Abstract
The purpose of this work was to determine whether changes in cholesterol profiles after interferon-β (IFN-β)1a treatment initiation following the first demyelinating event suggestive of multiple sclerosis are associated with clinical and MRI outcomes over 4 years. A group of 131 patients (age: 27.9 ± 7.8 years, 63% female) with serial 3-monthly clinical and 12-monthly MRI follow-ups over 4 years were investigated. Serum cholesterol profiles, including total cholesterol (TC), HDL cholesterol (HDL-C), and LDL cholesterol (LDL-C) were obtained at baseline, 1 month, 3 months, and every 6 months thereafter. IFN-β1a initiation caused rapid decreases in serum HDL-C, LDL-C, and TC within 1 month of IFN-β1a initiation (all P < 0.001) that returned slowly toward baseline. In predictive mixed model analyses, greater percent decreases in HDL-C after 3 months of IFN-β1a treatment initiation were associated with less brain atrophy over the 4 year time course, as assessed by percent brain volume change (P < 0.001), percent gray matter volume change (P < 0.001), and percent lateral ventricle volume change (P = 0.005). Decreases in cholesterol biomarkers following IFN-β1a treatment are associated with brain atrophy outcomes over 4 years. Pharmacological interventions targeting lipid homeostasis may be clinically beneficial for disrupting neurodegenerative processes.
Keywords: magnetic resonance imaging, cholesterol, brain atrophy
Interferon-β (IFN-β) treatment is one of the most widely used disease-modifying treatments for multiple sclerosis (MS). The efficacy of recombinant human IFN-β for relapsing MS has been established by multiple double-blind placebo-controlled multi-center trials (1–3). IFN-β can delay the conversion to clinically definite MS (CDMS) in patients with a first demyelinating event (4–6), which supports early intervention with IFN-β.
MRI atrophy measures of brain volume and gray matter, white matter, and lateral ventricle volumes provide quantitative measures of global and tissue-specific brain volumes (7–9). Brain atrophy is useful for evaluating disease progression and therapeutic efficacy in MS (7) because it is a predictor of physical disability, cognitive dysfunction, and quality of life (10). However, there is a lack of effective serum biomarkers capable of predicting atrophy in MS patients.
There is considerable inter-individual variability of IFN-β1a effectiveness among MS patients. Phase III studies of IFN-β indicate a 30–40% general clinical benefit, although the response varies significantly among MS patients. On MRI, approximately 40% of patients show complete suppression of new contrast-enhancing lesions (CELs), whereas 20% of patients have less than 70% suppression (11). Thus, the problem of predictively identifying nonresponders has gained greater clinical salience and urgency, given the availability of alternative disease-modifying treatments.
Anti-IFN-β neutralizing antibodies (NABs), which develop after 12–18 months of treatment in 2–25% of IFN-β1a-treated patients and up to 38% of IFN-β1b-treated patients (12, 13), provide a clinically useful biologically intuitive mechanistic explanation for partial responsiveness to IFN-β. The presence of persistently high levels of NAB abrogates IFN-induced signaling responses and is associated with decreases in clinical and MRI effectiveness of IFN-β therapy. The majority of MS patients who are partially responsive to IFN-β tend to be NAB-negative (2, 14, 15).
IFN-β has antiviral, anti-proliferative, and immunomodulatory effects (16, 17). Diverse biomarkers, ranging from single nucleotide polymorphisms, mRNA (e.g., MxA, Stat1, TRAIL, and others), proteins (e.g., oligoadenylate synthetase activity, Mx protein, and β2 microglobulin), and metabolites (e.g., neopterin) to immune cell populations, have been investigated as potential IFN-β biomarkers (18–20). However, they were not useful predictors of MRI and clinical outcomes.
We investigated cholesterol profiles as a potential biomarker for IFN-β therapy in MS based on a report that type 1 IFN treatment caused coordinated changes in the expression of genes involved in sterol synthesis (21). We found that each 10 mg/dl of greater baseline LDL cholesterol (LDL-C), total cholesterol (TC), and apoB was associated with a 7.4%, 5.9%, and 16% increase in the number of new T2 lesions over 2 years of IFN-β treatment, respectively (22, 23). Our findings on cholesterol and apolipoproteins in MS disease progression have since been independently confirmed (24–26).
The goals of this study were to investigate the effects of IFN-β therapy on cholesterol profile changes for a 72 month period following IFN-β1a initiation and to examine the associations, if any, of cholesterol changes with MRI measures over a 48 month period in IFN-β-treated MS patients.
METHODS
Study population
The SET study (27) was a prospective longitudinal observational clinical study that involved eight centers in the Czech Republic (clin.gov # NCT01592474). The Ethics Committees of all participating centers approved the study protocol. All patients gave their written informed consent.
Inclusion and exclusion criteria.
This multi-center study enrolled patients within 4 months after their first clinical event suggestive of MS. The inclusion criteria were: age 18–55 years, Expanded Disability Status Scale (EDSS) score of 3.5 or less, presence of two or more T2-hyperintense lesions on diagnostic MRI, and two or more oligoclonal bands in CSF obtained at the screening visit prior to steroid treatment. Exclusion criteria were occurrence of a second relapse before the baseline visit and pregnancy.
Study design and assessments
Assessments and treatment algorithms.
The study included clinical visits every 3 months during 48 months of follow-up in routine clinical practice. The clinical outcomes of the study were new relapses associated with conversion to CDMS and sustained disability progression, defined as an increase in EDSS by 1.0 point (if baseline EDSS > 0) or 1.5 points (if baseline EDSS = 0), confirmed after 12 months.
MRI was obtained using standardized protocols on a single 1.5 Tesla MRI scanner at baseline, at 6 months, and yearly thereafter until the 48 month time point. Information on the MRI acquisition and analysis protocols can be found in the supplemental data.
All patients started with 30 mcg of intramuscular IFN-β1a once-weekly (AVONEX®, Biogen-Idec) at baseline. The mean time between clinical onset and baseline was 81.8 ± 22.8 (SD) days (median 78.0 days; range 33–122 days). All patients were treated with 3–5 g of methylprednisolone for the first clinical symptoms before study entry. None of the patients were on statins.
Treatment change criteria.
The SET study protocol defines once-weekly IFN-β1a treatment failure as ≥2 relapses during 12 months or a 6 month sustained disability progression.
Treating physicians had full discretion to individualize treatment changes for once-weekly IFN-β1a treatment failures. Protocol-recommended options for treatment failures included the following: subcutaneous IFN-β1a (44 μg three times weekly), intravenous natalizumab (300 mg per month), or the addition of azathioprine (50 mg twice-daily to once-weekly IFN-β1a). Patients who failed the initial treatment change were treated with subcutaneous glatiramer acetate (20 mg daily) or mitoxantrone (10 mg).
Serum cholesterol profiles
For biochemical assessment, 5 ml of blood was obtained at screening, baseline, 1 month, every 3 months for the first year, and every 6 months thereafter. Serum for lipid analyses was obtained in the nonfasted state at the central site. Examination at screening was performed before any steroid treatment, and baseline examinations were at least 30 days after. Diagnostic reagent kits (Cholesterol Liquicolor and HDL Liquicolor; Human Gesellschaft fűr Biochemica und Diagnostica mbH, Germany) were used to measure serum TC, HDL cholesterol (HDL-C), and triglycerides. The intra-assay coefficient of variation for TC and HDL-C serum levels was 0.85%, and the inter-assay coefficient of variation was 1.46–1.86%. LDL-C was obtained from the Friedewald equation (28).
Statistical analysis
SPSS 22.0 (IBM, Armonk, NY) statistical software was used for statistical analyses.
Percent changes in TC (ΔTC%), HDL-C (ΔHDL-C%), and LDL-C (ΔLDL-C%) were computed at each time point using baseline TC, HDL-C, and LDL-C values as the reference. A zero value for percent change represents no change compared with baseline, a positive value represents an increase in levels, and a negative value represents a decrease in levels compared with baseline.
The statistical significance of ΔTC%, ΔHDL-C%, and ΔLDL-C% values at 1, 3, and 6 months was assessed using a one-sample t-test.
The time courses of clinical and MRI outcome measure-dependent variables [percent brain volume change (PBVC), percent gray matter volume change (PGMVC), percent lateral ventricle volume change (PLVVC), new/enlarging T2 and CEL, EDSS, and cumulative relapses] were analyzed with longitudinal adjusted linear mixed-effect model analysis with a random intercept for time and each patient. In the longitudinal analyses, the fixed effects included age, gender, BMI, and baseline values of the clinical or MRI outcome measure and the time course of the individual cholesterol variable (either ΔHDL-C%, ΔLDL-C%, or ΔTC%) of interest.
The same linear mixed-effect model method was used for the predictive analysis. The fixed effects included age, gender, BMI, baseline values of the clinical or MRI outcome measure, and individual cholesterol variable of interest (ΔHDL-C%, ΔLDL-C%, or ΔTC%) at a single early time point (either 1, 3, or 6 months).
The Mann-Whitney test was used to assess differences in cumulative relapses, EDSS change, PBVC, and ΔHDL-C% at 3 months between patients who remained on once-weekly intramuscular IFN-β1a or switched to no treatment versus patients who switched to subcutaneous IFN-β, glatiramer acetate, natalizumab, or other treatments.
A Benjamini-Hochberg correction with P < 0.05 was used to control the false discovery rate (q value) due to multiple testing for predictive associations.
RESULTS
Baseline and follow-up clinical characteristics
Of the 220 patients enrolled in the SET study, 131 (age: 27.9 ± 7.8 years, 63% female) had lipid, clinical, and MRI follow-up data at 4 years. A subset of patients also had lipid measures available over 6 years that were included in the analysis of the time course of lipid profile changes. Aside from a modest difference in baseline EDSS (P = 0.018, Mann-Whitney test) between the included patients (median EDSS ± interquartile range = 1.5 ± 0.5) and the excluded patients (median EDSS ± interquartile range = 1.5 ± 1.0), the subset of patients was representative of the larger SET study cohort on demographic, clinical, and MRI characteristics. Supplemental Table S1 summarizes the number of lipid profiles available at each time-point.
Table 1 summarizes patient demographic, clinical, laboratory, and MRI characteristics at baseline, 24 months, and 48 months. At 4 years, 13% of patients reached 12 month sustained disability progression.
TABLE 1.
Demographic, clinical, laboratory, and MRI characteristics
| Demographic Measures | Baseline | 24 Months | 48 Months |
| Number of females, n (%) | 86 (63) | — | — |
| Age, years at baseline | 27.9 ± 7.8 | — | — |
| Time to baseline in days | 81.9 ± 22.7 | — | — |
| Body mass index, kg/m2 | 24.2 ± 3.8 | — | — |
| Clinical measures | |||
| Median EDSS (range) | 1.5 (0.0–3.5) | 1.5 (0.0–4.0) | 1.5 (0.0–4.5) |
| Cumulative number of relapses | — | 0.9 ± 1.4 | 1.1 ± 1.7 |
| Number of patients with CDMS, n (%) | 0 (0) | 54 (40) | 59 (45) |
| Laboratory measures | |||
| Total cholesterol, mmol/l | 4.8 ± 0.9 | 4.5 ± 0.9 | 4.7 ± 0.9 |
| LDL-C, mmol/l | 2.8 ± 0.8 | 2.4 ± 0.7 | 2.6 ± 0.7 |
| HDL-C, mmol/l | 1.4 ± 0.4 | 1.4 ± 0.4 | 1.4 ± 0.4 |
| MRI measures | |||
| T2 lesion volume, ml | 4.8 ± 5.6 | 4.8 ± 7.5 | 4.9 ± 7.4 |
| Number of patients with CEL, n (%) | 31 (23) | 25 (18.7) | 9 (7) |
| Normalized brain volume, ml | 1,505 ± 70 | 1,485 ± 70 | 1,477 ± 72 |
| Gray matter volume, ml | 793 ± 46 | 777 ± 48 | 776 ± 45 |
| Lateral ventricle volume, ml | 36.5 ± 11 | 40.2 ± 12 | 41.9 ± 13 |
| Treatment changesa | |||
| Remaining on once-weekly IFN-β1a | — | 110 | 95 |
| Changed to no treatment | — | 2 | 3 |
| Changed to subcutaneous IFN-β | — | 17 | 22 |
| Changed to glatiramer acetate | — | 0 | 2 |
| Changed to natalizumab and other treatments | — | 3 | 8 |
Data are mean ± SD unless indicated.
Treatment change data not available for one patient at 2 years and one patient at 4 years.
Time course of cholesterol profile changes
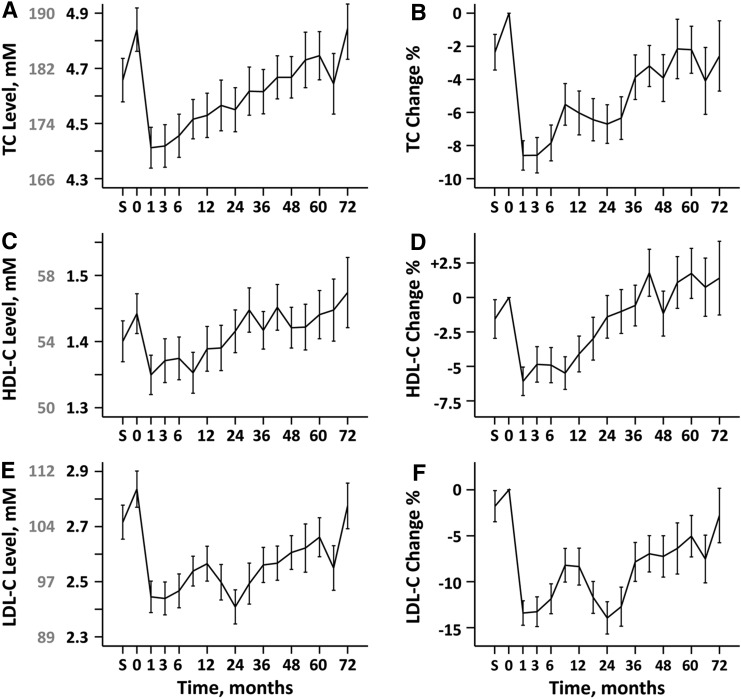
TC, HDL-C, and LDL-C levels declined from baseline at 1 month following IFN-β1a treatment and gradually returned to baseline over 72 months (Fig. 1). The corresponding percent changes, ΔTC%, ΔHDL-C%, and ΔLDL-C%, followed the same pattern.
Fig. 1.
The time course of TC levels and percent change in TC over 72 months are shown in (A) and (B), respectively. The time courses for HDL-C and percent change in HDL-C are shown in (C) and (D), respectively. E, F: Show the percent change in LDL-C. The units for TC, HDL-C, and LDL-C are in millimoles. The gray labels in (A), (C), and (E) show the corresponding levels in milligrams per deciliter.
At 1 month following IFN-β1a initiation, the average ΔTC% was an 8.7% decrease, the average ΔHDL-C% was a 6.1% decrease, and the average ΔLDL-C% was a 13.4% decrease. ΔTC%, ΔHDL-C%, and ΔLDL-C% at the 1, 3, and 6 month time points following IFN-β1a initiation were negative relative to baseline (all P < 0.001). The decreases in ΔTC% were sustained through 4 years (all P < 0.007) with a return to baseline occurring at month 54. ΔHDL-C% decreases remained significant compared with baseline through the first year (all P < 0.002), but returned to baseline levels at 18 months. The decreases in ΔLDL-C% were sustained over the observation period (all P < 0.05), with a return to baseline at the end of the 6 year period.
There was substantial inter-patient variability in the lipid changes over time. Supplemental Fig. S1 shows histograms of ΔTC%, ΔHDL-C%, and ΔLDL-C% at 1, 3, and 6 months of IFN-β1a treatment. The peaks of percent change were negative, indicating that the majority of patients exhibit decreases in ΔTC%, ΔHDL-C%, and ΔLDL-C% at 1, 3, and 6 months.
The time courses for the mean values of ΔTC%, ΔHDL-C%, and ΔLDL-C% by the quartiles of ΔHDL-C% at 3 months are summarized in supplemental Fig. S2. The lowest quartile of ΔHDL-C% at 3 months was associated with sustained decreases in mean ΔHDL-C% and ΔTC% over the 4 year period. The patterns of ΔLDL-C% were not sufficiently segregated by quartiles of ΔHDL-C%.
Longitudinal associations of cholesterol profile with MRI and clinical measures
We investigated the associations of cholesterol profiles at baseline and longitudinal ΔTC%, ΔHDL-C%, ΔLDL-C% changes occurring after IFN-β1a treatment with the longitudinal clinical and MRI measures. The results are summarized in Table 2.
TABLE 2.
Associations of longitudinal percent changes in cholesterol variables with MRI and clinical outcomes
| Clinical/MRI Variablea | Longitudinal Percent Changes in Cholesterol Variablesb | ||
| ΔTC% | ΔLDL-C% | ΔHDL-C% | |
| PBVC | −1.09 (< 0.001) | 0.0113 (0.95) | −0.835 (0.001) |
| PGMVC | 0.0137 (0.98) | 1.21 (< 0.001) | −1.34 (0.002) |
| PLVVC | 1.30 (0.51) | −4.48 (0.001) | 6.10 (0.001) |
| New T2 lesions | 2.90 (0.027) | 0.838 (0.35) | 3.29 (0.009) |
| New/enlarging T2 lesions | 3.69 (0.038) | 1.18 (0.33) | 4.47 (0.009) |
| CEL | 5.16 (< 0.001) | 3.51 (< 0.001) | 2.39 (0.023) |
| EDSS | 0.145 (0.36) | 0.105 (0.33) | 0.0054 (0.97) |
| Cumulative relapses | 0.289 (0.059) | 0.0458 (0.674) | 0.252 (0.089) |
Slope (P values) from mixed effect models are summarized.
In MS decreases in normalized brain volume, decreases in gray matter volume, increases in lateral ventricle volume, increases in the number of T2 lesions, increases in contrast enhancing lesions (CEL), increases in EDSS and increases in cumulative relapses are adverse MRI/clinical outcomes.
Percent changes in lipid variables (ΔTC%, ΔLDL-C%, ΔHDL-C%) are positive when the lipid level is increased relative to baseline, zero when the lipid variable is unchanged relative to baseline, and negative when the lipid variable is decreased relative to baseline.
Baseline cholesterol profiles.
Baseline LDL-C (P = 0.025) and TC (P = 0.001) levels were associated with a greater occurrence of new T2 lesions and a greater number of new/enlarging T2 lesions (P = 0.003 for TC and P = 0.015 for LDL-C). Increases in baseline TC were associated with a greater cumulative number of relapses (P = 0.039). We did not obtain evidence for associations for changes in brain volumes with baseline TC, LDL-C, and HDL-C (data not shown).
Longitudinal percent decreases.
Greater longitudinal decreases in ΔTC% and ΔHDL-C% were associated with a smaller number of new lesions (P = 0.027 for ΔTC%, P = 0.009 for ΔHDL-C%) and new/enlarging T2 lesions (P = 0.038 for ΔTC%, P = 0.009 for ΔHDL-C%).
Greater longitudinal decreases in ΔTC% (P < 0.001) and ΔHDL-C% (P = 0.001) were associated with smaller decreases in PBVC. Greater longitudinal decreases in ΔHDL-C% (P = 0.002) were associated with smaller decreases in PGMVC. In contrast, greater longitudinal decreases in ΔLDL-C% were associated with greater decreases in PGMVC (P < 0.001). Longitudinal decreases in ΔTC% were not associated with PGMVC. Thus the changes in ΔLDL-C% and ΔHDL-C% following IFN-β1a treatment have opposing effects on PGMVC that offset each other. Likewise, greater decreases in ΔHDL-C% were associated with smaller increases in PLVVC (P = 0.001).
These results suggest that greater decreases in ΔHDL-C% are associated with less brain atrophy.
Predictive associations of cholesterol profile changes with clinical and MRI measures
We assessed whether the associations of ΔHDL-C% changes at a single early time point (at 3 months) following IFN-β1a treatment initiation could be deployed predictively as a possible biomarker for longitudinal clinical and MRI measures.
For completeness, similar analyses were done with ΔHDL-C% changes at 1 and 6 months and for ΔLDL-C% and ΔTC% changes at 1, 3, and 6 months. The ΔHDL-C% at 1 and 3 months exhibited associations with a greater number of clinical measures than ΔLDL-C% and ΔTC% changes at 1, 3, and 6 months. In the interest of brevity, we report only our ΔHDL-C% results at 1 and 3 months in detail.
Lesion measures.
Table 3 summarizes the associations of MRI measures (number of new lesions, number of new/enlarging T2 lesions, and CEL number) with ΔHDL-C% after 1 and 3 months.
TABLE 3.
Predictive associations between HDL-C percent changes at 1 month and 3 months and clinical and brain imaging outcome measures
| Clinical/MRI Variablea | ΔHDL-C% 1 Monthb | ΔHDL-C% 3 Monthsb | ||||
| Slope | P | q | Slope | P | q | |
| PBVC | −2.55 | 1.2 × 10−8 | 1.2 × 10−7 | −1.90 | 4.9 × 10−8 | 4.9 × 10−7 |
| PGMVC | −1.67 | 0.004 | 0.02 | −1.70 | 1.4 × 10−4 | 4.7 × 10−4 |
| PLVVC | 3.39 | 0.16 | 0.23 | 5.5 | 0.005 | 0.013 |
| New T2 lesions | −3.13 | 0.16 | 0.27 | −0.644 | 0.71 | 0.71 |
| New/enlarging T2 lesions | −6.39 | 0.035 | 0.07 | −1.45 | 0.54 | 0.68 |
| CEL | 1.12 | 0.47 | 0.52 | 0.687 | 0.56 | 0.62 |
| EDSS | −0.01 | 0.96 | 0.96 | 0.324 | 0.054 | 0.077 |
| Cumulative relapses | 0.187 | 0.42 | 0.52 | 0.391 | 0.024 | 0.04 |
Slope, P value, and q value (false discovery rate) are provided.
In MS decreases in normalized brain volume, decreases in gray matter volume, increases in lateral ventricle volume, increases in the number of T2 lesions, increases in CEL, increases in EDSS, and increases in cumulative relapses are adverse MRI/clinical outcomes.
ΔHDL-C% at 1 month and ΔHDL-C% at 3 months are positive when HDL levels are increased relative to baseline, zero when HDL is unchanged relative to baseline, and negative when HDL is decreased relative to baseline.
Following correction for multiple testing, there were no significant associations of the ΔHDL-C% with these lesional measures.
Atrophy measures.
In adjusted mixed model analyses (Table 3), a significant association was observed between ΔHDL-C% after 1 month with PBVC (P < 0.001) and PGMVC (P = 0.004). Table 3 also shows the slopes that provide a measure of the strength of the associations with ΔHDL-C% at 1 month with all other variables held constant. A 1% decrease in HDL-C at 1 month was associated with a 2.55% improvement in brain volume and a 1.67% improvement in gray matter volume.
ΔHDL-C% at 3 months was associated with PBVC (P < 0.001), PGMVC (P < 0.001), and PLVVC (P = 0.005). The slopes in Table 3 indicate that a 1% decrease in HDL-C at 3 months is associated with a 1.9% improvement in brain volume, a 1.7% improvement in gray matter volume, and a 5.5% improvement in lateral ventricle volume.
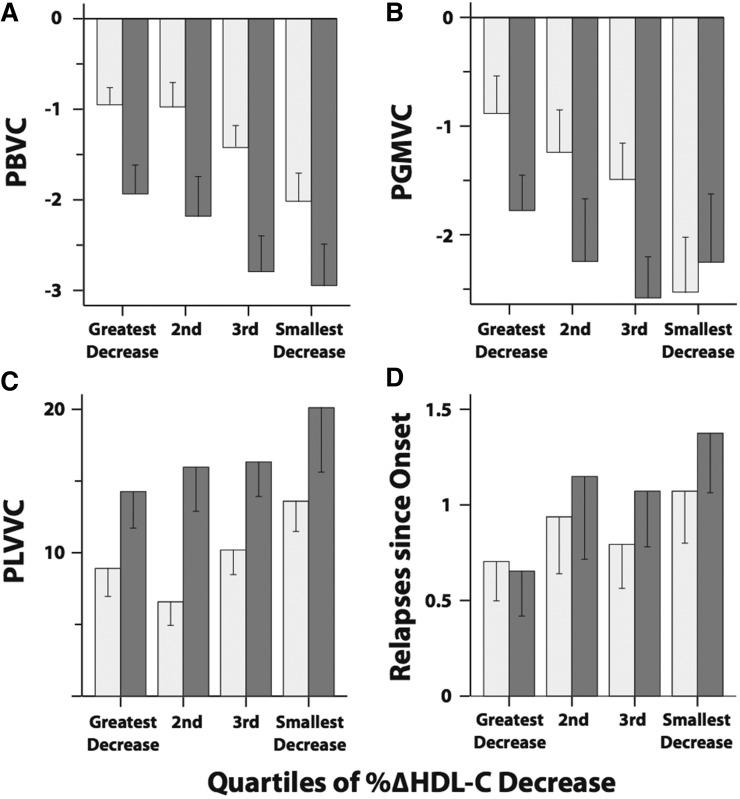
These results are graphically summarized by quartiles of ΔHDL-C% at 3 months in Fig. 2A–C. The lowest quartile represents the patients with the greatest decrease in ΔHDL-C% at 3 months, whereas the highest quartile represents the patients with the smallest decreases in ΔHDL-C% at 3 months. The lowest quartile subgroup shows better atrophy outcomes compared with the highest quartile subgroup.
Fig. 2.
Mean values of changes in clinical outcome measures at 2 years (light gray bars) and 4 years (dark bars) by quartiles of ΔHDL-C% at 3 months. The mean PBVC (A), PGMVC (B), PLVVC (C), and relapses since onset (D) are shown. The bars represent the standard error values.
For completeness, we also investigated the associations of ΔLDL-C% and ΔTC% at 1, 3, and 6 months with the same atrophy measures. PBVC, PGMVC, and PLVVC were not associated with ΔLDL-C% at 1, 3, or 6 months. PBVC was significantly associated with ΔTC% at 1 month (P = 0.001), ΔTC% at 3 months (P < 0.001), and ΔTC% at 6 months (P = 0.002). We attribute the associations of ΔTC% to the contributions of ΔHDL-C%.
Effects of T2 lesions on predictive associations.
In additional mixed effect analyses, we included baseline T2 lesion volume as an additional predictor to determine whether the associations of ΔHDL-C% at 3 months with atrophy measures remained significant. The associations of ΔHDL-C% at 3 months with PBVC, PGMVC, and PLVVC remained significant (all P ≤ 0.003, data not shown) in these analyses.
We also performed similar analyses that included the number of new T2 lesions as an additional predictor. Again, the associations of ΔHDL-C% at 3 months with PBVC, PGMVC, and PLVVC remained significant (all P ≤ 0.001).
The statistical significance of these associations indicates that ΔHDL-C% at 3 months explains variance in brain atrophy that is not explained by T2 lesions. Thus, the predictive associations of ΔHDL-C% at 3 months with brain atrophy may be clinically useful, because they complement MRI measures of lesion burden and activity.
Disability and relapses.
Greater decreases in ΔHDL-C% at 3 months were associated with a lower cumulative number of relapses (P = 0.024). ΔHDL-C% at 1 month was not associated with cumulative number of relapses (Table 3). Figure 2F compares the mean cumulative number of relapses for the quartiles of ΔHDL-C% at 3 months. The patients in the highest quartile of ΔHDL-C% at 3 months had a larger number of cumulative relapses compared with the lowest quartile. The ΔHDL-C% after 1 and 3 months was not associated with EDSS.
These findings are consistent with the hypothesis that the magnitude of decreases in ΔHDL-C% 3 months after IFN-β1a initiation may be a biomarker for individuals at risk for increased whole brain atrophy and relapses.
Cholesterol profiles in patients receiving treatment changes
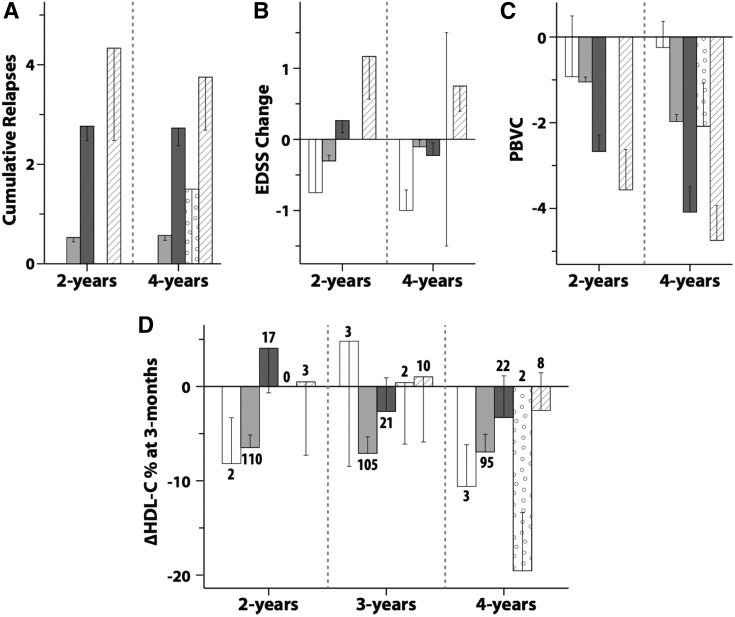
Group 1 comprised the patients who remained on once-weekly IFN-β1a and those who switched to no treatment. Group 2 consisted of patients whose treatment was changed to other treatments (subcutaneous IFN-β, glatiramer acetate, natalizumab, and other therapies). The number of patients requiring treatment change at 2, 3, and 4 years is summarized in Fig. 3D.
Fig. 3.
Mean values of cumulative relapses (A), EDSS change (B), and PBVC (C) at 2 years and at 4 years, and mean ΔHDL-C% at 3 months (D) at 2, 3, and 4 years for each treatment change group. The patients who switched to no treatment are shown with white bars, patients remaining on once-weekly IFN-β1a are in light gray bars, patients who switched to subcutaneous IFN-β are in dark gray bars, patients who switched to glatiramer acetate are in spotted bars, and patients who switched to natalizumab and other treatments are in striped bars. The number shown against each bar in (D) is the number of patients in that treatment change group. The error bars are standard errors.
Figure 3A–C shows the cumulative number of relapses, the EDSS change from baseline, and the PBVC for the treatment change groups at 2 and 4 years. At 2 years, Group 1 had lower values for mean number of relapses (P < 0.001), EDSS change (P = 0.002), and a smaller decrease in PBVC (P < 0.001) compared with Group 2. At 4 years, Group 1 had a lower mean number of relapses and a smaller decrease in PBVC (both P < 0.001). The results in Fig. 3A, B are not surprising because patients were changed to other therapies based on occurrence of relapses or EDSS changes. The PBVC findings in Fig. 3C mirror the clinical findings.
Figure 3D shows the ΔHDL-C% at 3 months for the treatment change subgroups at 2, 3, and 4 years. At 2 years, Group 1 had greater mean decreases in ΔHDL-C% at 3 months compared with Group 2 (P = 0.028).
We conducted sub-analyses including only data points at which patients were on once-weekly IFN-β1a. The associations of ΔHDL-C% at 3 months with PBVC, PGMVC, and PLVVC remained significant (all P ≤ 0.004, data not shown)
DISCUSSION
In this report, we investigated the effects of long-term IFNβ-1a treatment on cholesterol profiles following the first demyelinating event in patients enrolled in the SET study (23, 29–31). IFN-β treatment was associated with an initial decrease of serum cholesterol biomarkers followed by a gradual return to baseline. HDL-C returned to baseline within 18 months, TC returned to baseline within 54 months, but LDL-C did not return to baseline until the end of the 6 year observation period. Greater longitudinal decreases in ΔHDL-C% were associated with smaller decreases in PBVC and PGMVC, and greater increases in PLVVC. Changes in ΔLDL-C% and ΔHDL-C% following IFNβ-1a treatment have opposing effects on PGMVC that offset each other. ΔHDL-C% at 3 months was associated with the time course of atrophy outcomes over 4 years.
The strengths of the study include the prospective longitudinal study design and the availability of cholesterol biomarkers and clinical and MRI data over a 4 year period. This enabled us to investigate the role of cholesterol biomarkers at the early stages of MS in a relatively young patient cohort that was not on statin treatments. The limitations include the lack of a suitable control population that was not on IFNβ-1a treatment. We also did not have data on dietary and lifestyle changes in the patient population.
The mechanisms responsible for the associations between brain volume changes and early decreases in HDL-C levels following IFN initiation are unknown. A plausible explanation is that decreases in HDL-C are particularly effective as a clinically integrative measure of the same pathways that mediate IFN-β treatment effects on brain volume changes. Previously investigated biomarkers, e.g., MxA, reflect single pathways, whereas serum cholesterol levels are largely dependent on multiple biochemical homeostatic pathways. Cholesterol is also required for important brain functions, including myelin formation, immune functioning, neuronal signaling, and vascular function; therefore, changes may reflect not only treatment effects, but also pathophysiological effects. Interestingly, the MS-STAT study of the cholesterol-lowering drug, simvastatin, reported reduction of brain atrophy in secondary progressive MS (32). This may suggest that reductions in cholesterol may affect accumulation of atrophy in MS.
Vascular comorbidities were reported to be associated with more rapid disability progression in an MS patient-reported registry (33). However, the mean age of this study was 52.7 ± 10.4 years compared with our cohort, which had an average age of 27.9 ± 7.8 years. The venous insufficiency hypothesis for MS has been discredited (34). Thus, our operating hypothesis is that IFN treatment effects on serum cholesterol profiles, rather than vascular factors, are likely responsible for the observed associations with brain atrophy.
Our results confirm and extend the findings reported by Morra et al. (35), who reported that the percent decrease in TC at 2 months after IFN initiation was 8.2% and at 12 months following IFN initiation was 7%. We observed an 8.7% decrease in TC at 1 month, a 7.8% decrease at 6 months, and a 6% decrease at 12 months after IFN initiation. Morra et al. (35) did not fully assess whether the decreases in TC following IFN-β treatment were sustained over the long-term and did not include longitudinal outcome measures.
The IFN-β1a effects on cholesterol homeostasis that we report are likely shared by other type 1 IFNs. Once-daily intramuscular recombinant and leukocyte-derived IFN-α induced an approximately 25% decrease in cholesterol and a 16% decrease in HDL-C within a few days of initiating treatment in metastatic breast carcinoma and nodular lymphoma patients (36). Similar results were reported in patients treated with IFN-α2a for hepatitis (37). We anticipate that PEGylated-IFN (PEGinterferon-β1a, PLEGRIDY), which has been approved for MS, will also exhibit effects on cholesterol profiles.
The reason for the return of cholesterol levels to baseline remains unclear. The initial decrease may be attributed to the multiple mechanisms by which IFN-β acts on the cholesterol pathway. These include downregulation of the sterol pathway through decreases in SREBP2 (21), the primary transcription factor in sterol synthesis, as well as induction of 25-hydroxylase (38), which promotes the metabolism of cholesterol to 25-hydroxycholesterol. The return of cholesterol levels to baseline occurs more slowly compared with the IFN-β1a-induced decreases and may reflect compensatory mechanisms. It is also possible that patients are more susceptible to dyslipidemia as they age or that responses to IFN-β1a may be attenuated over time.
IFN-β remains an important first-line MS therapy alongside glatiramer acetate. The identification of HDL-C decreases as a potential biomarker of long-term atrophy following IFN-β treatment could find clinical utility if validated in larger well-controlled studies, because the underlying methodology is familiar and inexpensive. Our results could thus be a first step toward individualizing MS therapy by identifying IFN-β nonresponders who could be switched to other therapies.
Supplementary Material
Acknowledgments
The authors thank the patients who participated in this study. They thank the other clinical centers and investigators who participated in the SET study: i) M. Vachova, S. Machalicka, and J. Kotalova from KZ a.s. Hospital, Teplice; ii) Y. Benesova, P. Praksova, and P. Stourac from University Hospital, Brno, Bohunice; iii) M. Dufek from St. Anne’s University Hospital, Brno; iv) E. Meluzinova, J. Pikova, and E. Houzvickova from Charles University in Prague, Second Faculty of Medicine, Motol; v) D. Zimova from Charles University in Prague, Third Faculty of Medicine, Kralovske Vinohrady; vi) J. Sucha from University Hospital, Plzen; and vii) V. Sladkova and J. Mares from University Hospital, Olomouc.
Footnotes
Abbreviations:
- CDMS
- clinically definite multiple sclerosis
- CEL
- contrast-enhancing lesion
- EDSS
- Expanded Disability Status Scale
- HDL-C
- HDL cholesterol
- ΔHDL-C%
- percent changes in HDL cholesterol
- IFN-β
- interferon-β
- LDL-C
- LDL cholesterol
- ΔLDL-C%
- percent changes in LDL cholesterol
- MS
- multiple sclerosis
- NAB
- neutralizing antibody
- PBVC
- percent brain volume change
- PGMVC
- percent gray matter volume change
- PLVVC
- percent lateral ventricle volume change
- TC
- total cholesterol
- ΔTC%
- percent changes in total cholesterol
This work was supported by National Multiple Sclerosis Society Grant RG4836-A-5 to the Ramanathan Laboratory and Ministry of Health of the Czech Republic Grants PRVOUK-P26/LF1/4, RVO-VFN64165, NT13237-4/2012, and NT13108-4/2012 for the SET study. The National Institute of Neurological Disorders and Stroke (1R21NS098169) to the Ramanathan laboratory is gratefully acknowledged. This research was conducted while T.U. was an MSBase Fellow.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.1993. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. The IFNB Multiple Sclerosis Study Group. Neurology. 43: 655–661. [DOI] [PubMed] [Google Scholar]
- 2.Yong V. W., Chabot S., Stuve O., and Williams G.. 1998. Interferon beta in the treatment of multiple sclerosis: mechanisms of action. Neurology. 51: 682–689. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs L. D., Cookfair D. L., Rudick R. A., Herndon R. M., Richert J. R., Salazar A. M., Fischer J. S., Goodkin D. E., Granger C. V., Simon J. H., et al. 1996. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann. Neurol. 39: 285–294. [DOI] [PubMed] [Google Scholar]
- 4.Comi G., Filippi M., Barkhof F., Durelli L., Edan G., Fernandez O., Hartung H., Seeldrayers P., Sorensen P. S., Rovaris M., et al. 2001. Effect of early interferon treatment on conversion to definite multiple sclerosis: a randomised study. Lancet. 357: 1576–1582. [DOI] [PubMed] [Google Scholar]
- 5.Hartung H. P. 2005. Early treatment and dose optimisation BENEFIT and BEYOND. J. Neurol. 252(Suppl 3): iii44–iii50. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs L. D., Beck R. W., Simon J. H., Kinkel R. P., Brownscheidle C. M., Murray T. J., Simonian N. A., Slasor P. J., and Sandrock A. W.. 2000. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group. N. Engl. J. Med. 343: 898–904. [DOI] [PubMed] [Google Scholar]
- 7.Rudick R. A., Fisher E., Lee J. C., Simon J., and Jacobs L.. 1999. Use of the brain parenchymal fraction to measure whole brain atrophy in relapsing-remitting MS. Multiple Sclerosis Collaborative Research Group. Neurology. 53: 1698–1704. [DOI] [PubMed] [Google Scholar]
- 8.Benedict R. H., Bakshi R., Simon J. H., Priore R., Miller C., and Munschauer F.. 2002. Frontal cortex atrophy predicts cognitive impairment in multiple sclerosis. J. Neuropsychiatry Clin. Neurosci. 14: 44–51. [DOI] [PubMed] [Google Scholar]
- 9.Benedict R. H., Weinstock-Guttman B., Fishman I., Sharma J., Tjoa C. W., and Bakshi R.. 2004. Prediction of neuropsychological impairment in multiple sclerosis: comparison of conventional magnetic resonance imaging measures of atrophy and lesion burden. Arch. Neurol. 61: 226–230. [DOI] [PubMed] [Google Scholar]
- 10.Bakshi R., Czarnecki D., Shaikh Z. A., Priore R. L., Janardhan V., Kaliszky Z., and Kinkel P. R.. 2000. Brain MRI lesions and atrophy are related to depression in multiple sclerosis. Neuroreport. 11: 1153–1158. [DOI] [PubMed] [Google Scholar]
- 11.Stone L. A., Frank J. A., Albert P. S., Bash C. N., Calabresi P. A., Maloni H., and McFarland H. F.. 1997. Characterization of MRI response to treatment with interferon beta-1b: contrast-enhancing MRI lesion frequency as a primary outcome measure. Neurology. 49: 862–869. [DOI] [PubMed] [Google Scholar]
- 12.1996. Neutralizing antibodies during treatment of multiple sclerosis with interferon beta-1b: experience during the first three years. The IFNB Multiple Sclerosis Study Group and the University of British Columbia MS/MRI Analysis Group. Neurology. 47: 889–894. [DOI] [PubMed] [Google Scholar]
- 13.PRISMS Study Group and the University of British Columbia MS/MRI Analysis Group. 2001. PRISMS-4: Long-term efficacy of interferon-beta-1a in relapsing MS. Neurology. 56: 1628–1636. [DOI] [PubMed] [Google Scholar]
- 14.Filippini G., Munari L., Incorvaia B., Ebers G. C., Polman C., D’Amico R., and Rice G. P.. 2003. Interferons in relapsing remitting multiple sclerosis: a systematic review. Lancet. 361: 545–552. [DOI] [PubMed] [Google Scholar]
- 15.Sorensen P. S., Ross C., Clemmesen K. M., Bendtzen K., Frederiksen J. L., Jensen K., Kristensen O., Petersen T., Rasmussen S., Ravnborg M., et al. 2003. Clinical importance of neutralising antibodies against interferon beta in patients with relapsing-remitting multiple sclerosis. Lancet. 362: 1184–1191. [DOI] [PubMed] [Google Scholar]
- 16.Novick D., Cohen B., and Rubinstein M.. 1994. The human interferon alpha/beta receptor: characterization and molecular cloning. Cell. 77: 391–400. [DOI] [PubMed] [Google Scholar]
- 17.Colamonici O., Yan H., Domanski P., Handa R., Smalley D., Mullersman J., Witte M., Krishnan K., and Krolewski J.. 1994. Direct binding to and tyrosine phosphorylation of the alpha subunit of the type I interferon receptor by p135tyk2 tyrosine kinase. Mol. Cell. Biol. 14: 8133–8142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santos R., Weinstock-Guttman B., Tamano-Blanco M., Badgett D., Zivadinov R., Justinger T., Munschauer F. III, and Ramanathan M.. 2006. Dynamics of interferon-beta modulated mRNA biomarkers in multiple sclerosis patients with anti-interferon-beta neutralizing antibodies. J. Neuroimmunol. 176: 125–133. [DOI] [PubMed] [Google Scholar]
- 19.Bertolotto A., Sala A., Malucchi S., Marnetto F., Caldano M., Di Sapio A., Capobianco M., and Gilli F.. 2004. Biological activity of interferon betas in patients with multiple sclerosis is affected by treatment regimen and neutralising antibodies. J. Neurol. Neurosurg. Psychiatry. 75: 1294–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pachner A. R. 1997. Anticytokine antibodies in beta interferon-treated MS patients and the need for testing: plight of the practicing neurologist. Neurology. 49: 647–650. [DOI] [PubMed] [Google Scholar]
- 21.Blanc M., Hsieh W. Y., Robertson K. A., Watterson S., Shui G., Lacaze P., Khondoker M., Dickinson P., Sing G., Rodriguez-Martin S., et al. 2011. Host defense against viral infection involves interferon mediated down-regulation of sterol biosynthesis. PLoS Biol. 9: e1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Browne R. W., Weinstock-Guttman B., Horakova D., Zivadinov R., Bodziak M. L., Tamano-Blanco M., Badgett D., Tyblova M., Vaneckova M., Seidl Z., et al. 2014. Apolipoproteins are associated with new MRI lesions and deep grey matter atrophy in clinically isolated syndromes. J. Neurol. Neurosurg. Psychiatry. 85: 859–864. [DOI] [PubMed] [Google Scholar]
- 23.Weinstock-Guttman B., Zivadinov R., Horakova D., Havrdova E., Qu J., Shyh G., Lakota E., O’Connor K., Badgett D., Tamano-Blanco M., et al. 2013. Lipid profiles are associated with lesion formation over 24 months in interferon-beta treated patients following the first demyelinating event. J. Neurol. Neurosurg. Psychiatry. 84: 1186–1191. [DOI] [PubMed] [Google Scholar]
- 24.Tettey P., Simpson S. Jr., Taylor B., Blizzard L., Ponsonby A. L., Dwyer T., Kostner K., and van der Mei I.. 2014. An adverse lipid profile is associated with disability and progression in disability, in people with MS. Mult. Scler. 20: 1737–1744. [DOI] [PubMed] [Google Scholar]
- 25.Tettey P., Simpson S. Jr., Taylor B., Blizzard L., Ponsonby A. L., Dwyer T., Kostner K., and van der Mei I.. 2014. Adverse lipid profile is not associated with relapse risk in MS: results from an observational cohort study. J. Neurol. Sci. 340: 230–232. [DOI] [PubMed] [Google Scholar]
- 26.Tettey P., Simpson S. Jr., Taylor B. V., and van der Mei I. A.. 2014. Vascular comorbidities in the onset and progression of multiple sclerosis. J. Neurol. Sci. 347: 23–33. [DOI] [PubMed] [Google Scholar]
- 27.Horakova D., Zivadinov R., Weinstock-Guttman B., Havrdova E., Qu J., Tamano-Blanco M., Badgett D., Tyblova M., Bergsland N., Hussein S., et al. 2013. Environmental factors associated with disease progression after the first demyelinating event: results from the multi-center SET study. PLoS One. 8: e53996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedewald W. T., Levy R. I., and Fredrickson D. S.. 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18: 499–502. [PubMed] [Google Scholar]
- 29.Uher T., Horakova D., Bergsland N., Tyblova M., Ramasamy D. P., Seidl Z., Vaneckova M., Krasensky J., Havrdova E., and Zivadinov R.. 2014. MRI correlates of disability progression in patients with CIS over 48 months. Neuroimage Clin. 6: 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uher T., Horakova D., Kalincik T., Bergsland N., Tyblova M., Ramasamy D. P., Seidl Z., Vaneckova M., Krasensky J., Havrdova E. ,et al. 2015. Early magnetic resonance imaging predictors of clinical progression after 48 months in clinically isolated syndrome patients treated with intramuscular interferon beta-1a. Eur. J. Neurol. 22: 1113–1123. [DOI] [PubMed] [Google Scholar]
- 31.Weinstock-Guttman B., Horakova D., Zivadinov R., Tamano-Blanco M., Badgett D., Tyblova M., Vaneckova M., Seidl Z., Krasensky J., Bergsland N., et al. 2013. Interactions of serum cholesterol with anti-herpesvirus responses affect disease progression in clinically isolated syndromes. J. Neuroimmunol. 263: 121–127. [DOI] [PubMed] [Google Scholar]
- 32.Chataway J., Schuerer N., Alsanousi A., Chan D., MacManus D., Hunter K., Anderson V., Bangham C. R., Clegg S., Nielsen C., et al. 2014. Effect of high-dose simvastatin on brain atrophy and disability in secondary progressive multiple sclerosis (MS-STAT): a randomised, placebo-controlled, phase 2 trial. Lancet. 383: 2213–2221. [DOI] [PubMed] [Google Scholar]
- 33.Marrie R. A., Rudick R., Horwitz R., Cutter G., Tyry T., Campagnolo D., and Vollmer T.. 2010. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology. 74: 1041–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zivadinov R., Marr K., Cutter G., Ramanathan M., Benedict R. H., Kennedy C., Elfadil M., Yeh A. E., Reuther J., Brooks C., et al. 2011. Prevalence, sensitivity, and specificity of chronic cerebrospinal venous insufficiency in MS. Neurology. 77: 138–144. [DOI] [PubMed] [Google Scholar]
- 35.Morra V. B., Coppola G., Orefice G., De Michele G., Vacca G., Filla A., and Bonavita V.. 2004. Interferon-beta treatment decreases cholesterol plasma levels in multiple sclerosis patients. Neurology. 62: 829–830. [DOI] [PubMed] [Google Scholar]
- 36.Dixon R. M., Borden E. C., Keim N. L., Anderson S., Spennetta T. L., Tormey D. C., and Shrago E.. 1984. Decreases in serum high-density-lipoprotein cholesterol and total cholesterol resulting from naturally produced and recombinant DNA-derived leukocyte interferons. Metabolism. 33: 400–404. [DOI] [PubMed] [Google Scholar]
- 37.Shinohara E., Yamashita S., Kihara S., Hirano K., Ishigami M., Arai T., Nozaki S., Kameda-Takemura K., Kawata S., and Matsuzawa Y.. 1997. Interferon alpha induces disorder of lipid metabolism by lowering postheparin lipases and cholesteryl ester transfer protein activities in patients with chronic hepatitis C. Hepatology. 25: 1502–1506. [DOI] [PubMed] [Google Scholar]
- 38.Chen Y., Wang S., Yi Z., Tian H., Aliyari R., Li Y., Chen G., Liu P., Zhong J., Chen X., et al. 2014. Interferon-inducible cholesterol-25-hydroxylase inhibits hepatitis C virus replication via distinct mechanisms. Sci. Rep. 4: 7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.