Abstract
Background
Microbial production of monoterpenes provides a promising substitute for traditional chemical-based methods, but their production is lagging compared with sesquiterpenes. Geraniol, a valuable monoterpene alcohol, is widely used in cosmetic, perfume, pharmaceutical and it is also a potential gasoline alternative. Previously, we constructed a geraniol production strain by engineering the mevalonate pathway together with the expression of a high-activity geraniol synthase.
Results
In this study, we further improved the geraniol production through reducing the endogenous metabolism of geraniol and controlling the precursor geranyl diphosphate flux distribution. The deletion of OYE2 (encoding an NADPH oxidoreductase) or ATF1 (encoding an alcohol acetyltransferase) both involving endogenous conversion of geraniol to other terpenoids, improved geraniol production by 1.7-fold or 1.6-fold in batch fermentation, respectively. In addition, we found that direct down-regulation of ERG20 expression, the branch point regulating geranyl diphosphate flux, does not improve geraniol production. Therefore, we explored dynamic control of ERG20 expression to redistribute the precursor geranyl diphosphate flux and achieved a 3.4-fold increase in geraniol production after optimizing carbon source feeding. Furthermore, the combination of dynamic control of ERG20 expression and OYE2 deletion in LEU2 prototrophic strain increased geraniol production up to 1.69 g/L with pure ethanol feeding in fed-batch fermentation, which is the highest reported production in engineered yeast.
Conclusion
An efficient geraniol production platform was established by reducing the endogenous metabolism of geraniol and by controlling the flux distribution of the precursor geranyl diphosphate. The present work also provides a production basis to synthesis geraniol-derived chemicals, such as monoterpene indole alkaloids.
Electronic supplementary material
The online version of this article (doi:10.1186/s12934-017-0641-9) contains supplementary material, which is available to authorized users.
Keywords: Monoterpene, Geraniol, Geranyl diphosphate, ERG20, Saccharomyces cerevisiae
Background
In nature, isoprenoids are a large group of diverse compounds, that are biosynthesized via both the cytosolic mevalonate (MVA) pathway and the plastidial 2C-methyl-D-erythrtiol 4-phosphate (MEP) pathway in plants and most bacteria, or the mevalonate (MVA) pathway in animals and eukaryotes [1]. The universal precursors of isoprenoids, isopentenyl diphosphate (IPP) and dimethylallyl pyrophosphate (DMAPP), are derived from both pathways. Among them, monoterpenes are a particularly interesting subset of this family, which has been widely used as flavors, fragrances and pharmaceuticals as well as potential fuels [2, 3]. Traditionally, monoterpenes and their derivatives are produced from plants in low amounts, and the extraction process from plants is costly and highly dependent on the availability of raw materials. Engineering microbial organisms for monoterpene synthesis provides a potential effective route for their production.
Monoterpene geraniol (trans-3,7-dimethyl-2,6-octadien-1-ol; C10H18O), an acyclic monoterpene, is typically generated from aromatic plants and has many applications in perfume, pharmaceutical, and other chemical industries [4]. Geraniol is also considered as a promising biofuel due to its high energy content, low hygroscopicity and low volatility in comparison with ethanol [5]. In addition, a recent study reported that geraniol can undergo an 11-step heterologous biosynthetic pathway to form strictosidine, which is the common intermediate for production of the anticancer agent monoterpene indole alkaloids (MIAs) [6]. Given its low yield in aromatic plants, geraniol has been successfully synthesized in Escherichia coli and Saccharomyces cerevisiae through metabolic engineering strategies [7–11]. S. cerevisiae has been used widely as a cell factory to produce a diversity of terpenes, and many of them have achieved significant yields [12–15]. However, monoterpene production has been achieved only at low levels thus far [8, 16, 17]. There are two challenges for high level monoterpenes production. One is the availability of intracellular geranyl diphosphate (GPP) precursor, and the other is the toxicity of monoterpenes to cells [18, 19]. Most of monoterpenes are generated from the precursor GPP which is formed by condensation of one molecule of IPP with one molecule of its isomer DMAPP (Fig. 1). Unlike plants, S. cerevisiae does not supply enough GPP for production due to the lack of a specific GPP synthase (GPPS). In S. cerevisiae, the ERG20 gene of the endogenous MVA pathway encodes a farnesyl pyrophosphate synthase (FPPS) that, despite having both GPP and FPP synthase activity, only releases a very low amount of GPP from its catalytic site [20]. To increase the flux to GPP, a set of ERG20 mutants was constructed to screen for GPPS preference mutants. ERG20 K197G, the most effective mutant, improved geraniol production to 5 mg/L while expressing geraniol synthase (GES) from Ocimum basilicum in S. cerevisiae [8]. Liu et al. obtained 36 mg/L geraniol by overexpressing two key rate-limiting genes, tHMG1 encoding a truncated version of 3-hydroxy-3-methylglutaryl coenzyme A and IDI1 encoding IPP isomerase, to enhance MVA pathway flux and by overexpressing MAF1, a negative regulator of MOD5 encoding tRNA isopentenyltransferase, to decrease the flux to tRNA biosynthesis [10]. In addition, Ignea et al. demonstrated that the double mutant Erg20p (F96W-N127W) had a strong dominant negative ability to decrease the FPPS function of Erg20p, increasing production of the monoterpene sabinene by 10.4-fold to 0.53 mg/L in an industrial diploid strain [16]. In our previous work, we further improved geraniol production through enhancing MVA pathway flux, screening different sources of GESs and GPPSs, and designing fusion proteins of GES and GPPS. Geraniol of 293 mg/L production was achieved in fed-batch cultivation, which is the highest monoterpene production in S. cerevisiae ever reported [21]. However, it is still lower than the production of many other terpenes in S. cerevisiae [12, 22, 23].
Fig. 1.
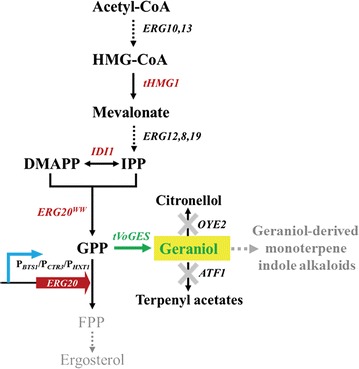
Schematic overview of geraniol biosynthesis based on the mevalonate (MVA) pathway in S. cerevisiae. The endogenous genes tHMG1, IDI1 and ERG20 WW and heterologous gene tVoGES encoding a truncated version of geraniol synthase from Valeriana officinalis were overexpressed to improve geraniol production in our previous work. The native promoter of ERG20 was replaced with the CTR3 promoter, BTS1 promoter or HXT1 promoter. Other engineered genes in this pathway: OYE2, NADPH oxidoreductase and ATF1, alcohol acetyltransferase. HMG-CoA 3-hydroxy-3-methylglutaryl coenzyme A, IPP isopentenyl pyrophosphate, DMAPP dimethylallyl pyrophosphate, GPP geranyl diphosphate, FPP farnesyl diphosphate
In addition to the low GPP pool, the toxicity to microorganisms of monoterpenes also largely limits their microbial production [2, 24]. To alleviate the toxicity of monoterpenes, a two-phase fermentation system has been extensively applied by adding a non-toxic extractive solvent (e.g., dodecane) [2]. In addition, monoterpenols, usually undergo biotransformation to other terpenoids in aromatic plants, as well as in some wine yeasts [25, 26]. A recent study demonstrated that geraniol is converted into citronellol under the catalysis of the enzyme OYE2, and it is acetylated by ATF1 encoding alcohol acetyltransferase during wine fermentation by S. cerevisiae [27]. Intracellular bioconversion of geraniol may be a self-defense response to avoid the toxicity of monoterpenes. In addition, introduction of a heterologous biosynthetic pathway and the overexpression of an endogenous pathway often lead to the accumulation of some intermediate metabolites, that may interfere with the native regulation of metabolic flux and increase metabolic burden, resulting in overproduction of some biosynthetic enzymes and accumulation of some toxic intermediate metabolites. Therefore, a dynamic control system that can sense environmental changes such as metabolic intermediates or nutrient concentrations can be used to avoid the toxicity of intermediate metabolites [28–30].
Previously, we achieved a significant increase in geraniol production through expressing geraniol synthase from Valeriana officinalis and regulating GPP synthesis [21]. In this study, we further engineered strains through dynamic control of ERG20 to fine-tune the GPP flux combined with minimized geraniol endogenous conversion. Combining these strategies together with LEU2 auxotrophic complementation, the final geraniol production reached 1.69 g/L in fed-batch fermentation, which is the highest reported production in yeast.
Results
Improving geraniol production by minimizing endogenous bioconversion
Geraniol is the main precursor of monoterpenoids in aromatic plants [4]. It also has many derivatives including nerol, neral, geranylgeraniol, geranial, citronellol and terpenyl acetates [31]. Previous studies have demonstrated that wine yeasts are able to convert geraniol into other monoterpenoids, which influence the sensory properties of wine [26, 32]. During S. cerevisiae fermentation, Oye2p is the main enzyme involved in the conversion of geraniol to citronellol, and Atf1p is the main contributor to synthesis of terpenyl acetates from geraniol [27]. In our early study, an efficient S. cerevisiae strain YZG13-GE1 was constructed to produce geraniol from glucose through engineering of geraniol synthesis and optimizing GPP synthesis. To further improve geraniol production, we attempted to minimize endogenous metabolism of geraniol in S. cerevisiae. Thus, we deleted OYE2 or ATF1 in YZG13-GE1 to create YZG14 or YZG15, respectively. Batch fermentation was carried out in a two-phase fermentation system using dodecane as extractive solvent. Geraniol was produced at 285.9 mg/L by YZG14 with OYE2 deletion, which was improved by 1.7-fold compared with the control strain (YZG13-GE1), and 259.8 mg/L was produced by YZG15 with ATF1 deletion, which was improved by 1.6-fold compared with the control strain (YZG13-GE1) (Fig. 2a). The conversion of geraniol to citronellol was decreased by 55% in YZG14 (Δoye2) (Additional file 1: Figure S1). In addition, we further investigated the effect of double deletion of OYE2 and ATF1 in YZG13-GE1 on geraniol production. The results showed that geraniol production dramatically decreased to 32.2 mg/L in YZG16 with OYE2-ATF1 deletions (Fig. 2a). Cell growth was not affected in either single gene deletion strain (Δoye2 or Δatf1) (Additional file 1: Figure S2), and geraniol yields were 57.8 mg/g DCW and 58.2 mg/g DCW, a 1.8-fold and 1.7-fold increase, respectively (Fig. 2b; Table 1). However, double deletion of OYE2 and ATF1 decreased the final biomass by 35%, and geraniol yield significantly dropped to 9.6 mg/g DCW (Fig. 2; Table 1).
Fig. 2.
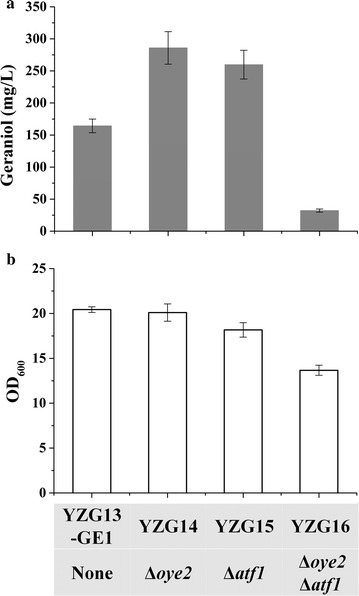
Production of geraniol by reducing endogenous conversion of geraniol in batch fermentation. a Production of geraniol in engineered strains. b Cell growth of engineered strains. The data shown are representative of duplicate experiments, and the error bars represent the standard deviation
Table 1.
Geraniol yield of the engineered strains in batch fermentation
| Strainsa | Dry cell weight (g/L) | Geraniol yield (mg/g DCW) |
|---|---|---|
| YZG13-GE1 | 5.02 ± 0.31 | 32.69 ± 2.01 |
| YZG14 (Δoye2) | 4.94 ± 0.28 | 57.83 ± 3.24 |
| YZG15 (Δatf1) | 4.47 ± 0.22 | 58.16 ± 2.59 |
| YZG16 (Δoye2Δatf1) | 3.36 ± 0.34 | 9.58 ± 0.96 |
| YZG17 (PBTS1-ERG20) | 1.96 ± 0.10 | 20.58 ± 0.91 |
| YZG18 (PCTR3-ERG20) | 2.94 ± 0.23 | 16.61 ± 1.21 |
| YZG19 (PHXT1-ERG20) | 3.92 ± 0.26 | 37.87 ± 2.55 |
aDuplicate experiments were performed for each strain, and the error bars the represented the standard deviation
We also compared geraniol production in YZG14 (Δoye2), YZG15 (Δatf1) and YZG16 (Δoye2Δatf1) in fed-batch cultivation using glucose as sole carbon source. The cell growth of YZG14 (Δoye2) and YZG15 (Δatf1) exhibited a similar growth profile compared with the reference strain YZG13-GE1. YZG14 (Δoye2) and YZG15 (Δatf1) produced 421.7 and 371.1 mg/L of geraniol, which represented approximately 2.5-fold and 2.2-fold increases relative to YZG13-GE1 (256.4 mg/L), respectively (Fig. 3). However, geraniol production was dramatically decreased in double deletion of OYE2 and ATF1 strain, only producing 46.1 mg/L geraniol (Fig. 3d).
Fig. 3.
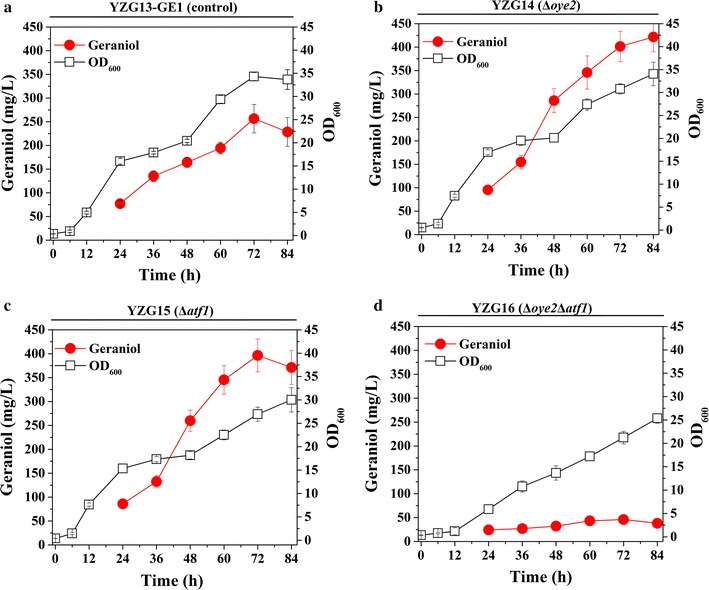
Fed-batch fermentation of YZG13-GE1 (control) (a), YZG14 (Δoye2) (b), YZG15 (Δatf1) (c) and YZG16 (Δoye2Δatf1) (d). Aerobic fed-batch fermentations were carried out by feeding 400 g/L glucose at 0.1/h feed rate. The data shown are representative of duplicate experiments, and the error bars represent the standard deviation
The effect of ERG20 expression control on geraniol production
In S. cerevisiae, synthesis of both the monoterpene precursor GPP and sesquiterpene precursor FPP is catalyzed by a single enzyme, Erg20p, with FPP synthase preference. In the absence of a specific GPP synthase, many efforts have been made to increase the intercellular GPP pool. Expression of heterologous GPP synthase does not increase the GPP-derived products significantly [16, 21]. Although engineering Erg20p into a GPP synthase increased sabinene and geraniol production by 10.4-fold and 2.8-fold, respectively, the titer is still much lower than that of FPP-derived sesquiterpenes, demonstrating the importance of down-regulating FPP synthesis [10, 16]. In this study, the native ERG20 promoter was first replaced with either the weak BTS1 promoter or the copper repressible CTR3 promoter. YZG13-GE1 containing the native ERG20 promoter was used as a reference strain. The transcription level of ERG20 using the BTS1 promoter or CTR3 promoter was reduced by 90 and 75% (Fig. 4a), respectively, in SD-URA-HIS medium with 2% glucose. Unexpectedly, the replacement of the ERG20 promoter with that of BTS1 or CTR3 decreased geraniol production by 75.6 and 70.3%, resulting in 40.37 and 48.85 mg/L, respectively (Fig. 4b). The BTS1 and CTR3 promoters also decreased the final biomass by 60 and 40% (Fig. 4c), repectively. Previously, we also found that overexpression of ERG20 reduced geraniol production [21]. These results demonstrated that both down-regulation and up-regulation of ERG20 have a negative impact on geraniol production. The dynamic control of ERG20 was then attempted by replacing the ERG20 promoter with the glucose-sensing HXT1 promoter. This promoter strength is higher than that of ERG20 promoter when glucose is present in the medium (Fig. 4a), but it is repressed when the glucose concentration is low or absent (Additional file 1: Figure S3). We found that the geraniol titer and final biomass were not markedly affected by HXT1 promoter replacement in batch cultivation, and the yield of geraniol increased slightly (37.9 mg/g DCW vs. 32.7 mg/g DCW) (Table 1). Considering that the HXT1 promoter provides both high-glucose induction and low-glucose repression, we attempted both glucose and glucose/ethanol (1:7) mixture feeding strategies to control ERG20 expression in fed-batch fermentation. Compared with the control strain, HXT1 promoter replacement did not increase geraniol production when pure glucose was fed; geraniol production only increased slightly (data not shown). In contrast, when a glucose/ethanol (1:7) mixture was fed, the geraniol production achieved a 176% increase in the HXT1 promoter replacement strain compared with the control strain, leading to 650.8 mg/L (Fig. 4d).
Fig. 4.
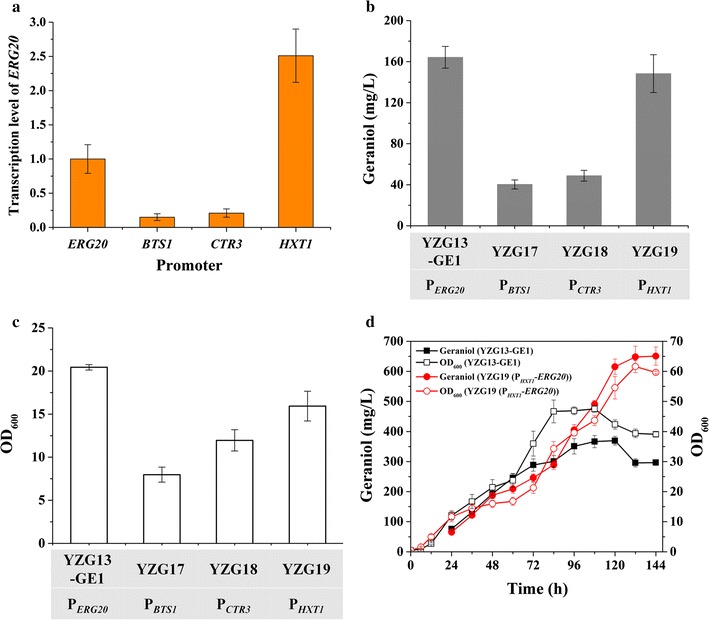
Production of geraniol in ERG20 promoter replacement strains. a Transcription level of ERG20 controlled by different promoters. b Production of geraniol in engineered strains in batch fermentation. c Cell growth of engineered strains in batch fermentation. d Geraniol production in YZG13-GE1 and HXT1 promoter replacement strain (YZG19) in fed-batch fermentation. All strains were grown in SD medium with 2% glucose, and the cells were collected when OD600 reached 0.6 to extract mRNA for ERG20 mRNA and transcription determination. Aerobic fed-batch fermentations were carried out by feeding a 50 g/L glucose and 350 g/L ethanol (1:7) mixture at 0.1/h feed rate. The data shown are representative of duplicate experiments, and the error bars represent the standard deviation
Previously, ethanol has been used as carbon for the production of hydrocortisone, amorpha-4,11-diene and resveratrol [33–35]. In this study, we further compared the impact of feeding different ratios of glucose and ethanol on geraniol production using YZG19 (PHXT1-ERG20). As shown in Fig. 5b, the cell growth of YZG19 (PHXT1-ERG20) gradually improved as the ethanol percentage in glucose/ethanol mixture increased, and the final production of geraniol also gradually increased. The highest production of geraniol was obtained when pure ethanol was fed, producing 867.7 mg/L of geraniol (Fig. 5a). Based on the results described above, the dynamic control of ERG20 expression under the HXT1 promoter together with optimizing the glucose and ethanol ratio improved the production of geraniol by 3.8-fold.
Fig. 5.
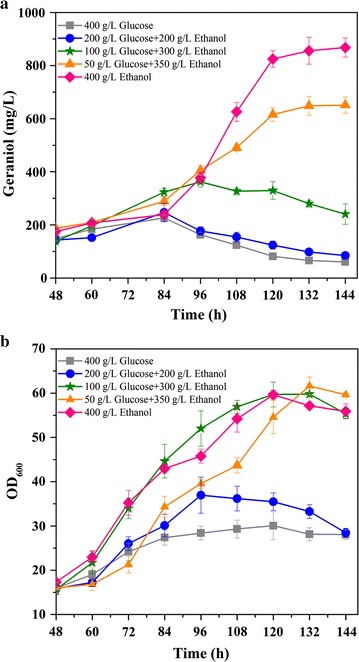
The effect of different carbon sources on geraniol production by YZG19 (PHXT1-ERG20) in fed-batch fermentation. a Production of geraniol by YZG19. b Cell growth of YZG19. Aerobic fed-batch fermentations were carried out by feeding different ratios of glucose and ethanol at 0.1/h feed rate. The data shown are representative of duplicate experiments, and the error bars represent the standard deviation
Combining the dynamic control of ERG20 and reduction of endogenous conversion together with LEU2 complementation enabled a further increase in geraniol production
To further improve geraniol production, we deleted the OYE2 gene in YZG19 (PHXT1-ERG20) to generate YZG20 (Δoye2, PHXT1-ERG20), which produced 984.6 mg/L of geraniol in fed-batch fermentation by feeding ethanol, a 14% increase over YZG19 (PHXT1-ERG20) (Fig. 6a). A recent study showed that leucine metabolism may be networked with isoprenoid biosynthesis, and leucine prototrophy was found to improve diterpenoid miltiradiene production [36]. We further engineered a strain with complemented LEU2 auxotrophy, and final geraniol production was enhanced up to 1.69 g/L, a further 71% increase over YZG20 (Δoye2, PHXT1-ERG20). Meanwhile, we noticed that LEU2 complementation also improved cell growth and geraniol yield (Fig. 6b). The final geraniol production in this study was improved by 5.8-fold compared with our previous study.
Fig. 6.
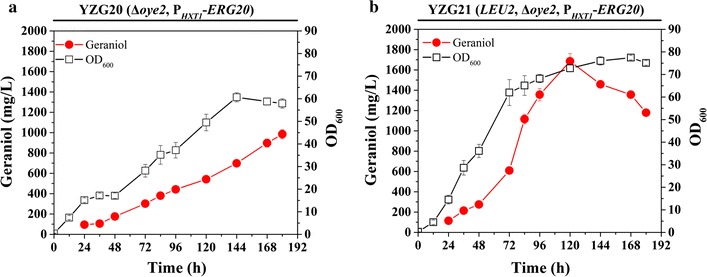
Fed-batch fermentation of YZG20 (Δoye2, PHXT1-ERG20) (a) and YZG21 (LEU2, Δoye2, PHXT1-ERG20) (b) using pure ethanol feeding. Aerobic fed-batch fermentations were carried out by feeding 400 g/L ethanol with 0.1/h feed rate. The data shown are representative of duplicate experiments, and the error bars represent standard deviations
Discussion
Engineering S. cerevisiae as a cell factory is a promising and attractive route for rapid and inexpensive biosynthesis of terpenoids [12, 13, 22, 23, 34]. Over the last few years, most work has focused on the production of sesquiterpenes in microorganisms including S. cerevisiae. For instance, production of the sesquiterpene amorpha-4,11-diene, the precursor for the antimalarial agent artemisinin, has reached 40 g/L using engineered S. cerevisiae in fed-batch fermentation [12]. Both monoterpenes and sesquiterpenes are derived from the MVA pathway. However, monoterpene production in S. cerevisiae is much lower than that of sesquiterpenes, mainly due to the poor GPP precursor supply and the toxicity of monoterpenes in yeast. In this work, we further engineered production of the monoterpene geraniol through reduction of endogenous geraniol conversion, dynamic control of ERG20 expression and leucine biosynthesis complementation based on YZG13-GE1, a geraniol-producing strain from our previous work [21]. Final geraniol production of 1.69 g/L was obtained, which to our knowledge is the highest level reported for engineered yeast.
Monoterpenes are highly toxic to many microorganisms. Cells may reduce their toxicity through the conversion of monoterpenes to other lower toxicity compounds by various specific or non-specific enzymes. In our previous study, we confirmed that 180 mg/L of geraniol in culture could strongly inhibit yeast cell growth [21]. Although two-phase extractive fermentation could effectively alleviate monoterpene toxicity in situ, the conversion of monoterpenes to other terpenoids is not avoided completely. In S. cerevisiae, a mechanism was proposed in which geraniol was first oxidized to geranial by OYE2 encoding an NADPH oxidoreductase and its homologous gene OYE3, belonging to the old yellow enzyme family, and then was reduced to citronellol [25, 27, 37]. However, the oxido-reductases involved in the reaction of geranial to citronellol are still unknown. Of the two old yellow enzymes, OYE2 was the main contributor to the reduction reaction. Except for citronellol, terpenyl acetates including citronellyl acetate and geranyl acetate are another group of geraniol-derived metabolites catalyzed by ATF1 encoding alcohol acetyltransferase. In our study, deletion of OYE2 or ATF1 led to a 1.8-fold increase of geraniol production. Interestingly, the ATF1 deletion strain increased citronellol production compared with the control strain (Additional file 1: Figure S1). A similar phenomenon was observed in a previous study, which indicated that there may be a relationship between the physiological functions of OYE2 and ATF1. Although the old yellow enzymes in yeast may have a physiological role in detoxification of unsaturated metabolites and reactive oxygen species, both OYE2 and ATF1 are thought to be involved in sterol metabolism [38, 39]. Therefore, functional complementation in the regulation of sterol metabolism between OYE2 and ATF1 may be a reason for the lower cell growth and geraniol production in the double deletion strain.
In the geraniol-producing engineered S. cerevisiae, the reaction catalyzed by ERG20 is a key branch point towards either geraniol synthesis or downstream ergosterol synthesis for cell growth. Although ERG20 mutations could improve monoterpene production, the reduction of intercellular FPP synthesis damaged cell growth [8, 16]. In this study, the weak promoter PBTS1 and the copper-repressible promoter PCTR3 were first tested for ERG20 down-regulation to redirect GPP flux. The transcriptional level of ERG20 in the promoter replacement strains was decreased to a one-fifth to one-sixth, and reduced cell growth was also observed when geraniol synthase and the MVA pathway were overexpressed in promoter replacement strains (Fig. 4; Additional file 1: Figure S4). The enhancement of the MVA pathway together with ERG20 down-regulation possibly led to the accumulation of toxic intermediates such as DMAPP or GPP. The toxicity of isoprenoid precursors has also been reported in E. coli and Bacillus subtilis [40–42]. Previously, we found that although overexpression of ERG20 did not affect growth, it decreased geraniol production. Therefore, neither up-regulation or down-regulation of ERG20 was able to increase geraniol production. A suitable ERG20 expression strategy that can weaken the downstream FPP flux but not impair cell growth to further improve geraniol production in engineered yeast is needed. To achieve this goal, dynamic control of ERG20 is proposed, which is likely to not only balance metabolism between product formation and cell growth, but also prevent the accumulation of toxic metabolites. Recently, several dynamic control systems have been developed and applied in metabolic engineering, i.e., a biosensor (sensor-regulator) system [43], a sequential control system based on the medication GAL regulation system combined with the HXT1 promoter [23] and a novel CRISPR-Cas9 interference system [44]. To avoid early accumulation of toxic intermediates and to better balance the utilization of GPP, PHXT1, a glucose-sensing promoter, was employed to dynamically control ERG20 expression in our study. An HXT1 promoter replacement strain (YZG19) did not contribute to improve geraniol production in batch or fed-batch fermentation using glucose as sole carbon, but its advantage appeared when the glucose concentration was low (feeding glucose/ethanol mixture carbon). The geraniol production improved stepwise when the glucose percentage decreased in the glucose/ethanol mixture (Figs. 4, 5). These results indicate that the dynamic control of ERG20 expression by the HXT1 promoter can balance the flux distribution between cell growth and monoterpene synthesis, thus improving geraniol production with low glucose feeding (Fig. 5). In addition, ethanol feeding possibly improved the geraniol production to some extent due to supplying acetyl-CoA precursors in a more direct metabolic pathway. An ethanol feeding strategy has also been successfully applied in amorphadiene and resveratrol production [34, 35].
Finally, strategies combining the OYE2 deletion and PHXT1-controlled ERG20 expression further improved geraniol production to 984.6 mg/L (Fig. 6). A previous report showed that leucine metabolism may be networked with sterol biosynthesis, and that leucine was disassimilated to form HMG-CoA in Leishmania mexicana [45]. In S. cerevisiae, the leucine biosynthetic pathway may act as a bypass pathway to supplement additional HMG-CoA. Therefore, we further constructed the prototrophic strain YZG21 by complementing the auxotrophic marker LEU2. The LEU2 complementation ultimately improved the geraniol production to 1.69 g/L, meanwhile also improving cell growth and yield. A similar result was observed in a previous study on miltiradiene production [36].
Although a relatively high level of geraniol was obtained in our work, the cytotoxicity of monoterpenes is still considered as a problem that hinders further improvement of monoterpene production. Improving the resistance of microorganisms to monoterpenes still needs to be addressed to further enhance the monoterpene production. In addition, diploidization is another potential strategy for improving biomass and productivity given that diploid strains generally grow faster and tolerate higher stresses compared with haploid strains [36, 46]. More importantly, constructing a plasmid-free geraniol overproducing strain by integration of all pathway components into the genome is necessary for application in industrial processes.
Conclusions
In summary, we engineered geraniol biosynthesis through the reduction of endogenous geraniol conversion and dynamic control of ERG20 expression. In addition, we further identified the importance of LEU2 complementation to geraniol synthesis. Ultimately, 1.69 g/L of geraniol was achieved when these approaches were combined in fed-batch fermentation with pure ethanol feeding. The present work provides a good platform for the production of geraniol and its derived chemicals.
Methods
Medium
The E. coli strain Tans5α was used for gene cloning and grown in Luria–Bertani medium (5 g/L yeast extract, 10 g/L tryptone, and 10 g/L NaCl) supplemented with 100 mg/L ampicillin at 37 °C. The engineered yeast strain YZG13-GE1 constructed in our previous work was used as the parent strain for further engineering [21]. Yeast cells were cultivated in yeast extract-peptone-dextrose (YPD) medium (20 g/L glucose, 20 g/L tryptone, and 10 g/L yeast extract), SD-URA or SD-URA-HIS medium (20 g/L glucose, 1.7 g/L yeast nitrogen base, and 5 g/L (NH4)2SO4; synthetic complete drop-out medium without uracil and/or histidine). Geneticin (G418) at 400 mg/L was added to the culture medium for gene deletion and promoter replacement.
DNA manipulation and strain construction
All yeast strains constructed in this study are listed in Table 2. The reference strain is YZG13-GE1, which is derived from CEN.PK102-5B and harbored pZGV6-GE1 (2µ ori, URA3, PTEF1-tVoGES-(GGGS)-ERG20 WW) and pZMVA4 (2µ ori, HIS3, PTEF1-tHMG1, PPGK1-IDI1, PTEF1-UPC2.1) [21]. The primers for all DNA fragment amplifications are listed in Additional file 1: Table S1. To replace the ERG20 promoter with different promoters, integration cassettes were constructed via fusion PCR using loxp-kanMX-loxp as the selection marker. The BTS1 promoter (from −1 to −333), CTR3 promoter (from +30 to −1116), and HXT1 promoter (from −1 to 1190) replaced the ERG20 promoter in the chromosome [23, 47, 48]. Similarly, the deletions of genes ATF1 and OYE2 were also performed through homologous recombination using loxp-kanMX-loxp as the selection marker. The LEU2 cassette was integrated into the YPRCτ3 site of the chromosome. The DNA fragments were transformed into S. cerevisiae CEN.PK102-5B by the standard lithium acetate method [49], and the transformants were selected on YPD agar plates supplemented with 400 mg/L geneticin (G418). The plasmid pSH47 (purchased from Euroscarf) was transformed into S. cerevisiae to remove the selection marker. The engineered strains were then transformed with plasmids pZGV6-GE1 and pZMVA4, resulting in a series of geraniol-producing strains (Table 2).
Table 2.
Strains used in this study
| Strain name | Parent strain | Plasmids/genotype | Source |
|---|---|---|---|
| CEN.PK102-5B | MATa ura3-52 his3Δ1 leu2-3,112 | Dr. P. Kötter, Frankfurt, Germany | |
| 5B-Δoye2 | CEN.PK102-5B | Δoye2::loxP | This study |
| 5B-Δatf1 | CEN.PK102-5B | Δatf1::loxP | This study |
| 5B-Δoye2Δatf1 | CEN.PK102-5B | Δoye2::loxP, Δatf1::loxP | This study |
| 5B-PBTS1 | CEN.PK102-5B | ΔP ERG20 ::loxP-P BTS1 | This study |
| 5B-PCTR3 | CEN.PK102-5B | ΔP ERG20 ::loxP-P CTR3 | This study |
| 5B-PHXT1 | CEN.PK102-5B | ΔP ERG20 ::loxP-P HXT1 | This study |
| 5B-Δoye2-PHXT1 | 5B-PHXT1 | ΔP ERG20 ::loxP-P HXT1, Δoye2::loxP | This study |
| 5B-LEU2-Δoye2-PHXT1 | 5B-Δoye2-PHXT1 | ΔP ERG20 ::loxP-P HXT1, ΔYPRCtau3::P LEU2-LEU2-T LEU2-loxP | This study |
| YZG13-GE1 | CEN.PK102-5B | pZGV6-GE1, pZMVA4 | [21] |
| YZG14 | 5B-Δoye2 | pZGV6-GE1, pZMVA4 | This study |
| YZG15 | 5B-Δatf1 | pZGV6-GE1, pZMVA4 | This study |
| YZG16 | 5B-Δoye2Δatf1 | pZGV6-GE1, pZMVA4 | This study |
| YZG17 | 5B-PBTS1 | pZGV6-GE1, pZMVA4 | This study |
| YZG18 | 5B-PCTR3 | pZGV6-GE1, pZMVA4 | This study |
| YZG19 | 5B-PHXT1 | pZGV6-GE1, pZMVA4 | This study |
| YZG20 | 5B-Δoye2-PHXT1 | pZGV6-GE1, pZMVA4 | This study |
| YZG21 | 5B-LEU2-Δoye2-PHXT1 | pZGV6-GE1, pZMVA4 | This study |
Batch and fed-batch fermentation for geraniol production
To determine the performance of the recombinant yeast strains, batch fermentation and fed-batch fermentation were performed in a 1-L Infors-HT fermenter (Infors AG, Bottmingen, Switzerland). To prepare seed cultures, the strains were grown for 24 h in 5 mL of SD-URA-HIS medium, and then inoculated into fresh SD-URA-HIS medium at an OD600 of 0.2 and cultivated for 12 h. For batch fermentation, a seed culture was inoculated into a 1-L Infors-HT fermenter (Infors AG, Bottmingen, Switzerland) containing 0.6 L of SD-URA-HIS medium at an initial OD600 of 0.2. Batch cultures were conducted at 30 °C, with the agitation rate at 600 rpm and an airflow rate of 1 vvm. The pH was maintained at 5.0 by automatic addition of 2.5 M NaOH. Dissolved oxygen was maintained above 30% saturation throughout cultivation by setting the stirring speed rate. For fed-batch cultivation, strains were first grown in a 400-mL batch culture at 30 °C with shaking at 600 rpm, an airflow rate of 1 vvm and pH 5.0. After the glucose and ethanol produced in the batch phase were depleted, feeding solution containing 400 g/L glucose, 100 g/L (NH4)2SO4, 34 g/L yeast nitrogen base, 1.3 g/L CSM-Ura-His, and 2 g/L leucine was fed to the fermenter at a controlled specific feed rate of 0.1/h. Feeding of a glucose/ethanol mixture or pure ethanol was also used for strains in which the ERG20 promoter was replaced with the HXT1 promoter to control ERG20 expression. In both batch and fed-batch cultures, 20% dodecane was added to the medium after cultivating for 12 h for geraniol extraction. Independent duplicate cultures were conducted for each strain.
RNA extraction and quantitative real-time PCR
Total RNA was prepared from 40 mL exponentially growing cell cultures using the UNIQ-10 Trizol RNA extraction kit (Sangon Biological Engineering, Shanghai, China). The samples were digested using DNase I treatment (TakaRa, Dalian, China) to avoid DNA contamination. The treated total RNA was used for cDNA synthesis using the PrimeScript RT-PCR Kit (TakaRa, Dalian, China). qPCR was performed using the SYBR Green Master Mix Kit (Roche Molecular Biochemicals, Germany). The ACT1 gene was chosen as the internal control gene. The relative transcription levels of genes were analyzed using the 2−ΔΔCT method.
Analysis of metabolites by HPLC
At each 12-h interval, the cultures were sampled and centrifuged at 12,000 rpm for 5 min. The filtered samples were analyzed using an HPLC equipped with an Aminex HPX-87H ion-exchange column (Bio-Rad, Hercules, CA, USA) at 45 °C with a mobile phase of 5 mM H2SO4 at a flow rate of 0.6 mL/min. The peaks of metabolites including glucose, ethanol, acetic acid and glycerol were detected by refractive index (RI) and ultraviolet (UV) detectors.
Characterization of geraniol and citronellol by GC–MS
To quantify titers of geraniol and citronellol in different cultures, 1 mL of the upper layer (dodecane/culture mixture) was sampled and concentrated at 13,000 rpm for 5 min to separate the dodecane phase, and then the dodecane layer was transferred into a GC vial and stored at −20 °C for analysis. The residual geraniol was extracted again by adding 10% (v/v) dodecane into the medium. Geraniol and citronellol were identified using a GC–MS system (Shimadzu Co., Kyoto, Japan) equipped with an HP-5 ms capillary column (30 m × 0.25 mm × 0.25 µm), an AOC-20i auto-injector, and a QP-2010 mass detector, and the operational conditions were as follows. One microliter of each dodecane sample was injected into the system with a split ratio of 10 and the carrier gas helium was set at a constant flow rate of 0.78 mL/min. The oven temperature was first maintained at 60 °C for 2 min, and then gradually increased to 150 °C at a rate of 10 °C/min, held for 10 min, and finally increased to 230 °C at a rate of 20 °C/min and held for 5 min. The mass spectrometer was set to SIM acquisition mode, scanning m/z ions within the range 40–500 for identification of geraniol and citronellol. The total run time was 30 min. Standard compounds of geraniol and citronellol (Sigma-Aldrich) were dissolved in dodecane and used to plot standard curves for quantification.
Biomass determination
Optical density at 600 nm (OD600) was measured using a spectrophotometer (Eppendorf AG, 22331 Hamburg, Germany). Dry cell weight (DCW) was obtained from OD600 measurement after calibration as indicated before () [50].
Authors’ contributions
JZ and JH designed experiments. JZ, CL and YZ carried out the experiments. JZ, XB, JH and YS analyzed data. JZ and JH wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its Additional file 1.
Funding
This work was supported by the National Natural Science Foundation of China (31470163), the National Key Technology R&D Program of China (2014BAD02B07), the Key R&D Program of Shandong Province (2015GSF121015) and State Key Laboratory of Microbial Metabolism, Shanghai Jiao Tong University (MMLKF16-06).
Additional file
Additional file 1: Table S1. Primers used in this study. Figure S1. The conversion of geraniol to citronellol in control strain and deletion strains. The data shown are representative of duplicate experiments, and the error bars represent the standard deviation. Figure S2. The effect of OYE2 or/and ATF1 deletion on cell growth in batch fermentation. All strains harbored pZGV6-GE1and pZMVA4 plasmids for geraniol production. The data shown are representative of duplicate experiments, and the error bars represent the standard deviation. Figure S3. Transcription level of ERG20 controlled by different promoters in SD-URA-HIS medium with different concentrations glucose. The cells were collected when OD600 reached 0.6 to extract mRNA for ERG20 mRNA and transcription determination. The data shown are representative of triplicate experiments, and the error bars represent the standard deviation. Figure S4. The effect of ERG20 expression controlled by different promoters on cell growth in batch fermentation. All strains harbored pZGV6-GE1and pZMVA4 plasmids for geraniol production. The data shown are representative of duplicate experiments, and the error bars represent the standard deviation.
Contributor Information
Jianzhi Zhao, Email: zhjzh_2006@126.com.
Chen Li, Email: lichen1015670145@126.com.
Yan Zhang, Email: 1152383371@qq.com.
Yu Shen, Email: shenyu@sdu.edu.cn.
Jin Hou, Phone: +86 531 8836 5826, Email: houjin@sdu.edu.cn.
Xiaoming Bao, Phone: +86 531 8836 5826, Email: bxm@sdu.edu.cn.
References
- 1.Kuzuyama T. Mevalonate and nonmevalonate pathways for the biosynthesis of isoprene units. Biosci Biotechnol Biochem. 2002;66:1619–1627. doi: 10.1271/bbb.66.1619. [DOI] [PubMed] [Google Scholar]
- 2.Brennan TC, Turner CD, Kromer JO, Nielsen LK. Alleviating monoterpene toxicity using a two-phase extractive fermentation for the bioproduction of jet fuel mixtures in Saccharomyces cerevisiae. Biotechnol Bioeng. 2012;109:2513–2522. doi: 10.1002/bit.24536. [DOI] [PubMed] [Google Scholar]
- 3.Ajikumar PK, Tyo K, Carlsen S, Mucha O, Phon TH, Stephanopoulos G. Terpenoids: opportunities for biosynthesis of natural product drugs using engineered microorganisms. Mol Pharm. 2008;5:167–190. doi: 10.1021/mp700151b. [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Viljoen AM. Geraniol—a review of a commercially important fragrance material. S Afr J Bot. 2010;76:643–651. doi: 10.1016/j.sajb.2010.05.008. [DOI] [Google Scholar]
- 5.Peralta-Yahya PP, Keasling JD. Advanced biofuel production in microbes. Biotechnol J. 2010;5:147–162. doi: 10.1002/biot.200900220. [DOI] [PubMed] [Google Scholar]
- 6.Brown S, Clastre M, Courdavault V, O’Connor SE. De novo production of the plant-derived alkaloid strictosidine in yeast. Proc Natl Acad Sci USA. 2015;112:3205–3210. doi: 10.1073/pnas.1423555112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou J, Wang C, Yoon SH, Jang HJ, Choi ES, Kim SW. Engineering Escherichia coli for selective geraniol production with minimized endogenous dehydrogenation. J Biotechnol. 2014;169:42–50. doi: 10.1016/j.jbiotec.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Fischer MJ, Meyer S, Claudel P, Bergdoll M, Karst F. Metabolic engineering of monoterpene synthesis in yeast. Biotechnol Bioeng. 2011;108:1883–1892. doi: 10.1002/bit.23129. [DOI] [PubMed] [Google Scholar]
- 9.Liu W, Xu X, Zhang R, Cheng T, Cao Y, Li X, Guo J, Liu H, Xian M. Engineering Escherichia coli for high-yield geraniol production with biotransformation of geranyl acetate to geraniol under fed-batch culture. Biotechnol Biofuels. 2016;9:58. doi: 10.1186/s13068-016-0466-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J, Zhang W, Du G, Chen J, Zhou J. Overproduction of geraniol by enhanced precursor supply in Saccharomyces cerevisiae. J Biotechnol. 2013;168:446–451. doi: 10.1016/j.jbiotec.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Javelot C, Girard P, Colonna-Ceccaldi B, Vladescu B. Introduction of terpene-producing ability in a wine strain of Saccharomyces cerevisiae. J Biotechnol. 1991;21:239–251. doi: 10.1016/0168-1656(91)90045-W. [DOI] [Google Scholar]
- 12.Paddon CJ, Westfall PJ, Pitera DJ, Benjamin K, Fisher K, McPhee D, Leavell MD, Tai A, Main A, Eng D, et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature. 2013;496:528–532. doi: 10.1038/nature12051. [DOI] [PubMed] [Google Scholar]
- 13.Tippmann S, Scalcinati G, Siewers V, Nielsen J. Production of farnesene and santalene by Saccharomyces cerevisiae using fed-batch cultivations with RQ-controlled feed. Biotechnol Bioeng. 2016;113:72–81. doi: 10.1002/bit.25683. [DOI] [PubMed] [Google Scholar]
- 14.Zhuang X, Chappell J. Building terpene production platforms in yeast. Biotechnol Bioeng. 2015;112:1854–1864. doi: 10.1002/bit.25588. [DOI] [PubMed] [Google Scholar]
- 15.Ignea C, Cvetkovic I, Loupassaki S, Kefalas P, Johnson CB, Kampranis SC, Makris AM. Improving yeast strains using recyclable integration cassettes, for the production of plant terpenoids. Microb Cell Fact. 2011;10:4. doi: 10.1186/1475-2859-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ignea C, Pontini M, Maffei ME, Makris AM, Kampranis SC. Engineering monoterpene production in yeast using a synthetic dominant negative geranyl diphosphate synthase. ACS Synth Biol. 2014;3:298–306. doi: 10.1021/sb400115e. [DOI] [PubMed] [Google Scholar]
- 17.Jongedijk E, Cankar K, Ranzijn J, van der Krol S, Bouwmeester H, Beekwilder J. Capturing of the monoterpene olefin limonene produced in Saccharomyces cerevisiae. Yeast. 2015;32:159–171. doi: 10.1002/yea.3038. [DOI] [PubMed] [Google Scholar]
- 18.Khor GK, Uzir MH. Saccharomyces cerevisiae: a potential stereospecific reduction tool for biotransformation of mono- and sesquiterpenoids. Yeast. 2011;28:93–107. doi: 10.1002/yea.1827. [DOI] [PubMed] [Google Scholar]
- 19.Brennan TC, Williams TC, Schulz BL, Palfreyman RW, Kromer JO, Nielsen LK. Evolutionary engineering improves tolerance for replacement jet fuels in Saccharomyces cerevisiae. Appl Environ Microbiol. 2015;81:3316–3325. doi: 10.1128/AEM.04144-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oswald M, Fischer M, Dirninger N, Karst F. Monoterpenoid biosynthesis in Saccharomyces cerevisiae. FEMS Yeast Res. 2007;7:413–421. doi: 10.1111/j.1567-1364.2006.00172.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhao J, Bao X, Li C, Shen Y, Hou J. Improving monoterpene geraniol production through geranyl diphosphate synthesis regulation in Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2016;100:4561–4571. doi: 10.1007/s00253-016-7375-1. [DOI] [PubMed] [Google Scholar]
- 22.Dai Z, Liu Y, Zhang X, Shi M, Wang B, Wang D, Huang L, Zhang X. Metabolic engineering of Saccharomyces cerevisiae for production of ginsenosides. Metab Eng. 2013;20:146–156. doi: 10.1016/j.ymben.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Xie W, Ye L, Lv X, Xu H, Yu H. Sequential control of biosynthetic pathways for balanced utilization of metabolic intermediates in Saccharomyces cerevisiae. Metab Eng. 2015;28:8–18. doi: 10.1016/j.ymben.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Brennan TC, Kromer JO, Nielsen LK. Physiological and transcriptional responses of Saccharomyces cerevisiae to d-limonene show changes to the cell wall but not to the plasma membrane. Appl Environ Microbiol. 2013;79:3590–3600. doi: 10.1128/AEM.00463-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marmulla R, Harder J. Microbial monoterpene transformations—a review. Front Microbiol. 2014;5:346. doi: 10.3389/fmicb.2014.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gamero A, Manzanares P, Querol A, Belloch C. Monoterpene alcohols release and bioconversion by Saccharomyces species and hybrids. Int J Food Microbiol. 2011;145:92–97. doi: 10.1016/j.ijfoodmicro.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 27.Steyer D, Erny C, Claudel P, Riveill G, Karst F, Legras JL. Genetic analysis of geraniol metabolism during fermentation. Food Microbiol. 2013;33:228–234. doi: 10.1016/j.fm.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 28.Liu D, Evans T, Zhang F. Applications and advances of metabolite biosensors for metabolic engineering. Metab Eng. 2015;31:35–43. doi: 10.1016/j.ymben.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Yuan J, Ching CB. Dynamic control of ERG9 expression for improved amorpha-4,11-diene production in Saccharomyces cerevisiae. Microb Cell Fact. 2015;14:38. doi: 10.1186/s12934-015-0220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams TC, Espinosa MI, Nielsen LK, Vickers CE. Dynamic regulation of gene expression using sucrose responsive promoters and RNA interference in Saccharomyces cerevisiae. Microb Cell Fact. 2015;14:43. doi: 10.1186/s12934-015-0223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King A, Richard Dickinson J. Biotransformation of monoterpene alcohols by Saccharomyces cerevisiae, Torulaspora delbrueckii and Kluyveromyces lactis. Yeast. 2000;16:499–506. doi: 10.1002/(SICI)1097-0061(200004)16:6<499::AID-YEA548>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 32.Pardo E, Rico J, Gil JV, Orejas M. De novo production of six key grape aroma monoterpenes by a geraniol synthase-engineered S. cerevisiae wine strain. Microb Cell Fact. 2015;14:136. doi: 10.1186/s12934-015-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szczebara FM, Chandelier C, Villeret C, Masurel A, Bourot S, Duport C, Blanchard S, Groisillier A, Testet E, Costaglioli P, et al. Total biosynthesis of hydrocortisone from a simple carbon source in yeast. Nat Biotechnol. 2003;21:143–149. doi: 10.1038/nbt775. [DOI] [PubMed] [Google Scholar]
- 34.Westfall PJ, Pitera DJ, Lenihan JR, Eng D, Woolard FX, Regentin R, Horning T, Tsuruta H, Melis DJ, Owens A, et al. Production of amorphadiene in yeast, and its conversion to dihydroartemisinic acid, precursor to the antimalarial agent artemisinin. Proc Natl Acad Sci USA. 2012;109:E111–E118. doi: 10.1073/pnas.1110740109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li M, Kildegaard KR, Chen Y, Rodriguez A, Borodina I, Nielsen J. De novo production of resveratrol from glucose or ethanol by engineered Saccharomyces cerevisiae. Metab Eng. 2015;32:1–11. doi: 10.1016/j.ymben.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Zhou YJ, Gao W, Rong Q, Jin G, Chu H, Liu W, Yang W, Zhu Z, Li G, Zhu G, et al. Modular pathway engineering of diterpenoid synthases and the mevalonic acid pathway for miltiradiene production. J Am Chem Soc. 2012;134:3234–3241. doi: 10.1021/ja2114486. [DOI] [PubMed] [Google Scholar]
- 37.Odat O, Matta S, Khalil H, Kampranis SC, Pfau R, Tsichlis PN, Makris AM. Old yellow enzymes, highly homologous FMN oxidoreductases with modulating roles in oxidative stress and programmed cell death in yeast. J Biol Chem. 2007;282:36010–36023. doi: 10.1074/jbc.M704058200. [DOI] [PubMed] [Google Scholar]
- 38.Trotter EW, Collinson EJ, Dawes IW, Grant CM. Old yellow enzymes protect against acrolein toxicity in the yeast Saccharomyces cerevisiae. Appl Environ Microbiol. 2006;72:4885–4892. doi: 10.1128/AEM.00526-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mason AB, Dufour JP. Alcohol acetyltransferases and the significance of ester synthesis in yeast. Yeast. 2000;16:1287–1298. doi: 10.1002/1097-0061(200010)16:14<1287::AID-YEA613>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 40.Martin VJ, Pitera DJ, Withers ST, Newman JD, Keasling JD. Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nat Biotechnol. 2003;21:796–802. doi: 10.1038/nbt833. [DOI] [PubMed] [Google Scholar]
- 41.Sivy TL, Fall R, Rosenstiel TN. Evidence of isoprenoid precursor toxicity in Bacillus subtilis. Biosci Biotechnol Biochem. 2011;75:2376–2383. doi: 10.1271/bbb.110572. [DOI] [PubMed] [Google Scholar]
- 42.Sarria S, Wong B, Garcia Martin H, Keasling JD, Peralta-Yahya P. Microbial synthesis of pinene. ACS Synth Biol. 2014;3:466–475. doi: 10.1021/sb4001382. [DOI] [PubMed] [Google Scholar]
- 43.Xu P, Li L, Zhang F, Stephanopoulos G, Koffas M. Improving fatty acids production by engineering dynamic pathway regulation and metabolic control. Proc Natl Acad Sci USA. 2014;111:11299–11304. doi: 10.1073/pnas.1406401111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim SK, Han GH, Seong W, Kim H, Kim SW, Lee DH, Lee SG. CRISPR interference-guided balancing of a biosynthetic mevalonate pathway increases terpenoid production. Metab Eng. 2016;38:228–240. doi: 10.1016/j.ymben.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 45.Ginger ML, Chance ML, Sadler IH, Goad LJ. The biosynthetic incorporation of the intact leucine skeleton into sterol by the trypanosomatid Leishmania mexicana. J Biol Chem. 2001;276:11674–11682. doi: 10.1074/jbc.M006850200. [DOI] [PubMed] [Google Scholar]
- 46.Lv X, Wang F, Zhou P, Ye L, Xie W, Xu H, Yu H. Dual regulation of cytoplasmic and mitochondrial acetyl-CoA utilization for improved isoprene production in Saccharomyces cerevisiae. Nat Commun. 2016;7:12851. doi: 10.1038/ncomms12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knight SA, Labbe S, Kwon LF, Kosman DJ, Thiele DJ. A widespread transposable element masks expression of a yeast copper transport gene. Genes Dev. 1996;10:1917–1929. doi: 10.1101/gad.10.15.1917. [DOI] [PubMed] [Google Scholar]
- 48.Chen Y, Partow S, Scalcinati G, Siewers V, Nielsen J. Enhancing the copy number of episomal plasmids in Saccharomyces cerevisiae for improved protein production. FEMS Yeast Res. 2012;12:598–607. doi: 10.1111/j.1567-1364.2012.00809.x. [DOI] [PubMed] [Google Scholar]
- 49.Gietz RD, Woods RA. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 2002;350:87–96. doi: 10.1016/S0076-6879(02)50957-5. [DOI] [PubMed] [Google Scholar]
- 50.Hou J, Suo F, Wang C, Li X, Shen Y, Bao X. Fine-tuning of NADH oxidase decreases byproduct accumulation in respiration deficient xylose metabolic Saccharomyces cerevisiae. BMC Biotechnol. 2014;14:13. doi: 10.1186/1472-6750-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its Additional file 1.


