Introduction
Cryptogenic organizing pneumonia (COP) is a distinct type of idiopathic interstitial pneumonia with a response rate of 65–85% on corticosteroid therapy. The difficulty of COP diagnosis is that the clinical features and the radiological findings are nonspecific. The pathological hallmark of organizing pneumonia (OP) needs to be confirmed. It can also occur in a variety of other interstitial pneumonia, infectious diseases, vasculitis, and so on.[1] To increase the pathological reliability, larger and more tissue samples are required. According to the current classification of interstitial lung disease and guidelines, the surgical lung biopsy is recommended and is considered to be the best way of obtaining a representative lung specimen.[2] However, the invasive diagnostic procedures require general anesthesia and also increase the morbidity and mortality risks; therefore, only few patients undergo such biopsy. Thus, safer and more acceptable methods for identifying COP are urgently needed.
Two other alternative methods include computed tomography (CT)-guided lung biopsy and transbronchoscopic lung biopsy (TBLB). CT-guided lung biopsy showed a current diagnostic accuracy of 87.96% for COP diagnosis.[3] However, OP can occur in a variety of other diseases, and thus, a small biopsy sample or only one lesion sampling is generally considered inconclusive. TBLB can allow doctors sample specimens from different lesions. However, being a “blind” procedure, low sensitivity has been reported.
Radial probe endobronchial ultrasound (RP-EBUS)-guided biopsy has been used in peripheral lung lesions with the density from ground glass opacity to consolidation.[4] Until now, there have been few reports showing whether RP-EBUS can be a reliable pathological evidence for COP diagnosis. To investigate the effectiveness for the diagnosis of COP using specimens produced by RP-EBUS-guided lung biopsy, a prospective study was conducted.
Methods
Subjects
From September 1, 2015, to November 30, 2015, a total of six patients were identified at the Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School. All of the patients had admission diagnosis of possible COP according to clinical features and CT examinations showing multiple alveolar filling shadows in both lungs. These patients were then examined by RP-EBUS. All of the patients have given informed consent, and the Ethics Committee of the Nanjing Drum Tower Hospital approved this study.
Bronchoscopy procedure
Lesions were located by studying the chest CT before procedure. The radial probe was introduced through the working channel of a conventional bronchoscope. RP-EBUS helped us obtain detailed images of the airway wall and surrounding structures. Depending on the absorption and scattering of the tissues and their interface, a processor generated ultrasound images. The probe was used to visualize the lesion and then withdrawn. A sheath was left in situ to localize and stabilize the lesion during the subsequent introduction of forceps for sampling. Samples were obtained from at least two different lesions from each patient. All pathological results were diagnosed by two experienced pathological doctors.
Results
The clinical features of the patients are listed in Table 1. A total of 6 patients with the final diagnosis of COP were identified. These patients’ presenting symptoms and signs included cough, sputum, dyspnea, fever, flu-like symptoms, chest pain, hemoptysis, weight loss, and crackle or wheezing on auscultation. Fifteen lesions were obtained from these patients. Three of the 15 lesions were obtained in right upper lesion, 1 in right middle lesion, 4 in right lower lesion, 5 in left upper lesion, and the other 2 in left lower lesion.
Table 1.
Demographic data describing the locations and results of lesions, treatment, and diagnosis of six patients with cryptogenic organizing pneumonia
| Patient number | Age (years) | Gender | Targeted lesions | Pathology | Treatment | Diagnosis |
|---|---|---|---|---|---|---|
| 1 | 46 | Female | Left lower lobe | OP | Glucocorticoid | COP |
| Right middle lobe | OP | |||||
| Right upper lobe | Negative | |||||
| 2 | 66 | Male | Left upper lobe | OP | Glucocorticoid | COP |
| Right lower lobe | Negative | |||||
| Right upper lobe | OP | |||||
| 3 | 52 | Female | Left upper lobe | OP | Glucocorticoid | COP |
| Right lower lobe | OP | |||||
| 4 | 48 | Male | Left upper lobe | OP | Glucocorticoid | COP |
| Right upper lobe | OP | |||||
| 5 | 69 | Male | Left upper lobe | OP | Glucocorticoid | COP |
| Right lower lobe | OP | |||||
| 6 | 64 | Male | Left upper lobe | Negative | Glucocorticoid | COP |
| Left lower lobe | OP | |||||
| Right upper lobe | OP |
COP: Cryptogenic organizing pneumonia; OP: Organizing pneumonia.
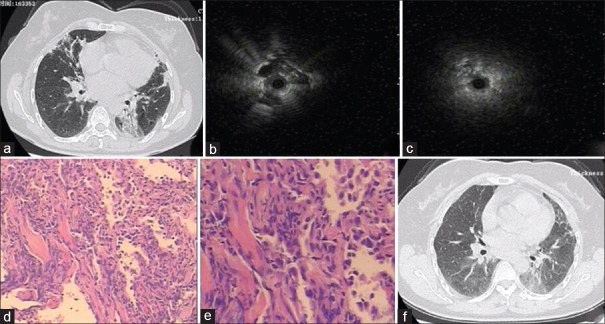
Take one patient, for example, CT scan of this 46-year-old female apparently showed bilateral multiple alveoli filling in her right middle lung and left lower lung. RP-EBUS showed the peripheral pulmonary lesion in left lower lobe as a hypoechoic structure and the same change in right middle lobe. OP was identified by RP-EBUS in both lesions. Pathological changes were evenly distributed. Masson bodies appeared in the alveoli, showing chronic inflammatory cell infiltration in the mesenchyma [Figure 1a-1e].
Figure 1.
Clinical and pathological images in a 46-year-old patient. (a) Computed tomography imaging showed bilateral multiple alveoli filling. (b) Radial probe endobronchial ultrasound showing the peripheral pulmonary lesion as a hypoechoic structure in the left lower and right middle lesion. (c) Radial probe endobronchial ultrasound showing the peripheral pulmonary lesion as a hypoechoic structure in the right middle lesion. (d) Organizing pneumonia identified in this patient (H and E, ×50). (e) Masson bodies apparent in the alveoli (H and E, ×100). (f) After 1-month follow-up, chest computed tomography scans showed that lesions in the right middle and left lower lobe were reduced.
RP-EBUS identified OP in 12 lesions. The results of the other 3 lesions were negative. The only complication of RP-EBUS was pneumothorax, which occurred in only one patient (1/6).
Based on the diagnosis obtained by RP-EBUS, all patients received corticosteroids treatment. A minimum of 1-month follow-up record was conducted to evaluate the effect of treatment. The CT images of four patients demonstrated that lung lesions were obviously decreased after corticosteroids therapy compared to before treatment [Figure 1f].
Discussion
Endobronchial ultrasound, which has been introduced about a decade ago, is becoming more and more popular nowadays. It is accurate, safe, and is being used for an increasing number of indications. In this study, an attempt has been made to identify the use of RP-EBUS in diagnosing COP, based on the fact that CT scanning exhibited multiple opacities accompanied by consolidation or ground-glass opacities. RP-EBUS can be used to sample several lesions in both two lungs even in subpleural and/or peribronchovascular distribution. With pathology of OP in two or more lesions in one patient, the diagnosis of COP would be more accurate. In this study, each of the six patients was sampled with different lesions that have the same pathology of OP and finally diagnosed COP by the use of RP-EBUS. Twelve of the 15 lesions sampled showed OP changes through the RP-EBUS. The diagnostic rate was even higher than previously reported 76% diagnosed through CT-guided lung biopsy.[4]
The only complication that occurred in our study was pneumothorax (1/6). The overall complication rate related to RP-EBUS was 1.3%:0.8% for pneumothorax and 0.5% for pulmonary infection.[5] The complication rate of RP-EBUS seems lower as compared to traditional CT-guided lung biopsy (16.7% vs. 18.5%).[3] Our study has several significant strengths. First, to the best of our knowledge, this is the first report that evaluates the role of RP-EBUS in the diagnosis of COP. Second, RP-EBUS can sample specimens from different lesions in one patient. Third, the diagnostic rate of the procedure is high and the complication rate is extremely low. Nevertheless, our study does have limitations. The sample size is not large enough. More studies are needed to evaluate the use of RP-EBUS in diagnosing COP which has multiple alveolar filling shadows in both two lungs.
Financial support and sponsorship
This study was supported by a grant from the key project of Nanjing Public Health Bureau (No. ZKX15020).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Qiang Shi
References
- 1.Cordier JF. Cryptogenic organising pneumonia. Eur Respir J. 2006;28:422–46. doi: 10.1183/09031936.06.00013505. doi:10.1183/09031936.06.00013505. [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society/European Respiratory Society. International multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. doi:10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 3.Yuan DM, Lü YL, Yao YW, Liu HB, Wang Q, Xiao XW, et al. Diagnostic efficiency and complication rate of CT-guided lung biopsy: A single center experience of the procedures conducted over a 10-year period. Chin Med J. 2011;124:3227–31. doi:10.3760/cma.j.issn.0366-6999. [PubMed] [Google Scholar]
- 4.Paone G, Nicastri E, Lucantoni G, Dello Iacono R, Battistoni P, D'Angeli AL, et al. Endobronchial ultrasound-driven biopsy in the diagnosis of peripheral lung lesions. Chest. 2005;128:3551–7. doi: 10.1378/chest.128.5.3551. doi:10.1378/chest.128.5.3551. [DOI] [PubMed] [Google Scholar]
- 5.Hayama M, Izumo T, Matsumoto Y, Chavez C, Tsuchida T, Sasada S. Complications with endobronchial ultrasound with a guide sheath for the diagnosis of peripheral pulmonary lesions. Respiration. 2015;90:129–35. doi: 10.1159/000431383. doi:10.1159/000431383. [DOI] [PubMed] [Google Scholar]