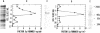Abstract
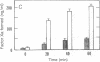
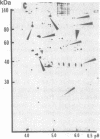
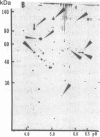
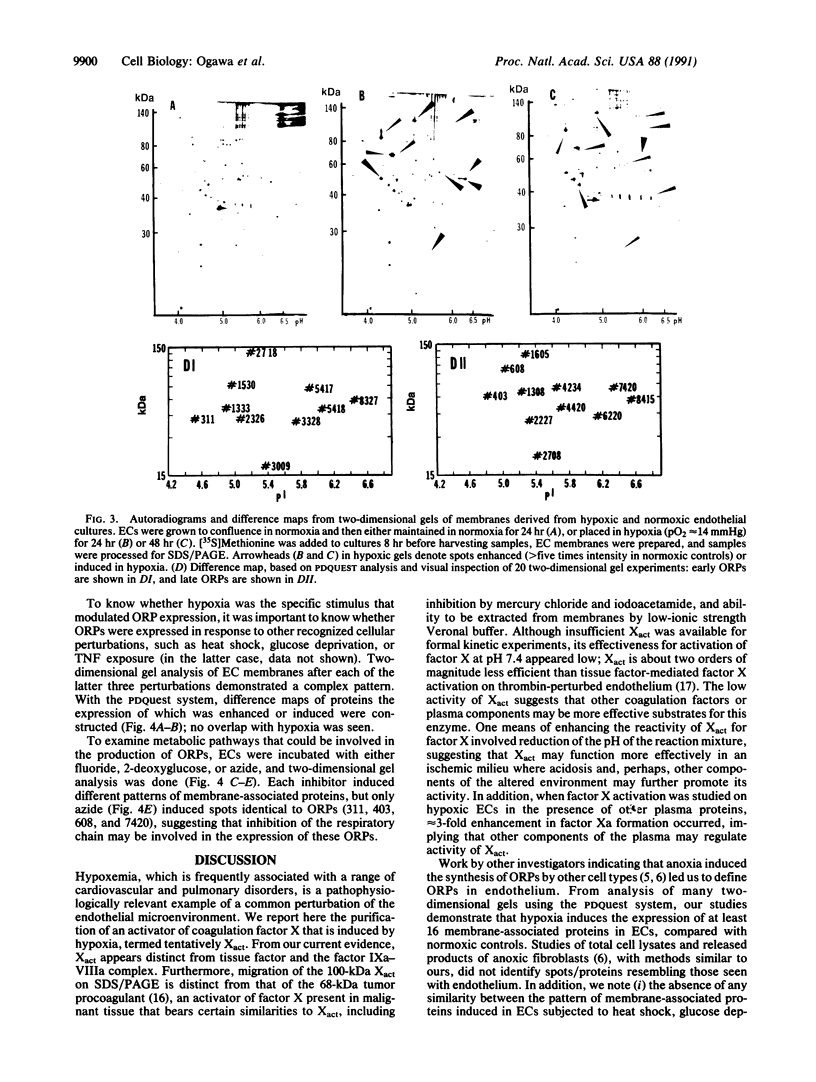
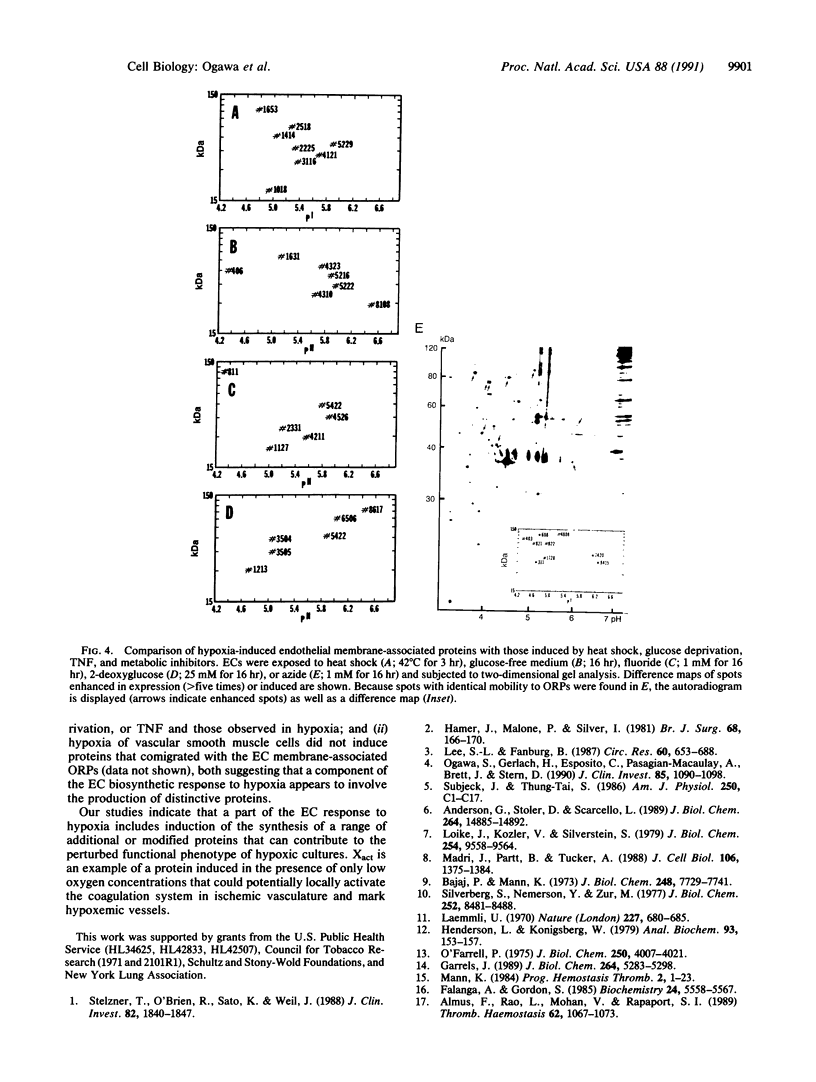
Hypoxemia is associated with a prothrombotic tendency. In this study we report the purification and partial characterization of an activator of a central coagulation component, factor X, induced in endothelium by exposure to hypoxia (hypoxia-induced factor X activator or Xact). Expression of Xact occurred in a reversible manner when endothelial cell cultures were exposed to hypoxia or sodium azide but not in response to a variety of other alterations in the cellular milieu, such as heat shock or glucose deprivation. The activity of Xact, which was not detected in normoxic endothelial cells, was maximal under acidic conditions, pH 6.0-6.8, which often coexist with hypoxia in an ischemic milieu. By sequential isoelectric focusing and preparative SDS/PAGE of endothelial membrane-rich fractions, Xact was purified approximately 19,000-fold and found to be a single-chain, approximately 100-kDa polypeptide with pI approximately 5.0. Activation of factor X by purified Xact was not affected by blocking antibodies to other coagulation proteins or by phenylmethylsulfonyl fluoride or leupeptin but was prevented by mercury chloride or iodoacetamide. In addition to the induction of Xact, two-dimensional gel analysis of membrane fractions from metabolically labeled hypoxic endothelial cultures revealed two groups of approximately 10 additional spots: (i) a group for which expression was maximal after 24 hr and (ii) a group for which expression continued to increase up to 48 hr. The pattern of hypoxia-mediated modulation of protein expression was distinct from that seen with other cellular stimuli but could be duplicated, in part, by sodium azide. These results indicate that hypoxia elicits a specific biosynthetic response, including the expression of endothelial cell-surface molecules that can alter cellular function and may potentially serve as markers of hypoxemic vessel-wall injury.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almus F. E., Rao L. V., Rapaport S. I. Functional properties of factor VIIa/tissue factor formed with purified tissue factor and with tissue factor expressed on cultured endothelial cells. Thromb Haemost. 1989 Dec 29;62(4):1067–1073. [PubMed] [Google Scholar]
- Anderson G. R., Stoler D. L., Scarcello L. A. Normal fibroblasts responding to anoxia exhibit features of the malignant phenotype. J Biol Chem. 1989 Sep 5;264(25):14885–14892. [PubMed] [Google Scholar]
- Bajaj S. P., Mann K. G. Simultaneous purification of bovine prothrombin and factor X. Activation of prothrombin by trypsin-activated factor X. J Biol Chem. 1973 Nov 25;248(22):7729–7741. [PubMed] [Google Scholar]
- Falanga A., Gordon S. G. Isolation and characterization of cancer procoagulant: a cysteine proteinase from malignant tissue. Biochemistry. 1985 Sep 24;24(20):5558–5567. doi: 10.1021/bi00341a041. [DOI] [PubMed] [Google Scholar]
- Garrels J. I., Franza B. R., Jr The REF52 protein database. Methods of database construction and analysis using the QUEST system and characterizations of protein patterns from proliferating and quiescent REF52 cells. J Biol Chem. 1989 Mar 25;264(9):5283–5298. [PubMed] [Google Scholar]
- Hamer J. D., Malone P. C., Silver I. A. The PO2 in venous valve pockets: its possible bearing on thrombogenesis. Br J Surg. 1981 Mar;68(3):166–170. doi: 10.1002/bjs.1800680308. [DOI] [PubMed] [Google Scholar]
- Henderson L. E., Oroszlan S., Konigsberg W. A micromethod for complete removal of dodecyl sulfate from proteins by ion-pair extraction. Anal Biochem. 1979 Feb;93(1):153–157. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee S. L., Fanburg B. L. Glycolytic activity and enhancement of serotonin uptake by endothelial cells exposed to hypoxia/anoxia. Circ Res. 1987 May;60(5):653–658. doi: 10.1161/01.res.60.5.653. [DOI] [PubMed] [Google Scholar]
- Loike J. D., Kozler V. F., Silverstein S. C. Increased ATP and creatine phosphate turnover in phagocytosing mouse peritoneal macrophages. J Biol Chem. 1979 Oct 10;254(19):9558–9564. [PubMed] [Google Scholar]
- Madri J. A., Pratt B. M., Tucker A. M. Phenotypic modulation of endothelial cells by transforming growth factor-beta depends upon the composition and organization of the extracellular matrix. J Cell Biol. 1988 Apr;106(4):1375–1384. doi: 10.1083/jcb.106.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K. G. Membrane-bound enzyme complexes in blood coagulation. Prog Hemost Thromb. 1984;7:1–23. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
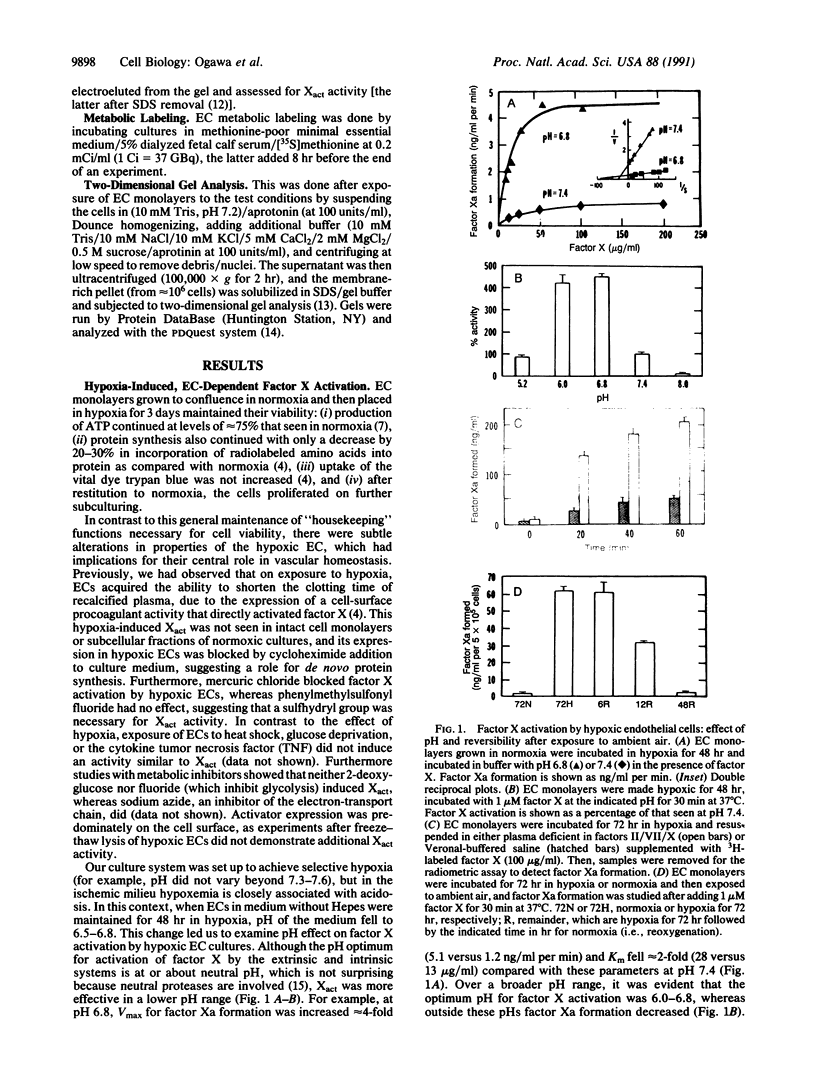
- Ogawa S., Gerlach H., Esposito C., Pasagian-Macaulay A., Brett J., Stern D. Hypoxia modulates the barrier and coagulant function of cultured bovine endothelium. Increased monolayer permeability and induction of procoagulant properties. J Clin Invest. 1990 Apr;85(4):1090–1098. doi: 10.1172/JCI114540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg S. A., Nemerson Y., Zur M. Kinetics of the activation of bovine coagulation factor X by components of the extrinsic pathway. Kinetic behavior of two-chain factor VII in the presence and absence of tissue factor. J Biol Chem. 1977 Dec 10;252(23):8481–8488. [PubMed] [Google Scholar]
- Stelzner T. J., O'Brien R. F., Sato K., Weil J. V. Hypoxia-induced increases in pulmonary transvascular protein escape in rats. Modulation by glucocorticoids. J Clin Invest. 1988 Dec;82(6):1840–1847. doi: 10.1172/JCI113800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subjeck J. R., Shyy T. T. Stress protein systems of mammalian cells. Am J Physiol. 1986 Jan;250(1 Pt 1):C1–17. doi: 10.1152/ajpcell.1986.250.1.C1. [DOI] [PubMed] [Google Scholar]