Abstract
Background:
Shensong Yangxin (SSYX), a traditional Chinese herbal medicine, has long been used clinically to treat arrhythmias in China. However, the mechanism of SSYX on atrial fibrillation (AF) is unknown. In this study, we tested the hypothesis that the effect of SSYX on the progression of paroxysmal AF is correlated with the regulation of autonomic nerve activity.
Methods:
Eighteen mongrel dogs were randomly divided into control group (n = 6), pacing group (n = 6), and pacing + SSYX group (n = 6). The control group was implanted with pacemakers without pacing; the pacing group was implanted with pacemakers with long-term intermittent atrial pacing; the pacing + SSYX group underwent long-term intermittent atrial pacing and SSYX oral administration.
Results:
Compared to the pacing group, the parameters of heart rate variability were lower after 8 weeks in the pacing + SSYX group (low-frequency [LF] component: 20.85 ± 3.14 vs. 15.3 ± 1.89 ms2, P = 0.004; LF component/high-frequency component: 1.34 ± 0.33 vs. 0.77 ± 0.15, P < 0.001). The atrial effective refractory period (AERP) was shorter and the dispersion of the AERP was higher after 8 weeks in the pacing group, while the changes were suppressed by SSYX intake. The dogs in the pacing group had more episodes and longer durations of AF than that in the pacing + SSYX group. SSYX markedly inhibited the increase in sympathetic nerves and upregulation of tumor necrosis factor-alpha and interleukin-6 expression in the pacing + SSYX group. Furthermore, SSYX suppressed the decrease of acetylcholine and α7 nicotinic acetylcholine receptor protein induced by long-term intermittent atrial pacing.
Conclusions:
SSYX substantially prevents atrial electrical remodeling and the progression of AF. These effects of SSYX may have association with regulating the imbalance of autonomic nerve activity and the cholinergic anti-inflammatory pathway.
Keywords: Atrial Fibrillation, Autonomic Nerve, Cholinergic Anti-inflammatory Pathway, Inflammatory Cytokines, Shensong Yangxin
Introduction
Studies have demonstrated that atrial fibrillation (AF) may progress from paroxysmal to persistent in up to 50% of patients despite pharmacological therapy.[1] In addition to increasing age, several other comorbidities, such as heart failure, hypertension, previous stroke or transient ischemic attack, and chronic obstructive pulmonary disease, have individually been identified as independent predictors that promote development of AF.[2] Attempts to curb AF progression have centered around the modulation of factors that are known to promote this development; however, no marked progress in the pharmaceutical cure of AF has been achieved.
Shensong Yangxin (SSYX), a Chinese herb extract, comprised 12 medicinal materials (Ginseng, Ophiopogonis, Cornus officinalis, Salvia miltiorrhiza, Semen Ziziphi spinosae, Taxilli herba, Paeoniae Radix Rubra, Eupolyphaga seu steleophaga, Nardostachys, Coptis chinensis, Schisandrae sphenantherae fructus, and Os draconis), is reported to be effective for the treatment of cardiac arrhythmias.[3,4,5] A recent systematic review showed that SSYX appears to be a relatively effective and safe treatment option for the treatment of paroxysmal AF.[6] Experimental studies indicated that SSYX is a multichannel blocker with a pronounced inhibition of inward Na+ and Ca2+ currents, which may change the action potential duration (APD) and contribute to some of its antiarrhythmic effects.[7,8] A previous study has shown that the effect of SSYX on the APD and atrial conducting capacity is attributed to an effect on the autonomic nervous system.[7] However, the mechanism of SSYX for the treatment of paroxysmal AF is unknown. The functional mechanisms underlying AF are characterized by a marked decrease in the APD as well as atrial effective refractory period (AERP).[9] Therefore, this study was designed to test the hypothesis that the effect of SSYX on the progression of paroxysmal AF is attributed to the regulation of autonomic nerve activity.
Methods
Animal model preparation
This study was approved by the Animal Studies Subcommittee of Wuhan University School of Medicine and complied with the guidelines of the National Institutes of Health for the care and use of laboratory animals. All surgeries were performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering. Eighteen adult mongrels, weighing an average of 18.2 ± 0.9 kg, were used in the study. Each dog was given an intramuscular injection of 25 mg/kg ketamine sulfate before being premedicated with pentobarbital sodium.
All the dogs were intubated and ventilated with room air supplemented with oxygen from a respirator (MAO01746, Harvard Apparatus, USA). Normal saline at 50–100 ml/h was infused to replace spontaneous fluid losses. Standard surface 12-lead electrocardiograms (ECGs, Lead 2007B, Jingjiang Inc., China) were monitored continuously throughout the procedure. Pacemakers were implanted as described previously.[10] All dogs were allowed to raise for eight weeks.
Experimental protocol
Eighteen dogs were assigned into three groups. The control group (n = 6) was implanted with pacemakers without pacing. The pacing group (n = 6) and pacing + SSYX group (n = 6) were implanted with pacemakers with long-term intermittent atrial pacing. After 3 days for recovery, the pacing group and pacing + SSYX group dogs were paced at 400 beats/min for 8 h a day and a total of 8 weeks. The pacing + SSYX group dogs were given solution of SSYX in 0.9% saline through oral administration for 8 weeks. SSYX capsules were provided by Yiling Pharmaceutical Corporation (Shijiazhuang, China). The control group and pacing group dogs were given 0.9% saline.
Heart rate variability analysis
Heart rate variability (HRV) measurements started at 10:00 a.m. The raw ECG data were extracted from the telemetric recording using a custom software (BI9800 Biomedical Instruments, China) at baseline and 8 weeks after cessation of pacing. The data were edited to manually remove technical and physiological artifacts. The following power spectral variables were determined: high-frequency (HF) component (0.15–0.4 Hz, a marker of the parasympathetic tone), low-frequency (LF) component (0.04–0.15 Hz, possibly correlated with sympathetic tone or to autonomic balance), and the ratio between LF and HF powers (LF/HF, index of the interaction between sympathetic and vagal activity).
Electrophysiological measurements
After the right- and left-sided atherectomy of the fourth intercostal space of the dog, six customized electrodes were sutured to the right atrium (RA), left atrium (LA), and four pulmonary veins, respectively. The AERP and dispersion of the AERP (dAERP) were determined as previously described.[11] An S1S1 (120, 100, and 75 ms cycle length, respectively, lasting for 5 s each) programmed stimulus method was used to assess the inducibility and duration of AF. AF was defined as irregular atrial rates faster than 500 beats/min, with irregular atrioventricular conduction lasting longer than 5 s. AF times and AF duration separately refer to the average number of times and length of time that AF was induced in every dog within a group, respectively.
Immunohistochemistry
All samples for histology were fixed in 4% paraformaldehyde until embedded in paraffin. Four-micrometer sections were cut from paraffin blocks of the LA and the RA. The sections were stained with tyrosine hydroxylase (TH, monoclonal rabbit anti-TH antibody, Abcam, Inc., UK; used at 1:200) to label sympathetic nerves and choline acetyltransferase (ChAT) (rabbit polyclonal anti-ChAT antibody, LSbio, Inc., USA; used at 1:200) to label parasympathetic nerves. The sections were then incubated with the secondary antibody (goat anti-mouse antibody, Aspen, Inc., China; used at 1:50). We determined the density by a computer-assisted Image-Pro Plus software (Media Cybernetics, Rockville, USA). Each slide was examined under a fluorescence microscope with ×400 objective to select three fields with the highest density of nerves. The computer automatically detected the stained nerves in these fields by their red colors and then calculated the nerve area occupied by the nerves in the field. The density was the positive area divided by the total area examined. The mean density in the three selected fields was used to represent the density of that slide.
Enzyme-linked immunosorbent assay
A 2 ml volume of venous blood was collected in ethylenediamine tetraacetic acid vacutainers and centrifuged at 3000 ×g for 10 minutes at 4°C from the three groups of dogs at baseline and after 8 weeks. The plasma was separately kept in microtubes and stored at −80°C until assay. The levels of tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and acetylcholine (Ach) were examined by enzyme-linked immunosorbent assay.
Western blotting
At the completion of the protocol, the animals were euthanized and the hearts were quickly excised. Tissue specimens were obtained from the LA and RA and were temporarily stored at −80°C until assay. Expression of TNF-α, IL-6, and α7 nicotinic acetylcholine receptor (α7nAChR) in the LA and RA free walls was measured by western blot. The membranes were incubated with the primary antibody TNF-α (mouse monoclonal anti-TNF-alpha antibody, Abcam Inc., UK; used at 1:100), IL-6 (rabbit polyclonal anti-IL6 antibody, LSbio, Inc., USA; used at 1:100), and a7nAChR (rabbit polyclonal anti-nicotinic Ach receptor alpha 7 antibody, Abcam, Inc., UK; used at 1:500). The membranes were blocked with 5% nonfat dry milk in tris-buffered saline with Tween 20 (TBST) for 1 h and incubated with the primary antibody overnight at 4°C. They were then washed in TBST three times, incubated with the secondary antibody for 1 h at 37°C, and imaged using Immun-Star horseradish peroxidase substrate. The relative expression levels of the protein were determined using image analyzer software (AlphaEase FC, San Leandro, CA, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation. A paired t-test was used for comparisons of continuous variables at the end of the study versus those at the baseline. One-way analysis of variance with Turkey tests was used to compare the means of continuous variables among multiple groups. All statistical tests were two-sided, and a value of P < 0.05 was required for statistical significance.
Results
Characteristics of animal models
There were no significant differences in heart rate and body weight at baseline among the three experimental groups. After 8 weeks, spontaneous AF was not observed in the three groups. There were no significant differences in body weight between baseline and after 8 weeks among the three groups. After 8 weeks, the heart rate was decreased from 167.8 ± 7.2 beats/min at baseline to 134.8 ± 3.5 beats/min (P < 0.001) and from 158.3 ± 10.9 beats/min at baseline to 133.3 ± 10.8 beats/min (P = 0.003) in the pacing and pacing + SSYX groups, respectively. However, there was no significant difference in heart rate between baseline and after 8 weeks in the control group (161.0 ± 10.3 beats/min vs. 157.2 ± 9.8 beats/min, P = 0.53). Compared with the control group dogs, the pacing and pacing + SSYX group dogs had a lower heart rate (134.8 ± 3.5 beats/min vs. 157.2 ± 9.8 beats/min, P < 0.001; and 133.3 ± 10.8 beats/min vs. 157.2 ± 9.8 beats/min, P = 0.003). At the end-point of the study, however, there were no significant difference in heart rate between pacing and pacing + SSYX groups (P = 0.75) [Table 1].
Table 1.
Changes of heart rate among the control, pacing, and pacing + SSYX groups at baseline and after long-term intermittent atrial pacing for 8 weeks
| Heart rate (beats/min) | Control (n = 6) | Pacing (n = 6) | Pacing + SSYX (n = 6) |
|---|---|---|---|
| Baseline | 161.0 ± 10.3 | 167.8 ± 7.2 | 158.3 ± 10.9 |
| After 8 weeks | 157.2 ± 9.8 | 134.8 ± 3. 5†,§ | 133.3 ± 10.8*,‡ |
*P<0.01 versus baseline; †P<0.001 versus baseline; ‡P<0.01 versus control group, §P<0.001 versus control group. SSYX: Shensong Yangxin.
Changes of heart rate variability
There were no significant differences in the parameters of HRV between baseline and after 8 weeks in the control group. After 8 weeks, the LF and LF/HF showed an increasing trend in the pacing + SSYX group, but this did not attain statistical significance. Compared to the parameters at baseline, LF and LF/HF increased and HF decreased significantly after 8 weeks in the pacing group (LF: 11.88 ± 2.54 ms2 vs. 20.85 ± 3.14 ms2, P = 0.003; LF/HF: 0.60 ± 0.17 ms2 vs. 1.34 ± 0.33 ms2, P = 0.001; HF: 20.59 ± 4.98 ms2 vs. 15.52 ± 3.45 ms2, P = 0.046). LF and LF/HF were lower, and HF was higher after 8 weeks in the control and pacing + SSYX groups than those in the pacing group (all P < 0.05) [Table 2].
Table 2.
Evaluation of LF, HF, and LF/HF ratio at baseline and at the end-point of the study in the control, pacing, and pacing + SSYX groups
| Groups | LF (ms2) | HF (ms2) | LF/HF |
|---|---|---|---|
| Control (n = 6) | |||
| Baseline | 13.18 ± 3.33 | 21.70 ± 2.36 | 0.60 ± 0.15 |
| End-point | 12.30 ± 3.36|| | 22.58 ± 4.45§ | 0.55 ± 0.14|| |
| Pacing (n = 6) | |||
| Baseline | 11.88 ± 2.54 | 20.59 ± 4.98 | 0.60 ± 0.17 |
| End-point | 20.85 ± 3.14† | 15.52 ± 3.45* | 1.34 ± 0.33† |
| Pacing + SSYX (n = 6) | |||
| Baseline | 12.49 ± 2.58 | 21.64 ± 4.61 | 0.62 ± 0.24 |
| End-point | 15.3 ± 1.89§ | 19.92 ± 2.75‡ | 0.77 ± 0.15|| |
*P<0.05 versus baseline; †P<0.01 versus baseline; ‡P<0.05 versus pacing group; §P<0.01 versus pacing group; ||P<0.001 versus pacing group. LF: Low-frequency component; HF: High-frequency component; SSYX: Shensong Yangxin.
Electrophysiological testing and atrial fibrillation induction
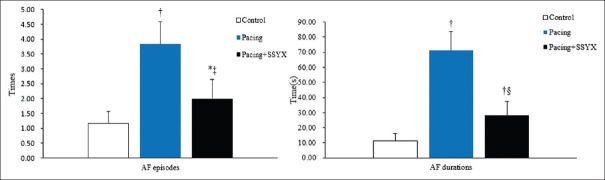
The AERP measurements in the three groups are shown in Figure 1. The AERPs were shorter in the pacing group than in the control and pacing + SSYX groups after 8 weeks (P < 0.05 for all), and the dAERP was higher in the pacing group than in the control group (13.67 ± 1.97ms vs. 8.33 ± 0.82 ms, P < 0.001) and pacing + SSYX group (13.67 ± 1.97 ms vs. 7.67 ± 1.97 ms, P < 0.001). As Figure 2 shows, compared with the control, the pacing and pacing + SSYX groups had significantly more episodes of AF (3.84 ± 0.75 vs. 1.17 ± 0.41, P < 0.01; 2.00 ± 0.63 vs. 1.17 ± 0.41, P < 0.001) and longer durations of AF (71.17 ± 12.51 s vs. 11.17 ± 4.79 s, P < 0.001; 28.33 ± 9.24 s vs. 11.17 ± 4.79 s, P < 0.001) during programmed stimulation after long-term intermittent atrial pacing. However, compared with the pacing group, the pacing + SSYX dogs had fewer episodes of AF (P = 0.033) and shorter durations of AF (P = 0.006).
Figure 1.
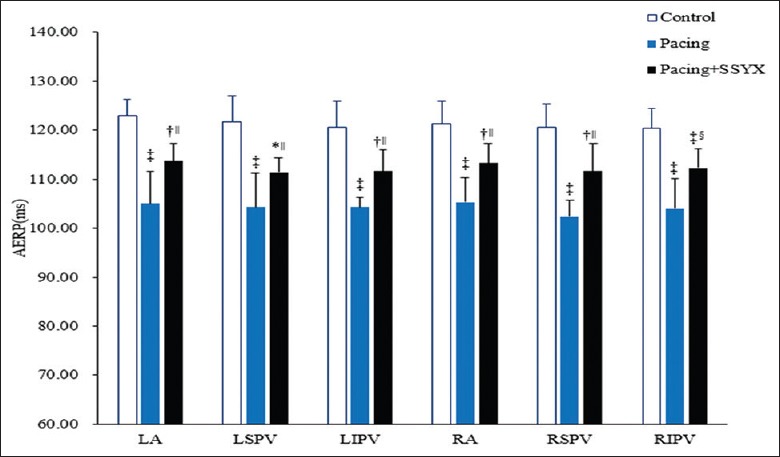
Changes of AERP in the control, pacing, and pacing + SSYX groups after long-term intermittent atrial pacing. *P < 0.05 versus control group, †P < 0.01 versus control group; ‡P < 0.001 versus control group; §P < 0.05, pacing + SSYX group versus pacing group; ||P < 0.01, pacing + SSYX group versus pacing group. SSYX: Shensong Yangxin; AERP: Atrial effective refractory period.
Figure 2.
Comparison of the induced times and durations of AF in control, pacing, and pacing + SSYX groups. *P < 0.01 versus control group, †P < 0.001 versus control group; ‡P < 0.05, pacing + SSYX group versus pacing group; §P < 0.01, pacing + SSYX group versus pacing group. AF: Atrial fibrillation; SSYX: Shensong Yangxin.
Histological findings
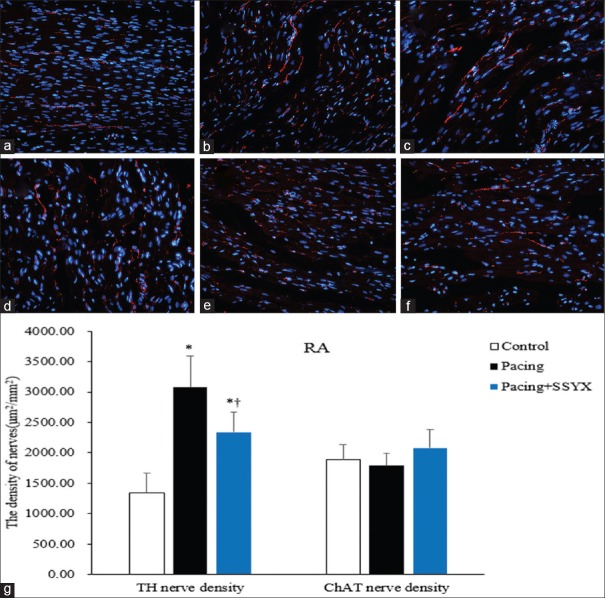
Representative RA sections stained with TH and ChAT are shown in Figure 3. The atrial nerve density for each group was expressed as the mean of the nerve densities in the RA. The densities of TH-positive nerves within the RA were significantly higher in the pacing group and pacing + SSYX group dogs than in the control dogs (3076.03 ± 515.08 μm2/mm2 vs. 1342.99 ± 319.02 μm2/mm2, P < 0.001; 2336.94 ± 336.92 μm2/mm2 vs. 1342.99 ± 319.02 μm2/mm2, P < 0.001), respectively. However, the densities of TH-positive nerves within the RA were significantly higher in the pacing group than in the pacing + SSYX group (3076.03 ± 515.08 μm2/mm2 vs. 2336.94 ± 336.92 μm2/mm2, P = 0.015).
Figure 3.
Histological findings of TH and ChAT RA nerves in all groups. Representative images of TH immunofluorescence staining (original magnification, ×400) of RA in the control group (a), the pacing group (b), and the pacing + SSYX group (c). Exemplary pictures of ChAT immunofluorescence staining of RA in the control group (d), the pacing group (e), and the pacing + SSYX group (f) and statistical chart of TH and ChAT RA nerve (g). *P < 0.001 versus control group; †P < 0.05 versus pacing group. Blue: Nucleus of cardiomyocyte; Red: TH-positive and ChAT-positive nerves. TH: Tyrosine hydroxylase; ChAT: Choline acetyltransferase; RA: Right atrium; SSYX: Shensong Yangxin.
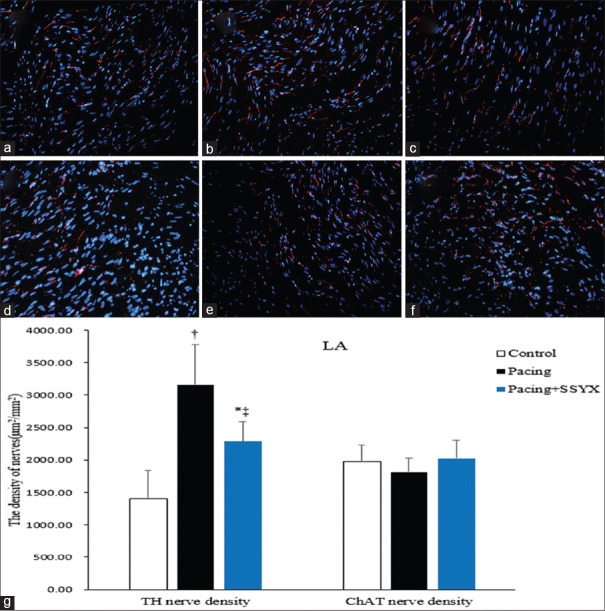
There were no significant differences between ChAT-positive nerve densities in control and pacing dogs (1890.89 ± 249.89 μm2/mm2 vs. 1785.86 ± 202.58 μm2/mm2, P = 0.363). Compared to the pacing group, there was an increasing in ChAT-positive nerve densities of the pacing + SSYX group dogs. However, this result was not statistically significant (1785.86 ± 202.58 μm2/mm2 vs. 2077.16 ± 303.42 μm2/mm2, P = 0.079). The densities of TH-positive and ChAT-positive nerves in the LA are similar to that in the RA [Figure 4].
Figure 4.
Histological sections of TH and ChAT in LA nerves in all groups. Exemplary pictures of TH immunofluorescence staining (original magnification, ×400) of LA in the control group (a), the pacing group (b), and the pacing + SSYX group (c). Exemplary pictures of CHAT immunofluorescence staining of LA in the control group (d), the pacing group (e), the pacing + SSYX group (f), and statistical chart of TH and ChAT in the LA nerve (g). Blue: Nucleus of cardiomyocyte; Red: TH-positive and ChAT-positive nerves. *P < 0.01 versus control group, †P < 0.001 versus control group; ‡P < 0.001, pacing + SSYX group versus pacing group. TH: Tyrosine hydroxylase; ChAT: Choline acetyltransferase; LA: Left atrium; SSYX: Shensong Yangxin.
Plasma levels of tumor necrosis factor-alpha, interleukin-6, and acetylcholine
There were no significant differences in the plasma TNF-α, IL-6, and Ach at baseline among the three experimental groups. After 8 weeks, the plasma TNF-α and IL-6 concentrations increased from 97.03 ± 13.76 pg/ml at baseline to 301.68 ± 39.38 pg/ml (P < 0.001) and from 200.23 ± 30.58 ng/ml at baseline to 607.39 ± 54.57 ng/ml (P < 0.001) in the pacing group, respectively. However, the levels of Ach decreased from 63.69 ± 10.67 μg/ml at baseline to 23.26 ± 4.07 μg/ml (P < 0.001). In the pacing + SSYX group, the plasma TNF-α and IL-6 concentrations increased from 96.55 ± 13.74 ng/ml at baseline to 219.25 ± 35.77 ng/ml (P < 0.001) and from 198.56 ± 37.75 ng/ml at baseline to 397.01 ± 47.58 ng/ml (P = 0.001), respectively, after 8 weeks. The levels of Ach decreased from 66.66 ± 10.35 ng/ml at baseline to 43.00 ± 5.84 ng/ml (P = 0.001). Compared with the control, the plasma TNF-α and IL-6 concentrations were higher in the pacing and the pacing + SSYX dogs (all P < 0.001) after 8 weeks. However, compared with that in the pacing group, the plasma TNF-α and IL-6 concentrations were lower in the pacing + SSYX dogs (P = 0.001 and P < 0.001, respectively), and the levels of Ach were higher in the pacing + SSYX dogs (P < 0.001) [Table 3].
Table 3.
Changes in the plasma levels of Ach, TNF-α, IL-6 among the control, pacing, and pacing + SSYX groups at baseline and after long-term intermittent atrial pacing for 8 weeks
| Groups | Ach (µg/ml) | TNF-α (pg/ml) | IL-6 (pg/ml) |
|---|---|---|---|
| Control (n = 6) | |||
| Baseline | 64.77 ± 9.84 | 98.49 ± 18.27 | 193.92 ± 20.99 |
| End-point | 65.94 ± 8.24§ | 100.65 ± 17.46‡ | 196.61 ± 34.63§ |
| Pacing (n = 6) | |||
| Baseline | 63.69 ± 10.67 | 97.03 ± 13.76 | 200.23 ± 30.58 |
| End-point | 23.26 ± 4.07† | 301.68 ± 39.38† | 607.39 ± 54.57† |
| Pacing + SSYX (n = 6) | |||
| Baseline | 66.66 ± 10.35 | 96.55 ± 13.76 | 198.56 ± 37.75 |
| End-point | 43.00 ± 5.84*,§ | 219.25 ± 35.77†,‡ | 397.01 ± 47.58*,§ |
*P<0.01 versus baseline; †P<0.001 versus baseline; ‡P<0.01 versus pacing group; §P<0.001 versus pacing group. TNF-α: Tumor necrosis factor-alpha; IL-6: Interleukin-6; Ach: Acetylcholine; SSYX: Shensong Yangxin.
Cardiac tissue levels of tumor necrosis factor-alpha, interleukin-6, and α-7 nicotinic acetylcholine receptor
As shown in Figures 5 and 6, Western blot results of atrial tissue samples from the three groups were compared. All immunoblot band intensity measurements were normalized to the intensity of the GADPH band in the loaded sample. As shown in Figures 5 and 6, the level of α7nAChR protein was reduced in RA and LA of the pacing group dogs compared with the control and the pacing + SSYX groups dogs, while TNF-α and IL-6 in LA and RA tissues were significantly higher in the pacing group than in the control and the pacing + SSYX group. Compared with the control group, the levels of TNF-α and IL-6 were higher, and the levels of α7nAChR were lower in the LA and RA tissues in the pacing + SSYX group.
Figure 5.
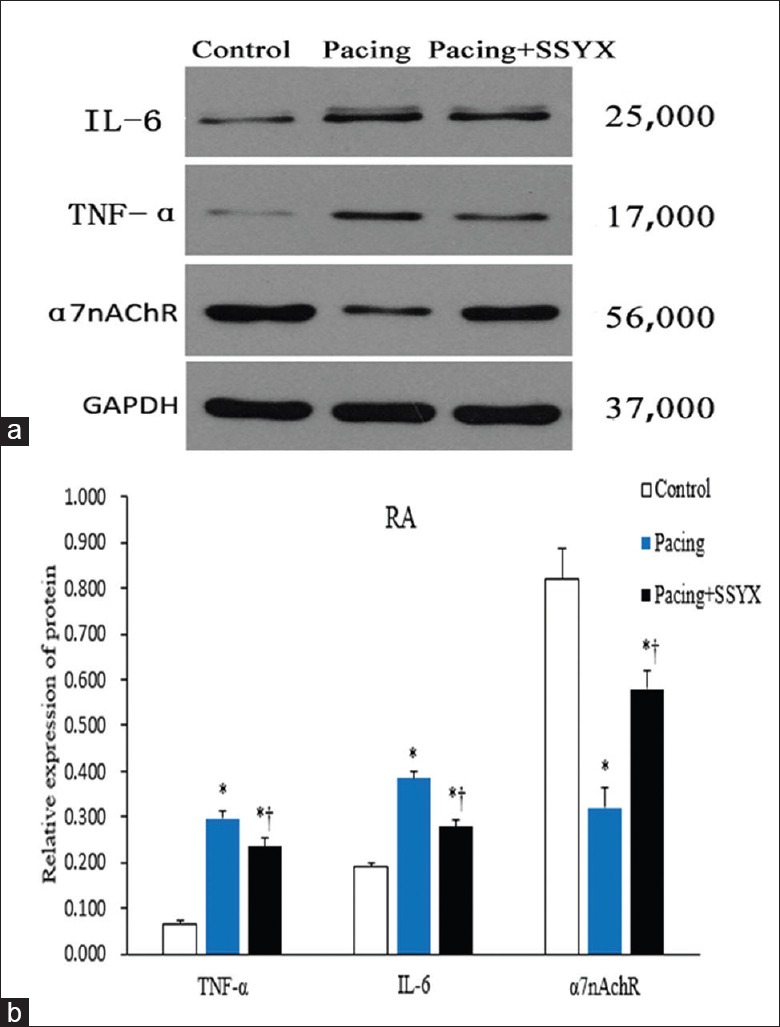
Western blot results of TNF-α, IL-6, and α7nAchR in samples extracts from the RA. (a) Representative images of Western blot results for IL-6 with a specific band at 25,000, for TNF-α with a specific band at 17,000, for α7nAchR with a specific band at 56,000 Western blot results analyses were performed for control, pacing, and pacing + SSYX groups. Antibodies for GAPDH with a specific band at 37,000 were used as a reference. (b) Statistical chart of Western blot results of RA. *P < 0.001 versus control group; †P < 0.001, pacing + SSYX group versus pacing group. IL-6: Interleukin-6; TNF-α: Tumor necrosis factor-alpha; α7nAChR: α7 nicotinic acetylcholine receptor protein; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; RA: Right atrium; SSYX: Shensong Yangxin.
Figure 6.
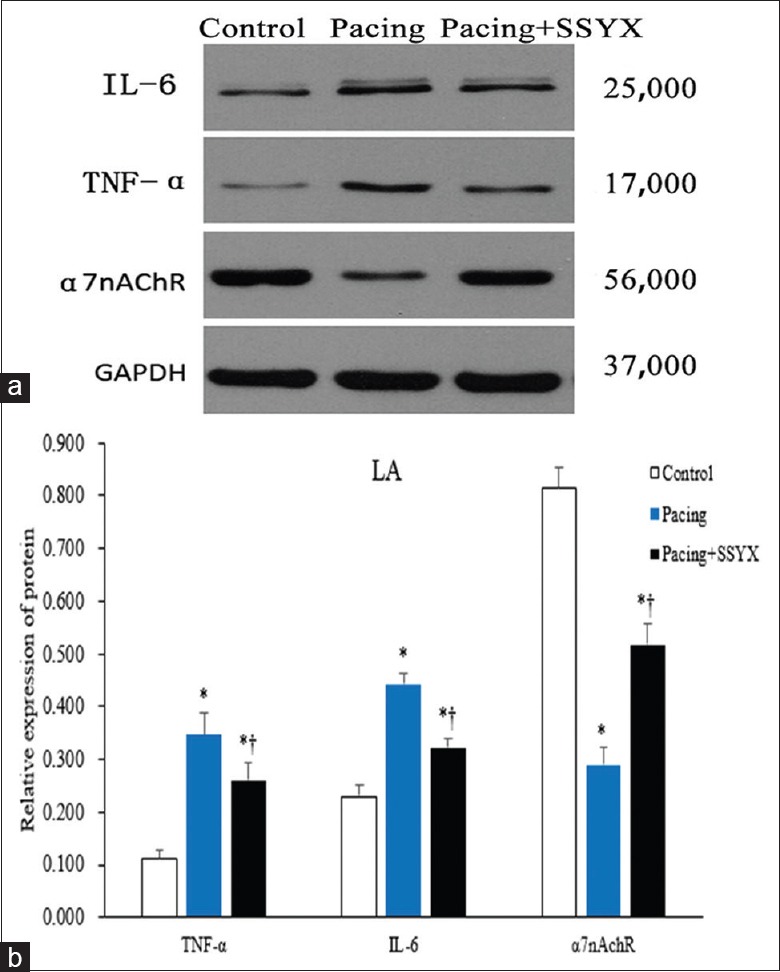
Western blot results of protein samples extracts from the LA. Representative images of Western blot results for IL-6 with a specific band at 25,000, for TNF-α with a specific band at 17,000, for α7nAchR with a specific band at 56,000 Western blot results analyses were performed for control, pacing, and pacing + SSYX groups. Antibodies for GAPDH with a specific band at 37,000 were used as a reference. (b) Statistical chart of Western blot results of LA. *P < 0.001 versus control group; †P < 0.001, pacing + SSYX group versus pacing group. IL-6: Interleukin-6; TNF-α: Tumor necrosis factor-alpha; α7nAChR: α7 nicotinic acetylcholine receptor protein; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; LA: Left atrium; SSYX: Shensong Yangxin.
Discussion
This study explored the effect of SSYX on AF vulnerability in experimental paroxysmal AF. We provide evidence for the following: (1) SSYX suppresses atrial electrical remodeling and AF vulnerability in experimental paroxysmal AF; (2) SSYX decreases sympathetic nerve activity and atrial sympathetic hyperinnervation after long-term intermittent atrial pacing in canines; (3) SSYX increases atrial α7nAChR level and decreases inflammatory cytokines after long-term intermittent atrial pacing in canines. Thus, these findings are the first to indicate that the effects of SSYX on AF vulnerability by modulating autonomic nerve activity.
SSYX, a traditional Chinese herbal medicine, has long been used clinically to treat arrhythmias in China. Recent studies have shown SSYX to be effective in the treatment of AF.[12,13] However, the mechanism of SSYX on AF vulnerability is unknown. Previous studies have shown that SSYX is a multichannel blocker.[8,14] In another study, Feng et al.[7] investigated the electrophysiological effects of SSYX on Chinese miniature swine hearts and isolated guinea pig ventricular myocytes. They found that SSYX increases heart rate and enhances the conducting capacity of the heart. After inhibition of the autonomic nervous system, SSYX had no effect on the intrinsic heart rate. Therefore, this study was carried out to test the hypothesis that SSYX can suppress atrial electrical remodeling and the occurrence and progression of paroxysmal AF in an experimental model of paroxysmal AF. The results of the present study showed that SSYX suppresses atrial electrical remodeling and AF vulnerability after long-term intermittent atrial pacing for 8 weeks. In the present study, we found that LF and LF/HF increased after 8 weeks in the pacing dogs, while the parameters were lower in the SSYX + pacing dogs than in the pacing dogs. These results showed that long-term intermittent atrial pacing induced an increase in sympathetic nerve activity and decrease in vagal nerve activity, while SSYX suppressed changes in autonomic nerve activity. We also found that long-term intermittent atrial pacing induced sympathetic hyperinnervation in the atrium. These results were similar to the previous studies.[15,16] Furthermore, we found that SSYX inhibited sympathetic hyperinnervation during long-term intermittent atrial pacing. Our study provided evidence that SSYX regulates the imbalance in autonomic nerve activity and inhibits sympathetic nerve remodeling during long-term intermittent atrial pacing. Taken together, these results suggest that SSYX suppresses atrial electrical remodeling by regulating autonomic nerve activity.
Inflammation is frequently associated with AF.[17,18] Inflammatory cytokines may be linked to fibrosis and the expression of current channel subunits that contribute to AF-associated electrical remodeling.[19,20] In the present study, we found that SSYX suppressed the increased levels of TNF-α and IL-6 observed after long-term intermittent atrial pacing in canines. We also found that SSYX inhibited the decrease in Ach that occurs after long-term intermittent atrial pacing. We speculated that the effects of SSYX on the levels of inflammatory cytokines were attributed to enhanced vagal nerve activity. Furthermore, we found that α7nAChR protein expression decreased after long-term intermittent atrial pacing, while SSYX treatment suppressed the decrease in α7nAChR protein expression in the atrium. Previous studies have demonstrated that vagus nerve stimulation or administration of α7nAChR antagonists not only inhibit TNF-α but also inhibit other inflammatory cytokines.[21,22] The efferent arm of the inflammatory reflex is now called the cholinergic anti-inflammatory pathway.[23] Our results indicated that SSYX elicits antiarrhythmic effects by regulating the imbalance in autonomic nerve activity as well as increasing vagal nerve activity, which enhance the cholinergic anti-inflammatory pathway, leading to a decrease in the levels of inflammatory cytokines and exuding a protective effect on AF.
Studies have demonstrated that AF may progress from paroxysmal to persistent in up to 50% of patients despite pharmacological therapy.[2,24] Attempts to curb AF progression have centered around the modulation of factors that are known to promote this development. Antiarrhythmic medications have been available for many years in the management of AF. The use of these drugs (such as propafenone, dofetilide, and amiodarone) has been limited by proarrhythmic and noncardiovascular toxicities as well as modest antiarrhythmic efficacy. SSYX, a traditional Chinese herbal medicine, has long been used clinically to treat arrhythmias in China. In this study, we demonstrated that SSYX suppressed the progression of AF by regulating the imbalance in autonomic nerve activity and activation of the cholinergic anti-inflammatory pathway. Recently, Wang et al.[13] demonstrated the efficacy and safety of SSYX in paroxysmal AF in a randomized controlled multicenter clinical study. Our findings further revealed the mechanisms of SSYX on preventing the progression of paroxysmal AF and provided new evidence for treatment of AF using SSYX.
This study has several limitations. First, we did not measure the changes in atrial ion currents such as Na+ currents and Ca2+ currents. Previous studies have shown that atrial electrical remodeling induced by rapid atrial pacing is mediated by rate-induced intracellular calcium overload. Therefore, it is unclear whether SSYX changed atrial ion currents in this study. Second, SSYX is a complex mixture of plant extracts and little is known about the effects of the individual components and their interaction with other medications. Therefore, the plasma therapeutic concentrations of active components are unknown. However, the oral dose of SSYX utilized in this study is similar to the concentration range in patients, as reported by clinical data. Third, in this study, we did not measure the structural changes such as the left atrial dimensions and the right dimensions using ultrasound. However, in our previous study,[10] we found that the left atrial diameter was increased significantly after 12 weeks of intermittent atrial pacing compared with baseline, whether there is any atrial structural change after 8 weeks of intermittent pacing remains unknown, which is also a limitation of the study.
Conclusively, in this study, we demonstrated that SSYX substantially prevents atrial electrical remodeling and the progression of AF induced by long-term intermittent atrial pacing. These effects of SSYX may be attributed to the regulation of imbalances in autonomic nerve activity and the cholinergic anti-inflammatory pathway.
Financial support and sponsorship
This study was supported by grants from the National Science and Technology Pillar Program of China (No. 2011BAI11B12), the National Key Basic Research Development Program of China (The “973” Program, No. 2012CB518604), the Ministry of Science and Technology of China with the Key Drug Discovery Project of 12th 5-year plan (No. 2011ZX09201-201-28), the Science and Technology Projects of Hubei Province (No. 2014CFB736), and the Fundamental Research Funds for the Central Universities (No. 2015301020201).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Qiang Shi
References
- 1.Jahangir A, Lee V, Friedman PA, Trusty JM, Hodge DO, Kopecky SL, et al. Long-term progression and outcomes with aging in patients with lone atrial fibrillation: A 30-year follow-up study. Circulation. 2007;115:3050–6. doi: 10.1161/CIRCULATIONAHA.106.644484. doi:10.1161/CIRCULATIONAHA.106.644484. [DOI] [PubMed] [Google Scholar]
- 2.de Vos CB, Pisters R, Nieuwlaat R, Prins MH, Tieleman RG, Coelen RJ, et al. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol. 2010;55:725–31. doi: 10.1016/j.jacc.2009.11.040. doi:10.1016/j.jacc.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y, Li N, Jia Z, Lu F, Pu J. Chinese medicine shensongyangxin is effective for patients with bradycardia: Results of a randomized, double-blind, placebo-controlled multicenter trial. Evid Based Complement Alternat Med 2014. 2014:605714. doi: 10.1155/2014/605714. doi:10.1155/2014/605714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun LP, Li N, Wu YL, Pu JL. Effects of shensong yangxin capsule on pacemaker channels encoded by human HCN4 gene. Chin Med J. 2010;123:3148–50. doi:10.3760/cma.j.issn.0366-6999.2010.21.036. [PubMed] [Google Scholar]
- 5.Gu CH, Wu YL, Tian SY, Gao X, Qi X, Jia Z, et al. Effect of shensong yangxin capsule on ventricular premature beat and cardiovascular autonomic nervous function in patients with coronary heart disease (In Chinese) Chin J Integr Tradit West Med. 2005;25:783–6. doi:10.3321/j.issn.1003-5370.2005.09.004. [PubMed] [Google Scholar]
- 6.Chen G, Wei B, Wang J, Feng B, Li Z, Zhang Z, et al. Shensongyangxin capsules for paroxysmal atrial fibrillation: A systematic review of randomized clinical trials. PLoS One. 2016;11:e0151880. doi: 10.1371/journal.pone.0151880. doi:10.1371/journal.pone.0151880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng L, Gong J, Jin ZY, Li N, Sun LP, Wu YL, et al. Electrophysiological effects of Chinese medicine shen song yang xin (SSYX) on Chinese miniature swine heart and isolated guinea pig ventricular myocytes. Chin Med J. 2009;122:1539–43. doi:10.3760/cma.j.issn.0366-6999.2009.13.012. [PubMed] [Google Scholar]
- 8.Li N, Ma KJ, Wu XF, Sun Q, Zhang YH, Pu JL. Effects of Chinese herbs on multiple ion channels in isolated ventricular myocytes. Chin Med J. 2007;120:1068–74. doi:10.1093/aob/mcr121. [PubMed] [Google Scholar]
- 9.Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: Implications for management. Circulation. 2011;124:2264–74. doi: 10.1161/CIRCULATIONAHA.111.019893. doi:10.1161/CIRCULATIONAHA.111.019893. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Huang C, Zhao Q, Huang H, Tang Y, Dai Z, et al. Effect of renal sympathetic denervation on the progression of paroxysmal atrial fibrillation in canines with long-term intermittent atrial pacing. Europace. 2015;17:647–54. doi: 10.1093/europace/euu212. doi:10.1093/europace/euu212. [DOI] [PubMed] [Google Scholar]
- 11.Huang CX, Zhao QY, Jiang H, Li JJ, Yang B. Experimental study of the effect of the vagus nerve on atrial electrical remodeling. J Electrocardiol. 2003;36:295–300. doi: 10.1016/j.jelectrocard.2003.08.004. doi:10.1016/j.jelectrocard.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Jin YW, Jin ZY, Ma L, Lv B. Effect of metoprolol combined with shensongyangxin capsule onPwave dispersion and plasma high sensitivity C-reactive protein in patients with paroxysmal atrial fibrillation (In Chinese) Chin Circ J. 2012;27:353–6. doi:10.3969/j.issn.1000-3614.2012.05.010. [Google Scholar]
- 13.Wang AH, Pu JL, Qi XY, Miao WL, Hou ZS, Cong HL, et al. Evaluation of shensong yangxin capsules in the treatment of paroxysmal atrial fibrillation: A randomized, double-blind and controlled multicenter trial (In Chinese) Natl Med J China. 2011;91:1677–81. doi:10.3760/cma.j.issn.0376-2491.2011.24.006. [PubMed] [Google Scholar]
- 14.Li N, Huo YP, Ma KJ, Sun Q, Pu JL. Effects of solution of dry power of shensong yangxin capsule on sodium current and L-type calcium current in ventricular myocytes: Experiment with guinea pig (In Chinese) Natl Med J China2007. 87:995–8. doi:10.1093/aob/mcr121. [PubMed] [Google Scholar]
- 15.Chang CM, Wu TJ, Zhou S, Doshi RN, Lee MH, Ohara T, et al. Nerve sprouting and sympathetic hyperinnervation in a canine model of atrial fibrillation produced by prolonged right atrial pacing. Circulation. 2001;103:22–5. doi: 10.1161/01.cir.103.1.22. doi:10.1161/01.CIR.103.1.22. [DOI] [PubMed] [Google Scholar]
- 16.Tan AY, Zhou S, Ogawa M, Song J, Chu M, Li H, et al. Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation. 2008;118:916–25. doi: 10.1161/CIRCULATIONAHA.108.776203. doi:10.1161/CIRCULATIONAHA.108.776203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012;60:2263–70. doi: 10.1016/j.jacc.2012.04.063. doi:10.1016/j.jacc.2012.04.063. [DOI] [PubMed] [Google Scholar]
- 18.Issac TT, Dokainish H, Lakkis NM. Role of inflammation in initiation and perpetuation of atrial fibrillation: A systematic review of the published data. J Am Coll Cardiol. 2007;50:2021–8. doi: 10.1016/j.jacc.2007.06.054. doi:10.1016/j.jacc.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 19.Shang LL, Sanyal S, Pfahnl AE, Jiao Z, Allen J, Liu H, et al. NF-kappaB-dependent transcriptional regulation of the cardiac scn5a sodium channel by angiotensin II. Am J Physiol Cell Physiol. 2008;294:C372–9. doi: 10.1152/ajpcell.00186.2007. doi:10.1152/ajpcell.00186.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. 2015;12:230–43. doi: 10.1038/nrcardio.2015.2. doi:10.1038/nrcardio.2015.2. [DOI] [PubMed] [Google Scholar]
- 21.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–62. doi: 10.1038/35013070. doi:10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 22.de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6:844–51. doi: 10.1038/ni1229. doi:10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 23.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117:289–96. doi: 10.1172/JCI30555. doi:10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jahangir A, Lee V, Friedman PA, Trusty JM, Hodge DO, Kopecky SL, et al. Long-term progression and outcomes with aging in patients with lone atrial fibrillation: A 30-year follow-up study. Circulation. 2007;115:3050–6. doi: 10.1161/CIRCULATIONAHA.106.644484. doi:10.1161/CIRCULATIONAHA.106.644484. [DOI] [PubMed] [Google Scholar]