Abstract
α-Chlorofatty aldehydes are generated from myeloperoxidase-derived HOCl targeting plasmalogens, and are subsequently oxidized to α-chlorofatty acids (α-ClFAs). The catabolic pathway for α-ClFA is initiated by ω-oxidation. Here, we examine PPAR-α activation as a mechanism to increase α-ClFA catabolism. Pretreating both HepG2 cells and primary mouse hepatocytes with the PPAR-α agonist, pirinixic acid (Wy 14643), increased the production of α-chlorodicarboxylic acids (α-ClDCAs) in cells treated with exogenous α-ClFA. Additionally, α-ClDCA production in Wy 14643-pretreated wild-type mouse hepatocytes was accompanied by a reduction in cellular free α-ClFA. The dependence of PPAR-α-accelerated α-ClFA catabolism was further demonstrated by both impaired metabolism in mouse PPAR-α−/− hepatocytes and decreased clearance of plasma α-ClFA in PPAR-α−/− mice. Furthermore, Wy 14643 treatments decreased plasma 2-chlorohexadecanoic acid levels in wild-type mice. Additional studies showed that α-ClFA increases PPAR-α, PPAR-δ, and PPAR-γ activities, as well as mRNA expression of the PPAR-α target genes, CD36, CPT1a, Cyp4a10, and CIDEC. Collectively, these results indicate that PPAR-α accelerates important pathways for the clearance of α-ClFA, and α-ClFA may, in part, accelerate its catabolism by serving as a ligand for PPAR-α.
Keywords: myeloperoxidase, lipid biochemistry, fatty acid, fatty acid/oxidation, liver metabolism, nuclear receptors/peroxisome proliferator-activated receptor
Myeloperoxidase (MPO) is an important enzyme mediating inflammatory reactions in leukocytes and neighboring cells. Upon activation, neutrophils and monocytes release MPO resulting in the formation of the reactive chlorinating species, HOCl, which can target biomolecules (1–4). Plasmalogen molecular species are a predominant phospholipid subclass present in the plasma membrane of tissues of the cardiovascular system, including myocardium, smooth muscle, endothelium, and leukocytes (5–8). The vinyl ether bond of plasmalogens is a preferential target for HOCl (9–11). The oxidation of plasmalogen by HOCl leads to the production of α-chlorofatty aldehyde (α-ClFALD) as well as its metabolites, α-chlorofatty acid (α-ClFA), and α-chlorofatty alcohol (12, 13). These chlorinated lipids exert several adverse biological effects. α-ClFALD can cause endothelial dysfunction by inhibiting endothelial nitric oxide synthase expression and brain-blood barrier dysfunction by inducing apoptosis (14, 15). Both α-ClFALD and α-ClFA induce cyclooxygenase 2 expression in human coronary artery endothelial cells (16). In addition, α-ClFA accumulates in activated monocytes and, in turn, induces monocyte apoptosis (17). Accordingly, the metabolic clearance of chlorinated lipids is important to limit their deleterious impact and potential signaling mediated by chlorinated lipids. Indeed, α-ClFA can be further catabolized by ω-oxidation and subsequent β-oxidation from the ω-end in the liver resulting in the eventual production of 2-chloroadipic acid (2-ClAdA), which is excreted in the urine (18).
PPAR-α is a ligand-activated transcription factor, which belongs to the nuclear hormone receptor superfamily (19, 20). PPAR-α activity can be modulated by a variety of endogenous ligands, such as long-chain polyunsaturated fatty acids and leukotriene B4 (21, 22). PPAR-α is also regulated by synthetic ligands, such as fibrates and pirinixic acid (Wy 14643). In the liver, PPAR-α regulates fatty acid oxidation by directly promoting the expression of multiple genes involved in the control of lipid metabolism (23). It modulates the activities of all three fatty acid oxidation systems, namely mitochondrial and peroxisomal β-oxidation and microsomal ω-oxidation (20). Accordingly, because α-ClFA catabolism is initiated by ω-oxidation, it seems likely that PPAR-α activity might decrease α-ClFA levels.
Here, we tested the hypothesis that the PPAR-α agonist, Wy 14643, accelerates the catabolism of the α-ClFA, 2-chlorohexadecanoic acid (2-ClHA), in hepatocytes and the clearance of 2-ClHA from the body. We show that Wy 14643 increases 2-ClAdA production and decreases intracellular levels of 2-ClHA in both HepG2 cells and primary mouse hepatocytes treated with exogenous 2-ClHA. The effects of Wy 14643 are likely mediated by augmenting fatty acid oxidation because Wy 14643, indeed, upregulates the expression of many genes involved in β-oxidation in both HepG2 cells and primary mouse hepatocytes. Moreover, we show that 2-ClHA activates PPAR-α, stimulating the mechanism responsible for its catabolism. Finally, Wy 14643 treatments reduced plasma levels of 2-ClHA in mice.
MATERIALS AND METHODS
Reagents
Synthetic 2-ClHA was prepared as described previously using hexadecanoic acid (HA) as precursor (12). Wy14643 and GW6471 were purchased from Cayman Chemical. The primers for real-time PCR were synthesized by IDT Inc. Information about primers is included in supplemental Table S1.
Incubation of 2-ClHA with HepG2 cell line
HepG2 cells were cultured in 10% FBS containing DMEM medium. The cells were pretreated with vehicle (DMSO) or Wy14643 (10 μM) overnight. Then the cells were incubated with 2-ClHA (BSA-conjugated, 50 μM) for 0, 3, 6, 12, and 24 h time points in the presence of 2% FBS. At the end of the incubation period, cell culture medium was collected following centrifugation at 200 g for 5 min to remove any floating cells and stored at −20°C. The cells were washed twice with PBS and then scraped in PBS and stored at -20°C.
Incubation of 2-ClHA with primary mouse hepatocytes
Cells were isolated from 10-week-old male wild-type C57BL/6J and PPAR-α−/− mice fed chow, using perfusion and digest buffers (Invitrogen) (24). Cells were resuspended in William’s E medium (Invitrogen) supplemented with plating supplements (Invitrogen), plated in 6-well BioCoat collagen I plates (BD), and incubated at 37°C and 5% CO2 for 6 h. Next, the cells were pretreated with vehicle or Wy14643 (10 μM) overnight in William’s E medium supplemented with maintenance supplements (Invitrogen) and 2-ClHA (BSA-conjugated, 10 and 50 μM) was added for another 24 h. The medium and cells were collected as described above for HepG2 cells.
Lipid extraction and analysis
For primary mouse hepatocytes, after washing the cells with PBS five times, the lipids were first extracted using a modified Bligh and Dyer extraction with 20 pmol of 2-chloro-[d4-7,7,8,8]HA (2-Cl−[d4]HA) added as the standard. Mouse tissues were also extracted using a modified Bligh and Dyer method prior to analysis of α-ClFA. The extracted lipids in chloroform were dried under nitrogen and then suspended in 180 μl of water for hydrolysis. For plasma samples, 10 μl of plasma was spiked with 103 fmol 2-Cl−[d4]HA followed by the addition of 155 μl of water prior to base-catalyzed hydrolysis. To initiate hydrolysis, 200 μl of 1 M NaOH was added to the samples and the samples were incubated for 2 h at 60°C. After incubation, reactions were stopped by the addition of 120 μl of 2 M HCl. After 10 min, lipids were extracted using a modified Dole extraction (25, 26). Finally, the lipid extracts were resuspended in 150 μl of methanol/water (85/15, v/v) containing 0.1% formic acid for 2-ClHA analysis by LC-MS using ESI and selected reaction monitoring following previously described methods on a Thermo Fisher Quantum triple quadrupole instrument (25, 26). Liver triglycerides (TAGs) were quantified by ESI-MS and liver FFAs were quantified following derivatization to pentafluorobenzyl esters by gas chromatography-MS as previously described (27–30).
Analysis of α-chlorodicarboxylic acid
For α-chlorodicarboxylic acid (α-ClDCA) analysis, [d4-3,3,4,4]adipic acid ([d4]AdA) was added as the internal standard. Cell culture medium (0.8 ml) was added with 0.2 ml of 6 N HCl. Then, the samples were extracted with 6 ml of ethyl acetate/diethyl ether (1:1, v/v) twice. After evaporating the organic extract under N2, the extract was suspended in 0.2 ml water containing 5 mM ammonium acetate and 0.25% acetic acid for α-ClDCA analyses by LC-MS using selected reaction monitoring (see supplemental Table S2). This analysis was based on a modification of the previously described method for 2-ClAdA analysis (18, 25). Twenty-five microliters of sample were injected onto a Supelcosil LC-18 DB column (150 × 3 mm, 5 μm) that was equilibrated in mobile phase A (5 mM ammonium acetate and 0.25% acetic acid in water) at 300 μl/min. The 2-ClDCA molecular species and internal standard were eluted from the column using discontinuous linear gradients from 100% mobile phase A to 100% mobile phase B (5 mM ammonium acetate and 0.25% acetic acid in methanol). This gradient was initiated by a linear gradient from 100% A to 90% A from time = 0 min to 4 min, followed by a linear gradient from 90% A to 20% A over the next 8 min, and then a linear gradient from 20% A to 0% A (i.e., 100% B) over the next 6 min. The mobile phase was then held at 100% B for the next 10 min followed by equilibration to initial conditions (100% A). Levels of 2-ClAdA were determined by isotope dilution (18). Levels of α-ClDCA molecular species were measured as a ratio of peak area of the target molecule compared with that of the internal standard, [d4]AdA.
Quantitative RT-PCR
Total RNA was extracted using TRIZOL reagent. CDNAs were generated from 1 μg of DNase1-treated RNA using Superscript III (Invitrogen). Real-time PCR was done with Power SybrGreen reagent (Applied Biosystems), using a LightCycler-480 (Roche).
In vivo metabolism of 2-ClHA
All animal experiments were conducted with the approval of the Institutional Animal Care and Use Committee at Saint Louis University. Wild-type male C57BL/6J mice were administered vehicle or Wy 14643 (10 mg/kg/day ip) for 2 days. Then, the mice were injected with 1 mg of 2-chloro-[d2-4,4]HA (2-Cl−[d2-4,4]HA) per 100 g of body weight (ip), and blood was collected 3 h later for α-ClFA analysis. A second mouse study compared C57BL/6J and PPAR-α−/− mice injected with 350 μg of 2-Cl−[d2-4,4]HA per 100 g of body weight (ip), and blood was collected at 2, 6, and 24 h for α-ClFA analysis. Additionally, urine was collected over 24 h. At the end of the 24 h experimental interval, fat pads, liver, and heart were collected and were immediately frozen at the temperature of liquid nitrogen. White blood cell differentials were also examined in C57BL/6J and PPAR-α−/− mice, which showed no significant differences in total white blood cell counts and neutrophils in these cell lines (supplemental Fig. S1). A third mouse study included analysis of livers from fed, fasted, or refed C57BL/6J mice that were fed chow diet (LabDiet, PicoLab Rodent 20) for 8 weeks and then were either euthanized at 9:00 AM (fed group), fasted for 24 h (9:00 AM to 9:00 AM the next day, fasted group), or fasted for 24 h and subsequently refed for 6 h (9:00 AM to 3:00 PM on the following day, refed group).
Transient transfection of HEK293 cells
All the transfection experiments were performed in triplicate in HEK293 cells. Cells were transfected in 10% serum medium using lipofectamine, with a mixture of plasmids that contained, in addition to the firefly luciferase reporter vector and the PPAR-α expression vector, a β-GAL expression vector as a control for the transfection efficiency. Medium was replaced 6 h after transfection. The next day, cells were incubated for 24 h with the indicated stimuli dissolved in DMSO at the indicated concentrations in serum-free medium. After incubation, cells were collected and resuspended in the reporter lysis buffer. Part of the sample was used to measure β-galactosidase activity. Ortho-nitrophenyl-β-galactoside (2.5 mg/ml) was added to the solution in a 96-well plate and the activity was measured by monitoring the formation of colorimetric substrate. The remaining sample was used to test luciferase activity by mixing the cell lysate and luciferase assay reagent and measuring the light produced (Promega, E4530). Expression vectors containing the ligand binding domain of human PPAR-α, PPAR-γ, or PPAR-δ fused to the DNA binding domain of GAL4 were provided by Dr. Brian Finck (Washington University) (31–33). Reporter luciferase vectors (UAS-TK-LUC) contained GAL4 binding sequences upstream of the TK promoter (31). For the normalization, we used pSV-β-galactosidase control vector.
Primary murine hepatocyte studies
All the experiments were performed in triplicate in primary murine hepatocytes. Cells were isolated and plated in 12-well plates. Cells were then incubated with the indicated stimuli and the indicated concentrations for 18 h in serum-free medium. RNA was then purified and the expression level of PPAR-α target genes was investigated through real-time PCR, where the GAPDH expression level was used for the normalization.
Statistical analysis
All data are presented as mean ± SE. Two-tailed Student’s t-test was performed for comparing two groups. For multiple groups, two-way ANOVA followed by the post hoc Dunnett test was employed. Differences were considered statistically significant at the P < 0.05 level.
RESULTS
Activation of PPAR-α by Wy 14643 increases the production of α-ClDCAs in HepG2 cells
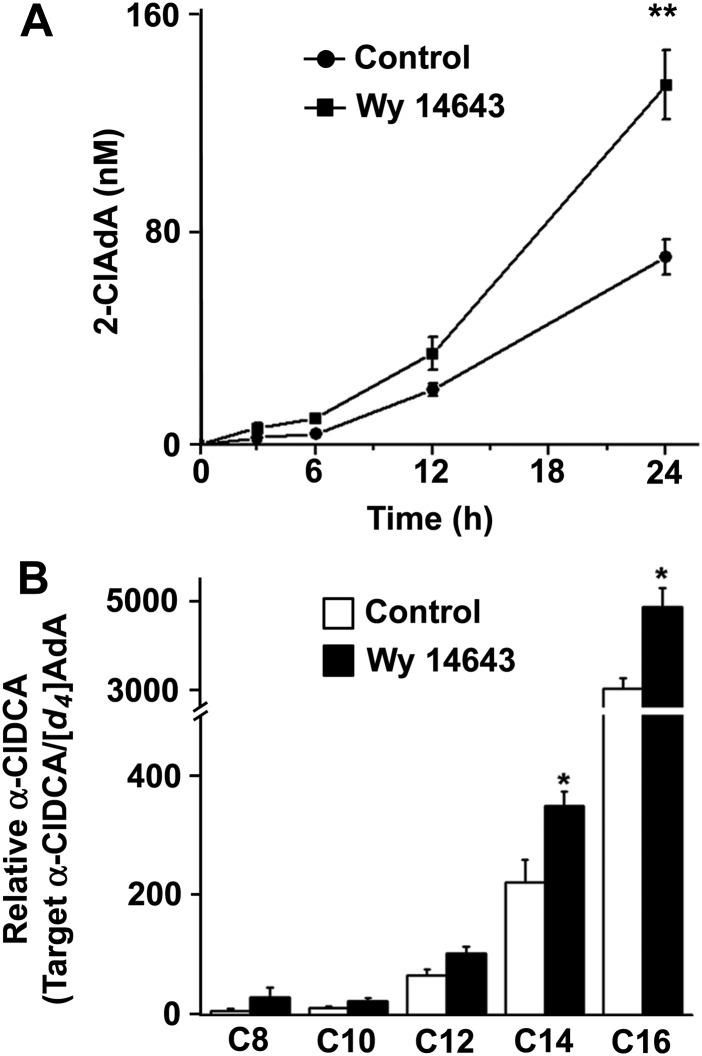
Because chlorinated lipids are produced under inflammatory conditions (13, 18, 34–37) and mediate some pro-inflammatory processes (14–17), it was of great interest to examine mechanisms to accelerate the metabolic removal of these lipids. α-ClFA is catabolized by ω-oxidation and subsequent β-oxidation from the ω-end in the liver, resulting in the eventual production of 2-ClAdA (18). To test whether PPAR-α activation alters the catabolism of α-ClFA, the human hepatoma cell line, HepG2, was pretreated with the selective PPAR-α agonist, Wy 14643, overnight before 2-ClHA was added to cell culture medium. Initial studies showed nearly equal levels of intracellular 2-ClFA in the presence and absence of WY 14643 following a 60 min incubation with 50 μM α-ClFA by HepG2 cells (intracellular levels of 2-ClHA under these conditions were 7.68 ± 0.41 nmol/mg protein and 7.22 ± 0.62 nmol/mg protein for no treatment and Wy 14643 treatment, respectively). As shown in Fig. 1A, the final product of α-ClFA catabolism, 2-ClAdA, was produced and detected in the cell culture medium in a time-dependent manner, and Wy 14643 pretreatment significantly increased its production. In addition to 2-ClAdA, other α-ClDCAs with intermediate acyl chain lengths were evaluated following 24 h incubations with 2-ClHA. Besides 2-ClAdA, the C14 and C16 α-ClDCA were also significantly increased in the cell culture medium with Wy 14643 pretreatment (Fig. 1B). These α-ClDCA species were only detected in cell culture medium, and were not present at detectable levels associated with cells.
Fig. 1.
Wy 14643 accelerates the catabolism of 2-ClHA in HepG2 cells. HepG2 cells were pretreated with or without Wy14643 (10 μM) overnight and then incubated with 50 μM 2-ClHA for 0, 3, 6, 12, and 24 h. α-ClDCA present in cell culture medium was analyzed as described in the Materials and Methods. The 2-ClAdA levels (A) at indicated incubation intervals and α-ClDCA (B) at 24 h in the culture medium. In (B) C(x) refers to the number of carbons in individual α-ClDCA molecular species. Values represent mean ± SEM for n = 3. *P < 0.05 versus control.
Effects of Wy 14643 on primary mouse hepatocytes
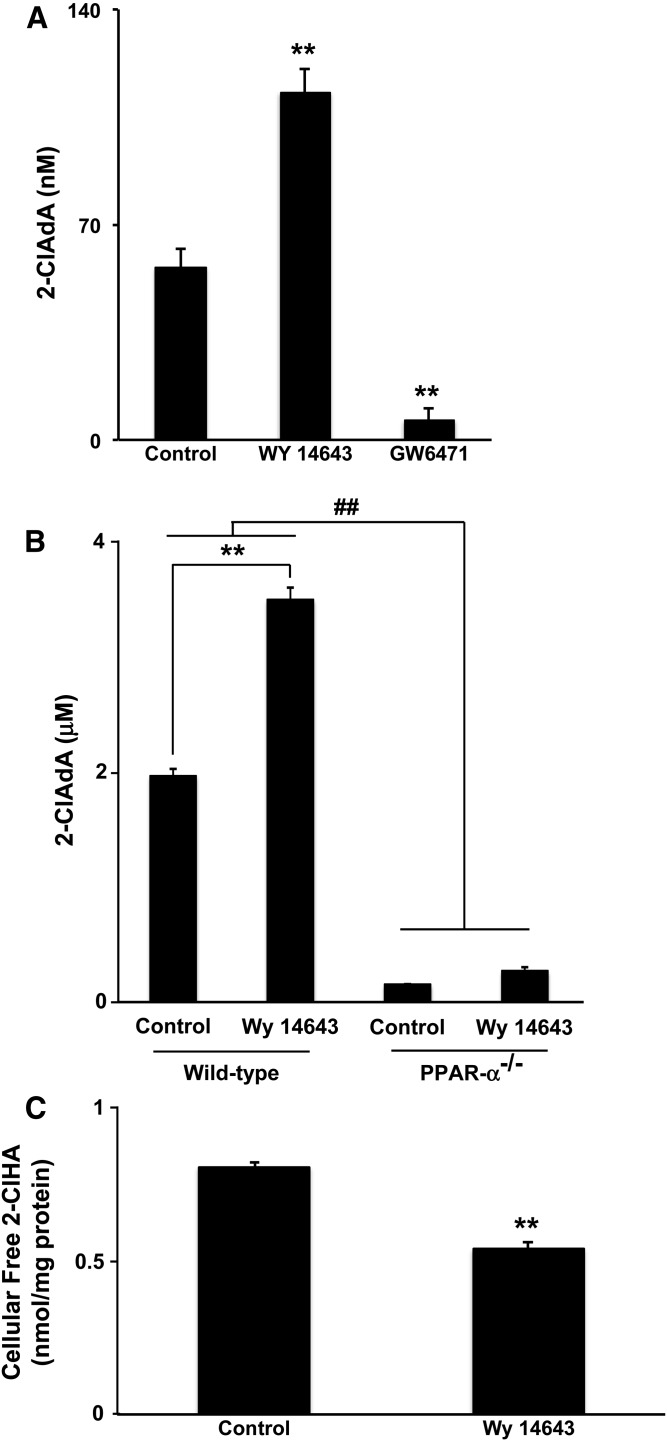
The effect of PPAR-α activation on the catabolism of α-ClFA was next determined in primary mouse hepatocytes. Wild-type hepatocytes metabolized both 10 μM and 50 μM 2-ClHA resulting in the accumulation of 2-ClAdA in the media (Fig. 2A, B). Consistent with our previous study in HepG2 cells (18), more 2-ClAdA was produced in primary hepatocytes treated with 50 μM 2-ClHA compared with 10 μM. In wild-type mouse hepatocytes, Wy 14643 pretreatment resulted in increased 2-ClAdA production in cells incubated with both 10 μM and 50 μM 2-ClHA (Fig. 2A, B). In contrast, the PPAR-α antagonist, GW6471, significantly decreased the catabolism of 10 μM 2-ClHA (Fig. 2A). To further test the specificity of PPAR-α activation on this process, the catabolism of 2-ClHA was also tested in PPAR-α−/− hepatocytes. The production of 2-ClAdA was significantly decreased in PPAR-α−/− hepatocytes compared with wild-type hepatocytes regardless of Wy 14643 pretreatment (Fig. 2B). In fact, Wy 14643 did not significantly increase 2-ClAdA production in PPAR-α−/− hepatocytes (Fig. 2B). The intracellular free 2-ClHA was determined by LC-MS in primary mouse hepatocytes to investigate whether Wy 14643 decreases the intracellular levels of free 2-ClHA, because it dramatically increases the catabolism of 2-ClHA in wild-type hepatocytes. The enhanced production of 2-ClAdA in Wy 14643-treated hepatocytes resulted in a concomitant decrease in intracellular 2-ClHA following the 24 h incubation time (Fig. 2C).
Fig. 2.
Effects of Wy 14643 on 2-ClHA catabolism in primary mouse hepatocytes. Mouse hepatocytes were prepared from either C57BL/6J or PPAR-α−/− mice, as indicated. Hepatocytes were pretreated with either Wy 14643 (10 μM), GW6471 (10 μM), or vehicle overnight and subsequently incubated with either 10 μM (A) or 50 μM (B) 2-ClHA for 24 h. The 2-ClAdA in the medium was analyzed as described in the Materials and Methods. Values represent mean ± SEM for n = 3. C: Primary hepatocytes from C57BL/6J mice were pretreated with or without Wy14643 (10 μM) overnight and then incubated with 50 μM of 2-ClHA for 24 h. Hepatocytes were collected for intracellular 2-ClHA analysis as described in the Materials and Methods. Values represent mean ± SEM for n = 3. **P < 0.01 for comparisons to control in (A) and (C). **P < 0.01 and ##P < 0.01 as indicated in (B).
Hepatic α-ClFA during fed, fasted, and refed state
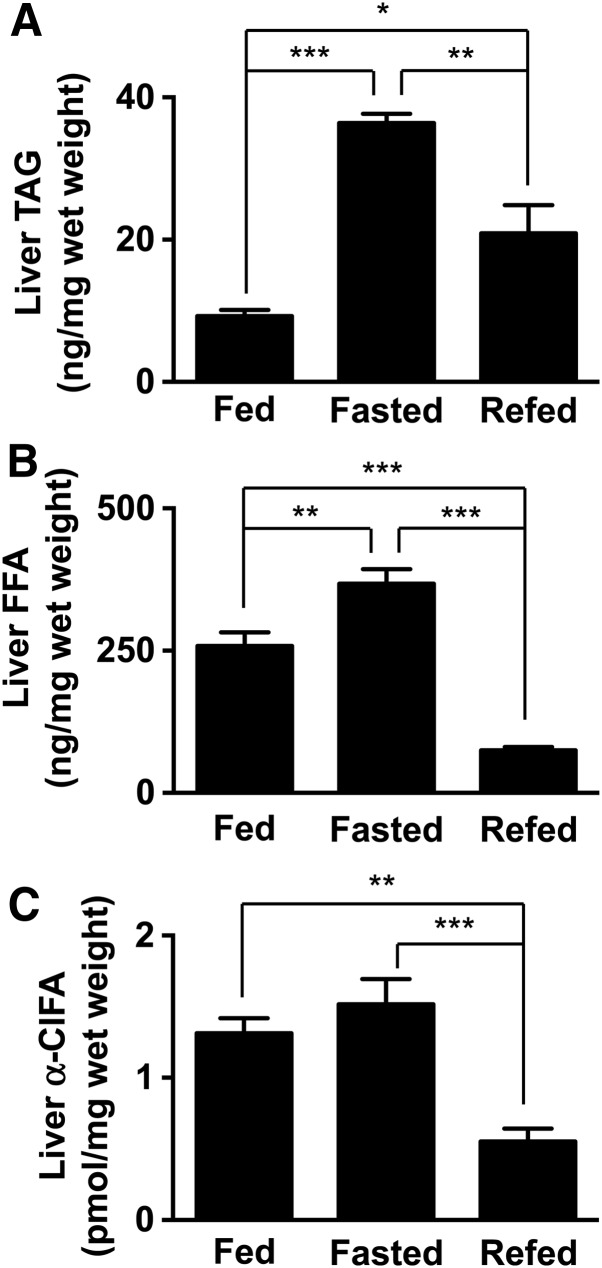
We next examined the dynamics of ClFA accumulation in the liver in response to fasting and fasting/refeeding. As expected, overnight fasting resulted in elevated hepatic TAG and FFA contents (Fig. 3A, B). These changes are likely due to a robust lipolysis in adipose tissue followed by hepatic re-esterification during fasting. Following a short-term refeeding, however, hepatic TAG and FFA levels were significantly reduced compared with the fasted state. Importantly, our data show that hepatic a-ClFA levels parallel the changes noted in total FFA (Fig. 3C).
Fig. 3.
Modulation of in vivo liver α-ClFA levels. Liver TAG (A), FFA (B), and α-ClFA (C) levels were determined in 8-week-old male C57BL/6J mice that were either fed chow (fed), fed chow and then fasted 24 h (fasted), or sequentially fed chow, fasted 24 h, and then refed 6 h (refed). *P < 0.05, **P < 0.01, and ***P < 0.001, as indicated. In each panel values are the mean ± SEM with n = 4–5 per group.
PPAR-α-mediated mechanisms accelerate in vivo 2-ClHA metabolism
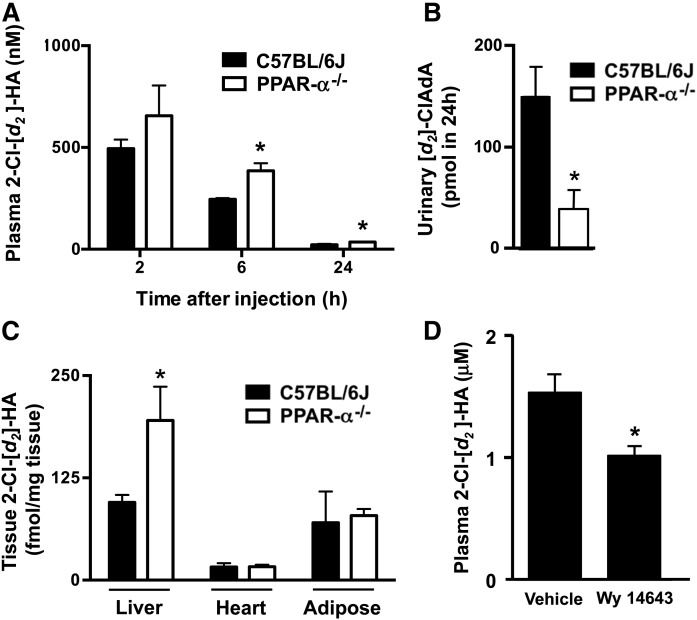
The effect of PPAR-α activity on 2-ClHA metabolism was evaluated in vivo. Studies compared 2-Cl−[d2-4,4]HA metabolism in C57BL/6J and PPAR-α−/− mice. In this study, plasma 2-Cl−[d2-4,4]HA was assessed at 2, 6, and 24 h following intraperitoneal injection of 2-Cl−[d2-4,4]HA. The 2-Cl−[d2-4,4]HA was significantly elevated in plasma at the 6 and 24 h time points and 24 h urinary [d2]ClAdA output was decreased in PPAR-α−/− mice compared with C57BL/6J mice (Fig. 4A, B). Also, 24 h following intraperitoneal injection of 2-Cl−[d2-4,4]HA, hepatic levels of 2-Cl−[d2-4,4]HA were elevated in PPAR-α−/− mice compared with C57BL/6J mice (Fig. 4C). In contrast, tissue 2-Cl−[d2-4,4]HA levels were not increased in heart and adipose tissue of PPAR-α−/− mice compared with C57BL/6J mice (Fig. 4C). In additional studies, wild-type C57BL/6J male mice were administered either vehicle or Wy 14643 (10 mg/kg/day ip) for 2 days before 2-Cl−[d2-4,4]HA (1 mg/100 g body weight) intraperitoneal injection. Three hours later, blood was collected from mice for 2-Cl−[d2-4,4]HA analysis. As seen in Fig. 4D, the plasma levels of total 2-Cl−[d2-4,4]HA were lower in the Wy 14643-treated group compared with the vehicle group, indicating that Wy 14643 reduces 2-ClHA plasma levels in wild-type mice.
Fig. 4.
Plasma 2-ClHA clearance is reduced in PPAR-α−/− mice. Male PPAR-α−/− mice (open bars) and wild-type C57BL/6J mice (closed bars) were treated with 2-Cl−[d2-4,4]HA (0.35 mg/100 g body weight) with plasma collected at indicated times (A) and urine collected over 24 h (B) post intraperitoneal treatment with subsequent analysis of 2-Cl−[d2-4,4]HA (A) and [d2]ClAdA (B), as described in the Materials and Methods. Similarly, tissue levels of 2-Cl−[d2-4,4]HA were analyzed (C). Values are the mean ± SEM with n = 5 per group. *P < 0.05 for comparisons between PPAR-α−/− mice and wild-type C57BL/6J mice. Male wild-type C57BL/6J mice were treated with vehicle or Wy 14643 (10 mg/kg/day ip) for 2 days before 2-Cl−[d2-4,4]HA (0.9 mg/100 g body weight) was administered (D). Three hours later, blood was collected and plasma 2-Cl−[d2-4,4]HA was analyzed as described in the Materials and Methods. *P < 0.05 for comparison between Wy 14643 treatment and vehicle.
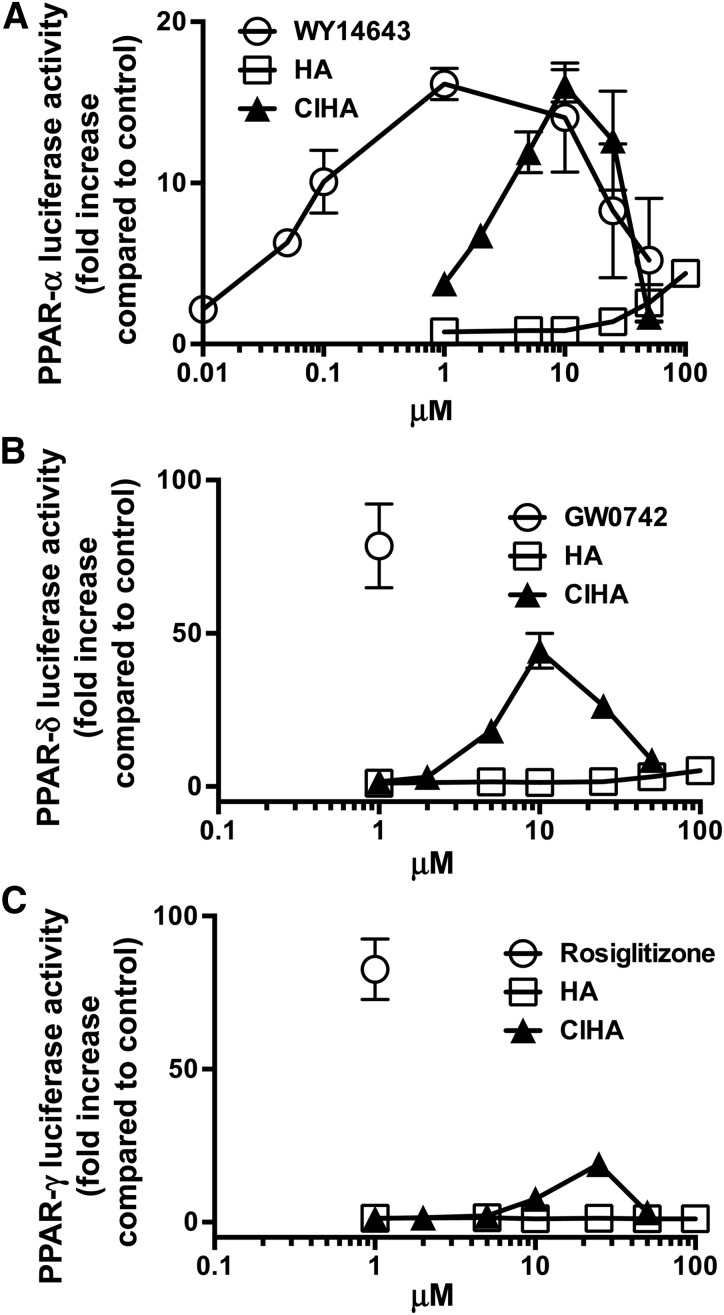
2-ClHA increases PPAR activity in HEK293 cells transfected with PPAR reporters
Because 1) PPAR-α activation can be induced by long-chain polyunsaturated fatty acids (22) and 2) our data indicated that α-ClFA catabolism at higher concentrations of α-ClFA was accelerated at rates that were higher than anticipated, we tested the possibility that α-ClFA alters the activity of PPAR-α, -δ, and -γ. HEK293 cells were transiently transfected with a reporter gene system that uses luciferase to monitor the activation of the ligand binding domain of specific subtypes of PPAR. Cells were treated with different concentrations of 2-ClHA, ranging between 0.1 and 50 μM (Fig. 5A). Compared with HA-stimulated cells, PPAR-α activity was significantly higher in 2-ClHA-stimulated cells, with only minimal PPAR-α activity observed at 100 μM HA (Fig. 5A). Increased PPAR-α activity by increasing 2-ClHA concentration was biphasic and decreased beyond 10 μM. This decrease may be due to toxic effects of 2-ClHA in transfected HEK293 cells at concentrations above 10 μM. The EC50 for 2-ClHA activation of PPAR-α was 4.5 μM. PPAR-α activity decreased at both 25 and 50 μM 2-ClHA. Also shown in Fig. 5A is the response to the synthetic PPAR-α ligand, Wy 14643, which elicits approximately the same maximal PPAR-α activity compared with 2-ClHA (2-ClHA maximal activation is 99% of Wy 14643 maximal activation), but at a much lower concentration compared with 2-ClHA (EC50 = 0.04 μM). Similar to 2-ClHA, increased PPAR-α activity elicited by increasing concentrations of Wy 14643 was biphasic with reduced activation at concentrations above 10 μM. Parallel studies were performed to test whether α-ClFA stimulates the activation of PPAR-γ and PPAR-δ we used the same reporter gene system to measure PPAR-γ and PPAR-δ activity in response to 2-ClHA treatment in HEK293 cells. Results shown in Fig. 5B, C demonstrate that 2-ClHA also activates PPAR-δ and PPAR-γ to a lesser extent compared with synthetic ligands, particularly in comparison of these responses to that of PPAR-α. Maximal 2-ClHA activation of PPAR-δ (Fig. 5B) was 56% that of the single concentration of synthetic ligand tested, GW0742. The 2-ClHA appears to be a weaker activator of PPAR-γ (Fig. 5C) with a maximal activation that is 23% that of the single concentration of synthetic ligand tested, rosiglitazone. The EC50s for 2-ClHA activation of PPAR-δ and -γ are 5.8 and 12.8 μM.
Fig. 5.
PPAR ligand analysis for 2-ClHA. In (A), (B), and (C), HEK293 cells were transfected with a luciferase reporter gene system to evaluate PPAR-α, PPAR-δ, and PPAR-γ activities, respectively. Cells were then treated for 24 h with the indicated concentrations of either HA, 2-ClHA, or isozyme-specific ligand (1 μM Wy 14643, GW0742, or rosiglitazone for PPAR-α, PPAR-δ, and PPAR-γ, respectively; all in closed circles). Values represent the mean ± SEM with n = 3. In some cases the error bars are within the symbol for the mean.
2-ClHA stimulates PPAR activity in primary mouse hepatocytes
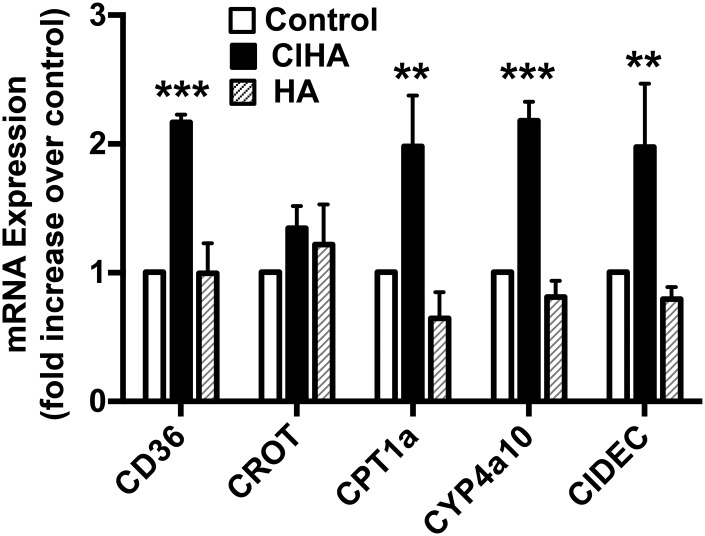
To evaluate endogenous PPAR activity, we examined mRNA levels of PPAR-α target genes in primary mouse hepatocytes treated with 2-ClHA for 18 h. The mRNA levels of the PPAR-α target genes, CD36, CPT1a, Cyp4a10, and CIDEC, were significantly increased (compared with controls) in hepatocytes treated with 20 μM 2-ClHA, but not with 20 μM HA (Fig. 6). In contrast, carnitine-O-octanoyl transferase mRNA levels were not significantly increased in response to 2-ClHA treatment.
Fig. 6.
Increased expression of PPAR-α mRNA targets in the presence of 2-ClHA. The expression level of PPAR-α target genes is compared between primary mouse hepatocytes treated with either 20 μM HA or 20 μM 2-ClHA. Values represent the mean ± SEM with n = 4–5. **P < 0.01 and ***P < 0.001 versus control. For comparison, fold-increases for WY 14643 in CD36, carnitine-O-octanoyl transferase (CROT), Cpt1a, Cyp4a10, and CIDEC were 6.3 ± 1.2, 2.1 ± 0.4, 3.3 ± 0.9, 5.3 ± 0.5, and 3.5 ± 0.4, respectively (mean ± SEM).
DISCUSSION
Evidence is evolving that chlorinated lipids generated from plasmalogens have significant biological roles. At sites of inflammation, chlorinated lipid levels, including 2-chlorohexadecanal and α-ClFA, reach concentrations as high as 90 μM (17, 35, 37). While concentrations of chlorinated lipids reach micromolar levels at sites of inflammation, the concentration in the systemic blood is lower, ranging from 1 to 100 nM, due to dilution by the whole animal blood volume. In endotoxemia models of inflammation, systemic plasma levels of α-ClFA rise from 1 nM (naïve rats) to 3 nM (LPS treated) (18). Systemic plasma levels of α-ClFA were recently shown to reach levels above 100 nM in mice subjected to a sublethal exposure of chlorine gas (38). It is likely that plasma levels of α-ClFA may be even higher in mice subjected to higher exposure levels of chlorine gas. The precursor of 2-ClHA, 2-chlorohexadecanal, induces the expression of many inflammatory mediators, such as cyclooxygenase-2, causes blood-brain barrier dysfunction, and is both a neutrophil chemoattractant and an inhibitor of endothelial nitric oxide synthase (14–17, 35). More recently, we reported that 2-ClHA accumulates in activated monocytes and elicits apoptosis, which implies that 2-ClHA may contribute to inflammatory diseases such as atherosclerosis (17). Taken together, it is important to consider mechanisms to decrease chlorinated lipids in biological systems. One strategy to potentially reduce the effects of 2-ClHA is to accelerate the removal and clearance of this lipid from the body. Previous studies have shown that 2-ClHA is catabolized in the liver through a pathway initiated by ω-fatty acid oxidation, which subsequently results in the production of 2-ClAdA that is excreted in urine (18). Accordingly, because PPAR-α is a well-known regulator of fatty acid oxidation, the role of PPAR-α activity in 2-ClHA catabolism was tested.
Initial studies explored the effects of PPAR-α agonist using two hepatic cell models. Activation of PPAR-α by Wy 14643 caused increased production of the final oxidation product of α-ClFA, 2-ClAdA, in both HepG2 cells and wild-type mouse primary hepatocytes. Notably, mouse primary hepatocytes have a greater capability to catabolize 2-ClHA compared with HepG2 cells because the 2-ClAdA produced under similar conditions (50 μM, comparing Figs. 1A, 2B) is much greater in primary mouse hepatocytes with and without PPAR-α activation. Furthermore, the regulation of 2-ClHA catabolism and clearance by PPAR-α-modulated pathways is supported by: 1) reduced clearance of exogenously administered 2-Cl−[d2-4,4]HA by PPAR-α−/− mice; 2) accelerated clearance of exogenously administered 2-Cl−[d2-4,4]HA in mice treated with the PPAR-α agonist, Wy 14643; and 3) reduced hepatic 2-ClHA levels following fasting-refeeding regimens. PPAR-α-modulated pathways that likely impact the clearance of 2-ClHA include CD36 expression (39) and multiple genes regulating fatty acid oxidation (23).
Similar to our previous studies in HepG2 cells (18), data shown in Fig. 2A, B demonstrate that mouse primary hepatocyte catabolism of 50 μM 2-ClHA is much greater than that of 10 μM 2-ClHA, suggesting that some pathways may be induced. Because higher concentrations of extracellular 2-ClHA led to greater than expected levels of 2-ClAdA, we considered the possibility that 2-ClHA has PPAR-α agonist activity. Indeed, reporter assays demonstrated that 2-ClHA is an activator of PPAR-α. The 2-ClHA elicited maximal PPAR-α activity similar to that of the synthetic agonist, Wy 14643, but at concentrations (EC50 = 4.8 μM) much higher than that of Wy 14643 (EC50 = 0.04 μM). In contrast, HA did not activate PPAR-α at similar concentrations compared with 2-ClHA. Interestingly, the reporter assays performed in HEK293 cells suggested that maximal PPAR-α would be achieved at 10 μM, but our catabolism data in primary mouse hepatocytes (Fig. 2) suggest induction of catabolism at concentrations above 10 μM. This disparity may simply reflect differences in responsiveness of primary cells compared with those of the transfected HEK293 cells used for reporter assays. The 2-ClHA (20 μM) treatments of primary mouse hepatocytes also led to increased CD36, CPT1a, Cyp4a10, and CIDEC mRNA. Importantly, Cyp4a10 mediates ω-oxidation, and this enzyme may be critical for 2-ClHA oxidation.
Plasma 2-Cl−[d2-4,4]HA levels were elevated and urinary [d2]ClAdA was reduced following 2-Cl−[d2-4,4]HA via intraperitoneal injection in PPAR-α−/− mice, compared with C57Bl/6J mice. Although plasma 2-Cl−[d2-4,4]HA levels were higher 24 h post intraperitoneal injection of 2-Cl−[d2-4,4]HA in PPAR-α−/− mice compared with C57Bl/6J mice, there were reduced plasma 2-Cl−[d2-4,4]HA levels in PPAR-α−/− mice 24 h post injection compared with earlier time points analyzed, despite reduced [d2]ClAdA in the urine collected over the entire 24 h interval from PPAR-α−/− mice compared with C57Bl/6J mice. A significant component of the removal of 2-Cl−[d2-4,4]HA from the plasma can be attributed to distribution of 2-Cl−[d2-4,4]HA in organs. For example, hepatic 2-Cl−[d2-4,4]HA levels are twice as high in PPAR-α−/− mice compared with C57Bl/6J mice in this study.
Taken together, our data show, for the first time, that activation of PPAR-α by a synthetic agonist, Wy 14643, accelerates the catabolism of 2-ClHA both in vitro and in vivo, resulting in the increased production of 2-ClAdA and the decrease of 2-ClHA in cultured hepatocytes and plasma of mice. Furthermore, 2-ClHA levels similar to those that are physiologically produced at sites of inflammation are capable of activating PPAR-α and, to a lesser degree, PPAR-γ and PPAR-δ. Thus, the use of PPAR-α agonists, such as fibrates, may provide a novel strategy to attenuate the adverse effects of chlorinated lipids.
Supplementary Material
Footnotes
Abbreviations:
- AdA
- adipic acid
- 2-ClAdA
- 2-chloroadipic acid
- 2-Cl−[d2]HA
- 2-chloro-[d2-4,4]hexadecanoic acid
- 2-Cl−[d4]HA
- 2-chloro-[d4-7,7,8,8]hexadecanoic acid
- 2-ClHA
- 2-chlorohexadecanoic acid
- α-ClDCA
- α-chlorodicarboxylic acid
- α-ClFA
- α-chlorofatty acid
- α-ClFALD
- α-chlorofatty aldehyde
- [d4]AdA
- [d4-3,3,4,4]adipic acid
- HA
- hexadecanoic acid
- MPO
- myeloperoxidase
- TAG
- triglyceride
- Wy 14643
- pirinixic acid
Research reported in this study was supported by National Institutes of Health Grants RO1 HL074214 (D.A.F.), GM115553 (D.A.F.), and HL107797 (Á.B.), as well as American Heart Association Grant-in-Aid 15GRNT25750022 (D.A.F.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Lampert M. B., and Weiss S. J.. 1983. The chlorinating potential of the human monocyte. Blood. 62: 645–651. [PubMed] [Google Scholar]
- 2.Nauseef W. M., Root R. K., Newman S. L., and Malech H. L.. 1983. Inhibition of zymosan activation of human neutrophil oxidative metabolism by a mouse monoclonal antibody. Blood. 62: 635–644. [PubMed] [Google Scholar]
- 3.Sepe S. M., and Clark R. A.. 1985. Oxidant membrane injury by the neutrophil myeloperoxidase system. II. Injury by stimulated neutrophils and protection by lipid-soluble antioxidants. J. Immunol. 134: 1896–1901. [PubMed] [Google Scholar]
- 4.Sepe S. M., and Clark R. A.. 1985. Oxidant membrane injury by the neutrophil myeloperoxidase system. I. Characterization of a liposome model and injury by myeloperoxidase, hydrogen peroxide, and halides. J. Immunol. 134: 1888–1895. [PubMed] [Google Scholar]
- 5.Chilton F. H., and Murphy R. C.. 1986. Remodeling of arachidonate-containing phosphoglycerides within the human neutrophil. J. Biol. Chem. 261: 7771–7777. [PubMed] [Google Scholar]
- 6.Ford D. A., and Gross R. W.. 1989. Plasmenylethanolamine is the major storage depot for arachidonic acid in rabbit vascular smooth muscle and is rapidly hydrolyzed after angiotensin ii stimulation. Proc. Natl. Acad. Sci. USA. 86: 3479–3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy E. J., Joseph L., Stephens R., and Horrocks L. A.. 1992. Phospholipid composition of cultured human endothelial cells. Lipids. 27: 150–153. [DOI] [PubMed] [Google Scholar]
- 8.Hazen S. L., Hall C. R., Ford D. A., and Gross R. W.. 1993. Isolation of a human myocardial cytosolic phospholipase A2 isoform. Fast atom bombardment mass spectroscopic and reverse-phase high pressure liquid chromatography identification of choline and ethanolamine glycerophospholipid substrates. J. Clin. Invest. 91: 2513–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ford D. A. 2010. Lipid oxidation by hypochlorous acid: chlorinated lipids in atherosclerosis and myocardial ischemia. Clin. Lipidol. 5: 835–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albert C. J., Crowley J. R., Hsu F. F., Thukkani A. K., and Ford D. A.. 2001. Reactive chlorinating species produced by myeloperoxidase target the vinyl ether bond of plasmalogens: identification of 2-chlorohexadecanal. J. Biol. Chem. 276: 23733–23741. [DOI] [PubMed] [Google Scholar]
- 11.Skaff O., Pattison D. I., and Davies M. J.. 2008. The vinyl ether linkages of plasmalogens are favored targets for myeloperoxidase-derived oxidants: a kinetic study. Biochemistry. 47: 8237–8245. [DOI] [PubMed] [Google Scholar]
- 12.Wildsmith K. R., Albert C. J., Anbukumar D. S., and Ford D. A.. 2006. Metabolism of myeloperoxidase-derived 2-chlorohexadecanal. J. Biol. Chem. 281: 16849–16860. [DOI] [PubMed] [Google Scholar]
- 13.Anbukumar D. S., Shornick L. P., Albert C. J., Steward M. M., Zoeller R. A., Neumann W. L., and Ford D. A.. 2010. Chlorinated lipid species in activated human neutrophils: lipid metabolites of 2-chlorohexadecanal. J. Lipid Res. 51: 1085–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marsche G., Heller R., Fauler G., Kovacevic A., Nuszkowski A., Graier W., Sattler W., and Malle E.. 2004. 2-chlorohexadecanal derived from hypochlorite-modified high-density lipoprotein-associated plasmalogen is a natural inhibitor of endothelial nitric oxide biosynthesis. Arterioscler. Thromb. Vasc. Biol. 24: 2302–2306. [DOI] [PubMed] [Google Scholar]
- 15.Ullen A., Fauler G., Bernhart E., Nusshold C., Reicher H., Leis H. J., Malle E., and Sattler W.. 2012. Phloretin ameliorates 2-chlorohexadecanal-mediated brain microvascular endothelial cell dysfunction in vitro. Free Radic. Biol. Med. 53: 1770–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Messner M. C., Albert C. J., and Ford D. A.. 2008. 2-chlorohexadecanal and 2-chlorohexadecanoic acid induce cox-2 expression in human coronary artery endothelial cells. Lipids. 43: 581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang W. Y., Albert C. J., and Ford D. A.. 2014. Alpha-chlorofatty acid accumulates in activated monocytes and causes apoptosis through reactive oxygen species production and endoplasmic reticulum stress. Arterioscler. Thromb. Vasc. Biol. 34: 526–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brahmbhatt V. V., Albert C. J., Anbukumar D. S., Cunningham B. A., Neumann W. L., and Ford D. A.. 2010. {omega}-oxidation of {alpha}-chlorinated fatty acids: identification of {alpha}-chlorinated dicarboxylic acids. J. Biol. Chem. 285: 41255–41269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chawla A., Repa J. J., Evans R. M., and Mangelsdorf D. J.. 2001. Nuclear receptors and lipid physiology: opening the x-files. Science. 294: 1866–1870. [DOI] [PubMed] [Google Scholar]
- 20.Pyper S. R., Viswakarma N., Yu S., and Reddy J. K.. 2010. PPARalpha: energy combustion, hypolipidemia, inflammation and cancer. Nucl. Recept. Signal. 8: e002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Devchand P. R., Keller H., Peters J. M., Vazquez M., Gonzalez F. J., and Wahli W.. 1996. The PPARalpha-leukotriene B4 pathway to inflammation control. Nature. 384: 39–43. [DOI] [PubMed] [Google Scholar]
- 22.Forman B. M., Chen J., and Evans R. M.. 1997. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc. Natl. Acad. Sci. USA. 94: 4312–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mandard S., Muller M., and Kersten S.. 2004. Peroxisome proliferator-activated receptor alpha target genes. Cell. Mol. Life Sci. 61: 393–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen R. M., Marquart T. J., Albert C. J., Suchy F. J., Wang D. Q., Ananthanarayanan M., Ford D. A., and Baldan A.. 2012. miR-33 controls the expression of biliary transporters, and mediates statin- and diet-induced hepatotoxicity. EMBO Mol. Med. 4: 882–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wacker B. K., Albert C. J., Ford B. A., and Ford D. A.. 2013. Strategies for the analysis of chlorinated lipids in biological systems. Free Radic. Biol. Med. 59: 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang W. Y., Albert C. J., and Ford D. A.. 2013. Approaches for the analysis of chlorinated lipids. Anal. Biochem. 443: 148–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aroor A. R., Habibi J., Ford D. A., Nistala R., Lastra G., Manrique C., Dunham M. M., Ford K. D., Thyfault J. P., Parks E. J., et al. 2015. Dipeptidyl peptidase-4 inhibition ameliorates Western diet-induced hepatic steatosis and insulin resistance through hepatic lipid remodeling and modulation of hepatic mitochondrial function. Diabetes. 64: 1988–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han X., and Gross R. W.. 2001. Quantitative analysis and molecular species fingerprinting of triacylglyceride molecular species directly from lipid extracts of biological samples by electrospray ionization tandem mass spectrometry. Anal. Biochem. 295: 88–100. [DOI] [PubMed] [Google Scholar]
- 29.Quehenberger O., Armando A., Dumlao D., Stephens D. L., and Dennis E. A.. 2008. Lipidomics analysis of essential fatty acids in macrophages. Prostaglandins Leukot. Essent. Fatty Acids. 79: 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rong X., Albert C. J., Hong C., Duerr M. A., Chamberlain B. T., Tarling E. J., Ito A., Gao J., Wang B., Edwards P. A., et al. 2013. LXRs regulate ER stress and inflammation through dynamic modulation of membrane phospholipid composition. Cell Metab. 18: 685–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forman B. M., Tzameli I., Choi H. S., Chen J., Simha D., Seol W., Evans R. M., and Moore D. D.. 1998. Androstane metabolites bind to and deactivate the nuclear receptor CAR-beta. Nature. 395: 612–615. [DOI] [PubMed] [Google Scholar]
- 32.Vega R. B., Huss J. M., and Kelly D. P.. 2000. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol. Cell. Biol. 20: 1868–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu Y., Qi C., Jain S., Rao M. S., and Reddy J. K.. 1997. Isolation and characterization of PBP, a protein that interacts with peroxisome proliferator-activated receptor. J. Biol. Chem. 272: 25500–25506. [DOI] [PubMed] [Google Scholar]
- 34.Thukkani A. K., Albert C. J., Wildsmith K. R., Messner M. C., Martinson B. D., Hsu F. F., and Ford D. A.. 2003. Myeloperoxidase-derived reactive chlorinating species from human monocytes target plasmalogens in low density lipoprotein. J. Biol. Chem. 278: 36365–36372. [DOI] [PubMed] [Google Scholar]
- 35.Thukkani A. K., Hsu F. F., Crowley J. R., Wysolmerski R. B., Albert C. J., and Ford D. A.. 2002. Reactive chlorinating species produced during neutrophil activation target tissue plasmalogens: production of the chemoattractant, 2-chlorohexadecanal. J. Biol. Chem. 277: 3842–3849. [DOI] [PubMed] [Google Scholar]
- 36.Thukkani A. K., Martinson B. D., Albert C. J., Vogler G. A., and Ford D. A.. 2005. Neutrophil-mediated accumulation of 2-CLHDA during myocardial infarction: 2-CLHDA-mediated myocardial injury. Am. J. Physiol. Heart Circ. Physiol. 288: H2955–H2964. [DOI] [PubMed] [Google Scholar]
- 37.Thukkani A. K., McHowat J., Hsu F. F., Brennan M. L., Hazen S. L., and Ford D. A.. 2003. Identification of alpha-chloro fatty aldehydes and unsaturated lysophosphatidylcholine molecular species in human atherosclerotic lesions. Circulation. 108: 3128–3133. [DOI] [PubMed] [Google Scholar]
- 38.Ford D. A., Honavar J., Albert C. J., Duerr M. A., Oh J. Y., Doran S., Matalon S., and Patel R. P.. 2016. Formation of chlorinated lipids post-chlorine gas exposure. J. Lipid Res. 57: 1529–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guerre-Millo M., Gervois P., Raspe E., Madsen L., Poulain P., Derudas B., Herbert J. M., Winegar D. A., Willson T. M., Fruchart J. C., et al. 2000. Peroxisome proliferator-activated receptor alpha activators improve insulin sensitivity and reduce adiposity. J. Biol. Chem. 275: 16638–16642. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.