Abstract
Truncated oxidized glycerophospholipids (ox-PLs) are bioactive lipids resulting from oxidative stress. The catabolic pathways for truncated ox-PLs are not fully understood. Lysosomal phospholipase A2 (LPLA2) with phospholipase A and transacylase activities is a key enzyme in phospholipid homeostasis. The present study assessed whether LPLA2 could hydrolyze truncated ox-PLs. Incubation of LPLA2 with liposomes consisting of 1,2-O-octadecenyl-sn-glycero-3-phosphocholine (DODPC)/1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) or truncated oxidized phosphatidylcholine (ox-PC)/N-acetylsphingosine (NAS) under acidic conditions resulted in the preferential deacylation at the sn-1 position of the truncated ox-PCs. Additionally, the release of free fatty acid from the truncated ox-PCs preferentially occurred compared with the NAS-acylation. Incubation of LPLA2 with the liposomes consisting of DODPC/DOPC/truncated ox-PC/NAS resulted in the same preferential fatty acid release from the truncated ox-PC. The cationic amphiphilic drug, amiodarone, did not inhibit such fatty acid release, indicating that truncated ox-PCs partition from the lipid membrane into the aqueous phase and react with free LPLA2. Consistent with this mechanism, the hydrolysis of some truncated ox-PCs, but not DOPC, by LPLA2 was detected at neutral pH. Additionally, LPLA2-overexpressed Chinese hamster ovary cells efficiently catabolized truncated ox-PC and were protected from growth inhibition. These findings support the existence of a novel catabolic pathway for truncated ox-PLs via LPLA2.
Keywords: lysosome, truncated oxidized phospholipid, catabolic pathway, positional specificity
Reactive oxygen species (ROS), generated enzymatically or nonenzymatically, modify proteins, nucleic acids, and lipids (1). Oxidative stress induced by an imbalance between generation and elimination of ROS affects cellular structure and function. Within lipid membranes and particles, unsaturated and polyunsaturated acyl groups conjugated at the sn-2 position of phospholipids (PLs) are susceptible to ROS inducing nonenzymatic fragmentation of the unsaturated acyl chains. As a result, various truncated oxidized glycerophospholipids (ox-PLs) are formed with an oxidized short or medium chain terminating in an aldehyde or carboxylic acid at the sn-2 position (2). The truncated ox-PLs produced under oxidative stress derive mainly from phosphatidylcholine (PC) and mediate inflammatory, apoptotic, and innate immune responses via cellular receptor-dependent or -independent pathways (3).
For example, 1-palmitoyl-2-(5′-oxo-valeroyl)-sn-glycero-3-phosphocholine (POVPC) and 1-palmitoyl-2-glutaryl-sn-glycero-3-phosphocholine (PGPC) are found in oxidized LDL and act on intracellular signaling systems associated with platelet activating factor (PAF) receptors (4), scavenger receptors (5), and Toll-like receptors (6). Truncated ox-PLs perturb the lipid bilayer and its physical properties (7, 8). Azelaoyl-PCs, including 1-palmitoyl-2-azelaoyl-sn-glycero-3-phosphocholine (PAzPC), can disrupt lipid membranes, inducing cytotoxicity in a receptor-independent manner (9, 10) and enhancing the interaction between Bax and mitochondrial membranes in an apoptotic pathway (11). The 1-palmitoyl-2-(9′-oxo-nonanoyl)-sn-glycero-3-phosphocholine (PONPC) accelerates the fibrillation of gelsolin associated with familial Finnish type amyloidosis (12), in which the PONPC-mediated fibrillation of gelsolin is dependent on both the concentration and the aggregation state of the PONPC-PL complex.
Despite accumulating evidence indicating that truncated ox-PLs mediate much of the pathology associated with oxidative stress, our understanding of the catabolic pathways for ox-PLs is still limited. Two enzymes with PLA2 activity, present in the circulation, have been shown to degrade truncated ox-PLs. PAF acetylhydrolase (PAF-AH), found in plasma, is classified as the group VII PLA2. PAF-AH removes the acetyl group from the sn-2 position of PAF (13). The enzyme is physically associated with lipoprotein and hydrolyzes not only PAF, but also truncated ox-PLs that are structurally similar to PAF (14). While there has been debate regarding the biological function of the plasma PAF-AH, some studies have shown a pro-atherogenic role of the enzyme in the inflammatory response elicited by oxidized LDL (15–17). LCAT also catalyzes the degradation of truncated ox-PLs resulting in the formation of oxidized truncated fatty acids and lysophosphatidylcholine (lyso-PC), supporting a novel role of LCAT in detoxification of truncated ox-PLs produced during lipoprotein oxidation (18). Thus both PAF-AH and LCAT may play a role in the degradation of truncated ox-PLs in plasma. Intracellular PAF-AH II also hydrolyzes PAF and truncated oxidized PC (ox-PC) (19). The overexpression of PAF-AH II in Chinese hamster ovary (CHO)-K1 cells renders them resistant to oxidative stress (20), suggesting that PAF-AH II functions as an anti-oxidant phospholipase within the cell.
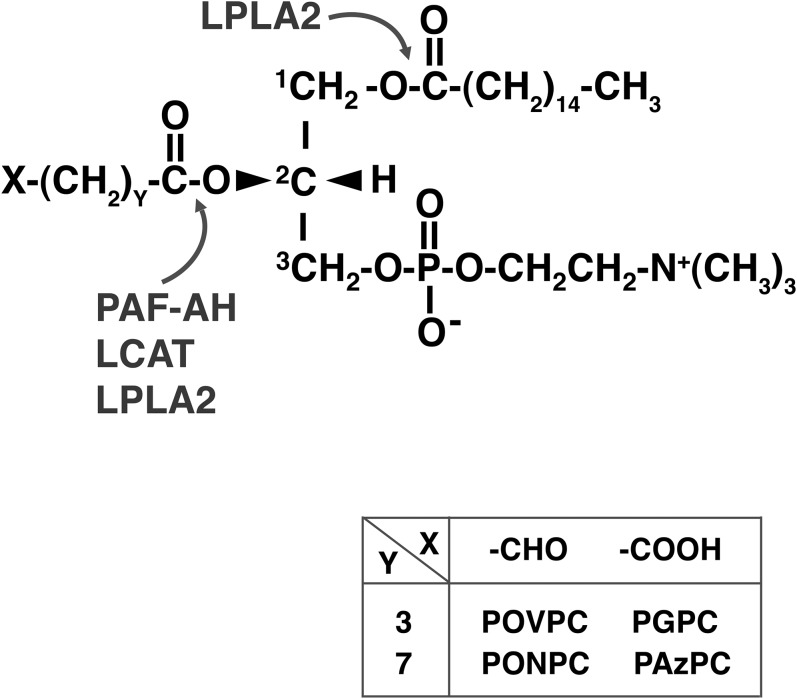
The phospholipase A2 in lysosomes is a major intracellular hydrolase that degrades PLs and plays a crucial role in cellular PL homeostasis (21). A deficiency of lysosomal phospholipase A2 (LPLA2) in mice results in phospholipidosis in alveolar macrophages (21). Some of the truncated ox-PLs produced intracellularly or extracellularly are probably transferred to lysosomes, raising the possibility that LPLA2 plays an important role in the intracellular catabolism of truncated ox-PLs. In the present study, we investigated the degradation of four well-known truncated ox-PLs, POVPC, PGPC, PONPC, and PAzPC (Fig. 1), by LPLA2 in vitro.
Fig. 1.
Chemical structures of truncated ox-PCs and possible cleavage sites by hydrolases with phospholipase A2 activity.
MATERIALS AND METHODS
Reagents
The 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-O-octadecenyl-sn-glycero-3-phosphocholine (DODPC), N-acetylsphingosine (NAS), N-oleoyl-sphingosine, POVPC, PGPC, PONPC, PAzPC, 1-palmitoyl-2-acetyl-sn-glycero-3-phosphocholine (PAcPC), and 1-nonanoyl-sn-glycero-3-phosphocholine were obtained from Avanti Polar Lipids Corp. (Alabaster, AL); recombinant mouse LPLA2 was from Proteos (Kalamazoo, MI); high-performance TLC (HPTLC) silica gel plates (10 × 20 cm) were from Merck (Darmstadt, Germany).
Argentation of the HPTLC plate
A HPTLC plate was immersed into 100 mM AgNO3 in acetonitrile and incubated for 10 min. The plate was dried in a fume hood and baked in an oven for 30 min at 100°C.
Transacylase and deacylase activities of LPLA2
For the preparation of liposomes, DODPC, truncated ox-PL, and NAS (molar ratio, 2.4:1:1.05) were added to a glass tube and dried down under a stream of nitrogen gas. The dried lipid mixture was dispersed into 50 mM sodium citrate (pH 4.5) by a probe-type sonicator for 8 min in an icy water bath.
LPLA2 transacylase and deacylase assays were conducted using a reaction mixture consisting of 49 mM sodium citrate (pH 4.5), 10 μg/ml BSA, and 40 μM NAS incorporated into PL liposomes containing 92 μM DODPC and 38 μM truncated ox-PL in the presence or absence of 40 or 80 ng/ml recombinant mouse LPLA2 in a total volume of 500 μl. The reaction was initiated by adding the enzyme. The mixture was incubated at 37°C and was terminated by adding and mixing 3 ml of chloroform/methanol (2:1) plus 0.3 ml of 0.9% (w/v) NaCl to the reaction mixture at the indicated time after initiating the reaction. The mixture was centrifuged at 800 g for 5 min at room temperature. The resultant lower organic layer was transferred into a second glass tube and dried down under a stream of nitrogen gas. The dried lipid was dissolved in 40 μl of chloroform/methanol (2:1), applied on an HPTLC plate, and developed in a solvent system consisting of chloroform/acetic acid (90:10, v/v). The plate was dried and soaked in 8% (w/v) CuSO4, 5H2O, 6.8% (v/v) H3PO4, and 32% (v/v) methanol. When an argentate HPLC plate was used, the dried plate was first immersed in a solvent consisting of methanol/acetic acid/water (20:0.5:79.5, v/v) and then soaked in the copper sulfate solution (22). The uniformly wet plate was briefly dried by a hair dryer and charred for 15 min in a 150°C oven. The plate was scanned and the content of the product (1-O-acyl-NAS and fatty acid) was estimated by National Institutes of Health ImageJ 1.37v with N-oleoyl-sphingosine, oleic acid, or palmitic acid as a standard.
To measure the hydrolysis of truncated ox-PLs by LPLA2 under acidic and neutral conditions, liposomes consisting of DODPC and truncated ox-PL (molar ratio, 2.4:1) were prepared using 50 mM sodium citrate (pH 4.5) or 50 mM HEPES (pH 7.4), as described above. The reaction was carried out using a reaction mixture consisting of 49 mM sodium citrate (pH 4.5) or 49 mM HEPES (pH 7.4), 10 μg/ml BSA, and DODPC/truncated ox-PL liposomes in the presence or absence of recombinant mouse LPLA2, as described above. The release of fatty acid and formation of lyso-PC from truncated ox-PLs by LPLA2 were examined by HPTLC using a solvent system consisting of chloroform/methanol/pyridine (98:2:0.5, v/v) or chloroform/methanol/water (60:35:8, v/v). These reaction products were detected and quantified as described above.
Establishment of human LPLA2 stable expressed cells
The Flp-In CHO-K1 cell line was obtained from Invitrogen (Carlsbad, CA) and maintained in Ham’s F12 medium (Sigma-Aldrich, St. Louis, MO) with 10% (v/v) fetal calf serum. To establish human LPLA2 stable expression in the cells, the Flp-In system (Invitrogen) was used. cDNA for human LPLA2 was subcloned into a pcDNA5/FRT expression vector and transfected into Flp-In CHO-K1 cells with pOG44 plasmids using LipofectAMINE 2000 (Invitrogen). Then, the cells were selected in the medium with 500 μg/ml hygromycin B (Calbiochem, San Diego, CA) for 3 weeks. In this report, CHO cells and CHO/LPLA2 cells denote Flp-In CHO-K1 cells and human LPLA2 stable expressed Flp-In CHO-K1 cells, respectively.
PAzPC treatment of CHO and LPLA2 stable expression CHO cells
CHO and CHO/LPLA2 cells were grown in Ham’s F12K medium (Thermo Fisher Scientific, Waltham, MA) supplemented with 10% fetal bovine serum. In the present study, 2.5 × 105 cells were seeded in each well of a 6-well plate. Upon reaching 80% confluence, the cells were washed three times with 2 ml of PBS and incubated with 2 ml of MEM (α) medium (Thermo Fisher Scientific) at 37°C, 5% CO2. The medium was removed after a 2 h incubation and replaced with 2 ml of MEM (α) medium with or without 15 μM PAzPC. The cells were treated for 1 or 4 h with or without PAzPC at 37°C, 5% CO2. The medium was then collected in a small conical tube and the cells were washed three times with 2 ml of PBS and fixed with 1 ml of cold methanol. The fixed cells were scraped and transferred into a glass tube. Another 1 ml of methanol was used to recover the remaining cells in the well. The 6-well plate was air dried in a hood.
Lipid extraction
One milliliter of chloroform was added to the glass tube and briefly sonicated in a water bath type sonicator. The cell suspension was centrifuged at 2,300 g for 30 min at 20°C. The resultant supernatant was transferred into a glass tube, dried down under a stream of nitrogen gas, and stored at −20°C as the cellular lipid fraction. The cell pellet was air dried in a hood and stored at room temperature as the cellular protein fraction. The lipid fraction was redissolved with 3 ml of chloroform/methanol (2:1, v/v) and partitioned by centrifugation at 800 g for 5 min at 20°C following the addition of 0.8 ml of 30 mM Na-citrate (pH 4.5). The organic layer was collected and used for TLC analysis.
For protein determination, the cell-protein fraction was treated with 1 ml of 0.2 N NaOH in a water bath type sonicator and the insoluble materials were removed by centrifugation. Also, 1 ml of 0.2 N NaOH was added to each well of the dried 6-well plate. The plate was gently shaken for 2 h at room temperature. The NaOH solutions (the cell-protein fraction’s NaOH and the dried well’s NaOH) were used to determine the cell protein content in each well by the method of BCA assay (23).
RESULTS
Degradation of truncated ox-PLs by LPLA2 under acidic conditions
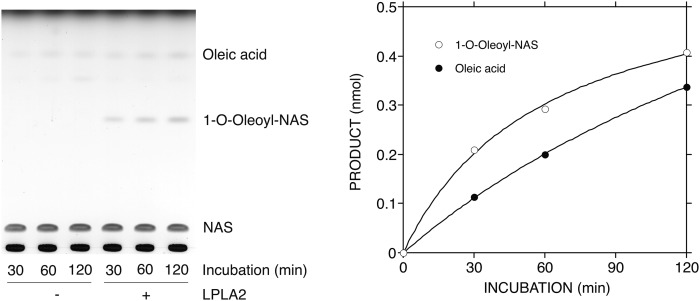
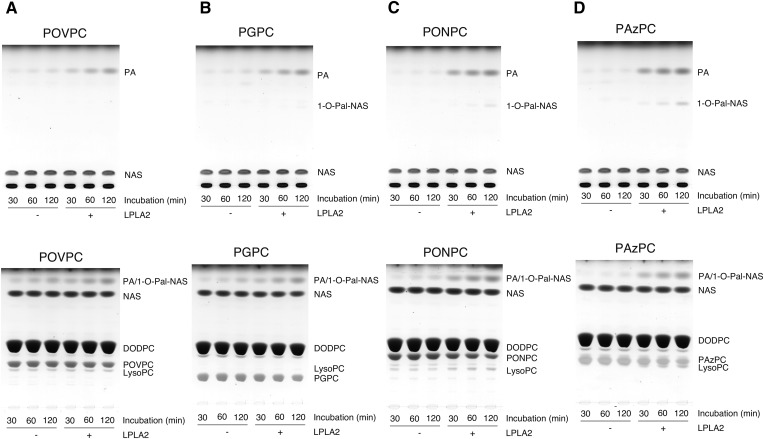
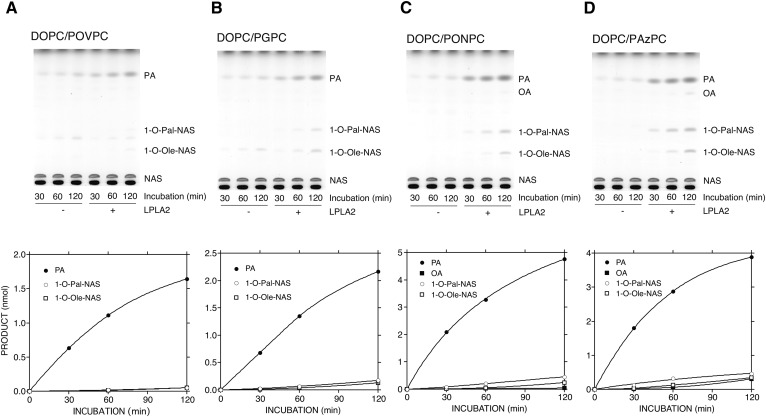
LPLA2 is an enzyme that exhibits both transacylase and phospholipase A activities under acidic conditions (24). In this study, DOPC and truncated ox-PC were loaded into DODPC-based liposomes. DODPC is a 1,2-O-dialkyl glycerophospholipid and not a substrate of LPLA2. As expected, when LPLA2 was incubated with DODPC/DOPC/NAS liposomes under acidic conditions, both the formation of 1-O-oleoyl-NAS and release of oleic acid by the enzyme were observed (Fig. 2). However, when ox-PCs instead of DOPC were used, the preferential release of palmitic acid by LPLA2 was observed in all cases (Figs. 3, 4). Interestingly, the enzyme activity measured as fatty acid release in the presence of ox-PCs was superior to the total activity (transacylase activity plus phospholipase A activity) by the enzyme in the presence of DOPC. However, the formation of 1-O-palmitoyl-NAS by the enzyme was much lower than the release of palmitic acid (Fig. 3, upper panels; Fig. 4). Although the short-chain ceramide acylation was visible in the presence of the medium-chain ox-PCs, including PONPC and PAzPC, it was much less apparent in the presence of the short-chain ox-PCs, POVPC, and PGPC (Fig. 3, upper panels; Fig. 4). The acylation of NAS by oxidized truncated acyl chain was not detected.
Fig. 2.
Degradation of nonoxidized two long-chain PC by LPLA2. The reaction mixture contained 49 mM sodium citrate (pH 4.5), 10 μg/ml BSA, liposomes (130 μM PL), and 40 ng/ml of recombinant mouse LPLA2 in 500 μl of total volume. The liposomes consisted of DODPC/DOPC/NAS (2.4:1:1.05, molar ratio). The reaction was initiated by the addition of recombinant LPLA2 and kept for 30 to 120 min at 37°C. The reaction products were extracted, separated with an HPTLC plate using a solvent system consisting of chloroform/acetic acid (9:1, v/v) (A), and plotted against the incubation time (B) as described in the Materials and Methods.
Fig. 3.
Degradation of truncated ox-PCs by LPLA2. The reaction mixture contained 49 mM sodium citrate (pH 4.5), 10 μg/ml BSA, liposomes (130 μM PL), and 40 ng/ml of recombinant mouse LPLA2 in 500 μl of total volume. The liposomes consisted of DODPC/truncated ox-PC/NAS (2.4:1:1.05, molar ratio). The reaction was initiated by the addition of recombinant LPLA2 and kept for 30 to 120 min at 37°C. The reaction products were extracted and separated with an HPTLC plate using a solvent system consisting of chloroform/acetic acid (9:1, v/v) (upper panels) or chloroform/methanol/water (60:35:8, v/v) (lower panels) as described in the Materials and Methods. 1-O-Pal-NAS, 1-O-palmitoyl-NAS; PA, palmitic acid; LysoPC, 1-palmitoyl-sn-glycero-3-phosphocholine. The truncated ox-PC tested was POVPC, PGPC, PONPC, and PAzPC in A, B, C, and D, respectively.
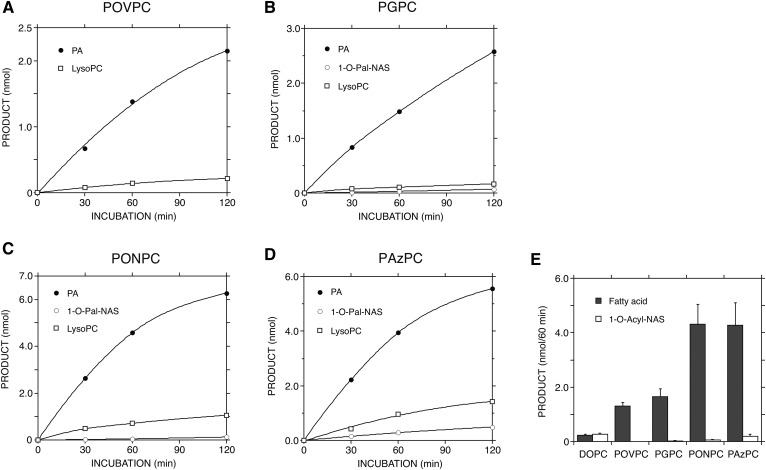
Fig. 4.
Reaction profiles of truncated ox-PCs by LPLA2. The reaction products of truncated ox-PCs by LPLA2 shown in Fig. 3 were quantified by scanning the plate, as described in the Materials and Methods, and plotted against the incubation time (A–D). In addition, liposomes consisting of DODPC/DOPC or truncated ox-PC/NAS (2.4:1:1.05, molar ratio) were incubated with the enzyme for 60 min at 37°C, as shown in Figs. 2, 3. The reaction products were extracted and separated with a HPTLC plate using a solvent system consisting of chloroform/acetic acid (9:1, v/v), as described in the Materials and Methods. The reaction products, long-chain fatty acid, and 1-O-acyl-NAS were plotted against the substrate (E). Error bars indicate standard deviation (n = 3).
In contrast to DOPC, the reaction products released from the sn-2 position of truncated ox-PCs are polar small molecules and soluble. The reaction products (1-lyso-2-truncated ox-PCs) formed by releasing at the sn-1 position of the ox-PCs are also polar molecules and can be recovered in the aqueous phase. By use of a short-chain lyso-PC, 1-nonanoyl-sn-glycero-3-phosphocholine, we confirmed that 80% of this lyso-PC was recovered in the aqueous phase, indicating that 1-lyso-2-truncated ox-PCs are more polar than 1-nonanoyl-sn-glycero-3-phosphocholine and are probably distributed in the aqueous phase during lipid extraction. In addition, lyso-PCs with a short acyl chain (C<15) are not visualized by the charring method used in this study. Thus, to better document the phospholipase A2 activity against the truncated ox-PC by LPLA2, the formation of 1-palmitoyl-sn-glycero-3-phosphocholine (2-lyso-PC) was followed during the reaction (Fig. 3, lower panels). The 2-lyso-PC formation by LPLA2 occurred in the presence of all ox-PC substrates. Lyso-PC levels were higher than the 1-O-acyl-NASs. Additionally, the lyso-PC levels were greater when the medium-chain ox-PCs (PONPC and PAzPC), as opposed to the short-chain ox-PCs (POVPC and PGPC), were used as substrates (Fig. 4). In the same reaction, however, the palmitic acid release was much greater than the lyso-PC formation (Fig. 4). Figure 4E compares the deacylase activity of long acyl chain from DOPC and four truncated ox-PCs by LPLA2.
Preferential degradation of truncated ox-PLs by LPLA2 under acidic conditions
To confirm that LPLA2 preferentially degrades truncated ox-PLs compared with the nonoxidized two long-chain PLs, DODPC/NAS liposomes loaded with DOPC/truncated ox-PC (1:1, molar ratio) were prepared and incubated with LPLA2 under the same conditions as the experiment in Fig. 3.
A silver-impregnated plate was employed to separate palmitic acid and 1-O-palmitoyl-NAS from oleic acid and 1-O-oleoyl-NAS, respectively (22). The deacylation of the truncated ox-PCs by LPLA2 was superior to that of DOPC by LPLA2 (Fig. 5). The pattern of palmitic acid release observed in this study was similar to that in the earlier experiments (Figs. 3, 4). Also, a slight increase in the formation of 1-O-acyl-NAS was noticed.
Fig. 5.
Preferential hydrolysis of truncated ox-PCs by LPLA2. The reaction mixture contained 49 mM sodium citrate (pH 4.5), 10 μg/ml BSA, liposomes (130 μM PL), and 40 ng/ml of recombinant mouse LPLA2 in 500 μl of total volume. The liposomes consisted of DODPC/DOPC/truncated ox-PC/NAS (2.4:1:1:1.05, molar ratio). The reaction was initiated by the addition of recombinant LPLA2 and kept for 30 to 120 min at 37°C. The reaction products were extracted and separated with an argentate HPTLC plate using a solvent system consisting of chloroform/acetic acid (9:1, v/v) (upper panels). The reaction products, long-chain fatty acid, and 1-O-acyl-NAS in each reaction were quantified as described in Fig. 4 and were plotted against the incubation time (lower panels). PA, palmitic acid; OA, oleic acid; 1-O-Pal-NAS, 1-O-palmitoyl-NAS; 1-O-Ole-NAS, 1-O-oleoyl-NAS. The truncated ox-PC tested was POVPC, PGPC, PONPC, and PAzPC in A, B, C, and D, respectively.
Effect of amiodarone on degradation of truncated ox-PLs by LPLA2
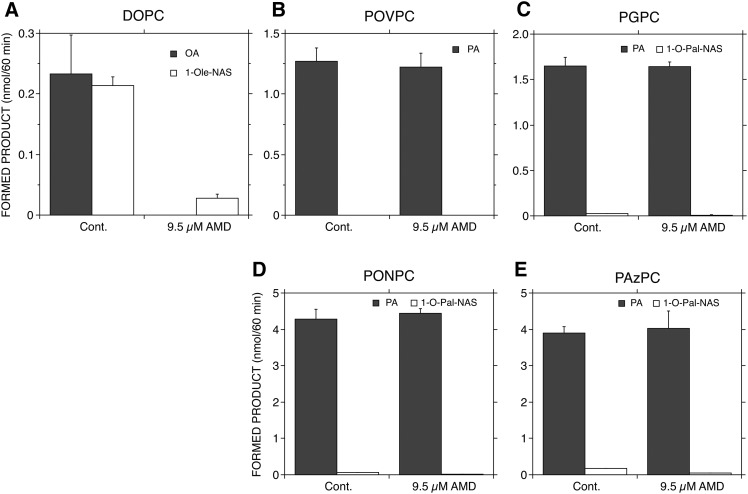
When the cationic amphiphilic drug, amiodarone (AMD), is loaded into the PL membrane under acidic conditions, the activity of LPLA2 is inhibited in a dose-dependent manner (25). The inhibition of LPLA2 activity is due to interference of the interfacial interaction of LPLA2 with the lipid membrane by AMD (25).
To understand whether the hydrolysis of truncated ox-PCs by LPLA2 is dependent on the interaction between the membrane and enzyme, 9.5 μM of AMD was loaded into DODPC/DOPC or truncated ox-PC/NAS liposomes under acidic conditions. In DODPC/DOPC/NAS liposomes, both phospholipase A and transacylase activities by LPLA2 were markedly reduced by AMD (Fig. 6A), consistent with our prior observations (25). Interestingly, in DODPC/truncated ox-PC/NAS liposomes, the transacylase activity was significantly inhibited in the presence of AMD, but the release of palmitic acid by LPLA2 was not significantly different in the presence or absence of AMD (Fig. 6B–E).
Fig. 6.
Effect of AMD on degradation of truncated ox-PC by LPLA2. The reaction mixture consisting of 50 mM sodium citrate (pH 4.5), 10 μg/ml BSA, and liposomes (131 μM PL) was preincubated with or without 9.7 μM AMD in 490 μl of total volume for 5 min at 37°C. The liposomes consisted of DODPC/DOPC or truncated ox-PC/NAS (2.4:1:1.05, molar ratio). The reaction was initiated by the addition of 10 μl of 2 μg/ml recombinant LPLA2 and kept for 60 min at 37°C. The reaction products were extracted and separated with an HPTLC plate using a solvent system consisting of chloroform/acetic acid (9:1, v/v), as described in the Materials and Methods. Formed 1-O-acyl-NAS and fatty acid were quantified as described in the Materials and Methods. Error bars indicate standard deviation (n = 3). DOPC (A), POVPC (B), PGPC (C), PONPC (D), or PAzPC (E) was loaded into DODPC-based liposomes. Cont. denotes control (without AMD).
Degradation of truncated ox-PLs by LPLA2 under neutral conditions
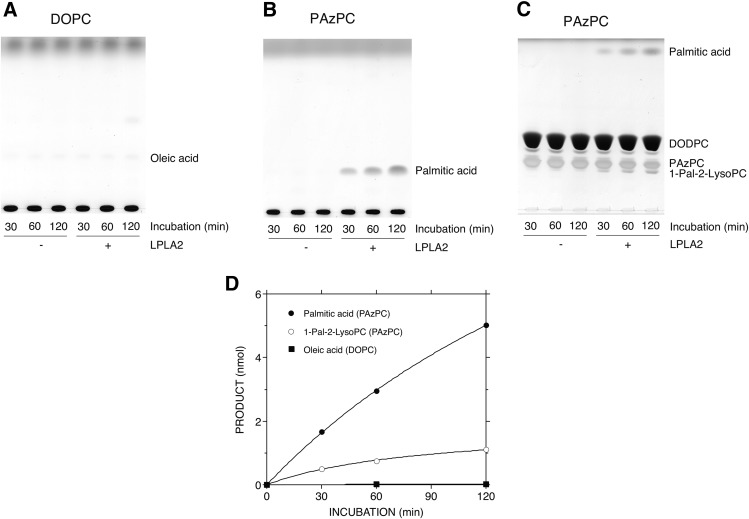
To further examine the hydrolysis of truncated ox-PCs by LPLA2, DODPC/DOPC and DODPC/truncated ox-PC liposomes were prepared in HEPES buffer (pH 7.4) and incubated with LPLA2 under neutral conditions. Because truncated ox-PLs with medium acyl chain lengths, PONPC and PAzPC, were better substrates for LPLA2 at acidic pH (Figs. 2–5), PAzPC was therefore first studied as a substrate of LPLA2 under neutral conditions.
DOPC was very poor as a substrate for LPLA2 at neutral pH (Fig. 7A). However, LPLA2 did hydrolyze PAzPC under the same neutral conditions (Fig. 7B, C). The enzyme not only released palmitic acid (Fig. 7B), but also produced 2-lyso-PC (Fig. 7C). As shown in Fig. 7D, the preferential selectivity at the sn-1 position of the enzyme to the substrate was still retained. Although LPLA2 showed the same positional selectivity against PGPC, PONPC, and POVPC as PAzPC (data not shown), the release rate of the sn-1 palmitic acid from PGPC, PONPC, and POVPC by LPLA2 was significantly lower than that of PAzPC (Fig. 8).
Fig. 7.
Hydrolysis of one medium-chain ox-PC by LPLA2 under neutral conditions. The reaction mixture contained 49 mM HEPES (pH 7.4), 10 μg/ml BSA, liposomes (130 μM PL), and 80 ng/ml of recombinant mouse LPLA2 in 500 μl of total volume. The liposomes consisted of DODPC/DOPC or one medium-chain ox-PC (PAzPC) (2.4:1, molar ratio). The reaction was initiated by the addition of recombinant LPLA2 and kept for 30 to 120 min at 37°C. The reaction products were extracted and separated with a HPTLC plate using a solvent system consisting of chloroform/methanol/pyridine (98:2:0.5, v/v) (A, B) or chloroform/methanol/water (60:35:8, v/v) (C), as described in the Materials and Methods. The reaction products produced by LPLA2 in (A–C) were quantified by scanning the plate, as described in the Materials and Methods, and were plotted against the incubation time (D). 1-Pal-2-LysoPC, 1-palmitoyl-sn-glycero-3-phosphocholine.
Fig. 8.
Hydrolysis of truncated ox-PCs by LPLA2 under acidic and neutral conditions. Liposomes consisting of DODPC/DOPC or truncated ox-PC (2.4:1, molar ratio) were incubated with the enzyme for 60 min at 37°C under acidic and neutral conditions as shown in Figs. 4, 7. The reaction products were extracted and separated with a HPTLC plate using a solvent system consisting of chloroform/methanol/pyridine (98:2:0.5, v/v), as described in the Materials and Methods. The reaction products, long-chain fatty acids, were plotted against the pH tested. Error bars indicate standard deviation (n = 3).
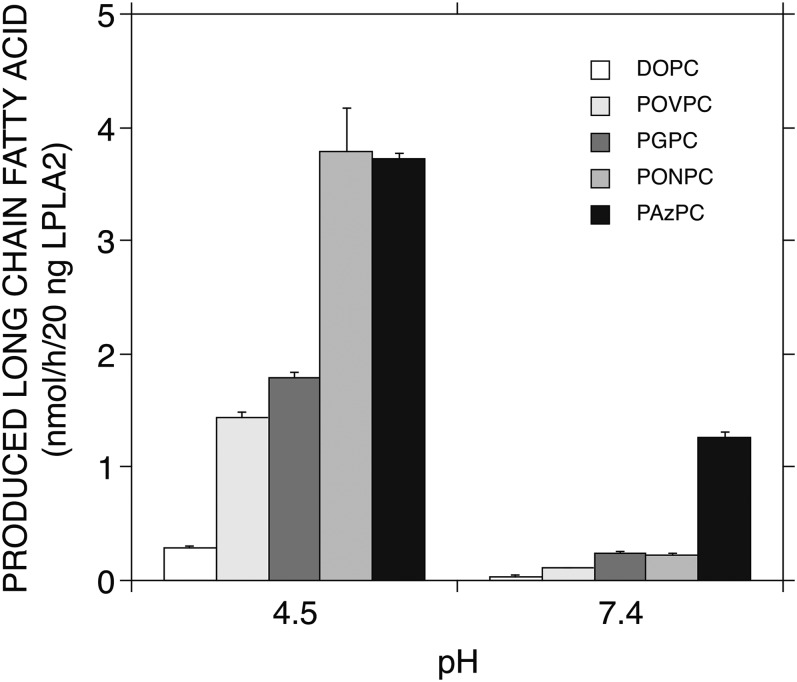
The release of palmitic acid from the truncated ox-PCs by LPLA2 under neutral conditions was compared with that under acidic conditions (Fig. 8). As shown in the earlier experiments (Figs. 2–4), the release rate of palmitic acid from the truncated ox-PCs under acidic conditions, which was superior to the release rate of oleic acid from DOPC, was dependent on the carbon chain length of the sn-2 position. POVPC/PONPC and PGPC/PAzPC have a formyl group and a carboxyl group, respectively, at the terminal of the sn-2 acyl chain. A significant reduction of palmitic acid release from the truncated ox-PCs by the enzyme was observed under neutral conditions. The degree of the reduction was significant by rank order of POVPC > PONPC > PGPC > PAzPC (Fig. 8) and was more pronounced for those ox-PCs with a formyl group than those with a carboxyl group.
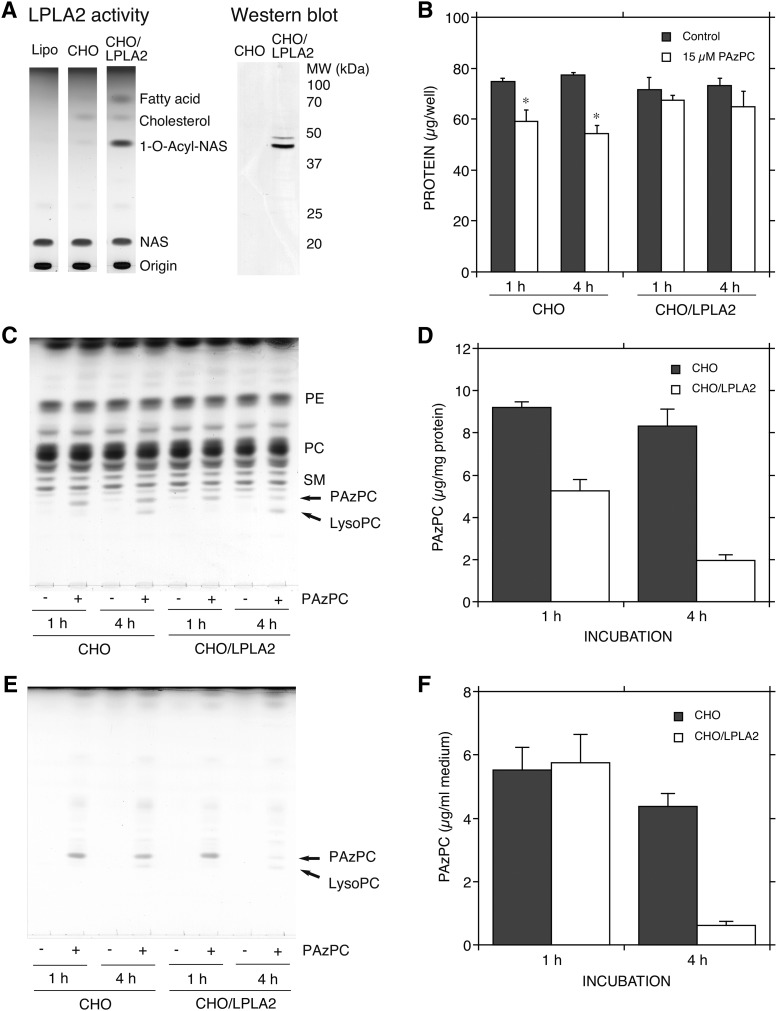
Clearance of PAzPC by LPLA2 in cell culture system
Exogenous PAzPC is rapidly internalized in mammalian cells, and induces cytotoxicity through disruption of intracellular targets (26). In the present study, CHO cells and CHO/LPLA2 cells in which LPLA2 was constitutively overexpressed were treated with 15 μM PAzPC, a concentration that is lower than the critical micellar concentration of PAzPC (27). The enzymatic activities and protein levels of LPLA2 in those cells were confirmed by using DOPC/sulfatide/NAS liposomes and anti-human LPLA2 polyclonal antibody, respectively (Fig. 9A). In the wild-type CHO cells, some cells were shrunken and detached from the culture plate after treatment with PAzPC (data not shown). PAzPC caused cell growth inhibition of CHO cells, but not CHO/LPLA2 cells (Fig. 9B). By lipid analysis, PAzPC was shown to be incorporated into both types of cells (Fig. 9C). There was no significant difference in the level of PAzPC in CHO cells in between the 1 and 4 h treatments (Fig. 9C, D). By contrast, after PAzPC treatment for 1 h, the level of PAzPC in CHO/LPLA2 cells was about two times lower than that in CHO cells, and after the treatment for 4 h, the level of PAzPC in CHO/LPLA2 cells was four times lower than that in CHO cells (Fig. 9C, D). There was no significant difference in PAzPC concentration in the medium between CHO and CHO/LPLA2 cells after treatment for 1 h with PAzPC (Fig. 9E, F). However, the PAzPC concentration in CHO/LPLA2 cell-cultured medium was markedly reduced after the treatment for 4 h, but not in CHO cell-cultured medium (Fig. 9E, F). LPLA2 is a secreted protein as well as a lysosomal protein (28–30). LPLA2 activity in the CHO/LPLA2 cell-cultured medium after a 4 h treatment with or without PAzPC was three times higher than that after a 1 h treatment (data not shown).
Fig. 9.
Removal of PAzPC by LPLA2 in mammalian cells. LPLA2 activity and protein expression in CHO cells stably transfected with LPLA2 (CHO/LPLA2 cells) and in CHO cells were confirmed by using liposome assay and anti-LPLA2 polyclonal antibody, respectively, as previously reported (42) (A). The cell culture, lipid, and protein analyses were carried out as described in the Materials and Methods. The cells (2.5 × 105 per well) were treated in the presence or absence of 15 μM PAzPC. After the treatment for 1 or 4 h, the media were collected and 200 μl were immediately used for lipid analysis (E). The PAzPC content in the medium was plotted against the incubation time (F). Error bars indicate standard deviation (n = 3). The cells were washed with PBS, fixed with methanol, and used for protein and lipid analysis. The protein content is shown in (B). Error bars indicate standard deviation (n = 3). Significance was determined using a nonpaired Student’s t-test. Asterisks indicate significant differences between the control and PAzPC-treated CHO cells (P < 0.005). The lipid extract obtained from 50 μg of the cell protein was applied to each lane in (C). The PAzPC content in the cell was plotted against the incubation time (D). Error bars indicate standard deviation (n = 3). CHO, CHO cells; CHO/LPLA2, LPLA2-overexpressed CHO cells. Similar results were also obtained from another independent experiment.
DISCUSSION
In the present study, we first found that LPLA2 preferentially cleaves and releases a long acyl chain at the sn-1 position of truncated ox-PCs and also preferentially hydrolyzes truncated ox-PCs compared with nonoxidized PLs with two long acyl chains (like DOPC) under acidic conditions (Figs. 2–4). Experimental conditions using DODPC/DOPC/truncated ox-PC/NAS liposomes further supported the substrate preference of the truncated ox-PCs compared with DOPC under acidic conditions (Fig. 5). The positional selectivity of LPLA2 against the acyl groups of PLs is broad and dependent on chain length and number of unsaturated bonds (22). In our earlier study, we found that when LPLA2 was incubated with DODPC/PAcPC/NAS liposomes (the same molar ratio as the present study) under acidic conditions, there was release of palmitic acid, but not formation of 1-O-palmitoyl-NAS or 2-lyso-PC (data not shown). The degree of palmitate release from PAcPC was similar to that of oleate release from DOPC. Also, the degradation of 1-palmitoyl-sn-glycero-3-phosphocholine loaded into DODPC/NAS liposomes by LPLA2 was not detectable in the present assay system (data not shown). Thus, the carbon chain length and polar group of the truncated aliphatic chain at the sn-2 position of the ox-PCs may play a substantial role in determining the preferential release of a long-chain acyl group at the sn-1 position of truncated ox-PCs by LPLA2. In addition, there is a helical wheel alignment between amino acid residues 138 and 155 of mouse LPLA2 that is located in proximity to the catalytic serine residue 165 (31, 32). This is an amphipathic helix, and may be involved in the binding of PL substrate at the active site (31, 32). Possibly, the palmitoyl group at the sn-1 position of the truncated ox-PC tested is more hydrophobic and more easily accessible to the catalytic site than the truncated acyl group at the sn-2 position, thus explaining its preference as a scissile fatty acyl group.
In the reaction of LPLA2 with nonoxidized two long-chain PLs, the binding of LPLA2 to the PL membrane is a critical step, and is based on an electrostatic interaction between the LPLA2 and membrane (25). The estimated isoelectric point of mouse LPLA2, estimated from its primary structure, is 5.9. Under acidic conditions, the lipid membrane is positively charged by the addition of AMD, inhibiting binding of LPLA2 to the membrane (25). Actually, AMD extensively inhibited both phospholipase A and transacylase activities of LPLA2 against two long-chain PLs, DOPC (Fig. 6A), indicating that the reaction of LPLA2 with DOPC occurs at the lipid-water interface. However, unlike nonoxidized two long-chain PCs, the truncated ox-PCs with an exposed carboxyl or formyl group at their sn-2 position are more polar and water-soluble, and spontaneously distribute from the lipid bilayer into the aqueous phase (33). With truncated oxidized lipids as substrates under acidic conditions, the transacylation by LPLA2 was a minor reaction (Figs. 3–5). AMD, without exception, suppressed the transacylation but failed to inhibit the hydrolysis of truncated ox-PCs by LPLA2 (Fig. 6B–E). Taken together, these observations indicate that the truncated ox-PCs transfer spontaneously from the lipid membrane into the aqueous phase and react with free LPLA2.
The binding of LPLA2 to PL membranes is markedly reduced under neutral conditions (25). It affects the enzyme activity against long-chain PLs. However, LPLA2 shows esterase activity to a water-soluble small molecule, p-nitrophenyl butyrate, over a wide pH range (25). This esterase reaction occurs in the aqueous phase akin to the reaction of LPLA2 against the truncated ox-PCs. In this study, PAzPC containing a carboxyl group at the end of an sn-2 medium acyl chain was an excellent substrate for LPLA2 under neutral conditions. By contrast, DOPC was a poor substrate for LPLA2 under the same conditions (Fig. 7). The positional selectivity of LPLA2 against PAzPC favored the sn-1 site, the same as at acidic pH (Figs. 4D, 7D). The level of this palmitate release activity at pH 7.4 was one-third of that at pH 4.5, but four times higher than that of oleate release at pH 4.5 in use of DOPC (Fig. 8). The other three ox-PCs tested were also hydrolyzed by LPLA2 under neutral conditions with the sn-1 positional preference. In using PGPC containing a carboxyl group at the end of an sn-2 short acyl chain, the degree of palmitate release by the enzyme at pH 7.4 was one-fifth of that at pH 4.5 (Fig. 8). However, it was mostly equal to that of oleate release at pH 4.5 with DOPC.
POVPC and PONPC containing a formyl group at the end of a short chain and medium chain of the sn-2 position, respectively, showed a very marked decrease in palmitate release under neutral conditions relative to acidic conditions (Fig. 8). A formyl group, as found in both POVPC and PONPC, is known to react with unprotonated primary amino groups of proteins and to form a Schiff base (34). Therefore, the formyl group of those truncated ox-PCs could modify the amino groups of LPLA2 under neutral, but not acidic, conditions. Such protein modifications might induce a conformational change or block substrate binding in the active site and thus explain their apparent lower efficiency as substrates at neutral pH. Indeed, there was no significant difference in the palmitate release activity of LPLA2 between formyl-type versus carboxyl-type oxidized truncated PCs under acidic conditions (Figs. 4E, 8).
The PAF-AH and LCAT found in the extracellular space are able to deacylate the truncated oxidized sn-2 acyl chains of ox-PLs. On this basis, these enzymes are believed to play a role in the elimination of truncated ox-PLs in plasma (18, 35). However, recent reports have shown that the truncated PLs, such as PAF and truncated ox-PCs, in the circulation are preliminarily removed by the liver and kidney via the PL transporter, TMEM30a (36, 37). Intracellular PAF-AH II is a cytosolic protein that translocates to the cell membrane under oxidative conditions and is thought to play a role in degradation of truncated ox-PLs produced during the oxidation, as well as PAF (20). It is possible that extracellular truncated PLs transported into the cell via the TMEN30a transporter are hydrolyzed by the intracellular PAH-AH. However, some ox-PLs produced intracellularly or extracellularly may reach the lysosome via endocytosis, phagocytosis, and autophagy. Momutzi et al. (38) reported that exogenously added fluorescent PGPC analogs are rapidly taken up by vascular smooth cells and are translocated into the lysosomes and metabolized. The present study showed that PAzPC incorporated into LPLA2-expressing CHO cells is more efficiently hydrolyzed in the cells compared with that into CHO cells (Fig. 9C, D). These results indicate that LPLA2 may represent a novel pathway by which the truncated ox-PLs are decomposed in the intracellular acidic compartments, including lysosomes.
Unlike nonoxidized PLs with two long-chain acyl groups, the truncated ox-PCs tested were hydrolyzed by LPLA2 even under neutral conditions (Fig. 8). Interestingly, LPLA2 activity found in the LPLA2-overexpressed CHO cell culture medium after 4 h treatment with or without PAzPC was three times higher than that after 1 h treatment. At the same time, the level of PAzPC in the medium was markedly reduced after 4 h treatment with the ox-PC (Fig. 9E, F). These results support a possibility that there is an alternative catabolic pathway of truncated ox-PLs via LPLA2 in the extracellular space. Possibly, both intracellular and extracellular LPLA2s are associated with rapid removal of truncated ox-PLs from the system and, as a result, the cytotoxicity by those lipids is limited.
Taniyama et al. (39) reported that LPLA2-deficient peritoneal macrophages are more sensitive to apoptosis following exposure to oxidized LDL, which contains various ox-PLs including truncated ox-PCs working as triggers of the inflammatory process in atherosclerosis (40), and suggested that LPLA2 is a link between macrophage apoptosis and atherogenesis (39). Experimental autoimmune uveitis in rats may be another model for discerning the relationship between LPLA2 activity and ox-PL metabolism at sites of inflammation in the eye (41).
Taken together, the present findings show that LPLA2 may play an important role in clearance and detoxification of truncated ox-PLs that act as mediators in various processes such as inflammation, immune response, and apoptosis.
Acknowledgments
The authors thank Dr. Motoko Takahashi, Department of Biochemistry, Sapporo Medical University School of Medicine, for her support and assistance during the present research, in particular CHO cell study.
Footnotes
Abbreviations:
- AMD
- amiodarone
- CHO
- Chinese hamster ovary
- DODPC
- 1,2-O-octadecenyl-sn-glycero-3-phosphocholine
- DOPC
- 1,2-dioleoyl-sn-glycero-3-phosphocholine
- LPLA2
- lysosomal phospholipase A2
- lyso-PC
- lysophosphatidylcholine
- 2-lyso-PC
- 1-palmitoyl-sn-glycero-3-phosphocholine
- NAS
- N-acetylsphingosine
- ox-PC
- oxidized phosphatidylcholine
- ox-PL
- oxidized glycerophospholipid
- PAcPC
- 1-palmitoyl-2-acetyl-sn-glycero-3-phosphocholine
- PAF
- platelet activating factor
- PAF-AH
- platelet activating factor acetylhydrolase
- PAzPC
- 1-palmitoyl-2-azelaoyl-sn-glycero-3-phosphocholine
- PC
- phosphatidylcholine
- PGPC
- 1-palmitoyl-2-glutaryl-sn-glycero-3-phosphocholine
- PL
- phospholipid
- PONPC
- 1-palmitoyl-2-(9′-oxo-nonanoyl)-sn-glycero-3-phosphocholine
- POVPC
- 1-palmitoyl-2-(5′-oxo-valeroyl)-sn-glycero-3-phosphocholine
- ROS
- reactive oxygen species
This work was supported by Japan Society for the Promotion of Science Grant 26462665 (M.H.), National Institutes of Health Grants RO1HL22416 and RO1AR056991, and a Department of Veterans Affairs Merit Review Award (J.A.S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Kohen R., and Nyska A.. 2002. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 30: 620–650. [DOI] [PubMed] [Google Scholar]
- 2.Fruhwirth G. O., Loidl A., and Hermetter A.. 2007. Oxidized phospholipids: from molecular properties to disease. Biochim. Biophys. Acta. 1772: 718–736. [DOI] [PubMed] [Google Scholar]
- 3.Bochkov V. N., Oskolkova O. V., Birukov K. G., Levonen A. L., Binder C. J., and Stockl J.. 2010. Generation and biological activities of oxidized phospholipids. Antioxid. Redox Signal. 12: 1009–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pégorier S., Stengel D., Durand H., Croset M., and Ninio E.. 2006. Oxidized phospholipid: POVPC binds to platelet-activating-factor receptor on human macrophages. Implications in atherosclerosis. Atherosclerosis. 188: 433–443. [DOI] [PubMed] [Google Scholar]
- 5.Gao D., Ashraf M. Z., Kar N. S., Lin D., Sayre L. M., and Podrez E. A.. 2010. Structural basis for the recognition of oxidized phospholipids in oxidized low density lipoproteins by class B scavenger receptors CD36 and SR-BI. J. Biol. Chem. 285: 4447–4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erridge C., Kennedy S., Spickett C. M., and Webb D. J.. 2008. Oxidized phospholipid inhibition of toll-like receptor (TLR) signaling is restricted to TLR2 and TLR4: roles for CD14, LPS-binding protein, and MD2 as targets for specificity of inhibition. J. Biol. Chem. 283: 24748–24759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabatini K., Mattila J. P., Megli F. M., and Kinnunen P. K.. 2006. Characterization of two oxidatively modified phospholipids in mixed monolayers with DPPC. Biophys. J. 90: 4488–4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beranova L., Cwiklik L., Jurkiewicz P., Hof M., and Jungwirth P.. 2010. Oxidation changes physical properties of phospholipid bilayers: fluorescence spectroscopy and molecular simulations. Langmuir. 26: 6140–6144. [DOI] [PubMed] [Google Scholar]
- 9.Itabe H., Kushi Y., Handa S., and Inoue K.. 1988. Identification of 2-azelaoylphosphatidylcholine as one of the cytotoxic products generated during oxyhemoglobin-induced peroxidation of phosphatidylcholine. Biochim. Biophys. Acta. 962: 8–15. [PubMed] [Google Scholar]
- 10.Chen R., Yang L., and McIntyre T. M.. 2007. Cytotoxic phospholipid oxidation products. Cell death from mitochondrial damage and the intrinsic caspase cascade. J. Biol. Chem. 282: 24842–24850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallgren M., Lidman M., Pham Q. D., Cyprych K., and Grobner G.. 2012. The oxidized phospholipid PazePC modulates interactions between Bax and mitochondrial membranes. Biochim. Biophys. Acta. 1818: 2718–2724. [DOI] [PubMed] [Google Scholar]
- 12.Mahalka A. K., Maury C. P., and Kinnunen P. K.. 2011. 1-Palmitoyl-2-(9′-oxononanoyl)-sn-glycero-3-phosphocholine, an oxidized phospholipid, accelerates Finnish type familial gelsolin amyloidosis in vitro. Biochemistry. 50: 4877–4889. [DOI] [PubMed] [Google Scholar]
- 13.Schaloske R. H., and Dennis E. A.. 2006. The phospholipase A2 superfamily and its group numbering system. Biochim. Biophys. Acta. 1761: 1246–1259. [DOI] [PubMed] [Google Scholar]
- 14.Stremler K. E., Stafforini D. M., Prescott S. M., and McIntyre T. M.. 1991. Human plasma platelet-activating factor acetylhydrolase. Oxidatively fragmented phospholipids as substrates. J. Biol. Chem. 266: 11095–11103. [PubMed] [Google Scholar]
- 15.Shi Y., Zhang P., Zhang L., Osman H., Mohler E. R. III, Macphee C., Zalewski A., Postle A., and Wilensky R. L.. 2007. Role of lipoprotein-associated phospholipase A2 in leukocyte activation and inflammatory responses. Atherosclerosis. 191: 54–62. [DOI] [PubMed] [Google Scholar]
- 16.MacPhee C. H., Moores K. E., Boyd H. F., Dhanak D., Ife R. J., Leach C. A., Leake D. S., Milliner K. J., Patterson R. A., Suckling K. E., et al. 1999. Lipoprotein-associated phospholipase A2, platelet-activating factor acetylhydrolase, generates two bioactive products during the oxidation of low-density lipoprotein: use of a novel inhibitor. Biochem. J. 338: 479–487. [PMC free article] [PubMed] [Google Scholar]
- 17.Lavi S., McConnell J. P., Rihal C. S., Prasad A., Mathew V., Lerman L. O., and Lerman A.. 2007. Local production of lipoprotein-associated phospholipase A2 and lysophosphatidylcholine in the coronary circulation: association with early coronary atherosclerosis and endothelial dysfunction in humans. Circulation. 115: 2715–2721. [DOI] [PubMed] [Google Scholar]
- 18.Goyal J., Wang K., Liu M., and Subbaiah P. V.. 1997. Novel function of lecithin-cholesterol acyltransferase. Hydrolysis of oxidized polar phospholipids generated during lipoprotein oxidation. J. Biol. Chem. 272: 16231–16239. [DOI] [PubMed] [Google Scholar]
- 19.Hattori M., Arai H., and Inoue K.. 1993. Purification and characterization of bovine brain platelet-activating factor acetylhydrolase. J. Biol. Chem. 268: 18748–18753. [PubMed] [Google Scholar]
- 20.Matsuzawa A., Hattori K., Aoki J., Arai H., and Inoue K.. 1997. Protection against oxidative stress-induced cell death by intracellular platelet-activating factor-acetylhydrolase II. J. Biol. Chem. 272: 32315–32320. [DOI] [PubMed] [Google Scholar]
- 21.Hiraoka M., Abe A., Lu Y., Yang K., Han X., Gross R. W., and Shayman J. A.. 2006. Lysosomal phospholipase A2 and phospholipidosis. Mol. Cell. Biol. 26: 6139–6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abe A., Hiraoka M., and Shayman J. A.. 2006. Positional specificity of lysosomal phospholipase A2. J. Lipid Res. 47: 2268–2279. [DOI] [PubMed] [Google Scholar]
- 23.Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., and Klenk D. C.. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150: 76–85. [DOI] [PubMed] [Google Scholar]
- 24.Abe A., and Shayman J. A.. 1998. Purification and characterization of 1-O-acylceramide synthase, a novel phospholipase A2 with transacylase activity. J. Biol. Chem. 273: 8467–8474. [DOI] [PubMed] [Google Scholar]
- 25.Abe A., and Shayman J. A.. 2009. The role of negatively charged lipids in lysosomal phospholipase A2 function. J. Lipid Res. 50: 2027–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McIntyre T. M. 2012. Bioactive oxidatively truncated phospholipids in inflammation and apoptosis: formation, targets, and inactivation. Biochim. Biophys. Acta. 1818: 2456–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pande A. H., Kar S., and Tripathy R. K.. 2010. Oxidatively modified fatty acyl chain determines physicochemical properties of aggregates of oxidized phospholipids. Biochim. Biophys. Acta. 1798: 442–452. [DOI] [PubMed] [Google Scholar]
- 28.Abe A., Kelly R., Kollmeyer J., Hiraoka M., Lu Y., and Shayman J. A.. 2008. The secretion and uptake of lysosomal phospholipase A2 by alveolar macrophages. J. Immunol. 181: 7873–7881. [DOI] [PubMed] [Google Scholar]
- 29.Abe A., Kelly R., and Shayman J. A.. 2010. The measurement of lysosomal phospholipase A2 activity in plasma. J. Lipid Res. 51: 2464–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abe A., Hiraoka M., Inatomi S., Ohguro I., and Ohguro H.. 2012. Lysosomal phospholipase A2 activity in pig aqueous humor. Invest. Ophthalmol. Vis. Sci. 53: 152–156. [DOI] [PubMed] [Google Scholar]
- 31.Hiraoka M., Abe A., and Shayman J. A.. 2005. Structure and function of lysosomal phospholipase A2: identification of the catalytic triad and the role of cysteine residues. J. Lipid Res. 46: 2441–2447. [DOI] [PubMed] [Google Scholar]
- 32.Glukhova A., Hinkovska-Galcheva V., Kelly R., Abe A., Shayman J. A., and Tesmer J. J.. 2015. Structure and function of lysosomal phospholipase A2 and lecithin:cholesterol acyltransferase. Nat. Commun. 6: 6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Min J. H., Jain M. K., Wilder C., Paul L., Apitz-Castro R., Aspleaf D. C., and Gelb M. H.. 1999. Membrane-bound plasma platelet activating factor acetylhydrolase acts on substrate in the aqueous phase. Biochemistry. 38: 12935–12942. [DOI] [PubMed] [Google Scholar]
- 34.Means G. E., and Feeney R. E.. 1968. Reductive alkylation of amino groups in proteins. Biochemistry. 7: 2192–2201. [DOI] [PubMed] [Google Scholar]
- 35.Stafforini D. M., McIntyre T. M., Zimmerman G. A., and Prescott S. M.. 1997. Platelet-activating factor acetylhydrolases. J. Biol. Chem. 272: 17895–17898. [DOI] [PubMed] [Google Scholar]
- 36.Chen R., Brady E., and McIntyre T. M.. 2011. Human TMEM30a promotes uptake of antitumor and bioactive choline phospholipids into mammalian cells. J. Immunol. 186: 3215–3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J., Chen R., Marathe G. K., Febbraio M., Zou W., and McIntyre T. M.. 2011. Circulating platelet-activating factor is primarily cleared by transport, not intravascular hydrolysis by lipoprotein-associated phospholipase A2/PAF acetylhydrolase. Circ. Res. 108: 469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moumtzi A., Trenker M., Flicker K., Zenzmaier E., Saf R., and Hermetter A.. 2007. Import and fate of fluorescent analogs of oxidized phospholipids in vascular smooth muscle cells. J. Lipid Res. 48: 565–582. [DOI] [PubMed] [Google Scholar]
- 39.Taniyama Y., Fuse H., Satomi T., Tozawa R., Yasuhara Y., Shimakawa K., Shibata S., Hattori M., Nakata M., and Taketomi S.. 2005. Loss of lysophospholipase 3 increases atherosclerosis in apolipoprotein E-deficient mice. Biochem. Biophys. Res. Commun. 330: 104–110. [DOI] [PubMed] [Google Scholar]
- 40.Berliner J. A., Leitinger N., and Tsimikas S.. 2009. The role of oxidized phospholipids in atherosclerosis. J. Lipid Res. 50(Suppl): S207–S212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohkawa E., Hiraoka M., Abe A., Murata M., and Ohguro H.. 2016. Fluctuation of lysosomal phospholipase A2 in experimental autoimmune uveitis in rats. Exp. Eye Res. 149: 66–74. [DOI] [PubMed] [Google Scholar]
- 42.Hiraoka M., Okamoto K., Ohguro H., and Abe A.. 2013. Role of N-glycosylation of human lysosomal phospholipase A2 for the formation of catalytically active enzyme. J. Lipid Res. 54: 3098–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]