Abstract
Purpose
Persistent oral human papillomavirus (HPV) infection increases risk for oropharyngeal carcinoma, and people living with HIV have higher rates of oral HPV infection and related cancers. Some prescription medications have immunomodulatory effects, but the impact of medication use on oral HPV natural history is unknown.
Methods
Scope® oral rinse-and-gargle samples were collected semi-annually from 1,666 participants and tested for 37 types of oral HPV DNA using PCR; 594 HPV-infected participants with 1,358 type-specific oral HPV infections were identified. Data were collected on recent (past 6 months) use of medications. The relationship between medication use and oral HPV clearance was evaluated using Wei-Lin-Weissfeld regression, adjusting for biologic sex, prevalent vs. incident infection, age, HIV status and CD4+ T-cell count.
Results
Out of 11 medications examined, oral HPV clearance was significantly reduced in participants reporting recent use of antipsychotics (HR=0.75, 95% CI=0.57–0.99), anxiolytics/sedatives (HR=0.78, 95% CI=0.63–0.96) and antidepressants (HR=0.82, 95% CI=0.67–0.999). Among antipsychotics users, effect modification by HIV status was observed, with reduced clearance in HIV-infected (HR=0.67, 95%: CI 0.49–0.91), but not HIV-uninfected participants (p-interaction=0.009). After adjusted analysis, antipsychotic use remained significantly associated with reduced oral HPV clearance overall (aHR=0.75, 95% CI=0.57–0.99), and when restricted to only HIV-infected participants (aHR=0.66, 95% CI=0.48–0.90). After adjustment, anxiolytic/sedative use and antidepressant use were no longer significantly associated with reduced oral HPV clearance.
Conclusions
Some medications were associated with decreased oral HPV clearance, most notably antipsychotic medications. These medications are prescribed for conditions that may have immunomodulating effects, so characteristics of underlying illness may have partially contributed to reduced oral HPV clearance.
Keywords: oral HPV, prescription medication, clearance, antipsychotic, HIV, immunomodulatory
Background and rationale
Persistent oncogenic oral human papillomavirus (HPV) infection is the presumed precursor for HPV-related oropharyngeal squamous cell carcinoma (OPSCC), but risk factors for oral HPV persistence are poorly understood [1]. Several commonly prescribed medication types have immunomodulatory effects, either as part of their primary therapeutic activity or as a side effect, including: antidepressants [2], antipsychotics [3], sedatives/anxiolytics [4], anti-asthmatics [5], antihypertensives [6], cholesterol-lowering medications[7], hormonal treatments [8], erectile dysfunction drugs [9], and diabetes medications [10]. It is therefore possible that these medications may impact oral HPV natural history, although this question has not been previously investigated.
People living with HIV (PLWH) have 2–3 times higher oral HPV prevalence than HIV-uninfected individuals, and are commonly taking multiple medications, in addition to antiretroviral therapy (ART) [11]. In a cross-sectional study of PLWH in the Southern U.S., 93% were receiving at least one non-ART medication, including antihypertensives (43%), lipid-lowering agents (34%) and antidepressants/antipsychotics/anxiolytics (34%), and the median number of medications per patient was 8 (IQR: 6–11) [12]. Indeed, as people are living longer with HIV, the diagnosis and treatment of age-related chronic conditions is increasing [13]. HIV-related immunosuppression, such as lower CD4+ T cell counts, may facilitate oral HPV persistence by attenuating immune responses to HPV [14]. However, it is unclear whether immunomodulatory medications may also play a role. Given the high prevalence of polypharmacy and oral HPV infection among PLWH, we conducted a study to investigate whether medication use may impact oral HPV natural history among individuals with and without HIV.
Methods
Study design and study population
From 2010 to 2014, 1,666 participants were enrolled in the “Persistence of Oral Papillomavirus Study” (POPS), a longitudinal study of oral HPV natural history. POPS was nested within two ongoing observational studies of men and women with or at risk for HIV: the Multicenter AIDS Cohort Study (MACS) and the Women’s Interagency HIV Study (WIHS) [15,16]. POPS participants were enrolled at 6 study locations: Baltimore/DC (MACS), Los Angeles (MACS), Pittsburgh (MACS), Brooklyn (WIHS), Bronx (WIHS), and Chicago (sites for MACS and WIHS). Each study center’s Institutional Review Board approved the study. All participants provided written informed consent.
Data collection
At each semi-annual POPS visit, a 30-second Scope® oral rinse-and-gargle sample was collected to obtain oral exfoliated cells which was tested for 37 types of HPV DNA. A blood sample was also obtained, from which HIV status and CD4+ T cell count were determined. At each visit, participants completed a structured interviewer-administered questionnaire about their medication use in the past 6 months [17]. Data were collected on recent medication use, which was then grouped into the following 11 medication types: antiasthmatics (steroidal and non-steroidal), antidepressants, antihypertensives, antipsychotics (used to treat schizophrenia, bipolar disorder and related psychotic disorders), anxiolytics/sedatives (used to treat anxiety, sleeping disorders, epilepsy, alcohol withdrawal, muscle spasms and other conditions), cholesterol-lowering medications, diabetes medications, hormones (including hormone replacement therapy or hormones used to treat thyroid disorders, but excluding birth control hormones), non-steroidal anti-inflammatory drugs (NSAIDs), and erectile dysfunction medications (men only). For analysis, anxiolytics and sedatives were combined into a single medication group. Other types of medications, such as HIV-related medication, were not explored as part of this analysis.
Oral HPV detection and genotyping
Oral rinse samples were tested for 37 types of HPV DNA using a polymerase chain reaction (PCR) assay, followed by reverse line-blot hybridization (Roche Molecular Systems, Pleasanton, CA) [18]. Each sample was evaluated for the presence of high-risk/oncogenic types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66) and low-risk/non-oncogenic types (6, 11, 26, 38, 40, 42, 53, 54, 55, 61, 62, 64, 67, 68, 69, 70, 71, 72, 73, 81, 83, 84, 89, IS39), classified according to criteria from the International Agency for Research on Cancer [19–21]. Prevalent oral HPV infection was defined as any type-specific oral HPV infection detected at study enrollment. Incident infection was defined as any newly detected HPV type-specific infection that was preceded by at least one visit where that HPV type was not detected.
Clearance was defined as two consecutive HPV-negative oral rinses, with time of clearance as the visit of the first negative oral rinse sample. A secondary less conservative definition of clearance was considered, requiring only one HPV-negative oral rinse sample, but as results were similar with both clearance definitions, these results are not shown.
Statistical analyses
The units of analysis were type-specific oral HPV infections. Some participants were infected with multiple HPV types at the same visit. The effect of each medication type on oral HPV clearance was modeled using Wei-Lin-Weissfeld regression models, accounting for within-participant clustering of HPV infections [22]. First, the effect of each medication was considered in separate univariable models. Then, concomitant use of medication pairs were modeled for each of the medications that were commonly used in this study, to assess whether use of both medications together was associated with time to clearance, as compared to using none or only one of the medications. For medications associated with clearance, the number of total medications concurrently used was evaluated to determine whether a greater number of medications used increased strength of association with clearance. The final multivariable model adjusted for biologic sex, prevalent vs. incident HPV infection, age (<45, 45–54, ≥55 years), HIV status, and CD4+ T cell count (≥500, <500 cells/µL). Analyses were performed using Stata 12 [23]. Statistical significance was defined by a two-sided p-value <0.05.
Results
Participant characteristics
Of 1,666 participants, 594 HPV-infected participants with 1,358 type-specific oral HPV infections were identified. Half of participants (54%) were men, 55% were of Black race, and the median age was 50 years (Table 1). The study included 168 (28%) HIV-uninfected individuals and 426 (72%) people living with HIV (PLWH), most (81%) of whom were on current antiretroviral therapy (ART) and had CD4+ T cell count ≥500 cells/µL (50%).
Table 1.
Participant characteristics at time of first oral HPV detection
| Participant characteristica | No. | % |
|---|---|---|
| Sex | ||
| Women (WIHS) | 274 | 46% |
| Men (MACS) | 320 | 54% |
| Infection typeb | ||
| Incident only | 189 | 32% |
| Any Prevalent (with or without incident) | 405 | 68% |
| Age (year) | ||
| <45 | 162 | 27% |
| 45–54 | 258 | 43% |
| ≥55 | 174 | 29% |
| Race/Ethnicity | ||
| White, non-Hispanic | 186 | 31% |
| Black, non-Hispanic | 328 | 55% |
| Hispanic (any race) | 80 | 14% |
| Study sitec | ||
| Baltimore, Maryland | 88 | 15% |
| Bronx, New York | 93 | 16% |
| Brooklyn, New York | 84 | 14% |
| Chicago, Illinois | 202 | 34% |
| Los Angeles, California | 24 | 4% |
| Pittsburgh, Pennsylvania | 103 | 17% |
| HIV status | ||
| Uninfected | 168 | 28% |
| Infected | 426 | 72% |
| AMONG HIV INFECTED | ||
| Current CD4+ T cell count (cells/µL) | ||
| ≥500 | 212 | 50% |
| <500 | 214 | 50% |
| Current HIV RNA viral load (copies/mL) | ||
| <200 | 286 | 68% |
| 200–19,999 | 78 | 19% |
| ≥20,000 | 57 | 14% |
| Current ART use | ||
| No | 83 | 20% |
| Yes | 343 | 81% |
All participant characteristics are from the time of oral HPV detection
Prevalent infection was defined as oral HPV detected at POPS enrollment. Incident infection was defined as newly detected oral HPV preceded by at least one HPV-negative oral rinse. Some participants had prevalent infection, followed by incident infection of another HPV type that was included in the analysis.
Baltimore, Los Angeles and Pittsburgh study sites enrolled men only (MACS). Bronx and Brooklyn study sites enrolled women only (WIHS). Chicago study site enrolled both men and women.
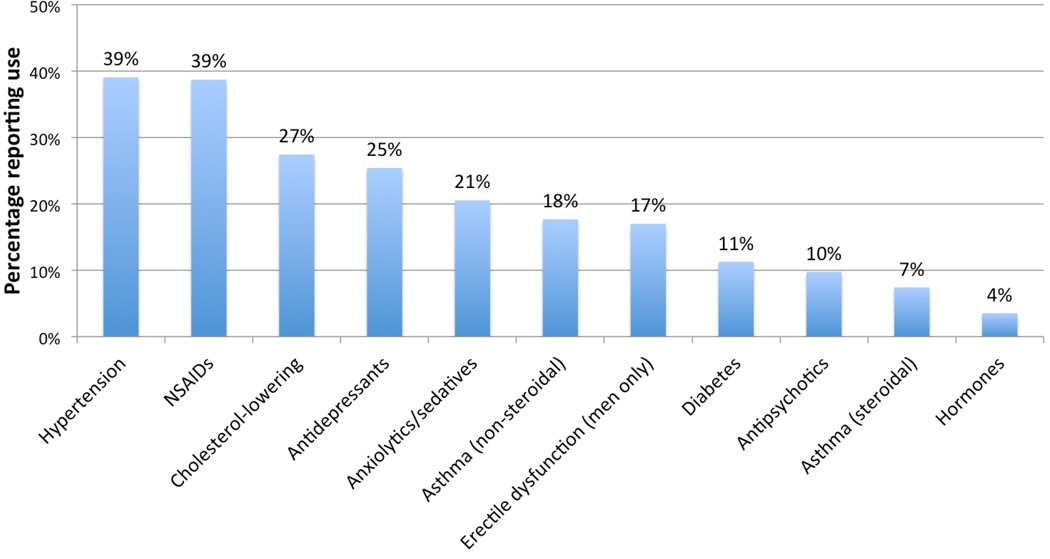
At time of first oral HPV detection, most participants (84%) reported using at least 1 medication, not counting ART, or prescription erectile dysfunction medications (which were used by 17% of men). Among 594 HPV-infected study participants, the most commonly used prescription drugs were anti-hypertensives (39% of participants), NSAIDs (39%), cholesterol-lowering drugs (27%), antidepressants (25%) and anxiolytics/sedatives (21%) (Figure 1).
Figure 1. Prevalence of medication use at time of first oral HPV detectiona.
a Medication use is shown for men (n=320) and women (n=274) together, with the exception of erectile dysfunction medications, which were assessed in men only.
NSAID = non-steroidal anti-inflammatory drugs
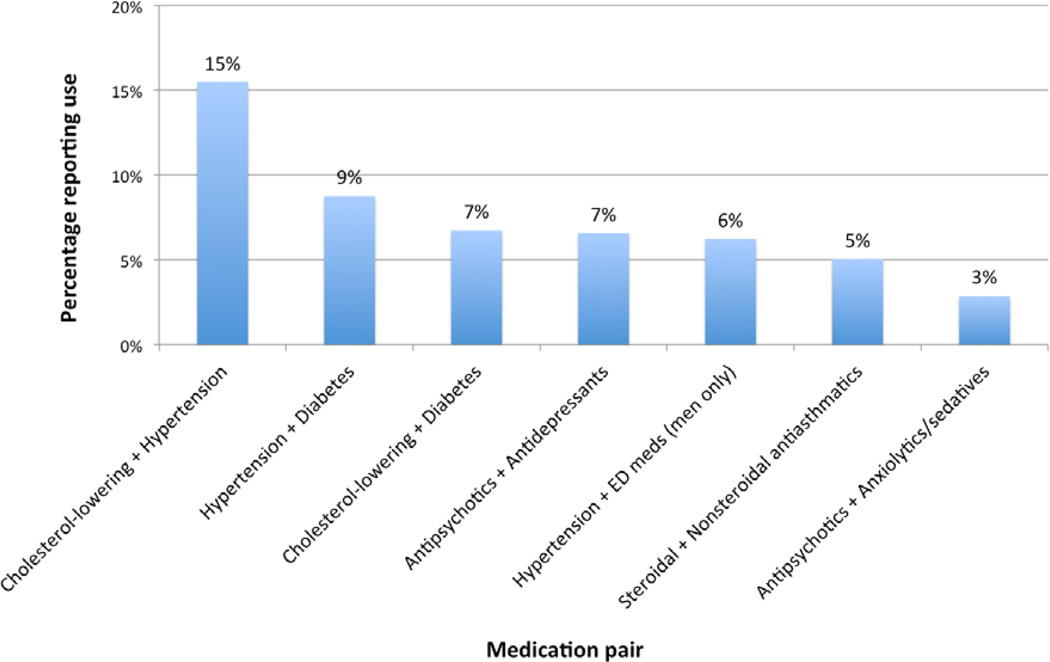
Concurrent use of two or more medications was reported by 34% of participants. The most commonly used medication classification pairs were: cholesterol-lowering and hypertension medications (15% of all study participants), hypertension and diabetes medications (9%), cholesterol-lowering and diabetes medications (7%) and antipsychotics and antidepressants (7%) (Figure 2).
Figure 2. Prevalence of concomitant medication use at time of oral HPV detectiona.
a Medication use is shown for men (n=320) and women (n=274) together, with the exception of erectile dysfunction medications, which were assessed in men only.
Effect of medication use on time to oral HPV clearance
Oral HPV clearance was evaluated over 18,465 person-months of follow-up. Median time to clearance of oral HPV infection was 6.3 months (IQR: 5.9–12.1). In unadjusted analyses, reduced oral HPV clearance was significantly associated with recent use of 3 of the 11 medication classes evaluated: antipsychotics (HR=0.75, 95% CI=0.57–0.99), anxiolytics/sedatives (HR=0.78, 95% CI=0.63–0.96) and antidepressants (HR=0.82, 95% CI=0.67–0.99) (Table 2). Use of erectile dysfunction medications was marginally associated with reduced oral HPV clearance (HR=0.77, 95% CI=0.58–1.01). No other medication class was significantly associated with oral HPV clearance.
Table 2.
Unadjusted associations of recent medication use with time to oral HPV clearance among 594 participants with 1,358 oral HPV infections
| Medication type used since last visita |
No. of cleared infections |
Person-months of observation |
HR (95% CI) |
|---|---|---|---|
| Antipsychoticsb | |||
| HIV-uninfected (n=299) | |||
| No | 122 | 3,968 | Ref |
| Yes | 13 | 273 | 1.38 (0.88–2.17) |
| HIV-infected (n=1,059) | |||
| No | 466 | 14,998 | Ref |
| Yes | 46 | 2,298 | 0.67 (0.49–0.91) |
| Anxiolytics/sedatives | |||
| No | 512 | 16,123 | Ref |
| Yes | 135 | 5,415 | 0.78 (0.63–0.96) |
| Antidepressants | |||
| No | 466 | 14,415 | Ref |
| Yes | 181 | 7,123 | 0.82 (0.67–0.99) |
| Asthma (steroidal) medications | |||
| No | 572 | 19,288 | Ref |
| Yes | 75 | 2,250 | 1.20 (0.89–1.63) |
|
Asthma (non-steroidal) medications |
|||
| No | 489 | 16,349 | Ref |
| Yes | 158 | 5,189 | 1.06 (0.87–1.28) |
|
Cholesterol-lowering medications |
|||
| No | 469 | 15,742 | Ref |
| Yes | 178 | 5,796 | 1.03 (0.86–1.25) |
| Diabetes medications | |||
| No | 567 | 19,231 | Ref |
| Yes | 80 | 2,307 | 1.19 (0.92–1.53) |
| Hormones | |||
| No | 618 | 20,665 | Ref |
| Yes | 29 | 872 | 1.10 (0.72–1.69) |
| Hypertension medications | |||
| No | 397 | 13,246 | Ref |
| Yes | 250 | 8,292 | 1.02 (0.86–1.22) |
| NSAIDs | |||
| No | 419 | 11,760 | Ref |
| Yes | 203 | 6,673 | 0.90 (0.76–1.07) |
|
Erectile dysfunction medications (men only) |
|||
| No | 296 | 9,952 | Ref |
| Yes | 56 | 2,419 | 0.77 (0.58–1.01) |
All medication use variables were time-updated (i.e. medication use reported at each visit was applied to the WLW model, and was allowed to change if the participant started/stopped medication use). If a participant missed a visit, the last reported medication use status was used in the analysis.
Statistically significant values are listed in bold. Statistical significance was defined as two-sided p-value <0.05.
Antipsychotic medication use was associated with reduced clearance in HIV-infected participants only (p-interaction=0.009)
Among users of antipsychotics, there appeared to be effect modification by HIV status (p-interaction=0.009), with reduced clearance in PLWH (HR=0.67, 95% CI=0.49–0.91), but not HIV-uninfected participants (HR=1.38, 95% CI=0.88–2.17) (Table 2). Although statistically significant, the numbers of HIV-uninfected participants reporting use of antipsychotics at time of oral HPV detection was small (13 infections among 10 people), which limits interpretation of this possible interaction by HIV status. Use of anxiolytics/sedatives and antidepressants were more common among HIV-uninfected than PLWH in this study, and the associations of these medications did not differ by HIV status (p-interaction=0.99 and 0.81, respectively).
After adjusting for the effects of biologic sex, prevalent vs. incident oral HPV infection, age, HIV status and CD4+ T cell count measured at the visit of oral HPV detection, antipsychotic use remained significantly associated with reduced oral HPV clearance (aHR=0.75, 95% CI=0.57–0.99) overall and when restricted to only HIV-infected participants (aHR=0.66, 95% CI=0.48–0.90), but not among HIV-uninfected participants (aHR=1.62, 95%CI=0.96–2.73, p=interaction=0.009). After adjustment, anxiolytics/sedatives (aHR=0.89, 95% CI=0.73–1.07), antidepressants (aHR=0.89, 95% CI=0.71–1.11) and erectile dysfunction medications (aHR=0.78, 95% CI=0.59–1.05) were no longer statistically significantly associated with oral HPV clearance. We were underpowered to explore risk factors separately for oncogenic and non-oncogenic oral HPV infection; when explored, with not statistically significant, all 3 medication classes associated with reduced overall oral HPV clearance (antipsychotic medications, antidepressants and anxiolytics/sedatives) remained associated with both reduced oncogenic and non-oncogenic oral HPV infection clearance
Concomitant medication use
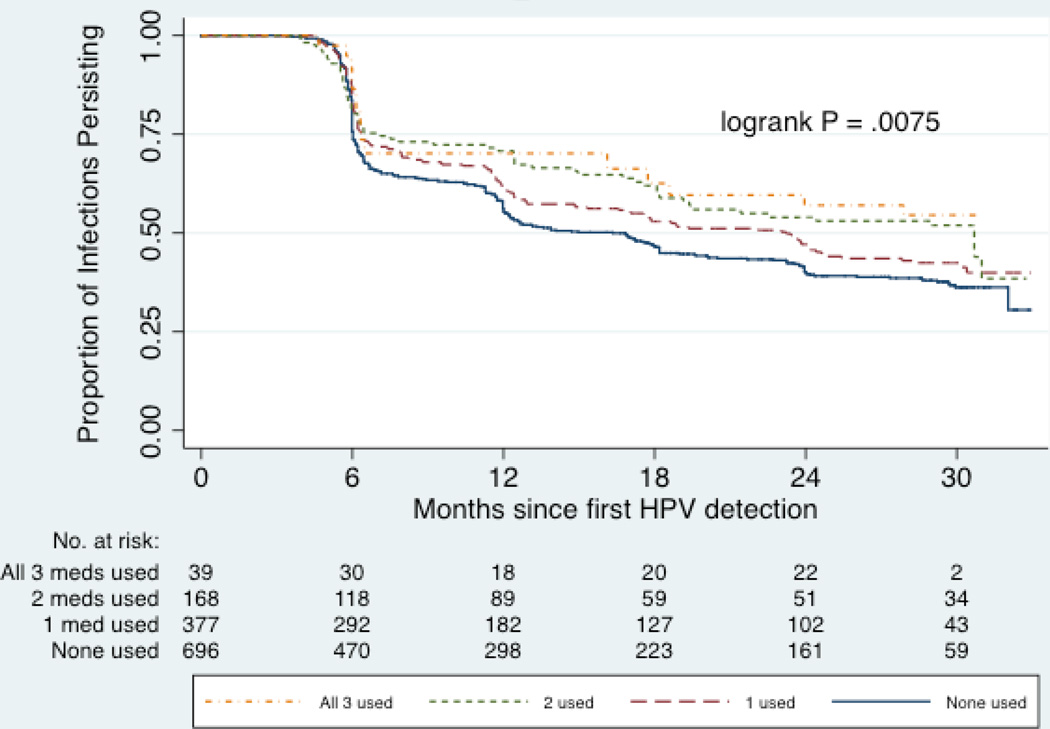
Of the participants using antipsychotics, 67% also reported use of antidepressants and 29% also reported use of anxiolytics/sedatives at time of oral HPV detection. When evaluating concurrent use of these 3 medication classes in unadjusted analyses, participants who reported recent use of all 3 medications had the greatest reduction in clearance (HR=0.57, 95% CI=0.35–0.94), followed by participants who used any 2 of these medications (HR=0.73, 95% CI=0.55–0.96) or only 1 of these three medications (HR=0.83, 95% CI=0.69–1.00), as compared to participants who used none of these medications (p-trend=0.002) (Figure 3). After adjustment for other risk factors, concomitant use of antipsychotics, antidepressants, and anxiolytics/sedatives remained marginally associated with reduced clearance, with increasing number of psychotropic medications used being associated with significantly higher likelihood of decreased clearance (p-trend=0.04), and no interaction by HIV in this association. Concomitant use of other types of medications (i.e. commonly paired medications) was not significantly associated with oral HPV clearance.
Figure 3.
Time to oral HPV clearance among 594 participants with 1,358 oral HPV infections, by use of antipsychotics, antidepressants and/or anxiolytics/sedatives
Discussion
While most medications did not affect oral HPV clearance, use of antipsychotics, antidepressants and anxiolytics/sedatives were observed to decrease oral HPV clearance with stronger effects when used concomitantly. The mechanisms by which these medications reduce oral HPV clearance remain unclear, but may be due to immunomodulatory effects of the medication or the underlying illness being treated by the medication. However, other immunomodulatory medications examined (i.e. steroidal antiasthmatics) did not affect oral HPV clearance, suggesting the possibility of other explanatory factors.
The immunomodulatory effects of antipsychotics, which are used for treatment of schizophrenia or bipolar disorder, have been consistently reported, and may explain the reduced oral HPV clearance observed among users of these medications [3]. It is also possible that reduced oral HPV clearance was associated with immune dysregulation, which is known to be associated with psychotic diagnoses such as schizophrenia and bipolar disorder [24], or by biological or behavioral factors unique to individuals with these disorders. This study assessed medication use only, and we were unable to confirm diagnoses or the specific disorders that these medications were used to treat.
In this study, recent use of antidepressants and benzodiazepines (a major class of anxiolytic/sedative medications) also appeared to reduce oral HPV clearance, although the effect was not as strong as that observed with use of antipsychotics. It is possible the immunomodulatory effects of these medications, or the inflammatory response from conditions treated with these medications, such as depression [25–29,2], or anxiety and stress-related disorders[30,4] may influence oral HPV clearance, although associations were not statistically significant in adjusted analysis.
This study had several strengths and limitations. Strengths of this study included the large population size, longitudinal follow-up and centralized testing of oral HPV infection. In addition, detailed information on medication use was collected prospectively on validated survey instruments administered by experienced research staff who participants knew well. Medication use was self-reported and we cannot exclude the possibility of misreporting/misclassification and heterogeneity in medications within classes. Data on dose, frequency, and adherence of medication use were not available. Our analysis does not take into consideration differences in drug formulations or specific medications within each drug class.
Conclusion
In summary, we found significantly reduced oral HPV clearance in participants using antipsychotic medication with potential differential effects by HIV status although these results should be interpreted with caution as the numbers were limited and mechanisms of action are unclear.
Acknowledgments
Financial support: This work was supported by grant R01DE021395 (NIDCR, NIH; Gypsyamber D’Souza). The MACS and WIHS cohorts receive primary funding from NIAID, with additional funding from NCI, NIDA, NIMH and NICHD.
Oral rinse samples were processed and tested in the laboratory of Dr. Maura Gillison at The Ohio State University. Data were collected by the Multicenter AIDS Cohort Study (MACS). MACS (Principal Investigators): Johns Hopkins University Bloomberg School of Public Health (Joseph Margolick), U01-AI35042; Northwestern University (Steven Wolinsky), U01-AI35039; University of California, Los Angeles (Roger Detels), U01-AI35040; University of Pittsburgh (Charles Rinaldo), U01-AI35041; the Center for Analysis and Management of MACS, Johns Hopkins University Bloomberg School of Public Health (Lisa Jacobson), UM1-AI35043. The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute of Mental Health (NIMH). The MACS website is located at http://aidscohortstudy.org/. Data in this manuscript were also collected by the Women’s Interagency HIV Study (WIHS). WIHS (Principal Investigators): U01-AI-103408; Bronx WIHS (Kathryn Anastos), U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago WIHS (Mardge Cohen and Audrey French), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590. The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH).
GD, DJW and RDC have/had research support from Merck & Co., Inc. DJW is a member of the speakers bureau for Merck & Co., Inc. MLG has been a consultant for Merck & Co., Inc and GSK. RDC also reports institutional grant funding and royalties from UptoDate (on HPV related topics).
Footnotes
Conflicts of Interest: JOL, EAS, KMW, RDB, SR, JBM, HDS, AW, LJ, CLC, JHB, AR, YG and WX have no conflicts of interest to report.
References
- 1.Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(29):3235–3242. doi: 10.1200/JCO.2015.61.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maes M. Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Progress in neuro-psychopharmacology & biological psychiatry. 2011;35(3):664–675. doi: 10.1016/j.pnpbp.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Tourjman V, Kouassi É, Koué M-È, Rocchetti M, Fortin-Fournier S, Fusar-Poli P, Potvin S. Antipsychotics' effects on blood levels of cytokines in schizophrenia: A meta-analysis. Schizophrenia research. 2013;151:43–47. doi: 10.1016/j.schres.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Covelli V, Maffione AB, Nacci C, Tatò E, Jirillo E. Stress, Neuropsychiatric Disorders and Immunological Effects Exerted by Benzodiazepines. Immunopharmacology and Immunotoxicology. 1998;20(2):199–209. doi: 10.3109/08923979809038539. [DOI] [PubMed] [Google Scholar]
- 5.Brusselle G, Bracke K. Targeting Immune Pathways for Therapy in Asthma and Chronic Obstructive Pulmonary Disease. Annals ATS. 2014;11((Supplement 5)):S322–S328. doi: 10.1513/AnnalsATS.201403-118AW. [DOI] [PubMed] [Google Scholar]
- 6.Deans KA, Sattar N. "Anti-Inflammatory" Drugs and Their Effects on Type 2 Diabetes. Diabetes Technology & Therapeutics. 2006;8(1):18–27. doi: 10.1089/dia.2006.8.18. [DOI] [PubMed] [Google Scholar]
- 7.Zhou Q, Liao JK. Pleiotropic Effects of Statins. Circulation Journal. 2010;74(5):818–826. doi: 10.1253/circj.cj-10-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan IJ, Peeva E, Zandman-Goddard G. Hormonal modulation of the immune system — A spotlight on the role of progestogens. Autoimmunity Reviews. 2015;14(6):536–542. doi: 10.1016/j.autrev.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Karakhanova S, Yang Y, Link J, Soltek S, von Ahn K, Umansky V, Werner J, Bazhin AV. Gender-specific immunological effects of the phosphodiesterase 5 inhibitor sildenafil in healthy mice. Molecular Immunology. 2013;56(4):649–659. doi: 10.1016/j.molimm.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Provinciali N, Lazzeroni M, Cazzaniga M, Gorlero F, Dunn BK, DeCensi A. Metformin: risk-benefit profile with a focus on cancer. Expert Opinion on Drug Safety. 2015;14(10):1573–1585. doi: 10.1517/14740338.2015.1084289. [DOI] [PubMed] [Google Scholar]
- 11.Harris CM, McKenzie R, Nayak S, Kiyatkin D, Baker D, Kisuule F. Graying of the HIV epidemic: a challenge for inpatient medicine providers. Journal of community hospital internal medicine perspectives. 2015;5(6):29428. doi: 10.3402/jchimp.v5.29428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou S, Martin K, Corbett A, Napravnik S, Eron J, Zhu Y, Casciere B, Boulton C, Loy B, Smith S, Woods A, Murray M, Ramsdell L, Wohl DA. Total daily pill burden in HIV-infected patients in the southern United States. AIDS patient care and STDs. 2014;28(6):311–317. doi: 10.1089/apc.2014.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.High KP, Brennan-Ing M, Clifford DB, Cohen MH, Currier J, Deeks SG, Deren S, Effros RB, Gebo K, Goronzy JJ, Justice AC, Landay A, Levin J, Miotti PG, Munk RJ, Nass H, Rinaldo CR, Jr, Shlipak MG, Tracy R, Valcour V, Vance DE, Walston JD, Volberding P. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. Journal of acquired immune deficiency syndromes. 2012;60(Suppl 1):S1–S18. doi: 10.1097/QAI.0b013e31825a3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Souza G, Fakhry C, Sugar EA, Seaberg EC, Weber K, Minkoff HL, Anastos K, Palefsky JM, Gillison ML. Six-month natural history of oral versus cervical human papillomavirus infection. International journal of cancer Journal international du cancer. 2007;121(1):143–150. doi: 10.1002/ijc.22667. [DOI] [PubMed] [Google Scholar]
- 15.Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, Young M, Greenblatt R, Sacks H, Feldman J. The Women's Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9(2):117–125. [PubMed] [Google Scholar]
- 16.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR., Jr The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. American journal of epidemiology. 1987;126(2):310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 17.Multicenter AIDS Cohort Study (MACS) - StatEpi Coordinating Center. [Accessed Dec 17 2015];2015 http://statepi.jhsph.edu/macs/ [Google Scholar]
- 18.Broutian TR, He X, Gillison ML. Automated high throughput DNA isolation for detection of human papillomavirus in oral rinse samples. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2011;50(4):270–275. doi: 10.1016/j.jcv.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, Snijders PJ, Meijer CJ International Agency for Research on Cancer Multicenter Cervical Cancer Study G. Epidemiologic classification of human papillomavirus types associated with cervical cancer. The New England journal of medicine. 2003;348(6):518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 20.Schiffman M, Clifford G, Buonaguro FM. Classification of weakly carcinogenic human papillomavirus types: addressing the limits of epidemiology at the borderline. Infectious agents and cancer. 2009;4:8. doi: 10.1186/1750-9378-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.International Agency for Research on Cancer. IARC Monographs on the evaluation of carcinogenic risks to humans, vol 100B. France, Lyon: International Agency for Research on Cancer; 2012. Human Papillomaviruses. [Google Scholar]
- 22.Wei L, Lin D, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. Journal of the American Statistical Association. 1989;84(408):1065–1073. [Google Scholar]
- 23.StataCorp. Stata Statistical Software. Release. (12) 2011 [Google Scholar]
- 24.Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biological psychiatry. 2011;70(7):663–671. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leonard BE. The immune system, depression and the action of antidepressants. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2001;25(4):767–780. doi: 10.1016/s0278-5846(01)00155-5. [DOI] [PubMed] [Google Scholar]
- 26.Maes M. Evidence for an immune response in major depression: A review and hypothesis. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1995;19(1):11–38. doi: 10.1016/0278-5846(94)00101-m. [DOI] [PubMed] [Google Scholar]
- 27.Miller AH, Maletic V, Raison CL. Inflammation and Its Discontents: The Role of Cytokines in the Pathophysiology of Major Depression. Biological psychiatry. 2009;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends in Immunology. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schiepers OJG, Wichers MC, Maes M. Cytokines and major depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2005;29(2):201–217. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Zavala F. Benzodiazepines, anxiety and immunity. Pharmacology & Therapeutics. 1997;75(3):199–216. doi: 10.1016/s0163-7258(97)00055-7. [DOI] [PubMed] [Google Scholar]