Abstract
The farnesoid X receptor (FXR) plays critical roles in plasma cholesterol metabolism, in particular HDL-cholesterol (HDL-C) homeostasis. Obeticholic acid (OCA) is a FXR agonist being developed for treating various chronic liver diseases. Previous studies reported inconsistent effects of OCA on regulating plasma cholesterol levels in different animal models and in different patient populations. The mechanisms underlying its divergent effects have not yet been thoroughly investigated. The scavenger receptor class B type I (SR-BI) is a FXR-modulated gene and the major receptor for HDL-C. We investigated the effects of OCA on hepatic SR-BI expression and correlated such effects with plasma HDL-C levels and hepatic cholesterol efflux in hyperlipidemic hamsters. We demonstrated that OCA induced a time-dependent reduction in serum HDL-C levels after 14 days of treatment, which was accompanied by a significant reduction of liver cholesterol content and increases in fecal cholesterol in OCA-treated hamsters. Importantly, hepatic SR-BI mRNA and protein levels in hamsters were increased to 1.9- and 1.8-fold of control by OCA treatment. Further investigations in normolipidemic hamsters did not reveal OCA-induced changes in serum HDL-C levels or hepatic SR-BI expression. We conclude that OCA reduces plasma HDL-C levels and promotes transhepatic cholesterol efflux in hyperlipidemic hamsters via a mechanism involving upregulation of hepatic SR-BI.
Keywords: farnesoid X receptor, scavenger receptor class B type I, high density lipoprotein cholesterol, hepatocyte nuclear factor 4 α, low density lipoprotein receptor, sterol regulatory element-binding protein 2
Bile acids (BAs) are the major metabolites of cholesterol and are predominantly produced in the liver and secreted into the small intestine. Secretion of biliary BAs and cholesterol into the intestine is the major route by which cholesterol is excreted from the body. BAs function both as detergents that facilitate lipid absorption and as endogenous ligands that regulate metabolic pathways through activation of the farnesoid X receptor (FXR).
FXR is expressed mainly in the liver, intestine, kidney, and adrenal glands. FXR forms a heterodimer with RXR to modulate expression of target genes by binding to DNA sequences referred to as FXR response elements that are typically composed of two inverted repeats separated by one nucleotide (IR1) (1–3). In addition to inducing gene expression directly, FXR mediates the repression of a number of genes involved in BA synthesis indirectly through the upregulation of small heterodimer partner (SHP) and V-Maf avian musculoaponeurotic fibrosarcoma oncogene homolog G (MAFG) that are FXR-induced transcriptional repressors (4). Activation of hepatic FXR modulates the expression of numerous hepatic genes involved in lipid homeostasis, including CYP7A1, CYP8B1, BSEP, and scavenger receptor class B type I (SR-BI).
SR-BI is a physiologically relevant HDL receptor and plays distinct roles in plasma HDL metabolism and transhepatic cholesterol efflux in preclinical animal models and in humans (5–9). SR-BI mediates selective uptake of cholesterol ester from HDL particles into cells. Consistent with this role, SR-BI expression is highest in the liver and steroidogenic tissues. Increased hepatic SR-BI expression by adenoviral-mediated overexpression in mice was associated with a reduction in plasma HDL-cholesterol (HDL-C) (10, 11). Conversely, targeted gene ablation of SR-BI in mice resulted in elevation of plasma HDL-C and reduced hepatic cholesterol excretion into feces (6, 12). Furthermore, additional studies in mice have established an inverse relationship between SR-BI expression and atherosclerosis, primarily via the mechanism of promoting reverse cholesterol transport (12, 13). The impacts of SR-BI on human HDL metabolism were demonstrated by the identification of a loss-of-function SR-BI variant that is associated with an extremely high plasma HDL-C level (9) and identification of human SR-BI variants (14, 15).
Obeticholic acid (OCA) is a first-in-class FXR agonist being developed for primary biliary cholangitis, nonalcoholic steatohepatitis (NASH), and nonalcoholic fatty liver disease (NAFLD) (16). In adult patients with NASH, OCA treatment significantly improved the biochemical and histological features of NASH and also affected plasma lipoprotein profiles in those patients with elevated total cholesterol (TC), LDL-cholesterol (LDL-C), and reduced HDL-C levels (17). The treatment-related elevations of serum TC and LDL-C were also observed in another study of NAFLD patients treated with OCA (18) and in healthy subjects (19). Interestingly, in a study of primary biliary cholangitis patients, OCA was shown to lower serum TC and HDL-C effectively without any impact on serum LDL-C (20). Currently, how OCA treatment differentially modulates plasma cholesterol metabolism in patients with various liver diseases is largely unknown.
In preclinical animal models, OCA treatment produced variable effects on plasma lipoprotein profiles. In Zucker (fa/fa) obese rats fed a normal chow diet (NCD), OCA effectively lowered plasma HDL-C and TG levels, which were accompanied by reduced expression of hepatic lipogenic genes (21). In LDL receptor (LDLR)−/− mice, OCA treatment did not alter plasma TC levels or plasma lipoprotein profile (22). In another study conducted in wild-type C57BL/6J mice fed a high-fat diet (HFD), OCA showed no effect on plasma TC level or LDL-C levels, but caused a small increase in HDL-C (23). In contrast to the lack of effect in reduction of plasma HDL-C in mice, administration of OCA to golden Syrian hamsters fed a HFD led to a reduction of HDL-C and a small increase in VLDL-cholesterol (VLDL-C), while total plasma cholesterol levels were unchanged (22, 24). Interestingly, despite the inconsistent changes on plasma HDL-C metabolism in these animal models after OCA treatment, OCA-modulated expression of typical FXR-regulated genes involved in the BA synthetic pathway, including CYP7Α1 and SHP, were consistently demonstrated in all those studies. Because SR-BI plays a key role in HDL-C uptake and the induction of hepatic SR-BI expression by OCA has not been thoroughly examined, in this current study, we investigated the effects of OCA on hepatic SR-BI expression and correlated such effects with plasma HDL-C levels and hepatic cholesterol efflux in dyslipidemic and normolipidemic hamsters. Our results demonstrate that OCA treatment effectively increased hepatic SR-BI mRNA and protein levels, which were associated with reduced plasma HDL-C levels and increased transhepatic cholesterol excretion into feces in hamsters fed a high-fat and high-cholesterol diet (HFHCD), but not in hamsters fed a NCD.
MATERIALS AND METHODS
Animals, diet, and drug treatment
All animal experiments were performed according to procedures approved by the Veterans Affairs Palo Alto Health Care System Institutional Animal Care and Use Committee. Six-week-old male golden Syrian hamsters were purchased from Harlan Sprague Dawley. Hamsters were housed (two animals per cage) under controlled temperature (22°C) and lighting (12 h light/dark cycle). Animals had free access to autoclaved water and food. After an acclimatization period of 7 days, hamsters were fed a HFHCD containing 40% calories from fat and 0.5% cholesterol (#D12107C; Research Diets, Inc., New Brunswick, NJ) for 2 weeks. OCA was suspended in 0.5% carboxyl-methyl cellulose (vehicle) at a concentration of 3 mg/ml and sonicated at 4°C in a Bioruptor 300 instrument (Diagenode, Inc.) for four to six cycles of 30 s ON/30 s OFF at a “medium” setting with intermittent vortexing. Continuous on the HFHCD, hamsters were then divided into two groups (n = 8 per group) and were given a daily dose of OCA at 10 mg/kg by oral gavage. The control group received vehicle. The drug treatment lasted 14 days. Serum samples were collected after 16 h fasting before and during the drug treatment.
In another in vivo study, male hamsters fed a NCD were treated with OCA (10 mg/kg, n = 6) or vehicle (n = 6) for 14 days and fasting serum samples were collected before and during the drug treatment. In the third in vivo study, male hamsters fed a NCD were gavaged with OCA at doses of 10, 20, or 30 mg/kg for 3 days (n = 5 per group). The control animals received vehicle (n = 5) for 3 days. Overnight fasting serum samples were collected before and after the drug treatment. In addition to fasting serum collection, fed serum samples were collected on day 13 of the drug treatment in the first and second studies. Health parameters, including body weight and food intake, were monitored and recorded throughout the experimental duration. After the last dosing, all animals were euthanized for collection of fasting serum and liver tissues. Livers were immediately removed, weighed, cut into small pieces, and stored at −80°C for RNA and protein isolations and lipid measurement. Fecal samples were collected over a 24 h period before the treatment and after 12 days of treatment from 14 day treatment studies.
Measurement of serum lipids
Standard enzymatic methods were used to determine TC, HDL-C, and TG with kits purchased from Stanbio Laboratory.
Measurement of serum total bilirubin and alanine transaminase
Serum total bilirubin concentration was measured using a bilirubin assay kit (Sigma-Aldrich, catalog number MAK126) following the instructions. Serum alanine transaminase (ALT) activity was measured using the ALT/SGPT Liqui-UV kit (Stanbio, catalog number 2930-430) following the instructions.
HPLC separation of serum lipoprotein cholesterol and TGs
For the hyperlipidemic hamster study, after 14 days of treatment, individual fasting serum samples from the OCA-treated group and vehicle control group were analyzed for cholesterol and TG levels of each of the major lipoprotein classes including chylomicron (CM; >80 nm), VLDL (30–80 nm), LDL (16–30 nm), and HDL (8–16 nm) with a dual detection HPLC system consisting of two tandem connected TSK gel Lipopropak XL columns (300 × 7.8 mm; Tosoh, Japan) at Skylight Biotech, Inc. (Tokyo, Japan), as we previously described (25). In addition, 50 μl of serum sample from two animals of the same treatment group of day 0, day 7, and day 13 were pooled together and analyzed for cholesterol and TG levels in different lipoprotein fractions after HPLC separation at Skylight Biotech, Inc.
Detection of hamster PCSK9 in serum
Levels of hamster serum proprotein convertase subtilisin/kexin type 9 (PCSK9) were measured using the Mouse PCSK9 Quantikine ELISA kit (R&D Systems) (26). Briefly, serum samples were diluted 1:10 in Calibrator diluent and allowed to bind for 2 h onto microplate wells that were precoated with the capture antibody. Samples were then sequentially incubated with PCSK9 conjugate followed by the PCSK9 substrate solution with extensive intermittent washes between each step. The amount of PCSK9 in serum was estimated colorimetrically using a standard microplate reader (MDS Analytical Technologies).
Measurement of hepatic and fecal lipids
Fifty milligrams of frozen liver tissue or 20 mg of dried feces were homogenized in 1 ml chloroform/methanol (2:1). After homogenization, lipids were further extracted by rocking samples overnight at room temperature, followed by centrifugation at 2,400 g for 10 min. Supernatant was transferred to a new tube and mixed with 0.2 ml 0.9% saline. The mixture was then centrifuged at 400 g for 5 min and the lower phase containing the lipids was transferred into a new tube. The lipid phase was dried overnight and dissolved in 0.25 ml isopropanol containing 10% Triton X-100. TC and TGs were measured using kits from Stanbio Laboratory.
Measurement of fecal total BAs
Twenty milligrams of dried feces were homogenized and extracted in 1 ml of 75% ethanol at 50°C for 2 h (27). The extract was centrifuged and the supernatant was used to measure total BAs using a kit from Diazyme, Poway, CA.
RNA isolation and real-time quantitative RT-PCR
Total RNA was extracted from liver tissue using the Quick RNA Mini Prep kit (Zymo Research) and was reverse-transcribed into cDNA. Real-time quantitative (q)RT-PCR was performed with 50 ng of cDNA template and specific primers using a SYBR Green PCR kit (Power SYBR® Green PCR Master Mix) and an ABI Prism 7700 system (Applied Biosystems® Life Technologies) according to the manufacturer’s protocols. qRT-PCR primers for each gene are listed in Table 1. Target mRNA expression in each sample was normalized to the housekeeping gene, actin. The 2−ΔΔCt method was used to calculate relative mRNA expression levels.
TABLE 1.
Primers used in qRT-PCR
| Forward | Reverse | |
| ABCA1 | AACAGTTTGTGGCCCTTTTG | AGTTCCAGGCTGGGGTACTT |
| ABCB4 | TCCTATGCACTGGCCTTCTG | GCCCCGATGAGGATTGAGAA |
| ABCG5 | ACTGGACTGCATGACTGCAA | AGTCAGGATGGCAATTTTGTCG |
| APOA1 | TGGCTGTGCTCTTCCTGACC | CTCTGCCGCTGTCTTTCACC |
| BSEP | AGGGCTCTCAACTCTCTCG | ATACAGGTCCGACCCTCTCTG |
| COL1A1 | GCTCCTCTTAGGGGCCACT | CCACGTCTCACCATTGGGG |
| CYP7A1 | TTCCTGCAACCTTCTGGAGC | GCCTCCTTGATGATGCTATCTAGT |
| CYP8B1 | GATGGCACCCGGAAAGTGGA | TAGTGGTGGATCTTCTTGGC |
| EL | ACGCTGGCAACTTTGTGAAA | AGGTATGCAGGACATCCACA |
| FASN | AGTCCTTGTCCAGGTTCGTG | CCACCTAAGCCACCAGTGAT |
| HMGCR | GACGGTGACACTTACCATCTGT | GATGCACCGTGTTATGGTGA |
| HMGCS1 | TTTGATGCAGCTGTTTGAGG | CCACCTGTAGGTCTGGCATT |
| HNF1α | GAGGTGGCTCAGCAATTCAC | CACTCCTCCACCAAGGTCTC |
| HNF4α | CGAGTGGGCCAAGTACATCC | CCGAGGGACGATGTAGTCATT |
| INSIG2A | TTCTCAGTTAGCTTGCGCCT | GTACCACATCTTGGCTGAACG |
| LDLR | TTGGGTTGATTCCAAACTCC | GATTGGCACTGAAAATGGCT |
| PCSK9 | TGCTCCAGAGGTCATCACAG | GTCCCACTCTGTGACATGAAG |
| PPARα | CCTGTCTGTTGGGATGTCAC | AGGTAGGCCTCGTGGATTCT |
| SHP | AGGGAGGCCTTGGATGTC | AGAAGGACGGCAGGTTCC |
| SR-BI | GCGTGGACCCTATGTCTACAG | GTCAGGCTGGAAATGGAGGC |
| SREBP1c | GCACTTTTTGACACGTTTCTTC | CTGTACAGGCTCTCCTGTGG |
| SREBP2 | GAGAGCTGTGAATTTTCCAGTG | CTACAGATGATATCCGGACCAA |
| TNF1α | ACTGAACTTCGGGGTGATCG | CTTGGTGGTTTGCTACGACG |
Western blot analysis
Approximately 50 mg of frozen liver tissue was homogenized in 0.3 ml RIPA buffer containing 1 mM PMSF and protease inhibitor cocktail (Roche). After protein quantitation using BCA protein assay reagent (Pierce), 50 μg of homogenate proteins from individual liver samples were resolved by SDS-PAGE and transferred to nitrocellulose membranes. Anti-SR-BI antibody was purchased from Abcam (Cambridge, MA). Anti-LDLR antibody was obtained from BioVision (Mountain View, CA). Anti-HNF4α, anti-FASN, and anti-stearoyl-CoA desaturase (SCD)1 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-hamster PCSK9 antibody that recognizes the C-terminal end of hamster PCSK9 (CRNRPSAKASWVHQ) was developed in our laboratory and previously reported (28). Rabbit anti-sterol regulatory element-binding protein (SREBP)2 antibodies were generously provided by Dr. Sahng Wook Park (Yonsei University College of Medicine, Seoul, Korea) and were used as previously described (29). Anti-β-actin antibody was purchased from Sigma-Aldrich. All primary antibodies were used at 1:1,000 dilution and the secondary antibody dilution was 1:10,000. Immunoreactive bands of predicted molecular mass were visualized using SuperSignal West Substrate (Thermo Scientific) and quantified with the Alpha View Software with normalization by signals of β-actin.
Statistical analysis
Values are presented as mean ± SEM. Significant differences between control and treatment groups were assessed by one-way ANOVA with Dunnett’s Multiple Comparison posttest or Student’s two-tailed t-test. Statistical significance is displayed as P < 0.05 (one asterisk), P < 0.01 (two asterisks), or P < 0.001 (three asterisks).
RESULTS
Time-dependent reduction of serum HDL-C levels by OCA treatment
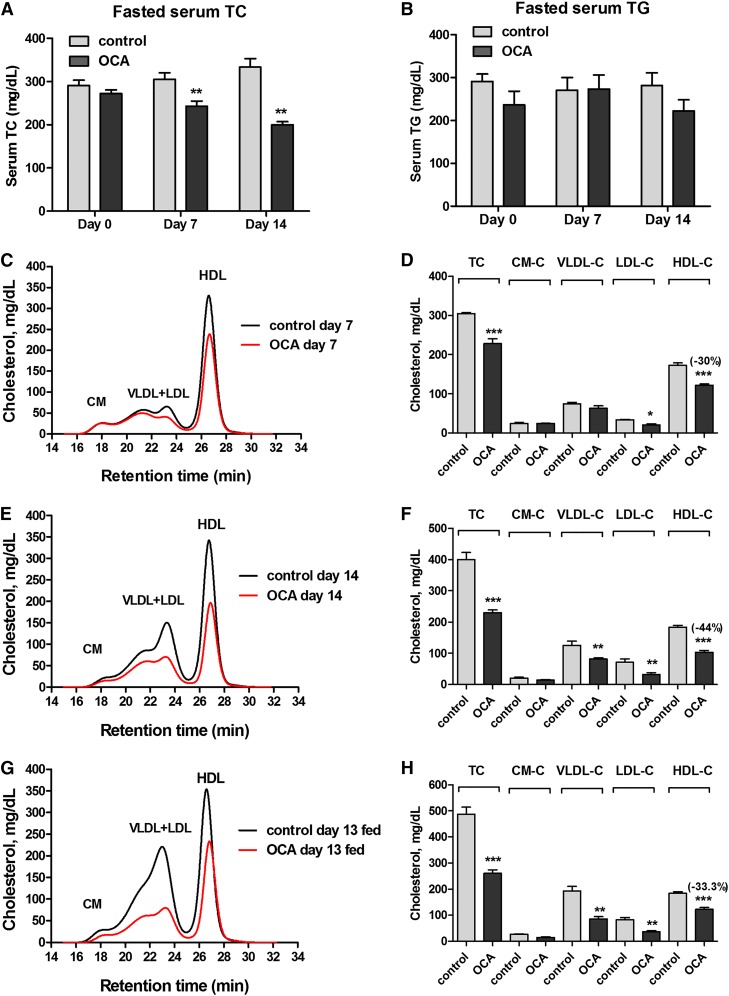
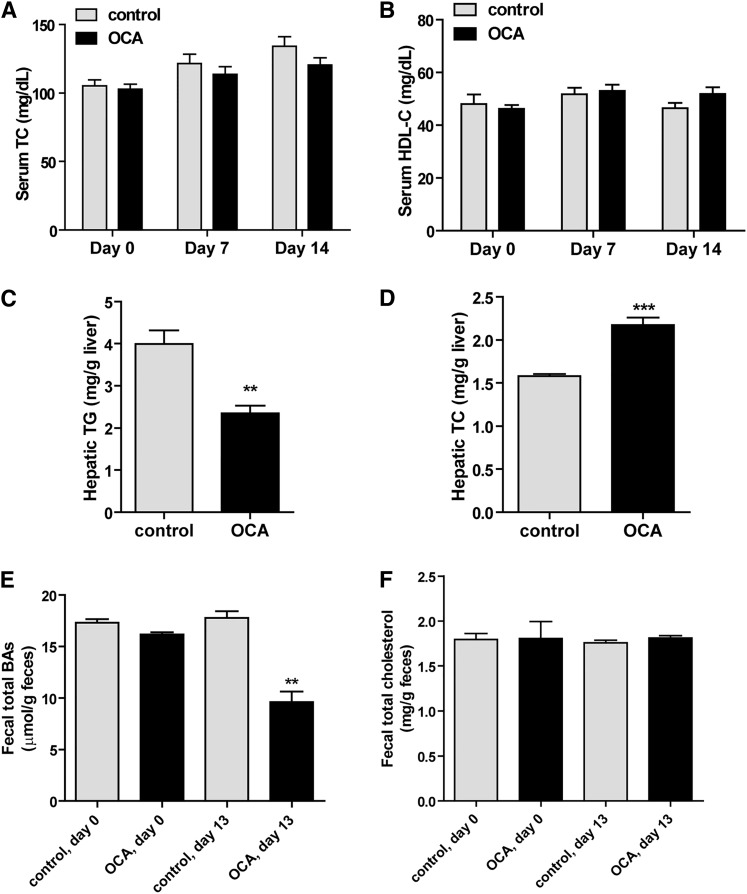
To determine the effects of OCA on plasma cholesterol metabolism, male hamsters fed a HFHCD for 2 weeks were orally treated with OCA (10 mg/kg/day) or vehicle as control for 14 days. Measurements of fasting serum TC and TG levels showed that OCA administration reduced TC levels to 80% of vehicle control (P < 0.01) after 7 days of treatment and TC levels further declined to 60% of control after 14 days in OCA-treated hamsters (P < 0.01) (Fig. 1A), while OCA did not significantly affect serum TG levels (Fig. 1B). We also examined the effects of OCA on serum lipids under fed conditions. Although there was a trend toward a reduction in nonfasting serum TG levels with OCA (vehicle, 350 ± 28 mg/dl; OCA, 272 ± 31 mg/dl), it did not reach statistical significance, whereas nonfasting TC levels were markedly decreased in OCA-treated hamsters (vehicle, 475 ± 36 mg/dl; OCA, 261 ± 7.2 mg/dl; P < 0.001), consistent with the effects of OCA under the fasted state. OCA treatment for 14 days slightly reduced body weight gain (8% versus 12.4% in the control group, P < 0.05) (supplemental Fig. S1A), which was likely caused by lower food intake in OCA-treated animals (supplemental Fig. S1B). The liver weights were slightly lower in OCA-treated hamsters, while liver index was not affected (supplemental Fig. S1C, D). Thus, the cholesterol lowering effects of OCA in these hyperlipidemic hamsters were time-dependent and were prominent.
Fig. 1.
OCA reduces serum TC and HDL-C in hamsters fed a HFHCD. Male hamsters fed a HFHCD for 2 weeks were treated by daily gavage with vehicle (n = 8) or 10 mg/kg OCA (n = 8) for 14 days. Fasted serum samples were collected before the treatment (day 0) and at day 7 and day 14, fed serum samples were collected on day 13. Fasted serum TC (A); fasted serum TG (B); cholesterol distribution in HPLC-separated lipoprotein factions from hamsters on a HFHCD treated with vehicle or OCA (C–H). C, D: Treatment day 7 fasted serum samples. E, F: Treatment day 14 fasted serum samples. G, H: Treatment day 13 fed serum samples. All values are expressed as mean ± SEM. Significance is indicated as *P < 0.05, ** P < 0.01, and *** P < 0.001 as compared with vehicle control group.
To gain a better understanding of the cholesterol-lowering effect of OCA and its impact on plasma lipoprotein cholesterol profiles, we performed HPLC separation of all serum samples individually (day 14 group) or pooled samples that combined two serum samples together from the same group (day 0, day 7, and day 13). Results showed that before the drug treatment, the control and OCA groups had identical lipoprotein fractions and cholesterol was largely carried in the HDL fraction in hamsters fed the HFHCD for 2 weeks (supplemental Fig. S2A, B). After 7 days of OCA treatment, the reduction of TC was driven nearly by the sole reduction of HDL-C, as cholesterol levels in the CM and VLDL fractions were unchanged and only a small decrease in the LDL fraction was observed (Fig. 1C, D). At the end of 14 days of treatment, cholesterol levels in the HDL fraction were further decreased by OCA treatment to 56% of control (−80.3 mg/dl, P < 0.001) (Fig. 1E, F). In addition, a 35% reduction in VLDL-C (−44 mg/dl) and a 54.5% reduction in LDL-C (−39 mg/dl) were also observed. Furthermore, we observed a consistent reduction in HDL-C and other lipoprotein cholesterol concentrations by OCA under the fed state (Fig. 1G, H). HPLC analysis of lipoprotein-TG fractions (supplemental Fig. S2C–G) revealed only a small reduction of VLDL-TG after 14 days of OCA treatment, which was consistent with the overall insignificant effects of OCA on serum TG levels in hyperlipidemic hamsters. In addition, we measured serum ALT levels and total bilirubin levels, which were in normal ranges of hamster values and were not significantly elevated by OCA treatment (supplemental Fig. S3), indicating that liver functions were not disturbed under the diet or treatment conditions. Altogether, these data demonstrate that FXR activation by OCA led to a strong cholesterol-lowering effect driven largely by reducing serum HDL-C levels in this hyperlipidemic animal model under both fasted and nonfasted conditions.
OCA treatment reduces hepatic cholesterol and increases fecal cholesterol contents
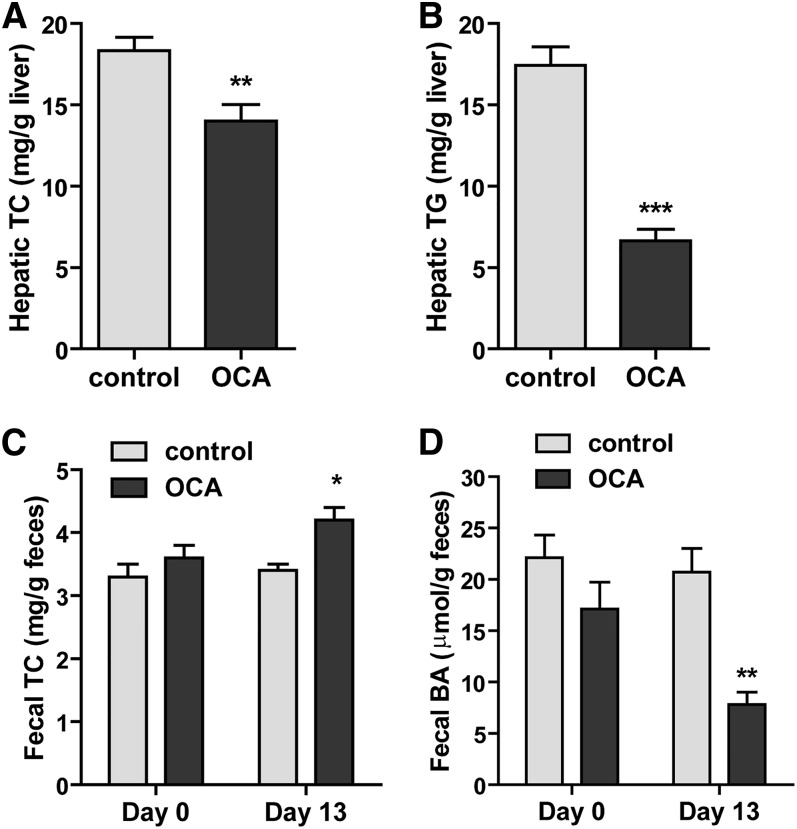
Next we investigated whether the plasma HDL-C-lowering effect of OCA was associated with changes in hepatic and fecal cholesterol contents. Measurement of hepatic lipids showed that OCA treatment of 14 days significantly reduced hepatic cholesterol contents by approximately 24% compared with vehicle control (P < 0.01) (Fig. 2A). OCA also reduced hepatic TG contents (Fig. 2B), which was consistent with the reported effects of other FXR agonists on lowering hepatic TG (23, 30, 31). The cholesterol contents of fecal samples collected on day 0 and day 13 of the control group were nearly identical, but cholesterol levels were significantly increased in fecal samples after 13 days of OCA treatment (Fig. 2C). We also detected a substantial reduction in fecal BA levels after OCA treatment (Fig. 2D). Collectively, these data demonstrated that the removal of HDL-C from the circulation by OCA was accompanied by an increase in transhepatic cholesterol movement into feces.
Fig. 2.
OCA treatment reduces hepatic cholesterol and increases fecal cholesterol contents along with reductions of hepatic TG and fecal BA. Male hamsters fed a HFHCD were orally administered vehicle (n = 8) or OCA at 10 mg/kg (n = 8) for 14 days. Feces were collected on day 0 and day 13 of treatment, dried, and weighed. Hamsters were euthanized and serum and livers were isolated at the termination of the experiment. A, B: Lipids were extracted from individual liver samples and TC and TG were measured. Values are mean ± SEM of eight hamsters per group. **P < 0.01 and ***P < 0.001 as compared with the vehicle control group. C, D: Lipids were also extracted from dried feces and TC and BA were measured. Values are mean ± SEM of four fecal samples per group. *P < 0.05 and **P < 0.01 as compared with the control group.
OCA upregulates hepatic SR-BI mRNA and protein levels
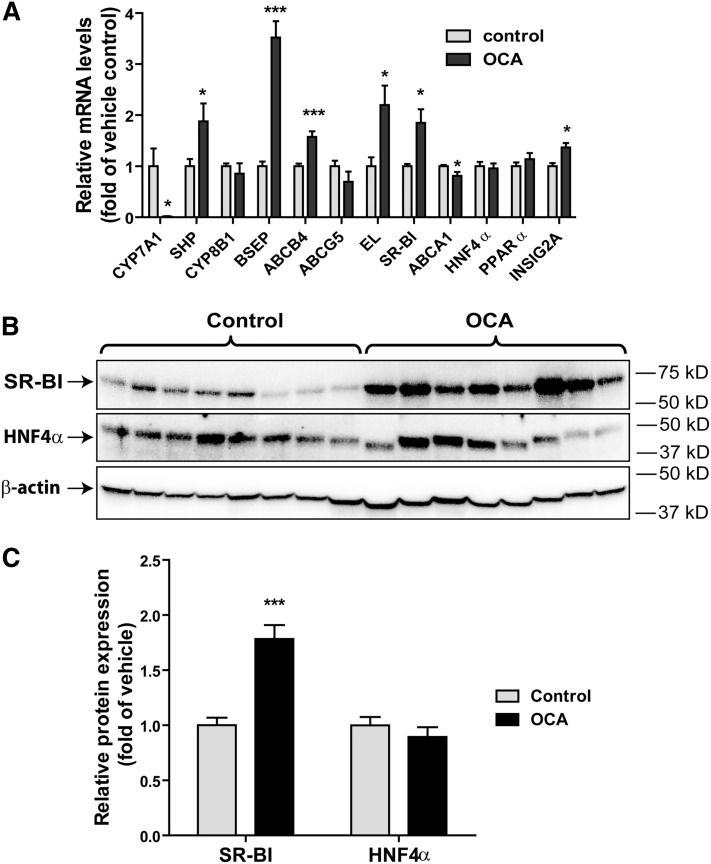
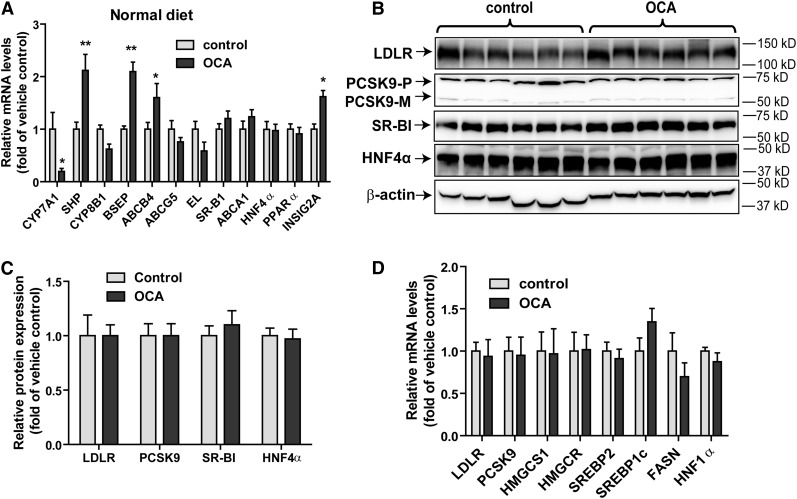
To gain a mechanistic insight into the OCA-mediated increases of fecal cholesterol levels and reductions of BA synthesis, we investigated the influence of FXR activation by OCA on the expression of hepatic genes that are involved in BA synthesis and transhepatic cholesterol efflux (Fig. 3A). Hepatic gene expression analysis by qRT-PCR showed that the mRNA expression of classical FXR-controlled genes in the BA synthetic pathway (CPY7A1, SHP, BSEP) was strongly modulated by OCA. The substantial reduction of CYP7A1 resulted in decreased fecal BA content via inhibition of BA synthesis in liver tissue. Interestingly, among the four genes involved in transhepatic cholesterol efflux, with the exception of ABCG5, mRNA levels of ABCB4, endothelial lipase (EL), and SR-BI were all elevated in OCA-treated hamsters. In addition, we measured mRNA levels of ABCA1, ApoA1, PPARα, and HNF4α. ABCA1 is involved in hepatic efflux of cholesterol and phospholipid to lipid-poor HDL particles (32), and we detected a small reduction (<20%) in ABCA1 mRNA levels by OCA treatment, whereas ApoA1, HNF4α, and PPARα mRNA levels were unchanged. Interestingly, a previous study has demonstrated a FXR-dependent upregulation of mouse Insig2a gene transcription (33). Using qPCR and specific hamster primers, we detected a nearly 40% increase (P < 0.05) in the mRNA levels of Insig2a in OCA-treated hamster livers, confirming the original findings made in mice.
Fig. 3.
Upregulation of hepatic SR-BI mRNA and protein expression by OCA in the liver of hyperlipidemic hamsters. Hamsters were euthanized and liver tissues were isolated after 14 days of drug treatment. A: Total RNA was isolated from individual livers and relative mRNA abundances of the indicated genes were determined by conducting qRT-PCR and normalized to actin. Values are mean ± SEM of eight hamsters per group. B: Individual liver homogenates were prepared and protein concentrations were determined. Fifty micrograms of homogenate proteins per liver sample were resolved by SDS-PAGE. SR-BI and HNF4α proteins were detected by immunoblotting using anti-SR-BI and anti-HNF4α antibodies. The membrane was reprobed with anti-β-actin antibody. C: The protein abundance of SR-BI and HNF4α was quantified with normalization by signals of β-actin using the Alpha View Software. Values are the mean ± SEM of eight samples per group. * P < 0.05, ***P < 0.001 as compared with the vehicle control group.
The function of SR-BI in reverse cholesterol transport is well-characterized and the increased expression of SR-BI protein in liver is linked to enhanced HDL-C uptake from plasma in mice (34). Western blotting of liver homogenates of all liver samples demonstrated a 1.8-fold increase (P < 0.001) in SR-BI protein levels in OCA-treated animals compared with control (Fig. 3B, C). Increased expression of HNF4α by FXR agonist, GW4064, was reported as a causal factor for FXR-mediated elevation of SR-BI expression in mice (35). However, in our study, HNF4α protein levels were unchanged in these samples, which were in line with the negative results of qRT-PCR. It was shown that activation of Janus N-terminal kinase (p-JNK) by GW4064 was responsible for increased HNF4α expression and subsequent SR-BI upregulation by this FXR ligand in mice (35). Thus, we examined p-JNK and total JNK protein levels in hamster livers and we did not detect differences between OCA-treated and control groups (supplemental Fig. S4). Thus, our data suggest that upregulation of SR-BI by OCA does not involve changes in hepatic HNF4α abundance. Altogether, these results of mRNA and protein analysis demonstrated that OCA upregulates hepatic SR-BI expression, which may account for the reduction of plasma HDL-C and the increase in fecal cholesterol in these dyslipidemic hamsters treated with OCA.
Effects of OCA treatment on the SREBP pathway
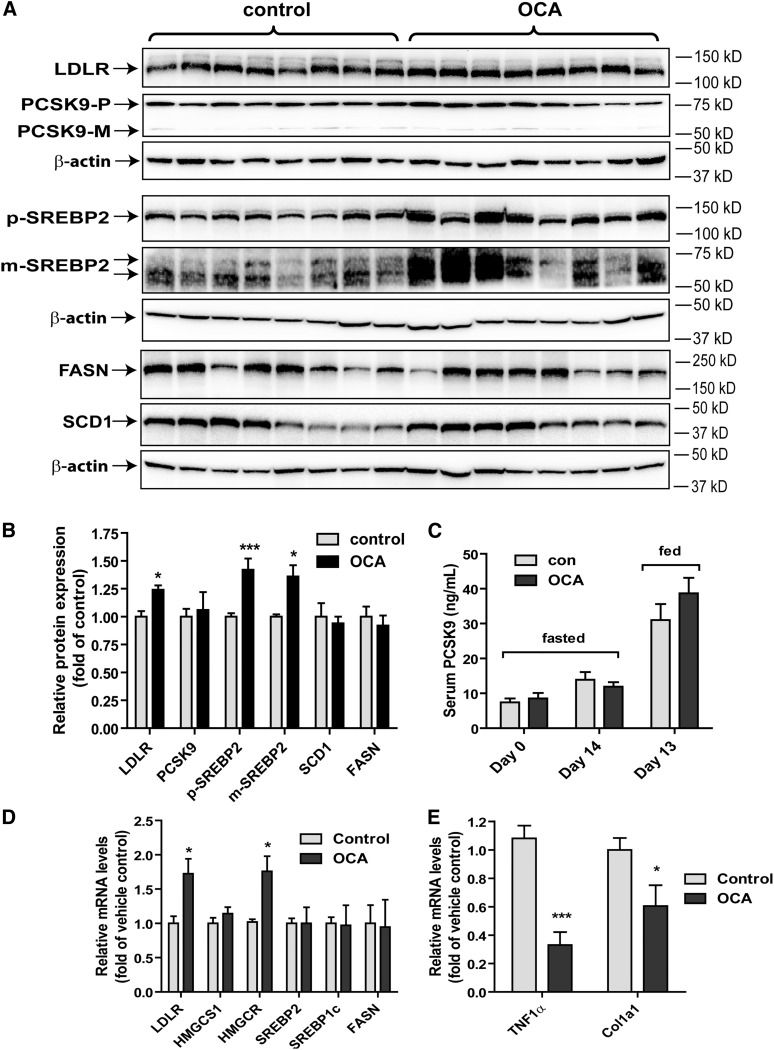
The plasma cholesterol metabolism is also critically influenced by the expression level of hepatic LDLR and its negative regulator, PCSK9, both of which are transcriptionally activated by the mature form of sterol regulatory element-binding protein 2 (m-SREBP2) via the sterol regulatory element (SRE)-1 sites in their gene regulatory region (36, 37). Using total liver homogenates, we analyzed hepatic LDLR, PCSK9, and SREBP2 precursor and mature protein levels by Western blotting (Fig. 4A, B) and serum PCSK9 levels by ELISA (Fig. 4C). OCA treatment produced small but significant increases in LDLR protein level to 24% over control and precursor (p-)SREBP2 and m-SREBP2 protein levels to approximately 40% over control, whereas serum and hepatic PCSK9 protein levels were not changed by OCA treatment. In addition, we examined the protein levels of FASN and SCD1 and no differences in their expression between the two groups were observed. The results of Western blotting were further corroborated by qRT-PCR analysis of hepatic gene expression (Fig. 4D). OCA treatment increased mRNA levels of LDLR by 72% compared with control. The mRNA level of HMG-CoA-reductase (HMGCR), another SREBP2-modulated gene was also elevated in OCA-treated liver tissues. Combined with the observation that hepatic cholesterol levels were reduced in liver tissues of OCA-treated hamsters, these data together suggest that a modest increase in hepatic LDLR abundance as the result of activated SREBP pathway may contribute to the reduction of serum LDL-C levels at the latter time point of OCA treatment. In addition, measurement of mRNA levels of TNF1α and collagen type I (Col1a1) (Fig. 4E) demonstrated significant mRNA reductions of these inflammatory and fibrosis-related marker genes, which were in line with the anti-inflammatory effects of this FXR agonist (16).
Fig. 4.
Effects of OCA treatment on the SREBP pathway. A: Individual liver homogenates were prepared and protein concentrations were determined. Fifty micrograms of homogenate proteins per liver sample were resolved by SDS-PAGE. LDLR, PCSK9, p-SREBP2, m-SREBP2, SCD1, FASN, and β-actin proteins were detected individually by immunoblotting using specific antibodies. B: The abundance of the indicated proteins was quantified with normalization by signals of β-actin using the Alpha View Software. Values are the mean ± SEM of seven to eight samples per group. C: Individual hamster serum PCSK9 levels were quantified by a mouse PCSK9 ELISA kit. Values are the mean ± SEM of eight samples per group. D, E: Total RNA was isolated from individual liver and relative mRNA abundance of the indicated genes was determined by conducting qRT-PCR and normalized to actin. Values are mean ± SEM of eight hamsters per group. *P < 0.05 and ***P < 0.001 as compared with the vehicle control group.
OCA treatment did not affect serum cholesterol and hepatic SR-BI expression levels in normolipidemic hamsters
We were interested in learning whether the effects of OCA on plasma cholesterol metabolism and SR-BI expression are affected by hepatic cholesterol levels. We treated hamsters fed a NCD with OCA at 10 mg/kg for 2 weeks. No obvious differences in food intake, body weight or liver weight were observed between OCA and the control groups (supplemental Fig. S5A–D). Measurement of serum lipids showed that administration of OCA to normolipidemic hamsters had no effects on serum TC and HDL-C levels (Fig. 5A, B) while serum TG levels were modestly increased owing to an increase in the VLDL-TG fraction (supplemental Fig. S5E, F). Hepatic TG content was significantly reduced, while hepatic cholesterol content was increased in OCA-treated animals by 38% compared with the control group (Fig. 5C, D). Measurement of fecal cholesterol and BAs demonstrated a reduction in fecal BAs, but no changes in fecal cholesterol contents after OCA treatment (Fig. 5E, F).
Fig. 5.
OCA treatment did not affect serum cholesterol levels and fecal cholesterol content in hamsters fed a NCD. Male hamsters fed a NCD were treated by daily gavage with vehicle (n = 6) or 10 mg/kg OCA (n = 6) for 14 days. Fasting serum samples were collected before the treatment (day 0) and at day 7 and day 14. Feces were collected on day 0 and day 13 of treatment, dried, and weighed. Hamsters were euthanized and serum and livers were isolated at the termination of the experiment. A, B: TC and HDL-C were measured from all serum samples. C, D: Lipids were extracted from individual liver samples, and TC and TG and were measured. Values are mean ± SEM of six hamsters per group. **P < 0.01 and ***P < 0.001 as compared with the vehicle control group. E, F: Lipids were also extracted from dried feces and TC and BAs were measured. Values are mean ± SEM of three fecal samples per group. **P < 0.01 as compared with the control group.
Hepatic gene expression analysis by qRT-PCR demonstrated that among FXR-modulated genes, CYP7A1, SHP, BSEP, INSIG2A, and ABCB4 were modulated by OCA to levels comparable to its effects seen in hamsters fed the HFHCD. However, the induction on EL and SR-BI gene expression by OCA were not observed in the normolipidemic hamsters (Fig. 6A). Furthermore, hepatic SR-BI protein levels were not increased by OCA treatment (Fig. 6B, C), which corroborated the results of qRT-PCR. Despite a small increase in hepatic cholesterol content, we did not detect differences in hepatic expressions of a panel of SREBP target genes, including LDLR mRNA and PCSK9 (Fig. 6D), as well as their proteins (Fig. 6B, C).
Fig. 6.
OCA modulated the expression of genes involved in the BA synthetic pathway without inducing the expression of SR-BI and EL in liver tissue of normolipidemic hamsters. A, D: qRT-PCR analysis of hepatic gene expression in the FXR and SREBP pathways. *P < 0.05 and **P < 0.01 as compared with the vehicle control group. B, C: Western blot analysis of hepatic protein expression. Values are the mean ± SEM of six samples per group.
To further examine dose-dependent effects of OCA on hepatic SR-BI and other FXR-modulated genes in normolipidemic hamsters, another cohort of hamsters fed a NCD were orally dosed with OCA for 3 days at 10, 20, or 30 mg/kg, while the control animals received the vehicle. Treatment of animals with OCA at these doses did not affect serum levels of TC and HDL-C (supplemental Fig. S6A, B). Health parameters, including serum bilirubin levels, food intake, and body weights of hamsters, were not significantly different among the groups (supplemental Fig. S6C–E). Measurements of liver weight showed a tendency of dose-dependent increase in liver weight, which was further manifested in comparisons of liver index (supplemental Fig. S6F, G). The liver index of the OCA 30 mg/kg group was 19% higher than the control group (P < 0.001), suggesting a mild adaptive changes in the liver.
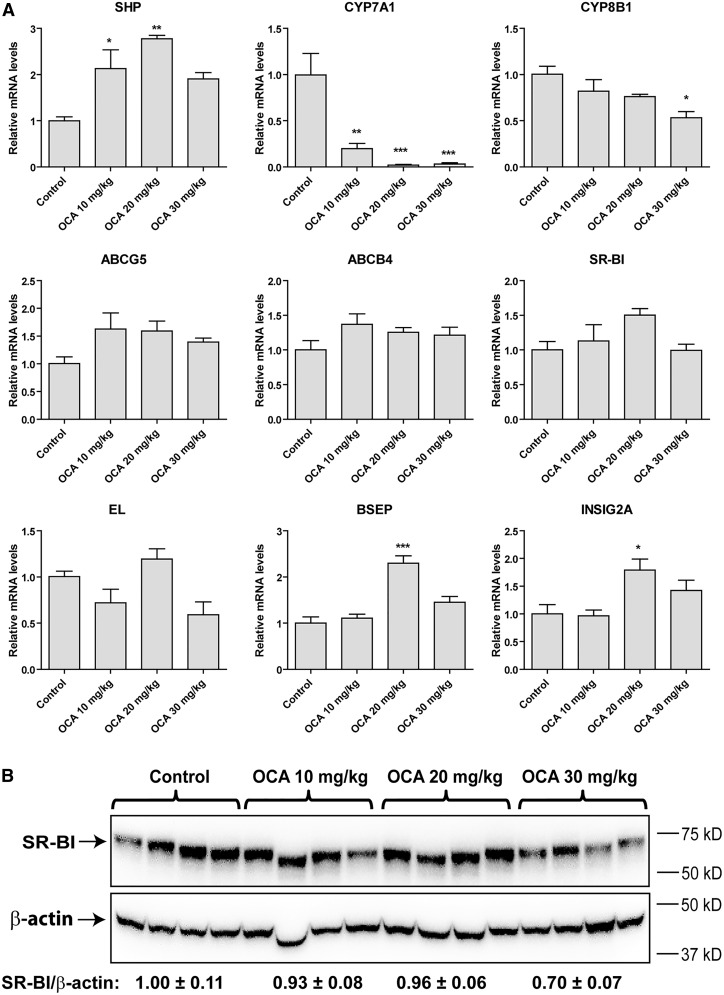
Hepatic gene expression analysis of a panel of FXR-modulated genes (Fig. 7A) demonstrated dose-dependent effects of OCA on mRNA levels of SHP, CYP7A1, CYP8B1, BSEP, and INSIG2A and showed that OCA at a dose of 20 mg/kg had a greater effect than 30 mg/kg. The relatively lower effect of 30 mg/kg OCA treatment on modulating FXR target genes including SHP, BSEP, and INSIG2A could reflect a mild liver toxicity at this higher dose. Importantly, we did not observe a significant upregulation of EL or SR-BI mRNA expression by OCA under these doses, which was further confirmed by Western blot analysis of liver SR-BI protein abundance (Fig. 7B). Overall, results of the 3 day treatment study with OCA at doses up to 30 mg/kg largely confirmed our results obtained from the 14 day OCA treatment of 10 mg/kg. Collectively, these results suggest that under normolipidemic conditions, FXR activation by OCA inhibited BA synthesis without inducing SR-BI-mediated transhepatic cholesterol movement.
Fig. 7.
Examination of dose-dependent effects of OCA on the expression of genes involved in BA synthetic pathway and on SR-BI protein abundances in hamsters fed a NCD and treated with OCA for 3 days. A: qRT-PCR analysis of hepatic gene expression in FXR pathway. Values are the mean ± SEM of 5 liver samples per group. *P < 0.05, **P < 0.01. and ***P < 0.001 as compared with the vehicle control group. B: Western blot analysis of hepatic SR-BI protein expression. Values are the mean ± SEM of four randomly chosen liver samples per group.
LXR activation alone did not induce hepatic expression of SR-BI in hamsters
One substantial difference between NCD- and HCHFD-fed hamsters is the hepatic cholesterol content. A higher hepatic cholesterol amount in HFHCD-fed hamsters might have a stimulating effect on SR-BI expression through LXR activation, as it was reported that SR-BI expression in human macrophages was induced by LXR agonists (38). To learn whether SR-BI expression in hamsters could be directly induced by LXR activation, we examined SR-BI protein levels in liver tissues of NCD-fed hamsters that were previously treated with a specific LXR agonist, GW3965 (30 mg/kg), or vehicle for 7 days (39). As shown in supplemental Fig. S7, hepatic SR-BI protein levels did not differ between the two groups. We further confirmed the lack of inducing effect of GW3965 treatment on hepatic SR-BI expression in hamsters fed a HFD or a dyslipidemic fructose diet (data not shown).
DISCUSSION
We set out this study to understand how OCA, a therapeutic FXR agonist, regulates plasma cholesterol metabolism under dyslipidemic conditions by utilizing hyperlipidemic hamsters, a model that has been used with increasing frequency in recent years to study lipoprotein metabolism, atherosclerosis, and to evaluate effects of hypolipidemic agents, including PPAR activators and LXR agonists (39–42). The important new findings of our current study are that activation of hepatic FXR by OCA increases SR-BI expression and accelerates the removal of circulating HDL-C with increased cholesterol fecal excretion in hyperlipidemic hamsters. Previous animal studies of OCA conducted in rats, wild-type mice, LDLR-deficient mice, and CETP-transgenic mice produced inconsistent effects on plasma cholesterol levels, in particular HDL-C levels. For example, Hambruch et al. (23) reported that in C57BL/6J mice fed a HFD, OCA treatment of 4 weeks at a dose of 30 mg/kg caused a small increase in HDL-C without significant effect on total plasma cholesterol; however, in the same study, it was reported that after 12 weeks of treatment, OCA at 10 mg/kg dose reduced plasma TC significantly and showed a trend in HDL-C lowering in CETPtg-LDLR(−/−) mice.
In this study, we demonstrated a specific and time-dependent reduction of HDL-C levels by treating hyperlipidemic hamsters with 10 mg/kg OCA. The facts that reduction of HDL-C preceded the decrease in LDL-C and VLDL-C levels and the majority of plasma cholesterol was carried in HDL particles suggest that enhanced HDL-C removal by OCA is likely the primary driving force for plasma cholesterol lowering observed in this animal model. It was previously reported that in a cohort of HFD-fed hamsters, OCA treatment of 11 days at a daily dose of 30 mg/kg reduced cumulative food intake by 13%, lowered HDL-C, and increased VLDL-C, resulting in unchanged total plasma cholesterol levels (22, 24). In our study, we observed a similar amount of reduction in cumulative food intake (∼14%), but we observed significant HDL-C reduction without any elevation of VLDL-C or LDL-C. It is not clear what factors contributed to the different effects of OCA on the VLDL-C fraction in these different hamster studies. The conflicting results might be due to different OCA doses, different diet compositions, or different OCA preparations.
In the previous report of HFD-fed hamsters, OCA reduced liver BA pool size and fecal BAs (22), which was in line with our current study of HFHCD-fed hamsters in which OCA treatment of 14 days reduced fecal BAs by 62%. Importantly, in addition to reduction of fecal BAs, the current study demonstrated a reduction of hepatic cholesterol contents and an increase in fecal cholesterol levels in OCA-treated hamsters. Among FXR-modulated genes that are involved in BA metabolism, the upregulation of SR-BI, ABCB4, ABCG5, and EL by FXR agonists, FXR-450, and PX20606 in mice were linked to the clearance of cholesteryl esters from HDL and their excretion into feces via the bile (23). In the same study, it was reported that OCA did not increase hepatic SR-BI, ABCB4, EL, or ABCG5 mRNA levels in mice, which was correlated with a lack of effect of OCA on cholesterol efflux in mice (23). However, we detected significant inductions of SR-BI, ABCB4, and EL mRNA levels in hamster livers after OCA treatment. We further demonstrated a 1.8-fold increase in hepatic SR-BI protein levels by OCA treatment. It has been suggested that the function of SR-BI in biliary cholesterol secretion is mediated through ABCG5/ABCG8-dependent as well as -independent mechanisms (43, 44). While ABCB4 is mainly involved in phospholipid secretion (45) and is shown not to be dependent on biliary sterol secretion (46), a model emerges in hyperlipidemic hamsters in which the increased hepatic uptake of cholesterol esters from HDL via SR-BI and enhanced HDL metabolism by EL, coupled with increased cholesterol excretion via ABCB4 lead to stimulated transhepatic cholesterol efflux in hyperlipidemic hamsters by OCA treatment. Further investigations using radioisotope-labeled cholesterol to demonstrate a direct effect of OCA on upregulation of HDL-C uptake in SR-BI wild-type and deficient animals will be required to validate this working model.
The results from our chow-fed hamster studies showed that OCA treatment of 14 days inhibited BA hepatic synthesis, which was evidenced by strong effects on hepatic CPY7A1 and SHP gene expression and reduction of fecal BAs. However, these OCA effects occurred in the absence of changes in serum HDL-C, fecal cholesterol, and hepatic expression of EL and SR-BI, indicating that the transhepatic cholesterol efflux was not induced by OCA in these normolipidemic hamsters, which might account for the small increase in hepatic cholesterol content. The lack of OCA effect on hepatic SR-BI expression under normolipidemic conditions was also consistently observed in chow-fed hamsters treated with OCA at daily doses up to 30 mg/kg.
It has been shown that the regulation of SR-BI expression by FXR involves different mechanisms. One study reported that FXR activation by synthetic agonist GW4064 increased transcription factor HNF4α protein levels that led to the transcriptional activation of SR-BI gene through HNF4α binding sequences embedded in the promoter region and intronic sequences of murine SR-BI gene (35). Another study identified multiple functional FXR binding sites (IR1) in the first intron of the murine SR-BI gene (47). Furthermore, it was reported that treating HepG2 cells with GW4064 increased SR-BI mRNA levels. This effect was linked to the binding of FXR to a putative FXR response element site (DR8) in the promoter region of the human SR-BI gene (48). In this current study, we observed increased SR-BI mRNA and protein expression to a similar extent by OCA treatment in the absence of changes in hepatic HNF4α abundance of hamsters fed a HFHCD. Thus, our data suggest that SR-BI gene transcription is directly induced by FXR activation in the hamster species under hyperlipidemic conditions, but not in the normolipidemic state. One major difference between NCD- and HCHFD-fed hamsters is the hepatic cholesterol content. Higher hepatic cholesterol in HFHCD-fed hamsters might have a stimulating effect on SR-BI expression through LXR activation, as SR-BI expression in human macrophages was induced by LXR agonists (38). However, our examination of SR-BI protein abundances in the livers of normolipidemic hamsters treated with LXR agonist GW3965 or vehicle failed to detect any differences in SR-BI expression levels (supplemental Fig. S7), suggesting that in the hamster species, SR-BI is not directly regulated by LXR alone. Thus, currently, it is unclear how different levels of hepatic cholesterol or cholesterol metabolites could impact the effect of OCA on SR-BI gene expression in the hamster species. Because activation of SR-BI transcription is associated with a favorable lipoprotein cholesterol profile, our findings warrant further investigations to better understand the influence of dietary cholesterol on the inducibility of SR-BI expression by FXR agonists, including OCA at the gene transcriptional level in different animal models and in humans.
In our hyperlipidemic hamster study, in addition to HDL-C reduction, OCA treatment lowered serum LDL-C and VLDL-C fractions at the later treatment time points. Previous in vitro studies reported that FXR activation in hepatic cells led to LDLR mRNA stabilization (49) or inhibition of PCSK9 transcription (50). Either of these effects could result in decreases in plasma LDL-C levels owing to increased hepatic LDLR abundance. As investigated here, while we did not observe changes in serum and hepatic PCSK9 protein levels, we did detect small but significant increases in hepatic LDLR mRNA and protein levels, which were accompanied by increased m-SREBP2 in the liver of OCA-treated hamsters fed a HFHCD (Fig. 4). Combined with the observation of reduced liver cholesterol content, our results suggest that in hamsters fed a cholesterol-enriched diet, the hepatic SREBP pathway was repressed; alleviation of this repression through SR-BI facilitated transhepatic cholesterol excretion into feces probably generated a positive yet modest impact on the intracellular proteolytic process that converts the inactive SREBP2 to the active form to enter the nucleus and turn on the LDLR gene transcription along with a subset of SREBP2-target genes. In the absence of changes in PCSK9 hepatic and serum levels, the increase in hepatic LDLR expression may contribute, at least in part, to the LDL-C reduction. Furthermore, previous in vitro and in vivo studies have suggested that SR-BI mediates selective uptake of cholesterol esters from LDL particles in addition to HDL particles (8, 51). Thus, the OCA-mediated reduction of serum LDL-C and VLDL-C could result from its combined activities in increasing SR-BI and LDLR abundances in liver tissue.
In summary, OCA treatment of hyperlipidemic hamsters elicited reductions of serum HDL-C levels with concomitant upregulation of hepatic SR-BI expression and increased cholesterol excretion into feces. Our findings in this hamster model suggest that induction of hepatic SR-BI expression may account for the hypocholesterolemic effect of OCA under hyperlipidemic states.
Supplementary Material
Footnotes
Abbreviations:
- ALT
- alanine transaminase
- BA
- bile acid
- CM
- chylomicron
- EL
- endothelial lipase
- FXR
- farnesoid X receptor
- HDL-C
- HDL-cholesterol
- HFD
- high-fat diet
- HFHCD
- high-fat and high-cholesterol diet
- JNK
- Janus N-terminal kinase
- LDL-C
- LDL-cholesterol
- LDLR
- LDL receptor
- m-SREBP2
- mature sterol regulatory element-binding protein 2
- NAFLD
- nonalcoholic fatty liver disease
- NASH
- nonalcoholic steatohepatitis
- NCD
- normal chow diet
- OCA
- obeticholic acid
- PCSK9
- proprotein convertase subtilisin/kexin type 9
- p-SREBP2
- precursor sterol regulatory element-binding protein 2
- SCD
- stearoyl-CoA desaturase
- SHP
- small heterodimer partner
- SR-BI
- scavenger receptor class B type I
- SRE
- sterol regulatory element
- SREBP
- sterol regulatory element-binding protein
- TC
- total cholesterol
- VLDL-C
- VLDL-cholesterol
This study was supported by the VA Office of Research and Development, by National Center for Complementary and Integrative Health Grant 1R01AT006336-01A1, and by a collaborative research grant from Intercept Pharmaceuticals.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Calkin A. C., and Tontonoz P.. 2012. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat. Rev. Mol. Cell Biol. 13: 213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas A. M., Hart S. N., Kong B., Fang J., Zhong X. B., and Guo G. L.. 2010. Genome-wide tissue-specific farnesoid X receptor binding in mouse liver and intestine. Hepatology. 51: 1410–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhan L., Liu H. X., Fang Y., Kong B., He Y., Zhong X. B., Fang J., Wan Y. J., and Guo G. L.. 2014. Genome-wide binding and transcriptome analysis of human farnesoid X receptor in primary human hepatocytes. PLoS One. 9: e105930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Aguiar Vallim T. Q., Tarling E. J., Ahn H., Hagey L. R., Romanoski C. E., Lee R. G., Graham M. J., Motohashi H., Yamamoto M., and Edwards P. A.. 2015. MAFG is a transcriptional repressor of bile acid synthesis and metabolism. Cell Metab. 21: 298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rigotti A., Trigatti B., Babitt J., Penman M., Xu S., and Krieger M.. 1997. Scavenger receptor BI-a cell surface receptor for high density lipoprotein. Curr. Opin. Lipidol. 8: 181–188. [DOI] [PubMed] [Google Scholar]
- 6.Rigotti A., Trigatti B. L., Penman M., Rayburn H., Herz J., and Krieger M.. 1997. A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism 8. Proc. Natl. Acad. Sci. USA. 94: 12610–12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen W. J., Hu J., Hu Z., Kraemer F. B., and Azhar S.. 2014. Scavenger receptor class B type I (SR-BI): a versatile receptor with multiple functions and actions. Metabolism. 63: 875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brodeur M. R., Luangrath V., Bourret G., Falstrault L., and Brissette L.. 2005. Physiological importance of SR-BI in the in vivo metabolism of human HDL and LDL in male and female mice. J. Lipid Res. 46: 687–696. [DOI] [PubMed] [Google Scholar]
- 9.Zanoni P., Khetarpal S. A., Larach D. B., Hancock-Cerutti W. F., Millar J. S., Cuchel M., DerOhannessian S., Kontush A., Surendran P., Saleheen D., et al. 2016. Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science. 351: 1166–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kozarsky K. F., Donahee M. H., Glick J. M., Krieger M., and Rader D. J.. 2000. Gene transfer and hepatic overexpression of the HDL receptor SR-BI reduces atherosclerosis in the cholesterol-fed LDL receptor-deficient mouse. Arterioscler. Thromb. Vasc. Biol. 20: 721–727. [DOI] [PubMed] [Google Scholar]
- 11.Kozarsky K. F., Donahee M. H., Rigotti A., Iqbal S. N., Edelman E. R., and Krieger M.. 1997. Overexpression of the HDL receptor SR-BI alters plasma HDL and bile cholesterol levels. Nature. 387: 414–417. [DOI] [PubMed] [Google Scholar]
- 12.Van Eck M., Twisk J., Hoekstra M., Van Rij B. T., Van der Lans C. A., Bos I. S., Kruijt J. K., Kuipers F., and Van Berkel T. J.. 2003. Differential effects of scavenger receptor BI deficiency on lipid metabolism in cells of the arterial wall and in the liver. J. Biol. Chem. 278: 23699–23705. [DOI] [PubMed] [Google Scholar]
- 13.Fuller M., Dadoo O., Serkis V., Abutouk D., MacDonald M., Dhingani N., Macri J., Igdoura S. A., and Trigatti B. L.. 2014. The effects of diet on occlusive coronary artery atherosclerosis and myocardial infarction in scavenger receptor class B, type 1/low-density lipoprotein receptor double knockout mice. Arterioscler. Thromb. Vasc. Biol. 34: 2394–2403. [DOI] [PubMed] [Google Scholar]
- 14.Vergeer M., Korporaal S. J., Franssen R., Meurs I., Out R., Hovingh G. K., Hoekstra M., Sierts J. A., Dallinga-Thie G. M., Motazacker M. M., et al. 2011. Genetic variant of the scavenger receptor BI in humans. N. Engl. J. Med. 364: 136–145. [DOI] [PubMed] [Google Scholar]
- 15.Brunham L. R., Tietjen I., Bochem A. E., Singaraja R. R., Franchini P. L., Radomski C., Mattice M., Legendre A., Hovingh G. K., Kastelein J. J., et al. 2011. Novel mutations in scavenger receptor BI associated with high HDL cholesterol in humans. Clin. Genet. 79: 575–581. [DOI] [PubMed] [Google Scholar]
- 16.Adorini L., Pruzanski M., and Shapiro D.. 2012. Farnesoid X receptor targeting to treat nonalcoholic steatohepatitis. Drug Discov. Today. 17: 988–997. [DOI] [PubMed] [Google Scholar]
- 17.Neuschwander-Tetri B. A., Loomba R., Sanyal A. J., Lavine J. E., Van Natta M. L., Abdelmalek M. F., Chalasani N., Dasarathy S., Diehl A. M., Hameed B., et al. 2015. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 385: 956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mudaliar S., Henry R. R., Sanyal A. J., Morrow L., Marschall H. U., Kipnes M., Adorini L., Sciacca C. I., Clopton P., Castelloe E., et al. 2013. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 145: 574–582. [DOI] [PubMed] [Google Scholar]
- 19.Pencek R., Marmon T., Roth J. D., Liberman A., Hooshmand-Rad R., and Young M.. 2016. Effects of obeticholic acid on lipoprotein metabolism in healthy volunteers. Diabetes Obes. Metab. 18: 936–940. [DOI] [PubMed] [Google Scholar]
- 20.Hirschfield G. M., Mason A., Luketic V., Lindor K., Gordon S. C., Mayo M., Kowdley K. V., Vincent C., Bodhenheimer H. C., Pares A., et al. 2015. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology. 148: 751–761. [DOI] [PubMed] [Google Scholar]
- 21.Cipriani S., Mencarelli A., Palladino G., and Fiorucci S.. 2010. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J. Lipid Res. 51: 771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gardès C., Chaput E., Staempfli A., Blum D., Richter H., and Benson G. M.. 2013. Differential regulation of bile acid and cholesterol metabolism by the farnesoid X receptor in Ldlr -/- mice versus hamsters. J. Lipid Res. 54: 1283–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hambruch E., Miyazaki-Anzai S., Hahn U., Matysik S., Boettcher A., Perovic-Ottstadt S., Schluter T., Kinzel O., Krol H. D., Deuschle U., et al. 2012. Synthetic farnesoid X receptor agonists induce high-density lipoprotein-mediated transhepatic cholesterol efflux in mice and monkeys and prevent atherosclerosis in cholesteryl ester transfer protein transgenic low-density lipoprotein receptor (-/-) mice. J. Pharmacol. Exp. Ther. 343: 556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gardès C., Blum D., Bleicher K., Chaput E., Ebeling M., Hartman P., Handschin C., Richter H., and Benson G. M.. 2011. Studies in mice, hamsters, and rats demonstrate that repression of hepatic apoA-I expression by taurocholic acid in mice is not mediated by the farnesoid-X-receptor. J. Lipid Res. 52: 1188–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong B., Singh A. B., Kan C. F. K., and Liu J.. 2014. CETP inhibitors downregulate hepatic LDL receptor and PCSK9 expression in vitro and in vivo through a SREBP2 dependent mechanism. Atherosclerosis. 235: 449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong B., Li H., Singh A. B., Cao A., and Liu J.. 2015. Inhibition of PCSK9 transcription by berberine involves down-regulation of hepatic HNF1alpha protein expression through the ubiquitin-proteasome degradation pathway. J. Biol. Chem. 290: 4047–4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu C., Wang F., Kan M., Jin C., Jones R. B., Weinstein M., Deng C. X., and McKeehan W. L.. 2000. Elevated cholesterol metabolism and bile acid synthesis in mice lacking membrane tyrosine kinase receptor FGFR4. J. Biol. Chem. 275: 15482–15489. [DOI] [PubMed] [Google Scholar]
- 28.Cao A., Wu M., Li H., and Liu J.. 2011. Janus kinase activation by cytokine oncostatin M decreases PCSK9 expression in liver cells. J. Lipid Res. 52: 518–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H., Dong B., Park S. W., Lee H. S., Chen W., and Liu J.. 2009. Hepatocyte nuclear factor 1alpha plays a critical role in PCSK9 gene transcription and regulation by the natural hypocholesterolemic compound berberine. J. Biol. Chem. 284: 28885–28895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evans M. J., Mahaney P. E., Borges-Marcucci L., Lai K., Wang S., Krueger J. A., Gardell S. J., Huard C., Martinez R., Vlasuk G. P., et al. 2009. A synthetic farnesoid X receptor (FXR) agonist promotes cholesterol lowering in models of dyslipidemia. Am. J. Physiol. Gastrointest. Liver Physiol. 296: G543–G552. [DOI] [PubMed] [Google Scholar]
- 31.Genin M. J., Bueno A. B., Agejas F. J., Manninen P. R., Bocchinfuso W. P., Montrose-Rafizadeh C., Cannady E. A., Jones T. M., Stille J. R., Raddad E., et al. 2015. Discovery of 6-(4-{[5-cyclopropyl-3-(2,6-dichlorophenyl)isoxazol-4-yl]methoxy}piperidin-1-yl)- 1-methyl-1H-indole-3-carboxylic acid: a novel FXR agonist for the treatment of dyslipidemia. J. Med. Chem. 58: 9768–9772. [DOI] [PubMed] [Google Scholar]
- 32.Rosenson R. S., Brewer H. B. Jr., Ansell B. J., Barter P., Chapman M. J., Heinecke J. W., Kontush A., Tall A., and Webb N. R.. 2016. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat. Rev. Cardiol. 13: 48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hubbert M. L., Zhang Y., Lee F. Y., and Edwards P. A.. 2007. Regulation of hepatic Insig-2 by the farnesoid X receptor. Mol. Endocrinol. 21: 1359–1369. [DOI] [PubMed] [Google Scholar]
- 34.Trigatti B., Rigotti A., and Krieger M.. 2000. The role of the high-density lipoprotein receptor SR-BI in cholesterol metabolism. Curr. Opin. Lipidol. 11: 123–131. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y., Yin L., Anderson J., Ma H., Gonzalez F. J., Willson T. M., and Edwards P. A.. 2010. Identification of novel pathways that control farnesoid X receptor-mediated hypocholesterolemia. J. Biol. Chem. 285: 3035–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horton J. D., Shah N. A., Warrington J. A., Anderson N. N., Park S. W., Brown M. S., and Goldstein J. L.. 2003. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc. Natl. Acad. Sci. USA. 100: 12027–12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horton J. D., Cohen J. C., and Hobbs H. H.. 2007. Molecular biology of PCSK9: its role in LDL metabolism. Trends Biochem. Sci. 32: 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma A. Z., Song Z. Y., and Zhang Q.. 2014. Cholesterol efflux is LXR alpha isoform-dependent in human macrophages. BMC Cardiovasc. Disord. 14: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong B., Kan C. F., Singh A. B., and Liu J.. 2013. High-fructose diet downregulates long-chain acyl-CoA synthetase 3 expression in liver of hamsters via impairing LXR/RXR signaling pathway. J. Lipid Res. 54: 1241–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Srivastava R. A., and He S.. 2010. Anti-hyperlipidemic and insulin sensitizing activities of fenofibrate reduces aortic lipid deposition in hyperlipidemic Golden Syrian hamster. Mol. Cell. Biochem. 345: 197–206. [DOI] [PubMed] [Google Scholar]
- 41.Srivastava R. A. 2011. Evaluation of anti-atherosclerotic activities of PPAR-alpha, PPAR-gamma, and LXR agonists in hyperlipidemic atherosclerosis-susceptible F(1)B hamsters. Atherosclerosis. 214: 86–93. [DOI] [PubMed] [Google Scholar]
- 42.Dong B., Wu M., Cao A., Li H., and Liu J.. 2011. Suppression of Idol expression is an additional mechanism underlying statin-induced up-regulation of hepatic LDL receptor expression. Int. J. Mol. Med. 27: 103–110. [DOI] [PubMed] [Google Scholar]
- 43.Wiersma H., Gatti A., Nijstad N., Oude Elferink R. P., Kuipers F., and Tietge U. J.. 2009. Scavenger receptor class B type I mediates biliary cholesterol secretion independent of ATP-binding cassette transporter g5/g8 in mice. Hepatology. 50: 1263–1272. [DOI] [PubMed] [Google Scholar]
- 44.Meyer J. M., Graf G. A., and Van der Westhuyzen D. R.. 2013. New developments in selective cholesteryl ester uptake. Curr. Opin. Lipidol. 24: 386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castro-Torres I. G., de Jesús Cárdenas-Vázquez R., Velazquez-Gonzalez C., Ventura-Martinez R., De la O-Arciniega M., Naranjo-Rodriguez E. B., and Martinez-Vazquez M.. 2015. Future therapeutic targets for the treatment and prevention of cholesterol gallstones. Eur. J. Pharmacol. 765: 366–374. [DOI] [PubMed] [Google Scholar]
- 46.Nijstad N., Gautier T., Briand F., Rader D. J., and Tietge U. J.. 2011. Biliary sterol secretion is required for functional in vivo reverse cholesterol transport in mice. Gastroenterology. 140: 1043–1051. [DOI] [PubMed] [Google Scholar]
- 47.Li G., Thomas A. M., Williams J. A., Kong B., Liu J., Inaba Y., Xie W., and Guo G. L.. 2012. Farnesoid X receptor induces murine scavenger receptor Class B type I via intron binding. PLoS One. 7: e35895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chao F., Gong W., Zheng Y., Li Y., Huang G., Gao M., Li J., Kuruba R., Gao X., Li S., et al. 2010. Upregulation of scavenger receptor class B type I expression by activation of FXR in hepatocyte. Atherosclerosis. 213: 443–448. [DOI] [PubMed] [Google Scholar]
- 49.Yashiro T., Yokoi Y., Shimizu M., Inoue J., and Sato R.. 2011. Chenodeoxycholic acid stabilization of LDL receptor mRNA depends on 3′-untranslated region and AU-rich element-binding protein. Biochem. Biophys. Res. Commun. 409: 155–159. [DOI] [PubMed] [Google Scholar]
- 50.Langhi C., Le M. C., Kourimate S., Caron S., Staels B., Krempf M., Costet P., and Cariou B.. 2008. Activation of the farnesoid X receptor represses PCSK9 expression in human hepatocytes. FEBS Lett. 582: 949–955. [DOI] [PubMed] [Google Scholar]
- 51.Ueda Y., Royer L., Gong E., Zhang J., Cooper P. N., Francone O., and Rubin E. M.. 1999. Lower plasma levels and accelerated clearance of high density lipoprotein (HDL) and non-HDL cholesterol in scavenger receptor class B type I transgenic mice. J. Biol. Chem. 274: 7165–7171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.