Abstract
Male sterility is an important tool for obtaining crop heterosis. A thermo-sensitive cytoplasmic male-sterile (TCMS) line was developed recently using a new method based on tiller regeneration. In the present study, we explored the critical growth stages required to maintain thermo-sensitive male sterility in TCMS lines and found that fertility is associated with abnormal tapetal and microspore development. We investigated the fertility and cytology of temperature-treated plant anthers at various developmental stages. TCMS line KTM3315A exhibited thermo-sensitive male sterility in Zadoks growth stages 41–49 and 58–59. Morphologically, the line exhibited thermo-sensitive male sterility at 3–9 days before heading and at 3–6 days before flowering, and it was partially restored in three locations during spring and summer. TCMS line KTM3315A plants exhibited premature tapetal programmed cell death (PCD) from the early uninucleate stage of microspore development until the tapetal cells degraded completely. Microspore development was then blocked and the pollen abortion type was stainable abortion. Thus, male fertility in the line KTM3315A is sensitive to temperature and premature tapetal PCD is the main cause of pollen abortion, where it determines the starting period and affects male fertility conversion in K-type TCMS lines at certain temperatures.
Keywords: Aegilops kotschyi, cytoplasm, hybrid wheat, programmed cell death, thermo-sensitive male sterility
Introduction
Wheat (Triticum aestivum L.) is the most widely planted and important food crop throughout the world. Heterosis can be exploited to increase the crop yield and it has been utilized widely in cross-pollinating crops (Duvick 1999). Hybrid maize and rice make tremendous contributions to the global food supply, but hybrid wheat has still not contributed significantly to worldwide wheat production (Dong et al. 2012, Edwards 2001, Zhou and Wang 2008). At present, two-line breeding systems for wheat are being studied, which depend on thermo/photoperiod-sensitive genic and cytoplasmic male sterility, to assess their potential use in hybrid wheat breeding. Indeed, two-line breeding systems can facilitate crop breeding and provide an effective alternative to the cytoplasmic male-sterile (CMS) system for hybrid seed production (Murai 2001, Sun et al. 2001). The currently available thermo/photoperiod-sensitive male-sterile lines include D2 (male-sterile at a photoperiod ≥15 h) (Murai 1998, Murai et al. 2008), C49S (male-sterile at a temperature ≤12.5°C) (Yang et al. 1998), ES (male-sterile at a photoperiod ≤11.5 h) (Zhou and He 1996), 337S (male-sterile at short day lengths plus low temperatures, or long day lengths plus high temperatures) (Rong et al. 2001), BNY-S (male-sterile at ≤10°C) (Xing et al. 2003), BS20 (male-sterile at ≤12°C) (Li et al. 2006a), BS210 (male-sterile at short day lengths plus low temperatures) (Zhang et al. 2007a), BNS (male-sterile at ≤11.4°C) (Li et al. 2009a), K78S (male-sterile at photoperiods ≤14.6°C) (Li et al. 2009b), and XN291S (male-sterile at long day lengths plus high temperatures) (Dong et al. 2012). However, it is been shown that these lines have limited applicability due to the narrow ranges of their fertility-restoring germplasm, lower critical male-sterility temperatures, and shorter critical male-sterility photoperiods.
Therefore, a thermo-sensitive cytoplasmic male-sterile (TCMS) line, KTM3315A, was developed using a novel breeding method, where its thermo-sensitive fertility depends on the recessive nuclear gene rfv1m from the 1BS chromosome of Triticum macha (He et al. 2008). According to our previous study, KTM3315A is completely male sterile at temperatures <18°C during Zadoks growth stages (GS) 45 to 52 and it is capable of producing self-pollinated seeds when the temperature exceeds 20°C during this growth period. KTM3315A performs well in practical hybrid wheat breeding and it is possible to produce its seeds in great quantities. Therefore, the TCMS-dependent two-line breeding system is likely to be adopted widely in hybrid wheat production. The development of TCMS wheat lines shows that there is great potential for producing hybrid wheat seed on a commercial scale.
In flowering plants, the development of the male gametophyte occurs in the anther, and it is a highly programmed and elaborate process. Pollen abortion can be classified according to four types: typical abortion, spherical abortion, stainable abortion, and pollen-free. The formation of fertile pollen in anther locules depends on nutritional contributions from the surrounding sporophytic tissues, which comprise four somatic layers from the exterior to interior, i.e., the epidermis, endothecium, middle layer, and tapetum. The tapetum is a layer of metabolically active sporophytic cells that surrounds the microspores within the anther (Sanders et al. 1999), which plays an important role in pollen development by supplying nutrients to support pollen maturation as well as providing enzymes and materials for pollen wall biosynthesis (Wilson and Yang 2004). During pollen maturation, the tapetum degenerates through a highly regulated process of programmed cell death (PCD). The premature or abrogated PCD of tapetal cells disrupts the supply of these nutrients to microspores, thereby resulting in sterile pollen (Kawanabe et al. 2006, Ku et al. 2003, Varnier et al. 2005). It is known that male sterility is associated with premature tapetal PCD, which has been described in CHA-SQ-1-induced male sterile wheat (Wang et al. 2015) as well as PET1-CMS in sunflower (Balk and Leaver 2001). However, little is known about tapetal and pollen development in TCMS line KTM3315A under different fertility conditions.
In this study, we investigated the characteristics of the TCMS line KTM3315A, where we compared the morphological changes in normal fertile anthers and male sterile anthers from TCMS line KTM3315A plants at different developmental stages using light microscopy, fluorescent microscopy, scanning (SEM) and transmission electron microscopy (TEM), and 4′,6-diamidino-2-phenylindole (DAPI) staining. We found that the TCMS line KTM3315A exhibited premature tapetal PCD, thereby resulting in the failure to form mature pollen grains. We fully elucidated the process of premature PCD in the anther tapetum.
In this study, we aimed to delimit the critical stages and cytological features of fertility conversion in TCMS lines. Furthermore, we obtained cytological insights into the mechanism that links PCD and male sterility at the cellular level.
Materials and Methods
Plant materials
The materials utilized in this study comprised the following. (1) KTP3314A and KTM3315A, which are two TCMS lines with Aegilops kotschyi cytoplasm. KTP3314A is a conventionally bred Ae. kotschyi cytoplasmic male-sterile line that carries the thermo-sensitive gene rfv1sp from the 1BS chromosome of Triticum spelta, which is characterized by later maturity (247 d, where “d” is defined as days after sowing) and greater plant height (114 cm) (Song et al. 2013). KTM3315A was bred by crossing KTP3314A with the elite wheat fertility line TM3315B (which carries the thermo-sensitive gene rfv1m from 1BS chromosome of T. macha L.) and it is characterized by early maturity (237 d) and dwarf plants (51 cm) (Fig. 1, Supplemental Figs. 1, 2), and thus its application was more promising than that of KTP3314A (He et al. 2008). (2) K3315A, a CMS line, was employed as the control. K3315A contains Ae. kotschyi cytoplasm and a wheat-rye 1BL/1RS translocation, but it lacks the restorer gene on 1BS due to the presence (occurrence) of the 1RS translocation, and thus this line is completely male sterile but with normal growth. The seeds for (1) were self-pollinated seeds and the seeds for (2) were backcrossed seeds obtained with its maintainer line 3315B. All of these materials were provided by the Northwest A&F University in Yangling, Shanxi, China.
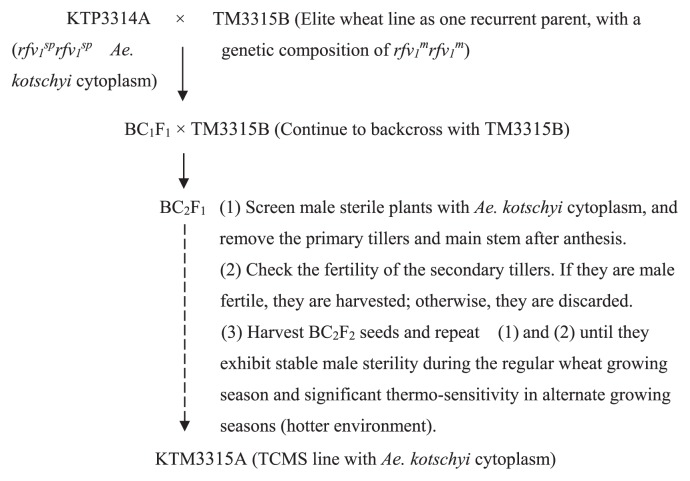
Fig. 1.
Breeding model of the TCMS line, where rfv1sp and rfv1m are a pair of alleles. For the K-CMS gene, rfv1sp and rfv1m are sensitive to temperature (He et al. 2008, Song et al. 2013).
Fertility evaluation of thermo-sensitive male sterility by the tiller regeneration technique
To develop an advanced thermo-sensitive male sterile wheat line, the existing wheat thermo-sensitive male sterile line was crossed by wheat cultivars with good performance, Tiller regeneration from the male sterile plants (F2 or BC2F1) screened above was performed as follows (Figs. 2, 3): one spike from one young tiller on each of the individual plants with completely sterile anthers was bagged to confirm sterility at anthesis. The main stems and other early tillers were removed from these plants after anthesis. The plants were then fertilized and irrigated to promote the formation of secondary tillers during the period with fertile temperature conditions. The spikes on the newly formed secondary tillers were bagged prior to anthesis. Mature seeds were harvested from the bagged spikes of the secondary tillers and the tillers were allowed to continue selfing until they exhibited stable fertility in the following year.
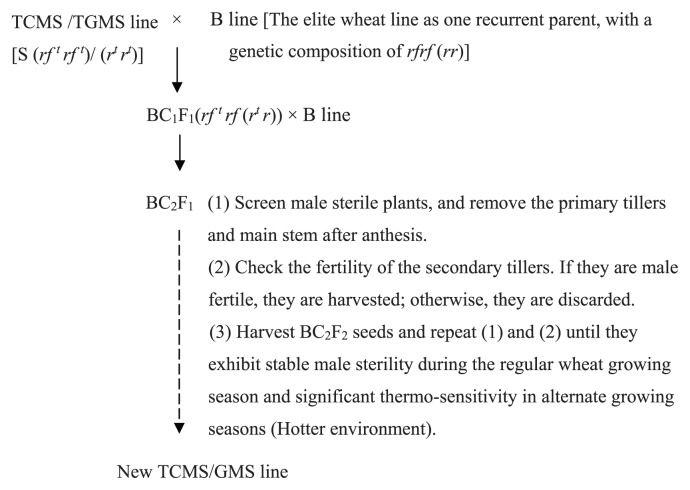
Fig. 2.
Breeding model of the TCMS/GMS line, where TCMS/TGMS line is a cytoplasmic/genic thermo-sensitive male sterile line, and B line is its Maintainer line. Gene rf trf t (r tr t) is sensitive to temperature, and S represent male sterile cytoplasm.
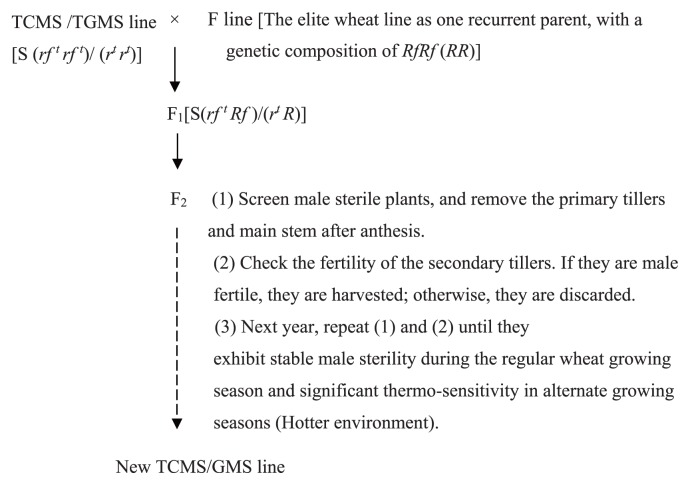
Fig. 3.
Breeding model of the TCMS/GMS line, where TCMS/TGMS line is a cytoplasmic/genic thermo-sensitive male sterile line, and F line is its restoration line. Gene rf trf t (r tr t) is sensitive to temperature, and S represent male sterile cytoplasm.
Fertility changes in KTM3315A in different wheat zones of China
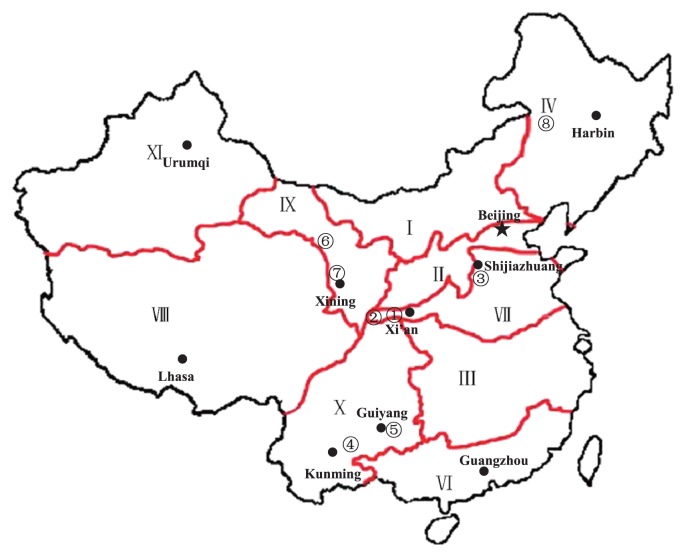
During the regular wheat growing season, KTM3315A was sown at the following five wheat zones of China (Fig. 4): Yangling and Longxian (34°91′N, 106°86′E), and Shijiazhuang (37°53′N, 114°30′E) in the wheat production zone located in Yellow and Huai River valleys Facultative Wheat Zone; Yuanmou (25°14′N, 108°08′E) and Guiyang (26°35′N, 106°42′E) in the wheat production zone located in Southern Autumn-Sown Spring Wheat Zone; Wuwei (37°55′N, 102°39′E) and Huzhu (36°49′N, 101°95′E) in the Northwestern Spring Wheat Zone; and Yi’an (47°88′N, 125°03′E) in the Northeastern Spring Wheat Zone. KTM3315A was also planted in these locations at later dates during 2010–2012 to determine whether the plants could set seeds at higher than normal temperatures, as well as to identify the optimum region for producing hybrid seeds of KTM3315A. After ripening, the seed setting rate (%) in KTM3315A was estimated based on the seed-setting rates of the first and second florets among all the spikelets from the bagged spikes of each plant. The results were analyzed by Duncan’s new multiple-range test using SAS 6.0 (SAS Institute, Cary, NC, USA).
Fig. 4.
Wheat zones of China. I Northern Spring Wheat Zone, II Northern Winter Wheat Zone, III Middle and Low Yangtze Valleys Autumn-Sown Spring Wheat Zone, IV Northeastern Spring Wheat Zone, VI Southwestern Autumn-Sown Spring Wheat Zone, VII Yellow and Huai River valleys Facultative Wheat Zone, VIII Qinghai-Tibetan plateau Spring-Winter Wheat Zone, IX Northwestern Spring Wheat Zone, X Southern Autumn-Sown Spring Wheat Zone, XI Xinjiang Winter-Spring Zone. ➀Yangling, ➁Longxian, ➂Shijiazhuang, ➃ Yuanmou, ➄Guiyang, ➅Wuwei, ➆Huzhu, ➇Yi’an.
Critical growth stages for thermo-sensitivity in the TCMS line KTM3315A
In October 2011, the K-type thermo-sensitive male-sterile wheat line KTM3315A and the non-thermo-sensitive male sterile wheat line K3315A were planted in pots (in 14 pots coded as A, B …M, N, and 1, 2 …13 and 14, respectively). The pots were 30 cm high and 30 cm in diameter. Topsoil was used to fill these pots and seven seeds of KTM3315A or K3315A were then sown in each of the pots. The pots were kept at the Irrigation Station of the Northwest A&F University and managed according to standard field wheat production practices. In 2012, when half of the potted plants began to joint (April 3), they were moved into a growth chamber, which was programmed with a day/night light period of 13/11 h and a day/night temperature of 22°C/20°C, with a light intensity of 10000 Lux. The last potted plants were treated when they entered the flowering stage on May 2. The method used is described in the following.
Two KTM3315A planted pots (Pots A and B) and two K3315A planted pots (Pots 1 and 2) were used as the growth chamber controls, which were moved into the growth chamber and subjected to cytological investigation between April 3 and May 2 (Supplemental Table 1). In addition, two KTM3315A planted pots (Pots M and N) and two K3315A planted pots (Pots 13 and 14) were used as the field controls, which were not moved into the growth chamber. Each of the other pots of KTM3315A and K3315A were moved separately into the growth chamber only once and they were kept there for three days, i.e., Pots C and 3, D and 4…, L and 12 (10 times in total). All of the potted plants from the different lines were treated under the day and night light period and temperature regimes described above, and then moved back to their original sites where they continued to grow. Before and after they were treated, the plants were examined and evaluated in terms of their growth, where they were tagged with their growth stages. The spikes of these plants were bagged before they flowered and their selfed seed setting percentages were examined when they ripened. Anther development was determined as described by Ba et al. (2014) and Zhang et al. (2013).
Phenotypic characterization and cytological observations
Photographs of plant materials were obtained using a Nikon E995 digital camera (Nikon, Tokyo, Japan) mounted on a Motic K400 dissecting microscope (Preiser Scientific, Louisville, KY, USA). The different anther development stages were identified by staining with 1% acetocarmine and the chromosomes were analyzed by staining with DAPI. To evaluate the viability of mature pollen grains, dehiscent anthers from mature flowers were stained using I2-KI (1 g iodine and 3 g potassium iodide in 100 mL water) (Chang et al. 2009). To obtain semi-thin sections, anthers were prefixed and embedded at various stages, and transverse sections measuring 1 μm were placed onto slides and then stained with toluidine blue. The anthers and microspores were analyzed by SEM, as described by Zhang et al. (2007b), and observed with a JSM-6360LV scanning electron microscope (JEOL, Tokyo, Japan). Anthers at various developmental stages were fixed, embedded, and stained for TEM, as described previously (Cheng et al. 2013). Observations and image capture were performed with a JEM-1230 transmission electron microscope (JEOL).
Results
Development and fertility changes in KTM3315A in different wheat zones of China
In order to optimize the conventional breeding system for the K-type TCMS (K-TCMS) lines (Song et al. 2013), we developed a novel seed production system for the TCMS line KTM3315A (Fig. 1, Supplemental Figs. 1, 2) based on the requirements of the K-TCMS wheat line. The system was operated as follows. In 2004, the TCMS line KTP3314A (rfv1sprfv1sp Ae. kotschyi cytoplasm) planted in the regular wheat growing season was backcrossed directly with an elite wheat line (TM3315B) as the recurrent parent, and then early maturing, dwarf male-sterile plants were screened from the BC2F1 generation.
Tiller regeneration from the male sterile plants screened above was performed as follows: one spike from one young tiller on each of the individual plants with completely sterile anthers was bagged at the end of April (during the last 10 days when the average daily temperature was 17°C) following anthesis to confirm sterility. The main stems and other early tillers were removed from these plants on May 4 when the average temperature was approximately 21°C. The plants were then fertilized and irrigated to promote the formation of secondary tillers during the period with higher average temperatures (23°C) in the early summer. The spikes on the newly formed secondary tillers were bagged prior to anthesis. Mature seeds were harvested from the bagged spikes of the secondary tillers and the tillers were allowed to continue selfing until they exhibited stable fertility in the following year. In 2008, an earlier mature and dwarf TCMS line, KTM3315A, was bred in the same manner. Compared with the conventional breeding system for hybrid wheat, this system had the advantages of simplifying and shortening the breeding process.
To investigate fertility changes in different wheat zones of China, we planted the line KTM3315A in the regions where it was planted during previous related trials. Table 1 shows that KTM3315A had a seed setting rate of zero in these regions during the regular wheat-growing season, but the rate was above 50% when it was planted in Guiyang, Yangling, and Longxian during the spring and summer. These results indicate that KTM3315A was completely male-sterile under conventional wheat-growing conditions, but it became male fertile and could produce seeds by self-pollination when it was planted in the typical hotter conditions of spring and summer.
Table 1.
Seed setting rates by KTM3315A and the average daily temperatures (Tm) and the average daily day length (Lm) during GS 37–60 at the eight planting locations
| Wheat production zones | Planting location | Sowing date | Lm (h) | Tm (°C) | Seed setting ratea (%) |
|---|---|---|---|---|---|
| Yellow and Huai River valleys Facultative Wheat Zone | Yangling, Shaanxi | Oct. 5, 2010 | 13.20 | 16.20 | 0D |
| Oct. 6, 2011 | 13.45 | 16.55 | 0D | ||
| Feb. 28, 2011 | 13.52 | 22.42 | 60.1 ± 7.32A | ||
| Feb. 27, 2012 | 13.50 | 23.70 | 66.8 ± 8.57A | ||
| Longxian, Shaanxi | Jul. 10, 2010 | 14.15 | 21.85 | 59.9 ± 7.86A | |
| Jul. 11, 2011 | 14.17 | 23.56 | 56.6 ± 10.21B | ||
| Jun. 28, 2012 | 14.18 | 22.78 | 50.8 ± 9.01B | ||
| Shijiazhuang, Hebei | Oct. 7, 2010 | 13.38 | 17.10 | 0D | |
| Oct. 8, 2011 | 13.42 | 17.70 | 0D | ||
|
| |||||
| Northwestern Spring Wheat Zone | Wuwei, Gansu | Apr. 6, 2010 | 14.60 | 15.73 | 0D |
| Apr. 13, 2011 | 14.70 | 16.56 | 0D | ||
| Huzhu, Qinhai | Apr. 15, 2010 | 14.55 | 16.10 | 0D | |
| Apr. 20, 2011 | 14.60 | 16.67 | 0D | ||
|
| |||||
| Northeastern Spring Wheat Zone | Yi’an, Heilongjiang | Apr. 5, 2010 | 15.68 | 16.42 | 0D |
| Apr. 6, 2011 | 15.70 | 16.78 | 0D | ||
|
| |||||
| Southern Autumn-Sown Spring Wheat Zone | Yuanmou, Yunnan | Nov. 9, 2010 | 10.31 | 16.56 | 0D |
| Nov. 7, 2011 | 10.22 | 17.10 | 0D | ||
| Guiyang, Guizhou | Nov. 8, 2010 | 10.60 | 14.01 | 0D | |
| Nov. 9, 2011 | 12.63 | 14.25 | 0D | ||
| Jul. 10, 2011 | 12.71 | 22.90 | 41.5 ± 9.65C | ||
| Jul. 20, 2012 | 12.80 | 23.50 | 64.2 ± 8.58A | ||
Data represent the means ± SD based on three replicates in eight independent experiments. The selfed seed setting rates for KTM3315A with the same superscripts do not differ significantly (p = 0.01) according to Duncan’s new multiple range test.
Crucial growth stages for fertility conversion by temperature
For the plants in pots M and N containing KTM3315A and pots 13 and 14 containing K3315A, which were not treated under the different day and night light period plus temperature regimes from the jointing stage until the flowering stage, the fertility of the plants did not vary when the average daily temperature ranged among 13.88–19.63°C in the field, where their selfed seed setting rates were zero. For the KTM3315A plants planted pots (Pots A and B) between April 3 and May 2, the seed setting rates ranged from 37.8% to 55.7%, but zero for K3315A (Pots 1 and 2) (Table 2). The temperature sensitivity of line KTM3315A was further verified by this study.
Table 2.
Seed setting percentages by KTM3315A and K3315A from April 3 to May 2 after treatment under the day/night light period of 13/11 h and day/night temperatures of 22°C/20°C
| Material | Codea | Anther developmental stage | Seed setting rateb (%) | |
|---|---|---|---|---|
|
| ||||
| April 3 | May 2 | |||
| KTM3315A | A1 | PMC | Milky maturity | 37.8 ± 1.42 |
| A2 | PMC | Mealy maturity | 41.2 ± 4.13 | |
| K3315A | 11 | PMC | Milky maturity | 0 |
| 12 | PMC | – | 0 | |
| KTM3315A | B1 | PMC | Milky maturity | 55.7 ± 4.37 |
| B2 | PMC | Milky maturity | 46.2 ± 3.39 | |
| K3315A | 21 | PMC | Milky maturity | 0 |
| 22 | PMC | Milky maturity | 0 | |
Two evenly growing plants were selected from different pots and designated as plants 1 and 2, where their full identification codes were A1, A2/11, 12, B1, B2/21 and 22.
–: no observations made; PMC: pollen mother cell.
Data represent the means ± SD based on the five plants each pot.
The seed setting rates of the plants from the two male sterile lines treated with the different day and night light periods and temperature regimes during specific growth stages indicated that KTM3315A did not convert to male fertility before the meiotic prophase of its pollen mother cells (PMCs). The seed setting rates in this line varied greatly from 6.7% to 54.5% between the PMC meiosis phase and early uninucleate phase, which indicates that the growing period between PMC meiosis and the early uninucleate phase is probably the crucial growing period for transformation to male fertility. By contrast, when the line was treated with different day and night light periods and temperature regimes at the growth stages after meiosis, the selfed seed setting rate was unchanged, i.e., it was equal to zero from the medium uninucleate phase to the medium binucleate phase, thereby indicating that this line did not convert to male fertility during the growing period between the two medium phases. The seed setting rate of this line varied greatly among 14.3–60.7% from the late binucleate phase to the late trinucleate phase, which indicates that its fertility increased. The seed setting rate of this line was zero after the trinucleate phase, thereby indicating that the late binucleate phase to the trinucleate phase was a second important thermo-sensitive stage for transformation from male sterility to fertility. When K3315A was treated under different day and night light periods and temperature regimes in the field or the growth chamber, its fertility was stable and always equal to zero, which further indicated that KTM3315A possessed thermo-sensitive male sterility. After treatment under the different day and night light periods of 13/11 h and temperatures of 22°C/20°C in the other growth stages, the seed setting percentage was zero for KTM3315A, thereby indicating that this line exhibited stable male sterility during these growth stages, i.e., this line was still unable to become fertile even at the temperatures required for fertility. Thus, we showed that the fertility of KTM3315A was thermosensitive during two growth stages: one between PMC meiosis and the early uninucleate phase (Zadocks GS 41–49), and another between the late binucleate phase and trinucleate phase (GS 58–59), which is about 10 days under natural conditions (Table 3, Supplemental Table 1). Morphological analyses showed that the fertility of wheat was thermosensitive for 3–9 days before heading and for 3–6 days before flowering (Supplemental Figs. 3, 4).
Table 3.
Seed setting rates by the thermo-sensitive male sterile line KTM3315A when treated at a day/night light period of 13/11 h and day/night temperature of 22°C/20°C
| Treatment date | Codea | Anther developmental stage | Seed setting rateb (%) | |
|---|---|---|---|---|
|
| ||||
| Before treatment with light and temperature | After treatment with light and temperature | |||
| April 3–6 | C1 | Pollen mother cell | Meiotic prophase I | 0 |
| C2 | Pollen mother cell | Meiotic late I | 0 | |
| April 6–9 | D1 | Pollen mother cell | Meiosis anaphase I | 0 |
| D2 | Meiotic prophase I | Tetrad | 6.7 ± 2.36 | |
| April 9–12 | E1 | Metaphase I | Early uninucleate | 26.9 ± 7.58 |
| E2 | Meiotic prophase I | Early uninucleate | 39.5 ± 6.87 | |
| April 12–15 | F1 | Meiotic Late I | Medium uninucleate | 30.8 ± 5.63 |
| F2 | Early uninucleate | Late uninucleate | 0 | |
| April 15–18 | G1 | Tetrad | Late uninucleate | 54.5 ± 2.63 |
| G2 | Medium uninucleate | Early binucleate | 0 | |
| April 18–21 | H1 | Early uninucleate | Early binucleate | 0 |
| H2 | Late uninucleate | Early binucleate | 0 | |
| April 21–24 | I1 | Early binucleate | Late binucleate | 60.7 ± 3.12 |
| I2 | Late binucleate | Trinucleate | 14.3 ± 6.58 | |
| April 24–27 | J1 | Medium uninucleate | Trinucleate | 57.1 ± 8.45 |
| J2 | Trinucleate | Mature | 0 | |
| April 27–30 | K1 | Trinucleate | Mature pollen grains | 0 |
| K2 | Late trinucleate | Mature pollen grains | 0 | |
| April 30–May 2 | L1 | Late trinucleate | Mature pollen grains | 0 |
| L2 | Trinucleate | Mature pollen grains | 0 | |
| April 3–May 2 | M1 | Pollen mother cell | Mature pollen grains | 0 |
| M2 | Pollen mother cell | Mature pollen grains | 0 | |
| April 3–May 2 | N1 | Pollen mother cell | Mature pollen grains | 0 |
| N2 | Pollen mother cell | Mature pollen grains | 0 | |
The plants in Pots M and N were placed in the field and they received no light and temperature treatments. The plants in the two pots were tagged as M1 and M2, and N1 and N2, respectively.
Data represent the means ± SD based on the all same developmental spikes during treatment.
Morphological features of the TCMS line KTM3315A
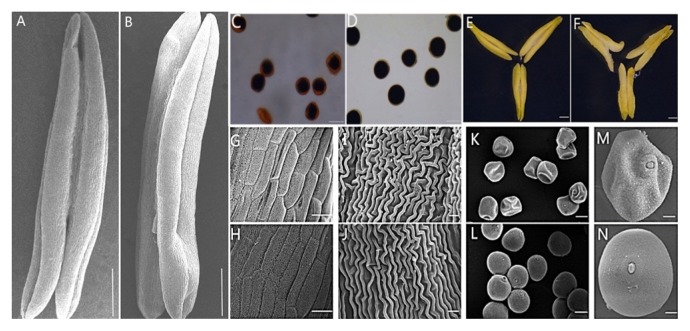
Based on morphological landmarks or cellular events visible by light microscopy and according to a previous classification of anther development (Ba et al. 2014, Zhang et al. 2013), we assigned wheat anther development to five stages. The anthers of sterile plants appeared to be normal during the first few stages (Fig. 5A–5D, 5F–5I). However, at the trinucleate stage, unlike the fertile plants with mature pollen, the pollen of the sterile plants could not be fully stained with 2% I2-KI, which demonstrated that the pollen was abortive and the pollen abortion type was stainable abortion (Fig. 6C, 6D). The anthers from fertile plants were fully plump and bright yellow. The upper and lower ends were slightly forked, with normal cracking and the shedding of loose powdery pollen (Figs. 5J, 6F). The anthers from sterile plants were light in color, empty, and flat. Anther wall cracking was not evident, and some sterile anthers were bent and tapered at the upper end, forked slightly at the base (Figs. 5E, 6E), and the anthers shed little or no pollen when mature. Moreover, the plant pistils in the TCMS line KTM3315A exhibited normal development (Fig. 5) and they were able to produce normal seeds when hybridized with fertile pollen. To obtain a more detailed understanding of the abnormalities in the sterile plant anthers during the trinucleate stage, we used SEM to study the outer epidermal surfaces of the anther patterns (Fig. 6A, 6B, 6G–6N). The outer epidermal cells of the anthers appeared to be smaller than those in fertile plant cells (Fig. 6G, 6H) and they were more irregular in shape (Fig. 6I, 6J). At the trinucleate stage, the fertile cells were rounded and plump, whereas the sterile cells appeared to be deformed and shrunken (Fig. 6K, 6L, 6M, 6N). These results indicate that the TCMS line KTM3315A exhibited major impacts on anther development, and that male fertility conversion could be induced.
Fig. 5.
Comparison of the stamens and pistils in sterile (A to E) and fertile wheat plants (F to J). A and F, tetrad stage; B and G, early uninucleate stage; C and H, later uninucleate stage; D and I, binucleate stage; E and J, trinucleate stage. Scale bars are 0.5 mm in A to J.
Fig. 6.
Comparison of scanning electron micrograph observations, I2-KI staining, and anther morphology in sterile (A, C, E, G, I, K, and M) and fertile (B, D, F, H, J, L, and N) wheat plants at the trinucleate stage. A, B, E, and F, anther; C and D, I2-KI staining; G, H, I, and J, outer epidermal cells; K, L, M, and N, trinucleate cells. Scale bars are 0.5 mm in A and B; 50 μm in C to F; 100 μm in G, H, K, and L; 10 μm in I to J; and 30 μm in M and N.
DAPI-staining analysis of microspore development in the TCMS line KTM3315A
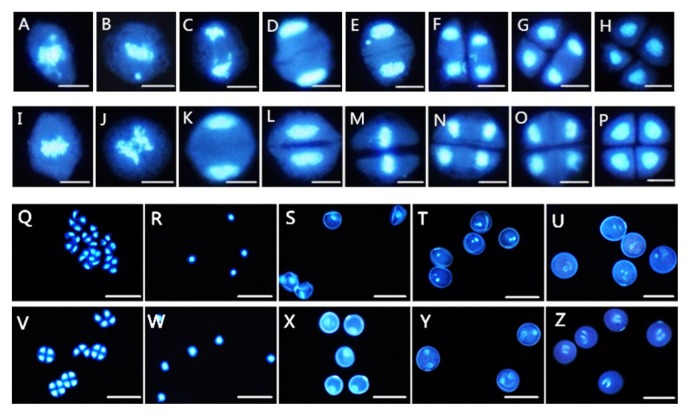
We also investigated the development of the microspores of sterile plants and fertile plants by fluorescence microscopy (Fig. 7). At entry into the meiotic cycle, the microsporocytes of the fertile and sterile plants appeared to exhibit normal development, where they underwent meiosis to generate tetrads of haploid microspores, but abnormal microspore chromosome behaviour was observed in the sterile plants (Fig. 7I–7P). At the early uninucleate stage, there were no significant differences between the fertile and sterile plants (Fig. 7R, 7W). At the later uninucleate stage, all of the microspores appeared to have germination apertures and they were round, where they also contained a very large vacuole with an increased microspore volume. The nucleus was displaced to the opposite side to the germination aperture in fertile plants (Fig. 7X), whereas cell development was obviously abnormal in the sterile plants (Fig. 7S). The microspores continued to increase in volume and they began to accumulate nutrients. They possessed dense cytoplasm and two distinct nuclei were visible in the fertile plants (Fig. 7Y), whereas the cell and nucleus had abnormal shapes at the binucleate stage in the sterile plants (Fig. 7T). At the trinucleate stage, the mature pollen grain contained two sperm nuclei and a vegetative nucleus in the fertile plants (Fig. 7Z), whereas the cell shape and cell nuclei were not normal at this stage in the sterile plants (Fig. 7U). Moreover, SEM analysis further confirmed that the sterile plants exhibited a completely misshapen and shrunken extine pattern (Fig. 6K, 6L, 6M, 6N).
Fig. 7.
DAPI staining showing microspore development in sterile (A to H and Q to U) and fertile (I to P and V to Z) wheat plants. A, Abnormal metaphase I, scattered chromosomes. B, Metaphase I, laggard chromosomes. C, Anaphase I, laggard chromosomes. D, Telophase I, abnormal dyad without the flat cell plate. E, Prophase II, abnormal micronucleus in cell. F, Anaphase II, laggard chromosomes. G, abnormal meiosis II, chromosomes separated asynchronous. H, Abnormal tetrad with different size. I, Metaphase I. J, Anaphase I. K, Telophase I. L, Prophase II. M, Metaphase II. N, Anaphase II. O, Telophase II. P, Tetrad stage. Q and V, Tetrad stage. R and W, Early uninucleate stage. S and X, Later uninucleate stage. T and Y, Binucleate stage. U and Z, Trinucleate stage. Scale bars are 10 μm in A to P and 50 μm in Q to Z.
Development of the tapetum in the TCMS line KTM3315A
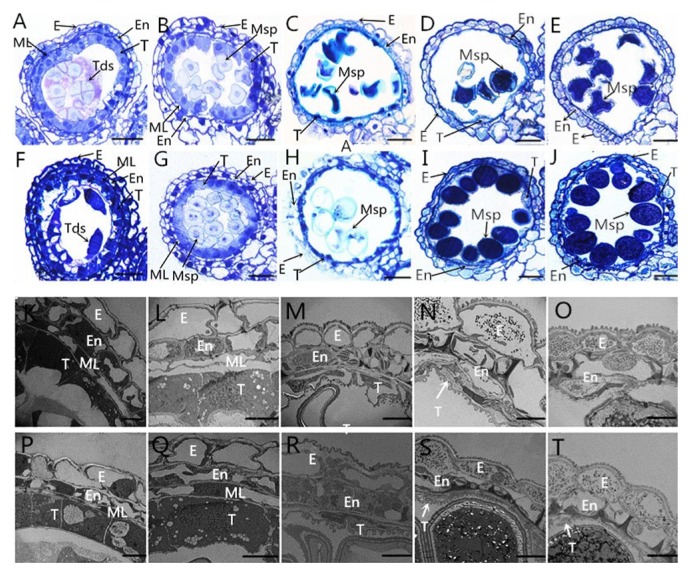
To further investigate cytological structural defects, we performed a detailed examination of anther development in sterile plants and fertile plants using light microscopy and TEM (Fig. 8). At the tetrad stage, the tapetal cells of the fertile plants and sterile plants were significantly larger than those at any other stage, and they were contained within an agglomerate that could not be disaggregated. The cells possessed dense cytoplasm and they could be deeply stained with toluidine blue O (Fig. 8A, 8F, 8K, 8P). In addition, the middle layer had a band-like shape. There were no obvious differences in anther cellular morphology in the fertile and sterile plants. Normal epidermis, endothecium, middle layer, and tapetum were found in both the fertile and sterile plants (Fig. 8A, 8F, 8K, 8P). During the early uninucleate stage, the fertile plant tapetal cells remained relatively thick and their cytoplasm stained strongly in the fertile plants (Fig. 8G, 8Q). By contrast, the tapetum became narrower in the sterile plants and began to degenerate (Fig. 8B, 8L). Moreover, the middle layers became very thin and they were still clearly visible in both the fertile and sterile plants (Fig. 8B, 8G, 8L, 8Q). The middle layers were barely visible in both the fertile and sterile plants until the later uninucleate stage (Fig. 8C, 8H, 8M, 8R). In the fertile plants, the tapetum began to degenerate to form a hill-like shape, but most of the tapetal cells still had a nuclear contour and relatively abundant cytoplasm in the fertile plants (Fig. 8H, 8R). These processes are crucial for supplying nutrition to the developing microspores. By contrast, in the sterile plants, the tapetal cells continued to degrade to yield an outline that was almost imperceptible, and they produced severely abnormal microspores with a typical falcate shape (Fig. 8C, 8M). At the binucleate stage, the epidermis and endothecium became thin in the fertile plants, and the tapetal cells remained relatively thick with an integral shape (Fig. 8I, 8S). However, the tapetal cells disintegrated into debris in the sterile plants (Fig. 8D, 8N). At the trinucleate stage, the tapetal cell outlines remained clear in the fertile plants, although the anther wall layers were thinner (Fig. 8J, 8T), where this helped the mature pollen grains to be released via anther dehiscence to pollinate female gametophytes. However, although the tapetum was fully degraded and invisible in the sterile plants (Fig. 8E, 8O), the epidermis and endothecium were thicker than normal at this stage (Fig. 8O, 8T). These observations suggest that the sterile plants developed defects during tapetal degeneration, which also affected microspore development.
Fig. 8.
Development of anthers in sterile (A to E and K to O) and fertile (F to J and P to T) wheat plants. A, F, K, and P, Tetrad stage. B, G, L, Q, and R, Early uninucleate stage. C, H, M, and R, Later uninucleate stage. D, I, N, and S, Binucleate stage. E, J, O, and T, Trinucleate stage. E, En, ML, T, Tds, and Msp indicate the epidermis, endothecium, middle layer, tapetum, and tetrads, respectively. Scale bars are 50 μm in A to J and 2 μm in K to T.
Discussion
Hybrids are now the predominant forms of many crop varieties or cultivars. However, at present, no practical cost-effective seed production system has been developed and implemented for hybrid wheat. As a result, the adoption of hybrid wheat is limited to a small niche market due to the complexity of hybrid wheat breeding, the strict temperature and day length requirements for hybrid seed production (Luo et al. 1998), and unstable male sterility (Murai and Tsunewaki 1993). Therefore, the most efficient way to address these problems is to simplify the breeding process for hybrid wheat.
In this study, we evaluated a two-line breeding system that depends on thermo-sensitive cytoplasmic male sterility. In this system, the male-sterile line KTM3315A, which was developed by a tiller regeneration technique, was readily maintained as sterile by self-pollination under conditions that allowed it to be fertile, and its selfed seed setting rate was above 50%. An important requirement for the success of hybrid wheat breeding programs is to screen for specific regions where higher temperatures prevail during GS 41–58, thereby ensuring high seed fertility. In China, we found that Guiyang in Guizhou, as well as Yangling and Longxian in Shaanxi satisfied this requirement when wheat was planted in these locations during spring and summer. Large amounts of KTM3315A seed could be produced in these three locations and they could be employed as male-sterile parents in other wheat producing regions.
Another important requirement for the success of a hybrid wheat breeding program is stable male sterility. Thus, it is necessary to employ a TCMS system for hybrid wheat breeding to develop a TCMS line that is completely male sterile under low temperature conditions. Therefore, we developed a TCMS wheat line, KTM3315A, which is completely male sterile at low temperatures. This line can be employed directly in breeding and hybrid wheat seed production in the winter and spring wheat production zones located in northern China, as well as in the winter wheat production zones located in Yunnan and Guizhou in China. As a consequence, the TCMS-dependent two-line breed system is more likely to be adopted widely for hybrid wheat production, thereby helping to reduce the imbalance between grain supply and demand. In addition, the most challenging problems during hybrid wheat seed production are low efficiency and the high production costs of hybrid seed, probably because male-sterile plants produce seeds in small quantities and they cannot be planted far from pollinator plants (D’Souza 1970, Lelley 1966). Indeed, male pollinators that disperse pollen should be planted close to sterile female lines so the latter can set seeds in great quantities. Hybrid wheat should allow the parental lines to be planted with their mixed seeds. The outcrossing frequency of the male-sterile lines will increase when a mixed seed production system (MSPS) (Supplemental Fig. 5) for hybrid wheat is employed compared with the conventional seed production system (CSPS) for hybrid wheat, where the male and female lines must be planted in alternate rows (Kempe and Gils 2011, Kim et al. 2007). We obtained similar results where the mean outcrossing frequency of the TCMS line was 68.03% under MSPS using hybrid wheat and 36.17% under CSPS (Supplemental Fig. 6) using hybrid wheat. In fact, MSPS increased the outcrossing frequencies of TCMS lines as well as the actual areas where the lines can be grown, but more importantly this method is simple to apply. In addition, in areas where a MSPS for hybrid wheat is adopted, when the restorer plants grow as high as the corresponding hybrid progeny plants and they disperse pollen, they are allowed to remain and they are harvested along with the hybrid F1 seed of the TCMS lines when both of them ripen, thereby increasing the yield of hybrid seeds. The application of MSPS for hybrid wheat using the two-line breeding system showed that MSPS is capable of simplifying the hybrid wheat seed production process, and the outcrossing frequency is increased due to the short distances between the TCMS and pollinator lines. Given these findings, we consider that the TCMS-dependent two-line breeding system should have a wide range of applications in hybrid wheat production.
The tapetum plays a vital secretory role during the development of microspores into pollen grains because it provides enzymes that facilitate the release of microspores from tetrads, nutrients for pollen development, and pollen wall components (Varnier et al. 2005). The premature or aborted death of tapetal cells disrupts the supply of nutrients to the microspores, thereby leading to male sterility (Li et al. 2011a, 2011b). Recently, there have been many reports that male sterility is associated with disrupted tapetal development and degeneration (Ahmadikhah and Karlov 2006, Li et al. 2006b, Luo et al. 2013, Zhang et al. 2010). Studies have also demonstrated that selective tapetal destruction can lead to pollen abortion (Cho et al. 2001, Lee et al. 2003). Moreover, in transgenic tobacco, premature callose wall degeneration causes male sterility (Worrall et al. 1992). It is known that male sterility is associated with premature tapetal PCD, which has been described in the Honglian CMS line of rice (Li et al. 2004) and in TAZ1-silenced plants (Kapoor et al. 2002), and these results were confirmed by our study. In addition, it has been demonstrated that tapetum-specific genes control tapetal development and affect pollen development, such as CEP1 (Zhang et al. 2014) and PR10 (Hsu et al. 2014). According to the results of the present study, we consider that the TCMS line KTM3315A undergoes early uninucleate abortion, which causes premature tapetal PCD and thus male sterility. Premature PCD of the tapetum is the main cause of pollen abortion. However, the molecular level regulation of tapetum PCD in plants remains poorly understood. In the present study, we obtained insights that may facilitate further investigations, which should explore the molecular mechanism that underlies anther degeneration.
Supplementary Information
Acknowledgements
This study was sponsored by the National Natural Science Foundation of China (31271792), the Shaanxi Province Agricultural Research Projects of China (2014K02-04-01), and the Zhongying Tang Breeding Foundation of the Northwest A&F University.
Literature Cited
- Ahmadikhah, A. and Karlov, G.I. (2006) Molecular mapping of the fertility-restoration gene Rf4 for WA-cytoplasmic male sterility in rice. Plant Breed. 125: 363–367. [Google Scholar]
- Ba, Q.S., Zhang, G.S., Wang, J.S., Niu, N., Ma, S.C. and Wang, J.W. (2014) Gene expression and DNA methylation alterations in chemically induced male sterility anthers in wheat (Triticum aestivum L.). Acta Physiol. Plant. 36: 503–512. [Google Scholar]
- Balk, J. and Leaver, C.J. (2001) The PET1-CMS mitochondrial mutation in sunflower is associated with premature programmed cell death and cytochrome c release. Plant Cell 13: 1803–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, Y.X., Gong, L., Yuan, W.Y., Li, X.W., Chen, G.X., Li, X.G., Zhang, Q.F. and Wu, C.Y. (2009) Replication protein A (RPA1a) is required for meiotic and somatic DNA repair but is dispensable for DNA replication and homologous recombination in rice. Plant Physiol. 151: 2162–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, Y.F., Wang, Q., Li, Z.J., Cui, J.M., Hu, S.W., Zhao, H.X. and Chen, M.S. (2013) Cytological and comparative proteomic analyses on male sterility in Brassica napus L. induced by the chemical hybridization agent monosulphuron ester sodium. PLoS ONE 8: e80191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, H.J., Kim, S., Kim, M. and Kim, B.D. (2001) Production of transgenic male sterile tobacco plants with the cDNA encoding a ribosome inactivating protein in Dianthus sinensis L. Mol. Cells 11: 326–333. [PubMed] [Google Scholar]
- Dong, P.H., Hu, Y.G., Guo, G.G., He, B.R., Wang, L.M. and Yuan, J.G. (2012) Inheritance and chromosome location of photoperiod-thermo sensitive male sterility in wheat line Xinong 291S. Plant Breed. 131: 695–699. [Google Scholar]
- D’Souza, L. (1970) Untersuchungen fiber die Eignung des Weizens als Pollenspender bei der Fremdbefruchtung, verglichen mit Roggen, Triticale and Secalotricum. Z. PfiZucht, pp. 63, 246–269. [Google Scholar]
- Duvick, D.N. (1999) Heterosis: feeding people and protecting natural resources. In: Coors, J.G. and Pandey S. (eds.) the genetics and exploitation of heterosis in crops. ASA-CSSA-SSSA, Madison, pp. 19–29. [Google Scholar]
- Edwards, I.B. (2001) Hybrid wheat. In: Bonjean, A.P. and Angus W.J. (eds.) The world wheat book, a history of wheat breeding. Lavoisier Publishing Inc., Paris, pp. 1017–1045. [Google Scholar]
- He, B.R., Hu, Y.G., Song, X.Y., Ma, L.J., Li, H.B., Dong, P.H. and Yu, L. (2008) Preliminary study on the fertility conversion of thermosensitive male-sterile wheat line YM3314 with the chromosome segments of T. Macha. J. Trit. Crops 282: 206–209. [Google Scholar]
- Hsu, S.W., Liu, M.C., Zen, K.C. and Wang, C.S. (2014) Identification of the tapetum/microspore-specific promoter of the pathogenesis-related 10 gene and its regulation in the anther of Lilium longiflorum. Plant Sci. 215–216: 124–133. [DOI] [PubMed] [Google Scholar]
- Kapoor, S., Kobayashi, A. and Takatsuji, H. (2002) Silencing of the tapetum-specific zinc finger gene TAZ1 causes premature degeneration of tapetum and pollen abortion in petunia. Plant Cell 14: 2353–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanabe, T., Ariizumi, T., Kawai-Yamada, M., Uchimiya, H. and Toriyama, K. (2006) Abolition of the tapetum suicide program ruins microsporogenesis. Plant Cell Physiol. 47: 784–787. [DOI] [PubMed] [Google Scholar]
- Kempe, K. and Gils, M. (2011) Pollination control technologies for hybrid breeding. Mol. Breed. 27: 417–437. [Google Scholar]
- Kim, S.S., Jung, J.Y., Jeong, S.K., Lee, D.S., Chen, L.J. and Suh, H.S. (2007) Use of herbicide-resistant genic male sterility in hybrid rice seed production. Euphytica 156: 297–303. [Google Scholar]
- Ku, S., Yoon, H., Suh, H.S. and Chung, Y.Y. (2003) Male-sterility of thermo-sensitive genic male-sterile rice is associated with premature programmed cell death of the tapetum. Planta 217: 559–565. [DOI] [PubMed] [Google Scholar]
- Lee, Y.H., Chung, K.H., Kim, H.U., Jin, Y.M., Kim, H.I. and Park, B.S. (2003) Induction of male sterile cabbage using a tapetum-specific promoter from Brassica campestris L. ssp. pekinensis. Plant Cell Rep. 22: 268–273. [DOI] [PubMed] [Google Scholar]
- Lelley, J. (1966) Befruchtungsbiologische Beobachtungen im Zusammenhang mit der Saatguterzeugung von Hybridweizen. Zuchter. 36: 314–317. [Google Scholar]
- Li, H., Yuan, Z., Vizcay-Barrena, G., Yang, C., Liang, W., Zong, J., Wilson, Z.A. and Zhang, D. (2011a) PERSISTENT TAPETAL CELL1 encodes a PHD-finger protein that is required for tapetal cell death and pollen development in rice. Plant Physiol. 156: 615–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L.J., Ru, Z.G., Gao, Q.R., Jiang, H., Guo, F.Z., Wu, S.W. and Sun, Z. (2009a) Male sterility and thermo-photosensitivity characteristics of BNS in wheat. Sci. Agric. Sin. 42: 3019–3027. [Google Scholar]
- Li, N., Zhang, D.S., Liu, H.S., Yin, C.S., Li, X.X., Liang, W.Q., Yuan, Z., Xu, B., Chu, H.W. and Wang, J. (2006b) The rice tapetum degeneration retardation gene is required for tapetum degradation and anther development. Plant Cell 18: 2999–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S., Gu, X., Liu, J.K., Tian, Y.X., Yang, H.X., Yang, M.J. and Ni, Z.F. (2009b) Genetic study on the sterility of thermo-photo sensitive genic male-sterile wheat K78S. J. Trit. Crops 29: 971–975. [Google Scholar]
- Li, S.Q., Wan, C.X., Kong, J., Zhang, Z.J., Li, Y.S. and Zhu, Y.G. (2004) Programmed cell death during microgenesis in a Honglian CMS line of rice is correlated with oxidative stress in mitochondria. Funct. Plant Biol. 31: 369–376. [DOI] [PubMed] [Google Scholar]
- Li, X.G., Gao, X.Q., Wei, Y., Deng, L., Ouyang, Y.D., Chen, G.X., Li, X.G., Zhang, Q.F. and Wu, C.Y. (2011b) Rice APOPTOSIS INHIBITOR5 coupled with two DEAD-box adenosine 5′-triphosphate-dependent RNA helicases regulates tapetum degeneration. Plant Cell 23: 1416–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y.F., Zhao, C.P., Zhang, F.T., Sun, H. and Sun, D.F. (2006a) Fertility alteration in the photo-thermo-sensitive male-sterile line BS20 of wheat (Triticum aestivum L.). Euphytica 151: 207–213. [Google Scholar]
- Luo, D.P., Xu, H., Liu, Z.L., Guo, J.X., Li, H.Y., Chen, L.T., Fang, C., Zhang, Q.Y., Bai, M., Yao, N.et al. (2013) A detrimental mitochondrial-nuclear interaction causes cytoplasmic male sterility in rice. Nat. Genet. 45: 573–577. [DOI] [PubMed] [Google Scholar]
- Luo, H.B., He, J.M., Dai, J.T., Liu, X.L. and Yang, Y.C. (1998) Studies on the characteristics of seed production of two ecological male-sterile lines in wheat. J. Hunan Agric. Univ. 24: 83–89. [Google Scholar]
- Murai, K. (1998) F1 seed production efficiency by using photoperiod-sensitive cytoplasmic male sterility and performance of F1 hybrid lines in wheat. Breed. Sci. 48: 35–40. [Google Scholar]
- Murai, K. (2001) Factors responsible for levels of male sterility in photoperiod-sensitive cytoplasmic male-sterile (PCMS) wheat lines. Euphytica 117: 111–116. [Google Scholar]
- Murai, K. and Tsunewaki, K. (1993) Photoperiod-sensitive cytoplasmic male sterility in wheat with Aegilops crassa cytoplasm. Euphytica 67: 41–48. [Google Scholar]
- Murai, K., Tsutui, I., Kawanishi, Y., Ikeguchi, S., Yanaka, M. and Ishikawa, N. (2008) Development of photoperiod-sensitive cytoplasmic male sterile (PCMS) wheat lines showing high male sterility under long-day conditions and high seed fertility under short-day conditions. Euphytica 159: 315–323. [Google Scholar]
- Rong, D.F., Li, S.H., Guo, Y.J. and Zhou, S.W. (2001) Breeding of “double pole photo-thermo-sensitive type” male-sterile line 337S of wheat. Hubei Agric. Sci. 40: 13–16. [Google Scholar]
- Sanders, P.M., Bui, A.Q., Weterings, K., McIntire, K.N., Hsu, Y.C., Lee, P.Y., Truong, M.T., Beals, T.P. and Goldberg, R.B. (1999) Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex. Plant Reprod. 11: 297–322. [Google Scholar]
- Song, X.Y., Zhang, L.L., Zeng, J.L., Qian, H.H., Li, H.B. and He, B.R. (2013) Development of thermo-sensitive cytoplasmic male sterile (TCMS) lines of wheat characterized by complete male sterility at lower-temperatures and partially restored fertility at higher-temperatures. Euphytica 192: 393–399. [Google Scholar]
- Sun, B., Zhang, A.M. and Bonjean, A.P. (2001) Chinese wheat pool. In: Bonjean, A.P. and Angus W.J. (eds.) The world wheat book, a history of wheat breeding. Lavoisier Publishing Inc., Paris, pp. 667–701. [Google Scholar]
- Varnier, A.L., Mazeyrat-Gourbeyre, F., Sangwan, R.S. and Clément, C. (2005) Programmed cell death progressively models the development of anther sporophytic tissues from the tapetum and is triggered in pollen grains during maturation. J. Struct. Biol. 152: 118–128. [DOI] [PubMed] [Google Scholar]
- Wang, S.P., Zhang, G.S., Song, Q.L., Zhang, Y.X., Li, Z., Guo, J.L., Niu, N., Ma, S.C. and Wang, J.W. (2015) Abnormal development of tapetum and microspores induced by chemical hybridization agent SQ-1 in wheat. PLoS ONE 10: e0119557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, Z.A. and Yang, C. (2004) Plant gametogenesis: conservation and contrasts in development. Reproduction 128: 483–492. [DOI] [PubMed] [Google Scholar]
- Worrall, D., Hird, D.L., Hodge, R., Paul, W., Draper, J. and Scott, R. (1992) Premature dissolution of the microsporocyte callose wall causes male-sterility in transgenic tobacco. Plant Cell 4: 759–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, Q.H., Ru, Z.G., Zhou, C.J., Xue, X., Liang, C.Y., Yang, D.E., Jin, D.M. and Wang, B. (2003) Genetic analysis, molecular tagging and mapping of the thermo-sensitive genic male-sterile gene (wtms1) in wheat. Theor. Appl. Genet. 107: 1500–1504. [DOI] [PubMed] [Google Scholar]
- Yang, Y.C., He, J.M., Liu, X.L. and Luo, H.B. (1998) Temperature and photoperiod effects on fertility transition of ecological sterile wheat ES-10. Acta Tritical Crops 18: 4–7. [Google Scholar]
- Zhang, D.D., Liu, D., Lv, X.M., Wang, Y., Xun, Z.L., Liu, Z.X., Li, F.L. and Lu, H. (2014) The cysteine Protease CEP1, a key executor involved in tapetal programmed cell death, regulates pollen development in Arabidopsis. Plant Cell 26: 2939–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H., Liang, W.Q., Yang, X.J., Luo, X., Jiang, N., Ma, H. and Zhang, D. (2010) Carbon starved anther encodes a MYB domain protein that regulates sugar partitioning required for rice pollen development. Plant Cell 22: 672–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L.P., Zhao, C.P., Shan, F.H., Zhang, F.T. and Ye, Z.J. (2007a) The mixed genetic analysis of photoperiod-temperature sensitive male sterility of BS210 in wheat. Acta Agron. Sin. 33: 1553–1557. [Google Scholar]
- Zhang, L.Y., Zhang, G.S., Zhao, X.L. and Yang, S.L. (2013) Screening and analysis of proteins interacting with TaPDK from physiological male sterility induced by CHA in wheat. J. Integr. Agric. 12: 941–950. [Google Scholar]
- Zhang, Z.B., Zhu, J., Gao, J.F., Wang, C., Li, H., Li, H., Zhang, H.Q., Zhang, S., Wang, D.M., Wang, Q.X.et al. (2007b) Transcription factor AtMYB103 is required for anther development by regulating tapetum development, callose dissolution and exine formation in Arabidopsis. Plant J. 52: 528–538. [DOI] [PubMed] [Google Scholar]
- Zhou, K.J. and Wang, S.H. (2008) Breeding method of nuclear male sterile hybrid wheat. CN, CN 101243770 A. [Google Scholar]
- Zhou, M.L. and He, J.M. (1996) A study on fertility transformation conditions of photoperiod-temperature sensitive genic male sterile wheat. J. Hunan Agric. Univ. 22: 231–235. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.