute cytomegalovirus, Epstein–Barr virus, or herpes simplex virus; (5) hepatolenticular degeneration; (6) α1-antitrypsin deficiency; (7) hemochromatosis; (8) and other types of liver and gallbladder diseases.
Liver biopsy
With the above examinations, there were 46 cases remained with uncertain diagnosis. Liver biopsy was then taken in these 46 patients for further DILI diagnosis. The liver specimens were 4% paraformaldehyde fixed, paraffin embedded, and routine hematoxylin and eosin staining was performed. Typical pathological changes of DILI include: (1) steatosis: divided into macrovesicular and/or microvesicular; (2) cholestasis: brown bile particles are present in cytoplasm of hepatocytes with bile capillary dilation, forming obvious bile plug; (3) cell apoptosis: apoptotic bodies are present in hepatic cords and sinusoids; (4) hepatocyte necrosis: including the states such as spotty and focal necrosis, piecemeal necrosis/interface inflammation, and submassive and massive necrosis; (5) leukocyte infiltration: eosinophilic leukocyte infiltration in necrotic areas and portal areas; (7) intraepithelial granuloma; (8) and iron deposition.[6,7] All these patients were confirmed with DILI by experienced liver pathologists.
Pathological characteristics of drug-induced liver injury
Pathological characteristics of DILI include: (1) hepatocellular damage type (hepatitis, steatohepatitis): focal or massive hepatocyte necrosis, collapsed mesh stent, inflammatory cell (lymphocyte, eosinophil, and neutrophil) infiltration in portal area and lobule, large fat deposits in hepatocytes, which is the most significant in centrilobular area, with necrosis, inflammation, and cholestasis; (2) intrahepatic cholestasis type: cholestasis in liver centrilobular area, bile plug formation in bile capillaries, accumulation of bilirubin pigment in hepatocytes and stellate cells, no inflammatory cell infiltration; (3) mixed: cholestasis in hepatocytes, bile capillaries and stellate cells, and focal hepatocyte necrosis with ballooning degeneration.[8,9]
Statistical analysis
Patients’ pathological and clinical characteristics were compared according to gender, age, disease courses, types of underlying diseases, oral drug categories, clinical cure rate, improvement rate, mortality rate, pathological type, and the pathogenesis using Chi-square test. Statistical analysis was performed using SPSS Statistical software (version 18.0, SPSS Inc., Chicago, IL, USA). P < 0.05 was considered statistically significant.
Results
In the 287 enrolled patients, the occurrence rate of DILI is slightly higher in males (54.36%) than in females (45.64%) but does not reach the statistically significant level [P > 0.05; Table 1]. However, the rate of DILI in different age groups is statistically significant [P < 0.05, Table 1] with the highest in patients between 21- and 40-year-old, which is in line with the findings from a previous study.[10] The underlying diseases were significantly associated with the increased rate of DILI (P < 0.05). Patients with liver diseases have higher morbidity of DILI than those without liver diseases [P < 0.05; Table 1]. The diseases include hypertension, diabetes, tuberculosis, connective tissue diseases, hyperthyroidism, tumor, gynecological diseases, mammary gland disease, psoriasis, leukoderma, and mental disorder.
Table 1.
Clinical characteristics of 287 patients with drug-induced liver injury
| Characteristics | n (%) | χ2 | P |
|---|---|---|---|
| Gender | |||
| Male | 156 (54.36) | 2.17 | >0.05 |
| Female | 131 (45.64) | ||
| Age (years) | |||
| <20 | 5 (1.74) | 16.20 | <0.05 |
| 21–40 | 99 (34.49) | ||
| 41–60 | 133 (46.34) | ||
| >60 | 50 (17.42) | ||
| Underlying diseases | |||
| Yes | 179 (62.37) | 16.55 | <0.05 |
| No | 108 (37.63) | ||
| Combined hepatopathy | |||
| Yes | 166 (57.84) | 6.89 | <0.05 |
| No | 121 (42.16) |
Age, whether the patients have underlying disease or hepatopathy is related to severe drug-induced liver injury, P<0.05.
We next analyzed the therapeutic categories of the drugs that cause liver injury. As shown in Table 2, Western medicines ranked as the top cause of DILI, accounting for 163 out of the 287 DILI patients (56.79%) in our study. Among the Western medicine, antituberculosis drugs were the highest cause of DILI with 53 cases (18.47%), and all patients were treated with a combination of antituberculosis drugs (isoniazid [INH], rifampin [RFP], pyrazinamide [PZA], etc.). Antibiotics (18 patients, 6.27%) and antithyroid (18 patients, 6.27%) drugs also ranked among the major causes of DILI. Chinese herbal medicines are another major cause of DILI, accounting for 36.59% of cases (105 patients). The Chinese herbal medicine included Chinese patent medicine, Chinese medicine decoction, and folk prescription, which were used to treat osteopathy, arthropathy, dermatosis, leukotrichia, alopecia, and gynecologic diseases, etc., [Table 2].
Table 2.
Therapeutic categories of causative drugs in the 287 patients with drug-induced liver injury
| Drug category | n (%) |
|---|---|
| Chinese herbal medicine | 105 (36.59) |
| Osteopathy, arthropathy | 24 (8.36) |
| Dermatosis | 18 (6.27) |
| Gastropathy | 13 (4.53) |
| Leukotrichia, alopecia | 11 (3.83) |
| Gynecologic disease | 11 (3.83) |
| Nephrosis, prostate disease | 9 (3.14) |
| Regulate sleep, improve physique | 5 (1.74) |
| Constipation | 3 (1.05) |
| Others | 11 (3.83) |
| Western medicine | 163 (56.79) |
| Antituberculosis drug | 53 (18.47) |
| Antibiotics | 18 (6.27) |
| Antithyroid drug | 18 (6.27) |
| Analgesic–antipyretic drug | 15 (5.23) |
| Psychiatric drug | 13 (4.53) |
| Antineoplastic drug | 10 (3.48) |
| Hypoglycemic drug | 10 (3.48) |
| Cardiovascular disease drug | 6 (2.09) |
| Healthcare product | 5 (1.74) |
| Immunosuppressive agent | 5 (1.74) |
| Antifungal agent | 4 (1.39) |
| Hair dyes | 4 (1.39) |
| Hypolipidemic | 2 (0.70) |
| Others | 19 (6.62) |
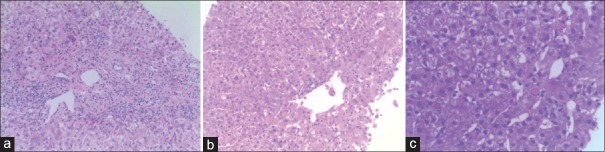
Liver tissue biopsy and pathological confirmation of DILI were performed in 46 patients. The pathological results showed that 37 patients had acute liver injury (80.43%) and nine patients had chronic liver injury (19.56%). Hydropic degeneration of liver cells was observed in all cases. Among the 46 patients, forty patients showed eosinophil infiltration (86.96%), 34 patients had spotty and focal necrosis (73.91%), thirty patients had inflammatory cell infiltration (65.22%), 26 patients had cholestasis in hepatocytes (56.52%), 22 patients had fatty degeneration of hepatocytes (47.83%), 16 patients had necrosis around the central vein (34.78%), 11 patients had apoptotic body (23.91%), ten patients had acidophilic degeneration of hepatocytes (21.74%), and four patients had central vein injury (8.70%). Fibrogenesis and interface hepatitis were observed in all the nine patients with chronic hepatitis, and there were significant changes in the portal area. Among these biopsied DILI cases, 27 were caused by Chinese herbal medicines, including 18 cases of hepatitis (66.67%), six cases of intrahepatic cholestasis (22.22%), and three cases of mixed characteristics (11.11%). There were 19 cases caused by Western medicines, including 11 cases of hepatitis (57.89%), six cases of intrahepatic cholestasis (36.84%), and three cases of mixed characteristics (5.26%). All the biopsied specimens showed hepatitis B surface antigen and hepatitis C virus antigen negative. The details are shown in Table 3, and typical pathological manifestations are shown in Figure 1a-1c.
Table 3.
Pathological characteristics of 46 patients with drug-induced liver injury, n (%)
| Drug category | n | Hepatitis | Intrahepatic cholestasis | Mixed |
|---|---|---|---|---|
| Chinese herbal medicine | 27 | 18 (66.67) | 6 (22.22) | 3 (11.11) |
| Western medicine | 19 | 11 (57.89) | 7 (36.84) | 1 (5.26) |
| Total | 46 | 29 (63.04) | 13 (28.26) | 4 (8.70) |
Figure 1.
Typical pathological manifestation observed in this study. (a) Hepatitis: focal or massive hepatocyte necrosis, collapsed mesh stent, and inflammatory cell (lymphocyte, eosinophil, and neutrophil) infiltration in portal area and lobule; (b) mixed: cholestasis in hepatocytes, bile capillaries and stellate cells, and focal hepatocyte necrosis with ballooning degeneration; (c) cholestasis: cholestasis in liver centrilobular area, bile plug formation in bile capillaries, accumulation of bilirubin pigment in hepatocytes and stellate cells, and no significant inflammatory cell infiltration, (H and E, original magnification, ×40).
Discussion
DILI, also known as drug-induced hepatitis, is liver damage caused by toxic effect and allergic reaction of drugs. DILI may occur in the healthy population or in patients with previous severe hepatopathy. Clinically, the manifestations may include various acute and chronic liver diseases. Most DILI patients may recover automatically, but some DILI patients have severe outcome and even result in death.
The pathogenesis of DILI is still largely unknown, but the related mechanisms could be direct toxic effect, metabolic disorder, or allergic reaction to drugs, i.e., metabolic and allergic idiosyncrasy. Idiosyncrasy might relate to the genetic polymorphism of cytochrome oxidase P450 (CYP450) and immune factors such as human leukocyte antigen.[11] Initial liver injury is generally caused by hepatic toxic metabolites, i.e., the result of combined activities of Phase I drug metabolism and CYP450 family.[11] CYP450 is a group of isoenzymes which belong to a multigene superfamily of enzymes responsible for the metabolism of a wide range of exogenous compounds such as drugs and environmental chemicals and of endogenous substances such as fatty acids and steroids. Some drugs are metabolized and converted into active toxic metabolites under the action of CYP450, such as electrophilic group, free radical, and oxygroup, which can covalently bind macromolecular substances including protein, nucleic acid, and lipid, causing lipid peroxidation and final hepatocyte necrosis.[12]
The interference or obstruction on an important metabolic pathway of hepatocytes or any step in synthesis or secretion of bile caused by metabolites of drugs can induce cholestasis.[13] On the cellular level, the main influences on bile secretion from drugs include cell receptors carrying cholate, fluidity of cell membrane, activity of Na+ -K+ -ATP enzyme, ion exchange, integrity changes of cytoskeleton, and cell lipid membrane. Immune response also plays a critical role in the development of DILI. Drugs or their metabolites are bound with liver-specific protein onto antigens, and then they are identified by immune competent cells postprocessing by macrophages, which lead to allergic abnormal reaction, inducing liver injury.[14,15]
In the current retrospective study, of the 287 patients with DILI, the rate of males is slightly higher than that of females but not statistically significant. The higher risk in males might relate the higher rate of smokers and drinkers, a greater living and working pressure, present slight discomfort not causing attention, or a difference in drug elimination among the male patients. These would attribute to the reduction of hepatocyte microsomal enzyme activity, hypohepatia, and renal insufficiency in the elderly. In addition, the incidence rate of severe DILI in those patients with combined hepatopathy or other underlying diseases is higher.
Chinese herbal medicines are extensively used in China. Due to the traditional viewpoint that these herbal medicines are less toxic than Western medicines, some clinicians and patients would neglect the adverse effects of Chinese herbal medicines to some extent. However, in recent years, greater attention has been given to the adverse effects of Chinese herbal medicines, particularly the DILI caused by Chinese herbal medicines with lethal events has been reported.[16] In the 287 cases of DILI in our study, there were 105 cases (36.59%) caused by Chinese herbal medicines, suggesting that Chinese herbal medicine is a major cause of DILI. Most of the Chinese herbal medicines associated with DILI were used to treat osteopathy, arthropathy, dermatosis, gastropathy, leukotrichia, alopecia, and gynecologic diseases, etc., The effective ingredients of these herbal medicines mainly include Rhizoma alismatis, tripterygium glycosides, Dioscorea bulbifera, Polygonum multiflorum, raw snake gallbladder, mylabris, centipede powder, ginger-processed Pinellia, and Fructus xanthii. The main mechanisms of liver injury induced by Chinese herbal medicines include direct toxicity, allergic reaction, improper drug processing, extended drug use, exceeding recommended dosages, and folk prescription. Therefore, the safety specifications and clinical monitoring of Chinese herbal medicine usage should be intensified.
In western medicine, antituberculosis drugs is a leading cause of DILI, accounting for the highest percentage up to 18.47%, followed by antibiotics and antithyroid drugs.[17] Several factors could relate to the high collected cases of liver injury induced by antituberculosis drugs, for example, the long current antithyroid course and the combined use of antituberculosis drugs that have significant hepatotoxic effects. The incidence rate of DILI related to RFP, INH, and PZA is up to 17.2–25.0%, and severe hepatitis can occur.[18] Therefore, clinicians should closely observe the potential adverse reactions by monitoring liver functions and enhance the awareness of diagnostics and therapies for the relevant diseases.
The diagnostics of the cause of DILI must exclude other related causes if no clinical manifestation of specificity and laboratory examination was observed. In clinical practice, patients often use a combination of multiple drugs, and therefore it is difficult to determine which drug is causative. Unfortunately, diagnostics of DILI is still in the absence of effective methods. Liver tissue biopsy can provide powerful evidence for the diagnostics of DILI, especially for those patients with unknown cause and unclear medication.[19] Because the manifestations of DILI can be fibrosis proliferation in portal areas, interface inflammation, etc., it is hard to be distinguished from liver injury induced by other reasons. Significant eosinophil infiltration of liver tissue and spotty and focal necrosis of hepatocytes around central veins are pathological characteristics of DILI. These pathological changes are different from those of viral hepatitis, alcoholic hepatitis, and autoimmune liver diseases. Therefore, they may be used as important evidences for pathological identification of DILI.
Acute liver injury caused by drug-induced hepatitis is a common clinical symptom. However, due to no specific manifestation and a long history of diseases, the diagnosis of DILI is difficult and easily ignored by clinicians. Due to no effective drug in the treatment of DILI at present, we should give priority to take precautions against it in clinic. It is suggested to avoid long-term and abundant usage of drugs at the greatest extent during medication. For the patients with underlying diseases or previous liver diseases, several practices will be helpful, including keeping greater vigilance on the occurrence of drug-induced hepatitis, strengthening the monitoring on blood concentration, performing liver function test periodically for early detection of drug-induced hepatitis, and taking treatment as soon as possible. Liver biopsy is necessary in the diagnosis of drug-induced hepatitis. For the patients with liver injury of unknown origin, liver biopsy should be done since it can provide the reliable evidence for clear diagnosis, especially for the patients with long-term medication.
The outcome of drug-induced hepatitis is generally good, and drug withdrawal is critical for the management of drug-induced hepatitis. Regardless of any kind of drug inducing liver injury, injured hepatocytes have great recoverability postdrug withdrawal. Chinese herbal medicine-induced liver injury cannot be neglected, and clinicians should avoid drug abuse and pay attention to monitor the adverse reactions of drugs and liver functions and prevent DILI, especially for Chinese herbal medicine.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Qiang Shi
References
- 1.Jakab L. The liver and the immune system. Orv Hetil. 2015;156:1203–13. doi: 10.1556/650.2015.30190. doi:10.1556/650.2015.30190. [DOI] [PubMed] [Google Scholar]
- 2.Chughlay MF, Kramer N, Spearman CW, Werfalli M, Cohen K. N-acetylcysteine for non-paracetamol drug-induced liver injury: A systematic review. Br J Clin Pharmacol. 2016;81:1021–9. doi: 10.1111/bcp.12880. doi:10.1111/bcp.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haque T, Sasatomi E, Hayashi PH. Drug-induced liver injury: Pattern recognition and future directions. Gut Liver. 2016;10:27–36. doi: 10.5009/gnl15114. doi:10.5009/gnl15114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tong J, Wang BY. Analysis on the diagnosis and treatment of 107 cases of drug hepatitis. Shandong Med J. 2011;51:79–80. doi:10.3969/j.issn.1002-266X.2011.40.039. [Google Scholar]
- 5.Zhao LL, Zhang Y. Risk assessment and advances in diagnosis and treatment of drug-induced liver injury. Int J Dig Dis. 2015;35:119–21. doi:10.3969/j.issn.1673-534X.2015.02.014. [Google Scholar]
- 6.Kleiner DE. The pathology of drug-induced liver injury. Semin Liver Dis. 2009;29:364–72. doi: 10.1055/s-0029-1240005. doi:10.1055/s-0029-1240005. [DOI] [PubMed] [Google Scholar]
- 7.Zhao D, Li HL, Diao ZY, Li CQ. Clinical analysis of 141 cases with drug hepatitis. Chin J Lab Clin Infect Dis (Electron Version) 2013;7:86–9. doi:10.3877/cma.j.issn.1674-1358.2013.02.021. [Google Scholar]
- 8.Tailor A, Faulkner L, Naisbitt DJ, Park BK. The chemical, genetic and immunological basis of idiosyncratic drug-induced liver injury. Hum Exp Toxicol. 2015;34:1310–7. doi: 10.1177/0960327115606529. doi:10.1177/0960327115606529. [DOI] [PubMed] [Google Scholar]
- 9.McGill MR, Du K, Weemhoff JL, Jaeschke H. Critical review of resveratrol in xenobiotic-induced hepatotoxicity. Food Chem Toxicol. 2015;86:309–18. doi: 10.1016/j.fct.2015.11.003. doi:10.1016/j.fct.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucena MI, Andrade RJ, Kaplowitz N, García-Cortes M, Fernández MC, Romero-Gomez M, et al. Phenotypic characterization of idiosyncratic drug-induced liver injury: The influence of age and sex. Hepatology. 2009;49:2001–9. doi: 10.1002/hep.22895. doi:10.1002/hep.22895. [DOI] [PubMed] [Google Scholar]
- 11.Feng S, He X. Mechanism-based inhibition of CYP450:An indicator of drug-induced hepatotoxicity. Curr Drug Metab. 2013;14:921–45. doi: 10.2174/138920021131400114. doi:10.2174/138920021131400114. [DOI] [PubMed] [Google Scholar]
- 12.Michaut A, Le Guillou D, Moreau C, Bucher S, McGill MR, Martinais S, et al. Acellular model to study drug-induced liver injury in nonalcoholic fatty liver disease: Application to acetaminophen. Toxicol Appl Pharmacol. 2016;292:40–55. doi: 10.1016/j.taap.2015.12.020. doi:10.1016/j.taap.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schadt HS, Wolf A, Pognan F, Chibout SD, Merz M, Kullak-Ublick GA. Bile acids in drug induced liver injury: Key players and surrogate markers. Clin Res Hepatol Gastroenterol. 2016;40:257–66. doi: 10.1016/j.clinre.2015.12.017. doi:10.1016/j.clinre.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 14.Dara L, Liu ZX, Kaplowitz N. Mechanisms of adaptation and progression in idiosyncratic drug induced liver injury, clinical implications. Liver Int. 2016;36:158–65. doi: 10.1111/liv.12988. doi:10.1111/liv.12988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrade RJ, Ortega-Alonso A, Lucena MI. Drug-induced liver injury clinical consortia: A global research response for a worldwide health challenge. Expert Opin Drug Metab Toxicol. 2016;12:589–93. doi: 10.1517/17425255.2016.1141896. doi:10.1517/17425255.2016. [DOI] [PubMed] [Google Scholar]
- 16.Yang J. Clinical analysis of HBsAg carrier's hepatic lesion caused by anti-tuberculosis treatment. J Clin Med Pract. 2003;7:370. doi:10.3969/j.issn.1672-2353.2003.04.036. [Google Scholar]
- 17.Devarbhavi H. Antituberculous drug-induced liver injury: Current perspective. Trop Gastroenterol. 2011;32:167–74. [PubMed] [Google Scholar]
- 18.Li XC. Clinical experience in treatment of drug-induced hepatitis. Jilin Med J. 2011;32:3015. doi:10.3969/j.issn.1004-0412.2011.15.056. [Google Scholar]
- 19.Teschke R, Frenzel C. Drug induced liver injury: Do we still need a routine liver biopsy for diagnosis today?Ann Hepatol. 2014;13:121–6. [PubMed] [Google Scholar]