Abstract
Background
Through functional screening of a fosmid library, generated from a phytopathogen-suppressive soil metagenome, the novel antifungal chitinase—named Chi18H8 and belonging to family 18 glycosyl hydrolases—was previously discovered. The initial extremely low yield of Chi18H8 recombinant production and purification from Escherichia coli cells (21 μg/g cell) limited its characterization, thus preventing further investigation on its biotechnological potential.
Results
We report on how we succeeded in producing hundreds of milligrams of pure and biologically active Chi18H8 by developing and scaling up to a high-yielding, 30 L bioreactor process, based on a novel method of mild solubilization of E. coli inclusion bodies in lactic acid aqueous solution, coupled with a single step purification by hydrophobic interaction chromatography. Chi18H8 was characterized as a Ca2+-dependent mesophilic chitobiosidase, active on chitin substrates at acidic pHs and possessing interesting features, such as solvent tolerance, long-term stability in acidic environment and antifungal activity against the phytopathogens Fusarium graminearum and Rhizoctonia solani. Additionally, Chi18H8 was found to operate according to a non-processive endomode of action on a water-soluble chitin-like substrate.
Conclusions
Expression screening of a metagenomic library may allow access to the functional diversity of uncultivable microbiota and to the discovery of novel enzymes useful for biotechnological applications. A persisting bottleneck, however, is the lack of methods for large scale production of metagenome-sourced enzymes from genes of unknown origin in the commonly used microbial hosts. To our knowledge, this is the first report on a novel metagenome-sourced enzyme produced in hundreds-of-milligram amount by recovering the protein in the biologically active form from recombinant E. coli inclusion bodies.
Electronic supplementary material
The online version of this article (doi:10.1186/s12934-017-0634-8) contains supplementary material, which is available to authorized users.
Keywords: Antifungal chitinase, Functional metagenomics, Heterologous expression, Inclusion bodies, Protein purification, Mode of action
Background
The existing repertoire of microbial-derived medically, agriculturally and industrially useful enzymes, and natural products, mostly originated from readily cultivable microorganisms. However, the vast majority of microorganisms in the environment is not cultivable under standard laboratory conditions, making this pool relatively untapped. For example, one gram of land soil may contain 1010 microbial cells [1] with an estimated species diversity from 104 [2] to nearly 107 species [3]. Today, metagenomics, that is the sequence- and/or function-based analysis of the collective genome assemblages of organisms, may provide a cultivation-independent access to such diversity and potential [4, 5].
We recently applied different genetic and/or activity-based screenings to metagenomes from suppressive and/or chitin-amended agricultural soils to access novel bacterial chitinolytic enzymes (CEs) [6–8]. After lignocellulose, chitin is the most abundant biopolymer in nature, widely distributed within exoskeletons of insects, fungal cell walls, marine diatoms, shells of crustaceans, eggs of nematodes and zooplankton [9, 10]. For example, there is a chitin and glycoprotein layer—the peritrophic matrix—that lines the midgut of most invertebrates [11]. Chitin is rather resistant to degradation and without the action of microbial CEs, it would be trapped in biomass as insoluble. Actually, bacterial CEs are crucial in the global biogeochemical recycling of carbon and nitrogen through the hydrolysis of chitin. CEs, including endochitinases (EC 3.2.1.14), chitobiosidases (EC 3.2.1.29), N-acetyl glucosaminidases (EC 3.2.1.30), chitin deacetylases (EC 3.5.1.14), and chitosanases (EC 3.2.1.132), process the unbranched biopolymer, which is composed of repeated units of N-acetylglucosamine (GlcNAc), in different synergic modes [10, 12]. Notably, CEs are attracting an increasing interest because of their potential in biotechnological applications [12–14]: e.g. as biocontrol agents that antagonize chitin-containing phytopathogenic fungi, insects and nematodes in integrated pest management strategies [7, 11, 13], or as industrial biocatalysts for the production of chitin derivatives that possess interesting nutraceutical and pharmaceutical properties [14]. For example, chitosan and chitooligosaccharides (COs) have been used for drug and gene delivery [15, 16]. COs have also been suggested to have a role as signal molecules in antagonism phenomena [7, 11, 13]. The mode of action of CEs is thus important in relation to the length, degree of acetylation and sequence of the COs they generate [17].
Several studies, both on aquatic and soil habitats, have specifically reported on the abundance of CE-encoding genes [6, 18–23]. To date, however, microbial CEs have been identified and isolated mostly by conventional molecular and/or functional screenings of microbial isolates from different environmental samples [24, 25]. Only very recently, the use of metagenomic tools has allowed the identification and isolation of novel bacterial CEs from naturally suppressive or chitin amended agricultural soil through expression screening [7], sequence screening [8] and gene synthesis [26]. In non-naturally suppressive soils, chitin amendment helps to enhance the suppressiveness against soil-borne pathogens by stimulating active chitinolytic microbial communities [22, 27–29].
Through expression screening of a fosmid library generated from a metagenome of a naturally suppressive field soil, we recently isolated the novel bacterial chitinase Chi18H8, which showed less than 45% amino acid sequence identity with known chitinases [7]. Sequence analysis revealed the presence of the consensus sequence of the 18 glycosyl hydrolases (GH) family (including the conserved active site’s motif, DXDXE of the catalytic domain) [30]. Unlike most of the other characterized chitinases of the 18GH family, Chi18H8 seemed to exert antagonistic activity against fungi, coherently with the suppressive nature of the soil from which it was isolated, envisaging its potential use as a biocontrol agent for crop protection [7]. In fact, when the recombinant Escherichia coli pGEX-6P-3::chi18H8 (expressing the novel chitinase) was co-cultivated with phytopathogenic fungi, it inhibited fungal growth. However, the extremely low production and purification yield of Chi18H8 (21 μg/g cell) at that time prevented any further investigation on its properties including its potential biotechnological applications.
Here we report how we succeeded in producing hundreds of milligrams of pure and biologically active Chi18H8 by developing and scaling up a high-yielding process in a 30 L bioreactor, based on the recovery of the recombinant protein from E. coli inclusion bodies (IBs). To our knowledge, this is the first report on a bacterial chitinase isolated through functional metagenomics brought to pre-industrial scale production.
Methods
chi18H8 cDNA sub-cloning
The nucleotide sequence of the chitinase Chi18H8 gene was previously deposited in the GenBank database under the accession number KC763366 [7]. The cDNA encoding for the chitinase was sub-cloned into the pET24b(+) expression plasmid (kanamycin resistance; Novagen Inc., Madison, USA) by using the primers chiEcoF (5′-ATAAAGAATTCCATGCGCCAGCTCACGCTTCTC-3′) and chiXhoR (5′-ATAAACTCGAGCTAATTGCCCCTATGCAGACTGG-3′), containing the underlined restriction sites for EcoRI and XhoI, respectively. The plasmid pGEX-6P-3::chi18H8, previously prepared [7], was used as DNA template. E. coli DH5α (Invitrogen-Life Technologies, Carlsbad, USA) was used as host for the sub-cloning procedures. The construct, following its control by DNA sequencing (BMR Genomics, Padua, Italy), was transformed into E. coli BL21 Star™(DE3) (Invitrogen-Life Technologies). Recombinant E. coli strains were maintained on Luria-Bertani broth (LB, Miller’s modification; Sigma-Aldrich, St. Louis, USA) agar plates supplemented with 50 µg/mL kanamycin.
Chi18H8 expression
All medium components and reagents were from Sigma-Aldrich, unless otherwise stated. Protein expression was carried out in the following media, supplemented with 50 µg/mL kanamycin: LB; terrific broth (TB); super broth (SB: 32 g/L tryptone, 20 g/L yeast extract, 5 g/L NaCl); autoinduction media A and B. Autoinduction medium A composition was based on [31]. Autoinduction medium B included: 10 g/L tryptone, 5 g/L yeast extract, 3.3 g/L (NH4)2SO4, 6.8 g/L KH2PO4, 7.1 g/L Na2HPO4, 0.5 g/L glucose, 2 g/L α-lactose, 0.15 g/L MgSO4, 2 mg/L CaCl2, 2 mg/L MnSO4 × H2O, 2 mg/L ZnSO4, 2 mg/L CoCl2, 2 mg/L CuCl2 × 2H2O, 2 mg/L NiCl2, 2 mg/L NH4MoO4, 2 mg/L FeCl3. Trace element (MgSO4, CaCl2, MnSO4 × H2O, ZnSO4, CoCl2, CuCl2 × 2H2O, NiCl2, NH4MoO4, FeCl3) stock solutions were sterilized by filtration (0.2 µm) and stored at 4 °C.
Starter cultures were prepared from a single recombinant E. coli colony inoculated in 10 mL LB medium supplemented with 50 µg/mL kanamycin, grown overnight at 37 °C and 200 revolutions per minute (rpm). Baffled 300 mL Erlenmeyer flasks containing 50 mL of the different media were inoculated with the starter culture (initial OD600nm = 0.1) and further incubated as above. For LB, TB and SB media, protein expression was induced by adding 0.4 mM isopropyl β-d-thiogalactopyranoside (IPTG) to cells at early- or late-exponential growth phase. After induction, cells were cultured at various temperatures (37, 25 or 20 °C, respectively) at 200 rpm and harvested by centrifugation (1900×g for 30 min at 4 °C) at different time intervals. Following centrifugation, total proteins in the supernatants (i.e. the cell-free fermentation broths) were concentrated by 10% (v/v) trichloroacetic acid precipitation and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE, see below). In parallel, cell pellets were sonicated on ice (3–5 cycles of 30 s each, with a 30-s interval, using a Branson Sonifier 250, Danbury, USA) in phosphate buffer saline (PBS) pH 7.3 (140 mM NaCl, 2.7 mM KCl, 10.1 mM Na2HPO4, 1.8 mM KH2PO4) containing 10 µg/mL deoxyribonuclease (DNase), 0.19 mg/mL phenylmethylsulfonylfluoride (PMSF) and 0.7 mg/mL pepstatin. Soluble (cytoplasmic) and insoluble (containing membranes and IBs) cell fractions were then separated by centrifugation at 20,000×g for 1 h at 4 °C. Insoluble fractions were re-suspended in a volume of PBS equal to the corresponding cytoplasmic soluble fraction (2 mL/g cell) for successive SDS-PAGE analyses. In every fraction, protein concentration was determined by the Biuret assay [32].
Chi18H8 production
Flask cultures of recombinant E. coli cells, grown overnight in LB medium supplemented with 50 µg/mL kanamycin, were used to inoculate (initial OD600nm = 0.1) 2 L Erlenmeyer flasks (containing 750 mL medium), or 3 L P-100 Applikon glass reactors (Applikon Biotechnology, Delft, The Netherlands) equipped with a AD1030 biocontroller and AD1032 motor (containing 2 L medium), or 30 L Bioengineering (Bioengineering AG, Wald, Switzerland) stirred fermenter (containing 27 L medium). The medium used throughout was LB medium supplemented with 50 µg/mL kanamycin. 2 L Erlenmeyer flasks were incubated at 37 °C and 200 rpm. Growth in fermentors was conducted at 37 °C and stirring at 500 rpm for 3 L bioreactor or at 300 rpm for the 30 L bioreactor, respectively, at constant 1.0 vvm aeration rate and pressure control set at 0.5 bar. Dissolved oxygen (measured as % of the initial pO2 value) and pH were monitored using an Ingold polarographic oxygen electrode and a pH meter, respectively. Foam production was monitored through an antifoam sensor and controlled by adding Antifoam SE-15. When cell culture reached an OD600nm = 0.6, protein production was induced by adding 0.4 mM IPTG: cultivation was then prolonged at 20 °C for further 24 h.
Recovery of IBs and solubilization of Chi18H8
Escherichia coli cells were harvested by centrifugation at 3220×g for 20 min, washed with sodium chloride-Tris-EDTA (STE buffer: 10 mM Tris–HCl pH 8.0, 1 mM EDTA [ethylenediaminetetraacetic acid], 100 mM NaCl). The different protocols for IB recovery and solubilization are described in Additional file 1: Table S1. In the case of the acid solubilization method, after centrifugation, the cell pellet was re-suspended in 50 mM Tris–HCl pH 8.0, 25% (w/v) sucrose, 1 mM EDTA, and incubated for 30 min at room temperature under vigorous shaking. After sonication on ice (6 cycles of 30 s each, with a 30-s interval), 0.2 M NaCl, 1% (w/v) sodium deoxycholate (DOC) and 1% (v/v) Nonidet P-40 were added. The sample was further incubated as above and centrifuged (20,000×g at 4 °C for 30 min). The pellet was washed with 1% (v/v) Triton X-100 and 1 mM EDTA, followed by centrifugation at 12,000×g at 4 °C for 10 min; the procedure was repeated twice. IBs were then washed twice with 10 mL/g cell deionized water, with centrifugation at 12,000×g at 4 °C for 10 min in-between, and stored overnight at −20 °C. To solubilize Chi18H8 from IBs, the frozen pellet was re-suspended in 10 mL/g cell of 10 or 100 mM HCl or lactic acid and incubated at 37 °C and 200 rpm for 5 h. Insoluble material was removed by centrifugation at 1900×g at 4 °C for 5 min. The solubilized protein was then dialyzed overnight against 100 mM sodium acetate buffer pH 5.0 or 100 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) pH 5.6.
Chi18H8 purification
Enzyme samples following dialysis were used for testing different chromatographic methods, described in details in Additional file 1: Table S2. Chi18H8 purification/enrichment was achieved by loading the protein sample after dialysis against 100 mM HEPES pH 5.6 onto the weak anionic exchanger Diaion WA11 resin (Resindion s.r.l., Milan, Italy), previously activated with methanol/water (1:1) and then equilibrated with 100 mM HEPES pH 5.6. Pilot experiments were performed with 1 mL resin (wet volume) or, for large scale purification, with 40 mL of WA11 resin (in a 4.5 cm diameter Amicon column loaded at a flow rate of 20 mL/min). The chromatography, run under hydrophobic interaction (HIC) conditions, allowed to fully recover the Chi18H8 protein in the flow-through, whereas other proteins retained by the WA11 resin were then recovered by an isocratic elution using 50% (v/v) ethanol in 100 mM HEPES pH 5.6. The Chi18H8 concentration was estimated using the theoretical extinction coefficient at 280 nm (77,015 M-1cm-1), based on the amino acid sequence of the protein.
SDS-PAGE electrophoresis and zymogram analysis
Chi18H8 size, production and solubilization were analyzed by SDS-PAGE with 12% (w/v) polyacrylamide gels [33] and estimated by densitometric analysis (Quantity One, Bio-Rad Laboratories, Hercules, USA) using known amount of His6-glycine oxidase (His6-GO) from Bacillus subtilis [34] as a standard. Chitinolytic activity was detected through zymogram analysis (semi native-PAGE) using 10% (w/v) polyacrylamide gels containing 0.7 mg/mL of carboxymethyl-chitin-remazol brilliant violet (CM-chitin-RBV) (Loewe Biochemica, Suerlach, Germany), as described in [7]. Briefly, the protein samples were diluted in a sample buffer lacking any reducing agent and incubated for 10 min at room temperature. Electrophoresis was conducted at 4 °C in Tris-glycine-SDS running buffer according to standard running conditions. The gel was rinsed twice in 2.5% (v/v) Triton X-100 for 30 min at room temperature to remove SDS and incubated in 100 mM sodium acetate pH 5.0 at 37 °C until the appearance of clear zones indicating chitinolytic activity. The chitinase from Trichoderma viride (Sigma-Aldrich) was used as positive control.
Chi18H8 activity assay
Chi18H8 activity was assayed with the fluorimetric chitooligosaccharide analogues 4-methylumbelliferyl N-acetyl-β-d-glucosaminide (4-MU-GlcNAc), 4-methylumbelliferyl N,N’-diacetyl-β-d-chitobioside (4-MU-(GlcNAc)2) and 4-methylumbelliferyl N,N’,N’’-triacetyl-β-d-chitotrioside (4-MU-(GlcNAc)3) as described previously [7]. Unless otherwise stated, Chi18H8 activity was assayed at 37 °C in 100 mM sodium acetate pH 5.0 [7]. Chitinolytic activity was also determined on colloidal chitin, prepared from chitin flakes from shrimp shells (Sigma-Aldrich), by the colorimetric method previously described [8]. One unit (U) of Chi18H8 activity was defined as the amount of enzyme that released 1 μmol of 4-MU or GlcNAc per min at 37 °C.
Effect of pH and temperature on Chi18H8 activity
pH influence on Chi18H8 activity on the substrate 4-MU-(GlcNAc)2 was determined by the fluorimetric assay in the following buffers (each 100 mM): glycine–HCl (pH 3.0), sodium acetate (pH 4.0 and 5.0), sodium phosphate (pH 6.0 and 7.0), Tris-HCl (pH 8.0) and sodium pyrophosphate (NaPPi, pH 9.0). Optimal temperature for the enzyme activity was determined by the same fluorescent assay, performing the reaction at various temperatures (from 5 to 70 °C). Stability of the enzyme was tested by incubating the recombinant protein at 30 °C at different pHs (in 100 mM sodium acetate pH 5.0 or 100 mM sodium phosphate pH 6.0 and 7.0): after different incubation times (from 0 to 144 h), the residual chitinolytic activity was assayed on 4-MU-(GlcNAc)2.
Effect of metal ions, organic solvents, detergents and other compounds on Chi18H8 activity
The effect of metal ions [Ca2+ (CaCl2 × 2H2O), Cu2+ (CuCl2 × 2H2O), Fe3+ (FeCl3 × 6H2O), K+ (KCl), Mg2+ (MgCl2 × 6H2O), Mn2+ (MnCl2 × 4H2O), Ni2+ (NiCl2 × 6H2O), NH4 (NH4Cl), Zn2+ (ZnCl2), Co2+ (CoCl2 × 6H2O)], reducing agents [dithiothreitol (DTT), 2-mercaptoethanol], the chelating agent EDTA, detergents [SDS, Triton X-100, Tween-20, DOC, N-lauroylsarcosine (NLS), Nonidet-40], organic solvents [ethanol, methanol, propanol, dimethyl sulfoxide (DMSO)], sugars (GlcNAc, chitobiose) and salt (NaCl) on Chi18H8 activity was investigated by adding each compound to the fluorimetric assay mixture. Final concentrations were: 20 mM for metal ions, EDTA and 2-mercaptoethanol, 10 mM for sugars and DTT, 10% (v/v) for organic solvents, and 1% (w/v) for detergents. In each case the residual activity was compared to the activity without additional compounds, set as 100%.
Circular dichroism
Far-UV CD spectra were recorded at 15 or 30 °C using a Jasco J-715 spectropolarimeter (Jasco, Cremello, Italy) equipped with temperature control, in the 190–250 nm wavelength range. Measurements were carried out in quartz cuvettes of path length 1 mm, employing a protein solution at 0.2 mg/mL in 10 mM HEPES pH 5.4, and corrected for buffer contribution. Secondary structure composition was calculated from deconvolution of the CD spectra with the program k2d3 (http://k2d3.ogic.ca//index.html) [35]. Binding of Ca2+ to purified Chi18H8 was investigated by recording the far-UV CD spectrum during the titration of the apoprotein form of the enzyme (obtained by incubation for 30 min with 20 mM EDTA), with increasing concentrations of CaCl2 (5, 10, 100, 1000 and 10,000 μM). The change in CD signals at 219 nm versus CaCl2 concentration were fitted to a classical 1:1 complex saturation equation to calculate the dissociation constant, Kd.
Determination of mode of action
A chitin-like substrate, i.e. a water-soluble high molecular weight chitosan with a degree of acetylation of 63%, was used as substrate for Chi18H8 to determine the enzyme’s mode of action as previously described [36]. The chitosan (30 mg) was dissolved in 3 mL of 80 mM sodium acetate, pH 5.5. To start the depolymerization reaction, 2.5, 7.5 or 26 µL of Chi18H8 (1.16 mg/mL) were added to 1 mL substrate. After incubating the mixture at 37 °C for 30 min, the reaction was stopped by adjusting the pH to 2.0 and boiling for 10 min. The reaction mixture containing the oligomers was injected and separated on three XK 26 columns in series, packed with Superdex™ 30 (Amersham Pharmacia Biotech, Piscataway, USA) using a refractive index (RI) detector (Shimadzu Schweiz GmbH, Reinach, Switzerland), as previously described [36].
Antifungal activity assay
Chi18H8 antifungal activity was evaluated by growth repression assays, in triplicate, on Fusarium graminearum ATCC 46779, Rhizoctonia solani ATCC 10183 and Botrytis cinerea ATCC 26943. Plate assays were carried out by centrally inoculating an agar plug, harboring an actively growing fungal culture, in 9-cm diameter Petri plates containing diluted potato dextrose agar (PDA). Sterilized paper discs (Munktell Filter ab, Falun, Sweden) 5 mm in diameter were soaked in 1000, 500, 100 or 10 µg/mL, respectively, of the solubilized Chi18H8 (in 100 mM sodium acetate, pH 5.0) and placed 25 mm around the fungal plug. A liquid assay system was also used. Wells (2 mL cellplate, Biofluidfocus, Largo, USA) containing fungal PDA agar plugs and 200 μL potato dextrose broth (PDB) were added with Chi18H8 (in 100 mM sodium acetate, pH 5.0) to a concentration of 1000, 100 or 10 µg/mL. Boiled enzyme in the same buffer was used as negative control in both assays. The systems were incubated at 23 °C in darkness for 48 h, and longer for F. graminearum due to slower mycelial growth rate. Growth of fungal hyphae was monitored daily using a microscope. For the liquid assay, dry weight biomass of the fungal plugs was measured seven days after inoculation.
Results
Heterologous expression of the metagenome-sourced Chi18H8
The complete chi18H8 gene sequence (G + C ratio 64.4%) was previously identified by genetic and expression screening of a high-molecular-weight DNA metagenomic library constructed from a soil in Uppsala (Sweden), characterized as suppressive to club-root disease of cabbage [6, 7, 29]. The gene, consisting of 1275 nucleotides and encoding a protein of 424 amino acids (predicted molecular mass of 45.96 kDa and isoelectric point of 7.75), was obtained by primer walking technique of a fosmid clone [7]. For this work, it was cloned into the pET24b(+) expression plasmid in E. coli BL21 Star™(DE3) cells, under the control of the IPTG-inducible T7 promoter.
Recombinant E. coli cells harboring pET24b(+)::chi18H8 and the control strain carrying the empty vector were grown at the following conditions: (1) five different growth media (LB, TB, SB, autoinduction media 1 and 2); (2) adding 0.4 mM IPTG at the early or at the late exponential phase of growth; (3) keeping temperature after induction at 37 °C or reducing it to 25 or 20 °C; (4) growing cells after induction for different time intervals before harvesting. In all the above experimental conditions, an intense protein band at the expected molecular mass for Chi18H8 was observed in SDS-PAGE only in the insoluble fractions from the recombinant strain harboring the chi18H8 gene (no signal was detected in cytoplasmic soluble fractions, nor in cell-free culture broths after protein precipitation with trichloroacetic acid). Figure 1 shows the SDS-PAGE when (a) cells were grown in LB, (b) protein expression was induced in the early exponential phase, (c) and temperature was maintained at 37 °C after induction. Similar results were achieved in SB and TB media; lower protein production was observed using the two autoinduction broths [31] or by inducing cells during the late exponential phase of growth (data not shown).
Fig. 1.
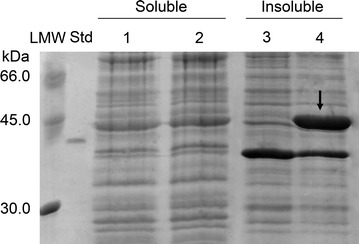
SDS-PAGE analysis of proteins from recombinant E. coli BL21 Star™(DE3) cells. E. coli BL21 Star™(DE3)/pET24b(+) cells: soluble (lane 1) and insoluble (lane 3) fractions; E. coli BL21 Star™(DE3)/pET24b(+)::chi18H8 cells: soluble (lane 2) and insoluble (lane 4) fractions. In each lane, samples corresponding to 0.5 mL of cell culture were loaded. Std reference protein, His6-GO (2.5 μg, 42.66 kDa), LMW standard proteins. Cells were grown in LB medium, protein expression was induced at the early exponential phase by adding 0.4 mM IPTG, and cells were harvested after 24 h incubation at 37 °C. Chi18H8 protein spot is indicated by an arrow
As shown in Fig. 2, accumulation of Chi18H8 into the insoluble fraction increased by prolonging the incubation time after induction, reaching 2.7 mg/g cell (corresponding to 32 mg/L culture in LB medium) after 24 h at 37 °C. Decreasing the incubation temperature to 25 or 20 °C favored the accumulation of recombinant protein into insoluble fractions (more than 9 mg/g cell, corresponding to 55 mg/L culture after 24 h incubation in LB at 20 °C). Fluorimetric enzyme assay of these insoluble fractions revealed that no chitinolytic activity was detectable from cells grown at 37 °C, whereas a weak enzyme activity appeared when growth temperature decreased to 25 and 20 °C (Fig. 2).
Fig. 2.
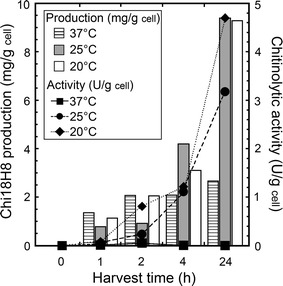
Chi18H8 expression levels in insoluble fractions from E. coli BL21 Star™(DE3)/pET24b(+)::chi18H8 grown in LB medium. Protein expression was induced at the early exponential phase of growth by adding 0.4 mM IPTG, cells were then incubated at 37, 25 or 20 °C, respectively, and harvested after 0, 1, 2, 4 or 24 h. Chi18H8 production (mg/g cell) was determined by densitometric analysis of SDS-PAGE; chitinase activity (U/g cell) was measured fluorimetrically using 4-MU-(GlcNAc)2 as a substrate. The values represent the mean of three experiments. Standard error never exceeded 5%
Taking all together, these results suggest that Chi18H8 accumulated at 37 °C largely into IBs in an inactive and insoluble form, as often occurs when E. coli is used to express metagenomics-sourced enzymes [7, 8]. Indeed, decreasing the expression temperature favored the formation of so-called non-classical IBs, i.e. those IBs that are, at least in part, biologically active since they contain an increasing portion of recombinant active protein molecules [37–39].
Solubilization of IBs and purification of Chi18H8
Considering the above results and those previously reported [7] on E. coli pGEX-6P-3::chi18H8 producing cytoplasmic Chi18H8 at an extremely low yield, a new approach was tested to recover the chitinase at a higher yield by solubilization of IBs produced by E. coli BL21 Star™(DE3)/pET24b(+)::chi18H8 cells grown in LB and incubated for 24 h at 20 °C after adding IPTG. These IBs, that likely contain some protein molecules in native-like active conformation, were recovered by centrifugation from cell lysate after mild sonication conditions. See Additional file 1: Table S1 for the different solubilization methods that were tested. Briefly, first we tried classical methods, re-suspending IBs in solutions containing strong denaturing agents and chaotropes, such as urea, guanidium hydrochloride (GdnHCl) or guanidium thiocyanate (GdnTC), alone or combined with the detergent Triton X-100, the reducing agent DTT, the chelating agent EDTA or NaCl salt. By increasing the denaturing agent concentration, an increased protein solubilization (up to 85–95%) was observed through SDS-PAGE. However, during the following refolding steps (removing the denaturant agents by dialysis), Chi18H8 aggregation and precipitation occurred and consequently the protein samples were biologically inactive (as checked by the fluorimetric enzyme activity assay). Milder solubilization conditions of IBs (Additional file 1: Table S1) were then evaluated for better preserving the inferred existing native-like secondary structure and reducing the risk of protein aggregation during the purification process. These mild solubilization protocols were based on IB incubation with detergents (Triton X-100, NLS and CHAPS [3-[(3-cholamidopropyl)dimethylammonium]-1-propanesulfonate]), or alkyl alcohol (n-propanol), or acid pH solutions (HCl, lactic acid, formic acid). Using the protocols based on detergents and n-propanol, the solubilization yield was very low (ca. 5–10%, Additional file 1: Table S1). Recovery of active Chi18H8 was only achieved in the case of IB solubilization in aqueous acid solutions of hydrochloric or lactic acid (but not in formic acid). As reported in Additional file 1: Table S1, this protocol of acid solubilization involved an osmotic shock pre-treatment of cell pellet with 25% (w/v) sucrose for disrupting the outer membrane of E. coli cells and removing periplasmic proteins. Additionally, by introducing different and sequential washing steps (with low concentrations of detergents like DOC, Triton X-100 and Nonidet P-40) after sonication of the IBs and prior to protein solubilization, IB purity increased and contaminants were reduced during the following steps of Chi18H8 purification; actually the washing steps contributed to the removal of membrane fragments, see [37, 40].
Thus, by re-suspending IBs in 10 mM hydrochloric or lactic acid we achieved solubilization yields of more than 90 or 80%, respectively, as shown in Table 1. However, in the case of IB solubilization in 10 mM lactic acid, the specific activity of the recovered Chi18H8 (40.7 U/mg protein) was definitely higher than in hydrochloric acid (29.6 U/mg protein). Increasing the acid concentration to 100 mM led to a reduction of both solubilization yield and specific activity. SDS-PAGE analysis revealed that the solubilized Chi18H8 in 10 mM lactic acid was 70% pure (Fig. 3a; Table 2).
Table 1.
Chi18H8 solubilization from IBs of E. coli BL21 Star™(DE3)/pET24b(+)::chi18H8 cells in acidic pH conditions
| Solubilization agent | Concentration (mM) | Solubilization yield (%) | Specific activity (U/mgChi18H8)a |
|---|---|---|---|
| HCl | 10 | 93 ± 4.0 | 29.6 ± 1.2 |
| 100 | 20 ± 2.0 | 15.9 ± 0.9 | |
| Lactic acid | 10 | 82 ± 3.5 | 40.7 ± 1.4 |
| 100 | 60 ± 6.5 | 30.7 ± 1.3 |
Solubilization yield was estimated by densitometric analysis of proteins separated by SDS-PAGE. Enzyme activity was measured by fluorimetric assay on 4-MU-(GlcNAc)2
aValues represent the mean of three independent experiments (mean ± standard error)
Fig. 3.
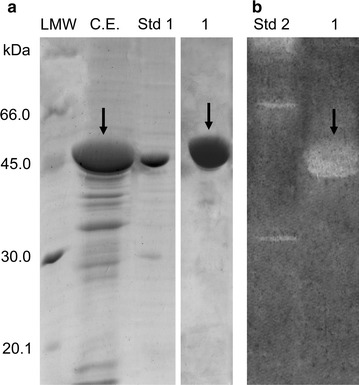
a SDS-PAGE and b zymogram analysis of Chi18H8 purification. C.E. crude extract, raw enzyme after solubilization from IBs in 10 mM lactic acid and dialysis against 100 mM HEPES pH 5.6 (loaded onto WA11 resin). 1 fraction corresponding to the flow-through. Std 1 reference protein, His6-GO (5 μg, 42.66 kDa). Std 2 zymogram reference protein, chitinases from Trichoderma viride (Sigma-Aldrich). LMW standards proteins. Chi18H8 protein spots are indicated by arrows
Table 2.
Purification of Chi18H8 from IBs of E. coli BL21 Star™(DE3)/pET24b(+)::chi18H8 cells
| Volume | Activity | Protein concentration | Specific activity | Purification | Recovery | ||
|---|---|---|---|---|---|---|---|
| (mL) | (U tot) | (mg/mL) | (U/mgprotein) | (fold) | (%) | (mg/gcell) | |
| IBs | 2.0 | 4.7 | 11.2 | 0.2 | 1.0 | 100.0 | 9.4 |
| Soluble fraction from IBs | 10.0 | 314.0 | 1.1 | 28.5 | 66.8 | 82.0 | 7.7 |
| Purified Chi18H8 | 25.3 | 355.7 | 0.2 | 63.9 | 75.7 | 57.4 | 5.4 |
Enzymatic activity was measured by fluorimetric assay on 4-MU-(GlcNAc)2. Protein concentration was determined by the Biuret assay
In order to further purify Chi18H8, different precipitation methods and chromatographic procedures were then evaluated. Salts, including NaCl, KCl, MgCl2 and NH4(SO4)2, were added to obtain a selective Chi18H8 precipitation, so were increasing concentrations of ethanol and the addition of CM-chitin-RBV. However, the enzyme co-precipitated with most of the contaminating proteins (data not shown). Different chromatographic methods were tested using sixteen resins, as listed in Additional file 1: Table S2. At most of the conditions, Chi18H8 did not interact with the resins and the chitinolytic activity was mainly found in the flow-through fractions, together with most of the contaminating proteins. In some cases the protein precipitated and, in general, it was inactive if the pH increased above 7.0. The weak cationic exchanger P11 and the hydrophobic resin HP20SS retained Chi18H8; however, from neither resin the enzyme could be eluted in a biologically active form (Additional file 1: Table S2). Interestingly, when the weak anionic exchanger WA11 resin was used at HIC conditions, most of the contaminating proteins were retained by the resin, allowing a partial purification of the chitinase from the flow-through in 100 mM HEPES pH 5.6. In this case, the purified protein was >95% pure (Fig. 3a) and the zymogram analysis in semi-denaturing conditions with CM-chitin-RBV confirmed the chitinolytic activity of the purified enzyme (Fig. 3b). Indeed, none chitinolytic activity was found when the contaminating proteins were eluted from WA11 resin by an isocratic elution using 50% (v/v) ethanol in 100 mM HEPES pH 5.6. As summarized in Table 2, the two-step lactic acid solubilization/HIC purification method allowed the recovery of more than 55% of the recombinant enzyme accumulated into IBs, with a 320-fold increase of specific activity (from 0.2 U/mg protein in the IBs, to 63.9 U/mg protein after HIC purification).
Chi18H8 production in 3 L bench- and 30 L industrial-bioreactors
Chi18H8 production and purification was scaled-up first in 3 L and then in 30 L bioreactors, where E. coli BL21 Star™(DE3)/pET24b(+)::chi18H8 cells were grown at the conditions optimized for flask fermentation. Figure 4 shows the time course of cell growth and Chi18H8 production in the 3 L bench-fermenter. Heterologous protein production was induced by adding IPTG to cells in the early stationary phase of growth. The maximum chitinase production in IBs after 24 h was 9.1 mg/g cell (corresponding to 75 mg/L) and 12.9 mg/g cell (equal to 80 mg/L) for the 3 L and the 30 L bioreactor, respectively. These amounts were comparable or even higher than what was obtained at flask level (Table 3).
Fig. 4.
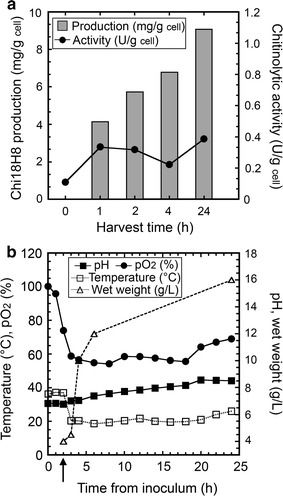
Chi18H8 production at 3 L bench-bioreactor scale. a Chi18H8 production (mg/g cell) into insoluble fractions was determined by densitometric analysis of proteins separated through SDS-PAGE. Chitinolytic activity was measured by fluorimetric assay on 4-MU-(GlcNAc)2. b Time course of pH, pO2, temperature and wet weight. Induction of protein expression with 0.4 mM IPTG is indicated by an arrow. The values represent the mean of three experiments. Standard error never exceeded 10%
Table 3.
Comparison of Chi18H8 production and IB solubilization in 2 L flasks, 3 L bench-bioreactor or 30 L industrial-bioreactor
| Cell weight (gcell/L) | Chi18H8 production (mg/gcell) | Solubilization yield (%) | Specific activity (U/mgprotein) | |
|---|---|---|---|---|
| Flask | 4.0 | 9.4 | >80 | 40.7 |
| 3 L bench-bioreactor | 5.3 | 9.1 | >65 | 52.1 |
| 30 L industrial-bioreactor | 6.2 | 12.9 | >65 | 48.4 |
Protein expression was induced in the early exponential growth phase (OD600nm ~0.6) with 0.4 mM IPTG and cells were harvested after 24 h of incubation. Chi18H8 production (mg protein/g cell) and solubilization yield in 10 mM lactic acid were estimated by densitometric analysis of proteins separated by SDS-PAGE. Specific activity of the solubilized enzyme was measured by the fluorimetric assay on 4-MU-(GlcNAc)2
The following IB solubilization in 10 mM lactic acid allowed, from cells grown at both reactor sizes, the recovery of active Chi18H8 with a >65% yield and a specific activity of 52.1 and 48.4 U/mg protein, respectively (Table 3). Solubilized protein was about 70% pure, as revealed by SDS-PAGE densitometric analysis, similarly to the results obtained with the protein produced in flasks (not shown). Also purification using HIC with the weak anionic exchanger WA11 resin turned out to be scalable and suitable for the production of Chi18H8. A volume of 135 mL solubilized Chi18H8 in 100 mM HEPES pH 5.6, corresponding to 2.2 L of culture, was loaded onto a column of WA11 resin (40 mL). Also in this case, the Chi18H8 protein was found in the flow-through, whereas contaminating proteins were retained by the resin. Chi18H8 recovery yield was approximately 70% and the purity was above 95%.
Chi18H8 characterization
Substrate specificity
Using the fluorimetric assay on three different-length analogues of natural chitooligosaccharides, pure Chi18H8 solubilized from IBs in lactic acid showed a prevalent chitobiosidase activity (40.7 U/mg), a weaker endochitinase activity (7.5 U/mg) and no β-N-acetyl-glucosaminidase activity, confirming what reported for the recombinant enzyme previously isolated [7]. Additionally, the enzyme was able to hydrolyze colloidal chitin, with an estimated activity of 0.41 U/mg.
Mode of action
Size-exclusion chromatograms revealing the length distribution of the COs when increasing amounts of Chi18H8 were added to a water-soluble chitin-like substrate, i.e. a high-molecular weight chitosan with a degree of acetylation of 63%, are shown in Fig. 5. The molecules eluting first in the chromatograms (after 7 h) are the largest molecules (e.g. chitosan with more than 50 units). The signal at the other end of the chromatograms (18 h) is the salt peak. Furthermore, the chitin dimer (GlcNAc–GlcNAc, labelled AA in Fig. 5) is the peak eluting between 15 and 15.5 h, the peaks eluting at 14 and 13.4 h are respectively the tetramer (labelled 4 in the figure) and the pentamer, and so on [36]. With increasing amounts of Chi18H8 added to the substrate, the chromatograms show that the chitosan peaks (eluting at 7 h) are gradually reduced and a continuum of oligomers appears as a result of the hydrolysis of the polymeric chitosan chain. The continuum of COs in the chromatograms with no preference for even-numbered oligomers (Fig. 5) indicates that Chi18H8 operates according to an endo-mode of action [36].
Fig. 5.
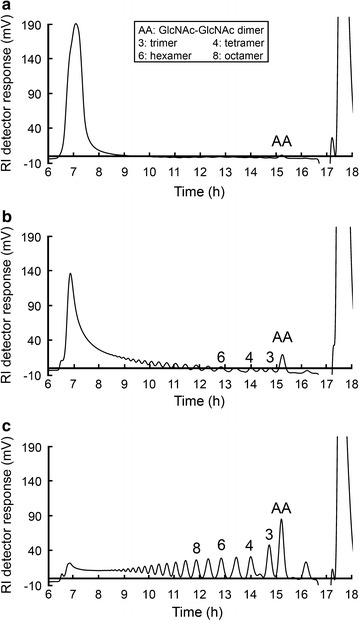
Size distribution of chitosan oligomers after Chi18H8 degradation of a water-soluble chitin-like substrate (chitosan with a 63% degree of acetylation). The chromatograms (using a refractive-index detector) show the size-distribution of the oligomers upon incubating the chitosan substrate (10 mg) with increasing amounts of Chi18H8 (1.16 mg/mL): 2.5 μL (a), 7.5 μL (b), and 26 μL (c). Peaks are labelled with the length of the oligomer or the sequence of the oligomer [AA is the chitin dimer (GlcNAc–GlcNAc)], and were identified by comparison with standard chitin/chitosan oligomers
pH and temperature profile
The optimum pH for the chitinolytic activity of the purified enzyme was between 5.0 and 6.0 and only 15.3% of the activity was maintained at pH 7.0, whereas no activity was detected at pH 3.0 and 4.0 and above pH 8.0 (Fig. 6a). Consistently, the chitinase was able to hydrolyze the CM-chitin-RBV only in the 5.0–7.0 pH range, and no activity was detectable in zymograms at pH 3.0, 4.0, 8.0 or 9.0 (Fig. 6c). The optimum temperature for chitobiosidase activity on 4-MU-(GlcNAc)2 was between 35 and 40 °C. More than 55% of the activity was retained at 25 °C (Fig. 6b); at 50 °C the enzyme was inactive. The stability of Chi18H8 was investigated by measuring the residual activity following 144 h of incubation in buffers at different pHs (5.0, 6.0 and 7.0) at the temperature of 30 °C. When Chi18H8 was incubated at pH 7.0, a complete loss of activity was observed within 24 h. At pH 6.0, the activity dramatically decreased to 5% of the initial activity within 144 h. At pH 5.0, however, Chi18H8 retained about 70% of its initial activity after 144 h (Fig. 6d).
Fig. 6.
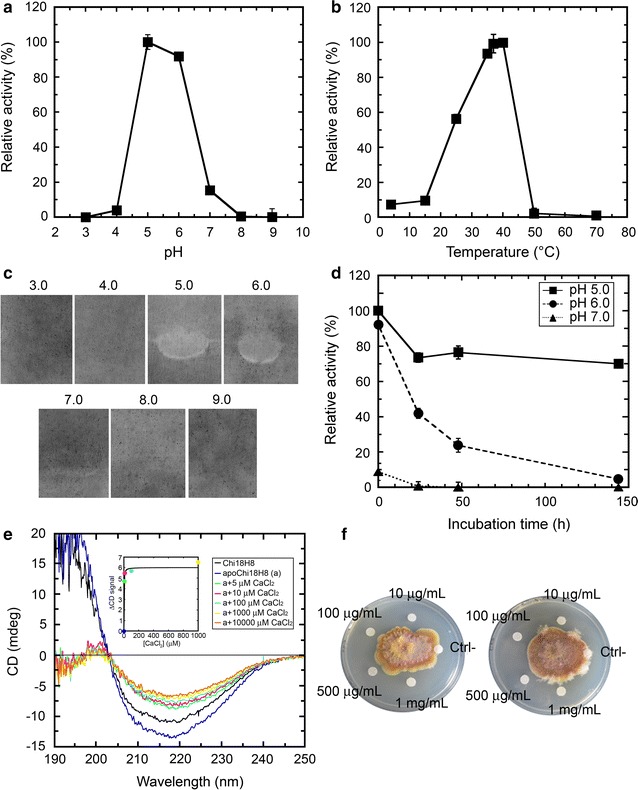
Characterization of Chi18H8 purified from IBs. a pH profile of Chi18H8 activity, using 4-MU-(GlcNAc)2 as substrate. b Temperature influence of Chi18H8 activity on 4-MU-(GlcNAc)2 measured in 100 mM sodium acetate buffer, pH 5.0. c Effect of pH on Chi18H8 activity on CM-chitin-RBV (zymograms in semi-denaturing conditions). d Time course of stability of Chi18H8 at different pHs. The enzyme was incubated in 100 mM sodium acetate pH 5.0, sodium phosphate pH 6.0 or sodium phosphate pH 7.0 for up to 144 h at 30 °C. At various time intervals, samples were withdrawn and the residual chitinolytic activity was fluorimetrically measured on 4-MU-(GlcNAc)2 under standard conditions. In panels a, b and d, the activity is expressed as a percentage of the maximum activity (set as 100%) and the values represent the mean of three experiments (mean ± standard error). e Circular dichroism (CD) far-UV spectra of the purified chitinase (indicated as ‘Chi18H8’), of the same preparation following EDTA addition and dialysis to eliminate divalent ions (‘apoChi18H8’), and after adding increasing concentrations of CaCl2. The concentration of Chi18H8 was 0.2 mg/mL in 10 mM HEPES pH 5.6. In the inset, the ΔCD signals at 219 nm vs. CaCl2 concentration used to extrapolate the dissociation constant Kd, are reported. f Antifungal activity of Chi18H8 repressing Fusarium graminearum growth on PDA plates. Reduction in radial growth of the centrally inoculated fungi was evident around discs embedded of Chi18H8 at 1000, 500, 100 or 10 µg/mL concentration. No growth inhibition was observed in the negative control (Ctrl−), i.e. boiled Chi18H8. The image shows two replicates (out of three)
Effect of metal ions, detergents, salts and other compounds
The effect of several compounds was tested on Chi18H8 enzyme activity (Table 4). The metal ions Cu2+, Fe2+, Mg2+, Mn2+, Ni2+, Zn2+, as well as the monovalent cation NH4 +, reduced the hydrolytic activity of Chi18H8. The strongest inhibition was caused by Cu2+ and Fe2+. On the contrary, Ca2+, K+ and Co2+ slightly increased the enzyme activity. The chelating agent EDTA gave an inhibitory effect, thus suggesting that Chi18H8 activity could be promoted by the presence of specific metal ions, in particular Ca2+ ions (see below).
Table 4.
Effect of different compounds on Chi18H8 activity
| Compounds | Final concentration | Relative activity (%) |
|---|---|---|
| Control | 100.0 | |
| Metal ions | mM | |
| Ca2+ | 20 | 126.8 ± 0.8 |
| Cu2+ | 20 | 0.1 |
| Fe2+ | 20 | 0.1 |
| K+ | 20 | 124.2 ± 3.1 |
| Mg2+ | 20 | 86.4 ± 1.4 |
| Mn2+ | 20 | 49.9 ± 0.1 |
| Ni2+ | 20 | 64.3 ± 0.1 |
| NH4 + | 20 | 68.0 ± 7.7 |
| Zn2+ | 20 | 16.6 ± 2.0 |
| Co2+ | 20 | 119.6 ± 1.5 |
| Chelating agents: | mM | |
| EDTA | 20 | 53.6 ± 0.1 |
| Reducing agents | mM | |
| 2-mercaptoethanol | 20 | 1.1 ± 2.1 |
| DTT | 10 | 20.5 ± 6.7 |
| Detergents | % (w/v) | |
| SDS | 1 | 0.0 |
| Triton X-100 | 1 | 72.2 ± 2.5 |
| Tween-20 | 1 | 24.0 ± 3.6 |
| DOC | 1 | 120.4 ± 0.1 |
| Nonidet P-40 | 1 | 91.0 ± 2.0 |
| NLS | 1 | 0.0 |
| Sugars | mM | |
| GlcNAc | 10 | 104.1 ± 3.3 |
| Chitobiose | 10 | 82.0 ± 1.9 |
| Organic solvents: | % (v/v) | |
| Ethanol | 10 | 90.9 ± 2.5 |
| Methanol | 10 | 88.6 ± 2.3 |
| n-Propanol | 10 | 69.4 ± 1.9 |
| DMSO | 10 | 97.7 ± 14.4 |
| Salt | M | |
| NaCl | 0.1 | 38.5 ± 1.8 |
| NaCl | 0.25 | 29.9 ± 8.1 |
| NaCl | 0.5 | 16.6 ± 0.1 |
| NaCl | 1 | 8.6 ± 0.4 |
| NaCl | 2 | 4.4 ± 2.5 |
The activity was measured by the fluorimetric assay on 4-MU-(GlcNAc)2. Activity values are reported as relative to the value of 40.7 U/mg protein (set as 100%) recorded for purified Chi18H8 at 37 °C in 100 mM sodium acetate, pH 5.0. Values represent the means of three independent experiments (mean ± standard error)
The reducing agents 2-mercaptoethanol and DTT strongly reduced the chitinase activity. Among the detergents, SDS, NLS, Tween-20 and Triton X-100 abolished or reduced the chitinase activity. Nonidet P-40 showed only a slight inhibitory effect on enzyme activity, whereas DOC stimulated the activity. Chi18H8 activity was only slightly affected in the presence of organic solvents (10% v/v final concentration). The presence of the monosaccharide GlcNAc did not influence the activity of Chi18H8, whereas 10 mM chitobiose was slightly inhibitory. High salt concentrations, as shown in Table 4, inhibited the chitinase, which retained only 4.4% of its activity when incubated with 2 M NaCl.
Secondary structure analysis
CD spectrum of the purified chitinase in the far-UV region (Fig. 6e, in 10 mM HEPES buffer pH 5.4) indicates a prevalence of β-sheets (~36.6%) and ~2.2% of α-helices. Following the incubation with 20 mM EDTA and dialysis, the spectrum of the enzyme was slightly changed, showing a more negative value of the peak at 219 nm, thus pointing to the generation of a metal-free enzyme form (i.e. the apoprotein, Fig. 6e). Notably, a significant alteration in far-UV CD spectrum was evident adding CaCl2 to the apoprotein of Chi18H8 (Fig. 6e). From the plot of ΔCD signal at 219 nm vs. CaCl2 concentration (inset in Fig. 6e), a Kd = 1.27 ± 0.33 μM was estimated, indicating a strong interaction of Ca2+-ions with the apoprotein moiety. The addition of Zn2+ also altered Chi18H8 CD spectrum similarly to Ca2+-ions (not shown) although Zn2+-ions were inhibitors of the enzymatic activity (see Table 4).
Antiphytopathogen activity
Chi18H8 was active against the phytopathogen fungus F. graminearum (Fig. 6f) and to a less extent against R. solani (Additional file 2: Figure S1). Reduction in radial growth of F. graminearum on PDA plates was evident around discs with purified Chi18H8 at concentrations of 1000, 500, 100 or 10 μg/mL (Fig. 6f). No growth inhibition was observed in the negative control (boiled enzyme). PDA plate assays with R. solani did not reveal a so clear growth inhibition effect as with F. graminearum, whereas no growth inhibition at all was observed in the case of the fast growing Botrytis cinerea (not shown). Liquid assays inoculated with F. graminearum and R. solani (Additional file 2: Figure S1), confirmed that growth of both fungi was reduced (even if at different extent) in the presence of Chi18H8, as measured as lower dry weight after incubation compared with control (boiled enzyme).
Discussion
Functional metagenomics is a powerful tool for accessing the genetic diversity encrypted in the environmental microbial communities, hence discovering novel enzymes useful for biotechnological applications, for example the broadly applicable chitinolytic enzymes [6–8, 41]. However, for any downstream industrial application, high-level production of the candidate enzyme for sustaining its chemical and biological characterization is central. Consequently, the development of suitable expression systems for the over-expression of proteins, together with the identification of efficient recovery and purification protocols, still represent the major bottlenecks for the biotechnological exploitation of microbial community’s genomes through the application of metagenomics.
Escherichia coli typically remains the first-choice host for library construction and recombinant protein production due to the convenience in cloning procedure, low cultivation costs, rapid growth and high expression level of many heterologous proteins. Unfortunately, one intrinsic limit of E. coli as a heterologous host for functional metagenomics depends on its poor secretory machinery; to limit toxicity and metabolic burden, E. coli tends to accumulate heterologous proteins into IBs. Consequently, E. coli is predicted to readily express no more than approximately 40% of the heterologous genes into biologically active proteins [42].
In this study, we developed a protein-tailored production and purification process for the novel metagenome-sourced chitinase Chi18H8 based on mild solubilization of recombinant E. coli IBs. Recombinant E. coli strains (E. coli pGEX-6P-3::chi18H8 used in [7] and E. coli pET24b(+)::chi18H8 used in this work) massively accumulated Chi18H8 into IBs. Notably, in the latter strain, a marginal chitinolytic activity was detectable when the temperature of growth was decreased from 37 to 20 °C after adding IPTG. As mentioned in the Results, biologically active IBs may be considered as non-classical IBs, presumably containing a loose arrangement of recombinant protein molecules co-existing in unfolded, partially folded or even native active structures [37, 38, 43, 44]. In the last decade, advanced structural techniques indicated that most IBs possess conformational heterogeneity, with amyloid structure building a network in which protein molecules with various conformations, including native, are trapped [45]. Consistently, we found that it was advantageous to adopt mild solubilization methods to recover Chi18H8 from partially active IBs, since these methods might preserve the existing native-like secondary structure of proteins, reducing the risk of protein re-aggregation during the refolding step [37, 46]. The protocol of mild solubilization that was developed for Chi18H8—without using denaturing and chaotropic agents—was based on a few IB washing steps, followed by freezing and thawing, and re-suspension in lactic acid. Solubilization of E. coli IBs using hydrochloric acid was introduced by Okumura et al. [47] for recovering the cytotoxic protein Cry45Aa of Bacillus thuringiensis. To our knowledge, our work is the first reporting on the use of lactic acid for IB solubilization.
An advantage of IBs is that these are specifically concentrated aggregates of the recombinant protein of interest that can be easily recovered from the cell milieu. In order to obtain a highly pure (≈95%) Chi18H8, IB solubilization in lactic acid was coupled to a further purification step based on hydrophobic-interaction chromatography, commonly employed for industrial purification of peptides and proteins [48]. The use of the weak anionic exchanger WA11 in a “negative mode” (i.e. retaining all the contaminating proteins on the resin) allowed the enzyme purification. Importantly, both upstream (cultivation and protein expression induction) and downstream (IB recovery and solubilization, and protein purification) processes were scalable from shaken flasks to 3 L bench- and to 30 L industrial pilot scale-bioreactors, giving comparable results in terms of recombinant E. coli biomass, IB solubilization and purification yields, and Chi18H8 biological activity. The process could be considered robust, reproducible and high-yielding: ca. 37 mg/L of pure active protein was obtained at the pilot scale of 30 L fermenter. This is the highest purification yield thus far obtained for a metagenome-sourced chitinase: actually, the two other chitinolytic enzymes identified by metagenomics and expressed in E. coli were recovered with a yield of ca. 0.6 mg/L for 53D1 [8] and ca. 8.3 mg/L for ChiA01 [26]. Notably, Chi18H8 purification yield also favorably compared with those obtained for other recombinant chitinolytic enzymes produced in E. coli: only few micrograms per liter of culture were obtained in the case of Bacillus circulans WL-12 ChiA (95 μg/L) [49], whereas milligrams of protein were prepared for HschiA1 from Halobacterium salinarum CECT 395 (1.4 mg/L) [50], for ChiCH and ChiCW from Bacillus cereus (4.6 and 2.3 mg/L, respectively) [51], for Bacillus licheniformis ChiA (20 mg/L) [52] and for Paecilomyces thermophila PtChiA (28 mg/L) [53].
Once Chi18H8 was purified, its prevalent chitobiosidase activity [7] was confirmed (specific activity of 40.7 U/mg on 4-MU-(GlcNAc)2): the pure enzyme was active not only on synthetic chitooligosaccharides used in the fluorimetric assay, but also on the complex substrate colloidal chitin (0.4 U/mg). Chi18H8 was found to operate according to a non-processive endomode of action on a water-soluble chitin-like substrate, in a similar mode of action as ChiC from Serratia marcescens, that, among hundreds of different microorganisms tested for chitinolytic activities, remains the most efficient one [54, 55]. Specific activity of pure Chi18H8 on fluorigenic analogues of chitooligosaccharides is within the range reported for other bacterial chitinases belonging to the 18GH family and for the commercial chitinolytic enzyme cocktail from the fungus Trichoderma viride (in this last case specific activity was of 35.9, 18.1 and 12.1 U/mg on 4-MU-GlcNAc, 4-MU-(GlcNAc)2 and 4-MU-(GlcNAc)3, respectively) [11]. We recently reported that the metagenome-sourced 53D1 showed a specific activity of 45.2 U/mg on 4-MU-(GlcNAc)2 and of 21.2 U/mg on 4-MU-(GlcNAc)3 [8]. The activities of PbChi70 from Paenicibacillus barengoltzii [56] and ChiA74 from B. thuringiensis [57] on 4-MU-(GlcNAc)3 were 13.5 and 75.2 U/mg, respectively. Indeed, Chi18H8 is less active on colloidal chitin if compared with other 18GH family-chitinases, such as 53D1 (2.3 U/mg) [8], B. licheniformis ChiA (1.5 U/mg) [52], Halobacterium salinarum CECT 395 HschiA1 (16.5 U/mg) [50], and Paenicibacillus berengoltzii PbChi70 (30.1 U/mg) [56].
Chi18H8 is a mesophilic enzyme, active and stable in acid pH conditions, consistently with the characteristics of the soil from which it was isolated [6, 7, 29]. Characterization suggests that Chi18H8 activity is stimulated by Ca2+ binding: its activity was significantly reduced in the presence of EDTA, possibly because of chelation of calcium ions necessary for correct protein structure and the catalytic site, as shown for some other chitinases [50, 58]. In accordance, the presence of calcium ions increased Chi18H8 activity and altered the protein secondary structure, according to circular dichroism analysis. Chi18H8 activity was strongly inhibited by sodium chloride, iron and copper and, to a minor extent, by manganese and nickel ions, as in the case of several other chitinolytic enzymes [59, 60]. A particularly relevant property of Chi18H8 for biotechnological application is the high solvent tolerance, indicating that it could be tested in non-aqueous solutions. Finally, growth inhibition assays conducted with the pure enzyme confirmed Chi18H8 antifungal activity on the important plant pathogen F. graminearum [61] and, to a less extent, on R. solani [62].
In conclusion, Chi18H8 shows interesting features including activity on complex chitin substrates, antifungal activity and stability at conditions compatible with possible in field applications, e.g. its range of activity is adequate for inhibiting phytopathogen fungal growth typically occurring in acidic and mesophilic environments. Additionally, this work contributes to expanding the methods for efficiently recovering active proteins by mild solubilization of E. coli IBs using lactic acid and coupling it with a single step purification by hydrophobic interaction chromatography.
Authors’ contributions
FrB conducted the experiments of cloning, expression trials and protein recovery from IBs, prepared tables and figures and co-wrote the paper; IP collaborated to the experimental work of cloning and in data analyses; FaB and MP conducted the experiments at 30 L fermenter scale and the screening of the resins for protein purification; KMV investigated the mode of action of the pure chitinase; LP contributed to enzyme assay and characterization; SS conceived the project on metagenome-source chitinase and its biotechnological application; FM managed the protein production project and wrote the paper. All authors read and approved the final manuscript.
Acknowledgements
MIUR fellowship and CIB contribution to FrB and IP are acknowledged. The authors thank Sadhna Alström at the Swedish University of Agricultural Sciences, Uppsala, Sweden for generously providing the fungal strains and advising on antifungal assays. Technical assistance by Elisa Binda and Laura Caldinelli from the University of Insubria, Varese, Italy is also acknowledged.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The authors declare that all relevant data supporting the findings of this study are available within the article and its Additional files 1 and 2.
Funding
This research was funded by the EU FP7/2007-2011 Metaexplore project, Grant Agreement No. 222625 to FM and SS.
Abbreviations
- CD
circular dichroism
- CEs
chitinolytic enzymes
- CHAPS
3-[(3-cholamidopropyl)dimethylammonium]-1-propanesulfonate
- CM-chitin-RBV
carboxymethyl-chitin-remazol-brilliant-violet
- COs
chitooligosaccharides
- DMSO
dimethyl sulfoxide
- DNase
deoxyribonuclease
- DOC
sodium deoxycholate
- DTT
dithiothreitol
- EDTA
ethylenediaminetetraacetic acid
- GdnHCl
guanidium hydrochloride
- GdnTC
guanidium thiocyanate
- GH
glycosyl hydrolase
- GlcNAc
N-acetylglucosamine
- GO
glycine oxidase
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- HIC
hydrophobic interaction chromatography
- IBs
inclusion bodies
- IPTG
isopropyl β-d-thiogalactopyranoside
- Kd
dissociation constant
- LB
Luria-Bertani broth
- 4-MU-GlcNAc
4-methylumbelliferyl N-acetyl-β-d-glucosaminide
- 4-MU-(GlcNAc)2
4-methylumbelliferyl N,N’-diacetyl-β-d-chitobioside
- 4-MU-(GlcNAc)3
4-methylumbelliferyl N,N’,N’’-triacetyl-β-d-chitotrioside
- NaPPi
sodium pyrophosphate
- NLS
N-lauroylsarcosine
- OD
optical density
- PBS
phosphate buffer saline
- PDA
potato dextrose agar
- PDB
potato dextrose broth
- PMSF
phenylmethylsulfonylfluoride
- RI
refractive index
- rpm
revolutions per minute
- SB
super broth
- SDS
sodium dodecyl sulfate
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- STE
sodium chloride-Tris-EDTA buffer
- TB
terrific broth
Additional files
Additional file 1. Evaluation of Chi18H8 antifungal activity in liquid assays. Figure S1. Growth in liquid assays of Fusarium graminearum ATCC 46779 and Rhizoctonia solani ATCC 10183 in the presence of increasing concentrations of Chi18H8.
Additional file 2. Screening of methods for recovery and solubilization of Chi18H8 from Escherichia coli inclusion bodies (IBs) and its purification. Table S1. Different protocols used for isolation and solubilization of IBs and, eventually, for refolding Chi18H8. Table S2. Affinity chromatography (AC), ion exchange chromatography (IEC) and hydrophobic interaction chromatography (HIC) pilot experiments for Chi18H8 purification.
Contributor Information
Francesca Berini, Email: f.berini@uninsubria.it.
Ilaria Presti, Email: ilaria.presti@chemogroup.it.
Fabrizio Beltrametti, Email: fbeltrametti@actygea.com.
Marco Pedroli, Email: mpedroli@actygea.com.
Kjell M. Vårum, Email: kjell.m.varum@ntnu.no
Loredano Pollegioni, Email: loredano.pollegioni@uninsubria.it.
Sara Sjöling, Email: sara.sjoling@sh.se.
Flavia Marinelli, Email: flavia.marinelli@uninsubria.it.
References
- 1.Portillo MC, Leff JW, Lauber CL, Fierer N. Cell size distributions of soil bacterial and archaeal taxa. Appl Environ Microbiol. 2013;79(24):7610–7617. doi: 10.1128/AEM.02710-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roesch LF, Fulthorpe RR, Riva A, Casella G, Hadwin AK, Kent AD, Daroub SH, Camargo FA, Farmerie WG, Triplett EW. Pyrosequencing enumerates and contrasts soil microbial diversity. ISME J. 2007;1(4):283–290. doi: 10.1038/ismej.2007.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gans J, Wolinsky M, Dunbar J. Computational improvements reveal great bacterial diversity and high metal toxicity in soil. Science. 2005;309(5739):1387–1390. doi: 10.1126/science.1112665. [DOI] [PubMed] [Google Scholar]
- 4.Ekkers DM, Cretoiu MS, Kielak AM, van Elsas JD. The great screen anomaly—a new frontier in product discovery through functional metagenomics. Appl Microbiol Biotechnol. 2012;93(3):1005–1020. doi: 10.1007/s00253-011-3804-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrer M, Martìnez-Martìnez M, Bargiela R, Streit WR, Golyshina OV, Golyshin PN. Estimating the success of enzyme bioprospecting through metagenomics: current status and future trends. Microb Biotechnol. 2016;9(1):22–34. doi: 10.1111/1751-7915.12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hjort K, Bergstrom M, Adesina MF, Jansson JK, Smalla K, Sjöling S. Chitinase genes revealed and compared in bacterial isolates, DNA extracts and a metagenomic library from a phytopathogen-suppressive soil. FEMS Microbiol Ecol. 2010;71(2):197–207. doi: 10.1111/j.1574-6941.2009.00801.x. [DOI] [PubMed] [Google Scholar]
- 7.Hjort K, Presti I, Elväng A, Marinelli F, Sjöling S. Bacterial chitinase with phytopathogen control capacity from suppressive soil revealed by functional metagenomics. Appl Microbiol Biotechnol. 2014;98:2819–2828. doi: 10.1007/s00253-013-5287-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cretoiu MS, Berini F, Kielak AM, Marinelli F, van Elsas JD. A novel salt-tolerant chitobiosidase discovered by genetic screening of a metagenomic library derived from chitin-amended agricultural soil. Appl Microbiol Biotechnol. 2015;99(19):8199–8215. doi: 10.1007/s00253-015-6639-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gooday GW. The ecology of chitin degradation. In: Marshall KC, editor. Advances in microbial ecology. US: Springer; 1990. pp. 387–430. [Google Scholar]
- 10.Karlsson M, Stenlid J. Evolution of microbial family 18 glycoside hydrolases (chitinases) J Mol Microbiol Biotechnol. 2009;16:208–223. doi: 10.1159/000151220. [DOI] [PubMed] [Google Scholar]
- 11.Berini F, Caccia S, Franzetti E, Congiu T, Marinelli F, Casartelli M, Tettamanti G. Effects of Trichoderma viride chitinases on the peritrophic matrix of Lepidoptera. Pest Manag Sci. 2016;72(5):980–989. doi: 10.1002/ps.4078. [DOI] [PubMed] [Google Scholar]
- 12.Adrangi S, Faramarzi MA. From bacteria to human: a journey into the world of chitinases. Biotechnol Adv. 2013;31(8):1786–1795. doi: 10.1016/j.biotechadv.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Neeraja C, Anil K, Purushotham P, Suma K, Sarma PVSRN, Moerschbacher BM, Podile AR. Biotechnological approaches to develop bacterial chitinases as a bioshield against fungal diseases of plants. Crit Rev Biotechnol. 2010;30:231–241. doi: 10.3109/07388551.2010.487258. [DOI] [PubMed] [Google Scholar]
- 14.Dahiya N, Tewari R, Hoondal GS. Biotechnological aspects of chitinolytic enzymes: a review. Appl Microbiol Biotechnol. 2006;71:773–782. doi: 10.1007/s00253-005-0183-7. [DOI] [PubMed] [Google Scholar]
- 15.Schipper NGM, Vårum KM, Artursson P. Chitosan as absorption enhancer for poorly absorbable drugs. 1: influence of molecular weight and degree of acetylation on drug transport across human intestinal epithelial (CaCo-2) cells. Pharm Res. 1996;13(11):1686–1692. doi: 10.1023/A:1016444808000. [DOI] [PubMed] [Google Scholar]
- 16.Strand SP, Danielsen S, Christensen BE, Vårum KM. Influence of chitosan structure on the formation and stability of DNA-chitosan polyelectrolyte complexes. Biomacromolecules. 2005;6(6):3357–3366. doi: 10.1021/bm0503726. [DOI] [PubMed] [Google Scholar]
- 17.Aam BB, Heggset EB, Norberg AL, Sørlie M, Vårum KM, Eijsink VGH. Production of chitooligosaccharides and their potential applications in medicine. Marine Drugs. 2010;8(5):1482–1517. doi: 10.3390/md8051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cottrell MT, Moore JA, Kirchman DL. Chitinases from uncultured marine microorganisms. Appl Environ Microb. 1999;65(6):2553–2557. doi: 10.1128/aem.65.6.2553-2557.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metcalfe AC, Krsek M, Gooday GW, Prosser JI, Wellington EMH. Molecular analysis of a bacterial chitinolytic community in an upland pasture. Appl Environ Microbiol. 2002;68:5042–5050. doi: 10.1128/AEM.68.10.5042-5050.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LeCleir GR, Buchan A, Hollibaugh JT. Chitinase gene sequences retrieved from diverse aquatic habitats reveal environment-specific distributions. Appl Environ Microbiol. 2004;70(12):6977–6983. doi: 10.1128/AEM.70.12.6977-6983.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cretoiu MS, Kielak AM, Abu Al-Soud W, Sorensen SJ, van Elsas JD. Mining of unexplored habitats for novel chitinases–chiA as a helper gene proxy in metagenomics. Appl Microbiol Biotechnol. 2012;94:1347–1358. doi: 10.1007/s00253-012-4057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kielak AM, Cretoiu MS, Semenov AV, Sorensen SJ, van Elsas JD. Bacterial chitinolytic communities respond to chitin and pH alteration in soil. Appl Environ Microbiol. 2013;79:263–272. doi: 10.1128/AEM.02546-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacquiod S, Demanèche S, Franqueville L, Ausec L, Xu Z, Delmont TO, Dunon V, Cagnon C, Mandic-Mulec I, Vogel TM, Simonet P. Characterization of new bacterial catabolic genes and mobile genetic elements by high throughput genetic screening of a soil metagenomics library. J Biotechnol. 2014;190:18–29. doi: 10.1016/j.jbiotec.2014.03.036. [DOI] [PubMed] [Google Scholar]
- 24.Howard MB, Ekborg NA, Weiner RM, Hutcheson SW. Detection and characterization of chitinases and other chitin-modifying enzymes. J Ind Microbiol Biotechnol. 2003;30(11):627–635. doi: 10.1007/s10295-003-0096-3. [DOI] [PubMed] [Google Scholar]
- 25.Hoster F, Schmitz JE, Daniel R. Enrichment of chitinolytic microorganisms: isolation and characterization of a chitinase exhibiting antifungal activity against phytopathogenic fungi from a novel Streptomyces strain. Appl Microbiol Biotechnol. 2005;66(4):434–442. doi: 10.1007/s00253-004-1664-9. [DOI] [PubMed] [Google Scholar]
- 26.Stoveken J, Singh R, Kolkenbrock S, Zakrzewski M, Wibberg D, Eikmeyer FG, Puhler A, Schluter A, Moerschbacher BM. Successful heterologous expression of a novel chitinase identified by sequence analyses of the metagenome from a chitin-enriched soil sample. J Biotechnol. 2015;201:60–68. doi: 10.1016/j.jbiotec.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Cretoiu MS, Korthals GW, Visser JH, van Elsas JD. Chitin amendment increases soil suppressiveness toward plant pathogens and modulates the actinobacterial and oxalobacteraceal communities in an experimental agricultural field. Appl Environ Microbiol. 2013;79(17):5291–5301. doi: 10.1128/AEM.01361-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacquiod S, Franqueville L, Cécillon S, Vogel TM, Simonet P. Soil bacterial community shifts after chitin enrichment: an integrative metagenomic approach. PLoS ONE. 2013;8(11):e79699. doi: 10.1371/journal.pone.0079699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hjort K, Lembke A, Speksnijder A, Smalla K, Jansson JK. Community structure of actively growing bacterial populations in plant pathogen suppressive soil. Microb Ecol. 2007;53:399–413. doi: 10.1007/s00248-006-9120-2. [DOI] [PubMed] [Google Scholar]
- 30.Henrissat B. A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem J. 1991;280(Pt 2):309–316. doi: 10.1042/bj2800309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Z, Kessler W, van den Heuvel J, Rinas U. Simple defined autoinduction medium for high-level recombinant protein production using T7-based Escherichia coli expression systems. Appl Microbiol Biotechnol. 2011;91(4):1203–1213. doi: 10.1007/s00253-011-3407-z. [DOI] [PubMed] [Google Scholar]
- 32.Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177(2):751–766. [PubMed] [Google Scholar]
- 33.Schägger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 34.Mörtl M, Diederichs K, Welte W, Molla G, Motteran L, Andriolo G, Pilone MS, Pollegioni L. Structure-function correlation in glycine oxidase from Bacillus subtilis. J Biol Chem. 2004;279(28):29718–29727. doi: 10.1074/jbc.M401224200. [DOI] [PubMed] [Google Scholar]
- 35.Louis-Jeune C, Andrade-Navarro MA, Perez-Iratxeta C. Prediction of protein secondary structure from circular dichroism using theoretically derived spectra. Proteins. 2012;80(2):374–381. doi: 10.1002/prot.23188. [DOI] [PubMed] [Google Scholar]
- 36.Sørbotten A, Horn SJ, Eijsink VGH, Vårum KM. Degradation of chitosans with Chitinase B from Serratia marcescens; production of chito-oligosaccharides and insight into enzyme processivity. FEBS J. 2005;272(2):538–549. doi: 10.1111/j.1742-4658.2004.04495.x. [DOI] [PubMed] [Google Scholar]
- 37.Singh A, Upadhyay V, Upadhyay AK, Singh SM, Panda AK. Protein recovery from inclusion bodies of Escherichia coli using mild solubilization process. Microb Cell Fact. 2015;25:14–41. doi: 10.1186/s12934-015-0222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jevsevar S, Gaberc-Porekar V, Fonda I, Podobnik B, Grdadolnik J, Menart V. Production of nonclassical inclusion bodies from which correctly folded protein can be extracted. Biotechnol Prog. 2005;21:632. doi: 10.1021/bp0497839. [DOI] [PubMed] [Google Scholar]
- 39.Peternel S, Grdadolnik J, Gaberc-Porekar V, Komel R. Engineering inclusion bodies for non denaturing extraction of functional proteins. Microb Cell Fact. 2008;7:34. doi: 10.1186/1475-2859-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patra AK, Mukhopadhyay R, Mukhija R, Krishnan A, Garg LC, Panda AK. Optimization of inclusion body solubilization and renaturation of recombinant human growth hormone from Escherichia coli. Protein Expr Purif. 2000;18:182–192. doi: 10.1006/prep.1999.1179. [DOI] [PubMed] [Google Scholar]
- 41.Sjöling S, Stafford W, Cowan DA. Soil metagenomics: exploring and exploiting the microbial gene pool. In: van Elsas JD, Jansson JT, Trevors JT, editors. Modern soil microbiology. Boca Raton: CRC Press; 2006. pp. 409–434. [Google Scholar]
- 42.Gabor EM, Alkema WB, Janssen DB. Quantifying the accessibility of the metagenome by random expression cloning techniques. Environ Microbiol. 2004;6(9):879–886. doi: 10.1111/j.1462-2920.2004.00640.x. [DOI] [PubMed] [Google Scholar]
- 43.Mitraki A, Fane B, Haase-Pettingell C, Sturtevant J, King J. Global suppression of protein folding defects and inclusion body formation. Science. 1991;253:54–58. doi: 10.1126/science.1648264. [DOI] [PubMed] [Google Scholar]
- 44.Ventura S, Villaverde A. Protein quality in bacterial inclusion bodies. Trends Biotechnol. 2006;24(4):179–185. doi: 10.1016/j.tibtech.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 45.de Groot NS, Sabate R, Ventura S. Amyloids in bacterial inclusion bodies. Trends Biochem Sci. 2009;34:408–416. doi: 10.1016/j.tibs.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 46.Singh SM, Sharma A, Upadhyay AK, Singh A, Garg LC, Panda AK. Solubilisation of inclusion body proteins using n-propanol and its refolding into bioactive form. Protein Expr Purif. 2012;81(1):75–82. doi: 10.1016/j.pep.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Okumura S, Saitoh H, Wasano N, Katayama H, Higuchi K, Mizuki E, Inouye K. Efficient solubilisation, activation, and purification of recombinant Cry45Aa of Bacillus thuringiensis expressed as inclusion bodies in Escherichia coli. Protein Expr Purif. 2006;47(1):144–151. doi: 10.1016/j.pep.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 48.Saraswat M, Musante L, Ravidá A, Shortt B, Byrne B, Holthofer H. Preparative purification of recombinant proteins: current status and future trends. Biomed Res Int. 2013;2013:312709. doi: 10.1155/2013/312709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen CT, Huang CJ, Wang YH, Chen CY. Two-step purification of Bacillus circulans chitinase A1 expressed in Escherichia coli periplasm. Protein Expr Purif. 2004;37(1):27–31. doi: 10.1016/j.pep.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 50.García-Fraga B, da Silva AF, López-Seijas J, Sieiro C. Functional expression and characterization of a chitinase from the marine archaeon Halobacterium salinarum CECT 395 in Escherichia coli. Appl Microbiol Biotechnol. 2014;98(5):2133–2143. doi: 10.1007/s00253-013-5124-2. [DOI] [PubMed] [Google Scholar]
- 51.Huang CJ, Chen CY. High-level expression and characterization of two chitinases, ChiCH and ChiCW, of Bacillus cereus 28-9 in Escherichia coli. Biochem Biophys Res Commun. 2005;327(1):8–17. doi: 10.1016/j.bbrc.2004.11.140. [DOI] [PubMed] [Google Scholar]
- 52.Songsiriritthigul C, Lapboonrueng S, Pechsrichuang P, Pesatcha P, Yamabhai M. Expression and characterization of Bacillus licheniformis chitinase (ChiA), suitable for bioconversion of chitin waste. Bioresour Technol. 2010;101(11):4096–4103. doi: 10.1016/j.biortech.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 53.Kopparapu NK, Zhou P, Zhang S, Yan Q, Liu Z, Jiang Z. Purification and characterization of a novel chitinase gene from Paecilomyces thermophila expressed in Escherichia coli. Carbohydr Res. 2012;347(1):155–160. doi: 10.1016/j.carres.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 54.Horn SJ, Sørlie M, Vaaje-Kolstad G, Norberg AL, Synstad B, Vårum KM, Eijsink VGH. Comparative studies of chitinases A, B and C from Serratia marcescens. Biocatal Biotransform. 2006;24:39–53. doi: 10.1080/10242420500518482. [DOI] [Google Scholar]
- 55.Gupta RD. Bio-control potential of designed bacterial chitinase. Austin J Biotechnol Bioeng. 2014;1(6):2. [Google Scholar]
- 56.Yang S, Fu X, Yan Q, Guo Y, Liu Z, Jiang Z. Cloning, expression, purification and application of a novel chitinase from a thermophilic marine bacterium Paenibacillus barengoltzii. Food Chem. 2016;192:1041–1048. doi: 10.1016/j.foodchem.2015.07.092. [DOI] [PubMed] [Google Scholar]
- 57.Casados-Vàazquex LE, Avila-Cabrera S, Bideshi DK, Barboza-Corona JE. Heterologous expression, purification and biochemical characterization of endochitinase ChiA74 from Bacillus thuringiensis. Protein Express Purif. 2015;106:99–105. doi: 10.1016/j.pep.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 58.Bendt A, Hüller H, Kammel U, Helmke E, Schweder T. Cloning, expression, and characterization of a chitinase gene from the Antarctic psychrotolerant bacterium Vibrio sp. strain Fi:7. Extremophiles. 2001;5(2):119–126. doi: 10.1007/s007920100179. [DOI] [PubMed] [Google Scholar]
- 59.Barghini P, Moscatelli D, Garzillo AM, Crognale S, Fenice M. High production of cold-tolerant chitinases on shrimp wastes in bench-top bioreactor by the Antarctic fungus Lecanicillium muscarium CCFEE 5003: bioprocess optimization and characterization of two main enzymes. Enzyme Microb Technol. 2013;53(5):331–338. doi: 10.1016/j.enzmictec.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 60.Deane EE, Whipps JM, Lynch JM, Peberdy JF. The purification and characterization of a Trichoderma harzianum exochitinase. Biochim Biophys Acta. 1998;1383(1):101–110. doi: 10.1016/S0167-4838(97)00183-0. [DOI] [PubMed] [Google Scholar]
- 61.King R, Urban M, Hammond-Kosack MCU, Hassani-Pak K, Hammond-Kosack KE. The completed genome sequence of the pathogenic ascomycete fungus Fusarium graminearum. BMC Genom. 2015;16:544. doi: 10.1186/s12864-015-1756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hane JK, Anderson JP, Williams AH, Sperchneider J, Singh KB. Genome sequencing and comparative genomics of the broad host-range pathogen Rhizoctonia solani AG8. PLoS Genet. 2014;10(5):e1004281. doi: 10.1371/journal.pgen.1004281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that all relevant data supporting the findings of this study are available within the article and its Additional files 1 and 2.


