Abstract
Lysophosphatidic acids (LysoPAs) and lysophosphatidylserine (LysoPS) are emerging lipid mediators proposed to be involved in the pathogenesis of acute coronary syndrome (ACS). In this study, we attempted to elucidate how LysoPA and LysoPS become elevated in ACS using human blood samples collected simultaneously from culprit coronary arteries and peripheral arteries in ACS subjects. We found that: 1) the plasma LysoPA, LysoPS, and lysophosphatidylglycerol levels were not different, while the lysophosphatidylcholine (LysoPC), lysophosphatidylinositol, and lysophosphatidylethanolamine (LysoPE) levels were significantly lower in the culprit coronary arteries; 2) the serum autotaxin (ATX) level was lower and the serum phosphatidylserine-specific phospholipase A1 (PS-PLA1) level was higher in the culprit coronary arteries; 3) the LysoPE and ATX levels were significant explanatory factors for the mainly elevated species of LysoPA, except for 22:6 LysoPA, in the peripheral arteries, while the LysoPC and LysoPE levels, but not the ATX level, were explanatory factors in the culprit coronary arteries; and 4) 18:0 and 18:1 LysoPS were significantly correlated with PS-PLA1 only in the culprit coronary arteries. In conclusion, the origins of LysoPA and LysoPS might differ between culprit coronary arteries and peripheral arteries, and substrates for ATX, such as LysoPC and LysoPE, might be important for the generation of LysoPA in ACS.
Keywords: acute coronary disease, lysophosphatidic acids, lysophosphatidylcholine, lysophosphatidylethanolamine, lysophosphatidylserine
Lysophosphatidic acids (LysoPAs) are potent lipid mediators, especially in the field of vascular biology (1), and many basic studies have suggested that LysoPA might be involved in the pathogenesis of atherosclerotic diseases in terms of both the formation of atheromatous plaques and the rupture of such plaques; for example, LysoPA induces the expression of adhesion molecules and chemokines in endothelial cells (2, 3), the migration of smooth muscle cells (4, 5), and the activation of platelets (6, 7).
Recently, we have obtained evidence of the involvement of LysoPA in human acute coronary syndrome (ACS); LysoPA, as measured using an enzymatic method (8), was elevated in the plasma of subjects with ACS, compared with the levels in plasma samples from subjects with normal coronary and stable angina pectoris (9), and LysoPA was also higher in plasma samples collected from culprit coronary arteries than in those from peripheral arteries (10). We also investigated the origins of the elevated LysoPA levels in ACS using LC-MS/MS and found that the levels of long-chain unsaturated LysoPAs (22:6, 20:4, 18:2), in particular, were elevated in ACS patients and were strongly correlated with the levels of corresponding species of lysophosphatidylinositol (LysoPI), lysophosphatidylcholine (LysoPC), and lysophosphatidylethanolamine (LysoPE), but were only weakly correlated with the level of autotaxin (ATX), which produces LysoPA through its lysophospholipase D activity, suggesting that increased levels of precursor glycero-lysophospholipids (glycero-LPLs) might be involved in the generation of LysoPA (11).
In addition to the possible roles of glycero-LPLs in the generation of LysoPA in ACS, several glycero-LPLs other than LysoPA might also be directly involved in the pathogenesis of ACS. Among the glycero-LPLs with elevated levels in ACS, lysophosphatidylserine (LysoPS) might possess some important physiological roles. Although evidence from both clinical and basic studies remains insufficient, LysoPS seems to be correlated specifically with serotonin, a biomarker for platelet activation, in human subjects (12). Basic research has reportedly shown LysoPS to possess several biological activities, such as the attenuation of the expressions of inflammatory mediators in macrophages (13), the suppression of T lymphocyte proliferation (14), and the constraint of regulatory T lymphocyte development and functions (15).
As described above, however, although the origins and functions of LysoPA and LysoPS have been elucidated from both basic and clinical studies, evidence of the involvement of LysoPA and LysoPS in atherosclerotic diseases, especially in human subjects, remains insufficient. Therefore, the present study aimed to elucidate the origins of LysoPA and LysoPS in ACS using human samples. For this purpose, we used an LC-MS/MS method to measure the levels of glycero-LPLs and their producing enzymes, ATX and its isoforms and phosphatidylserine-specific phospholipase A1 (PS-PLA1), in plasma samples collected simultaneously from both culprit coronary arteries and peripheral arteries (10). We then investigated differences in the levels of possible explanatory factors for LysoPA and LysoPS between these samples. In this context, LysoPA is reportedly abundant in human atherosclerotic plaque lesions (16) and, after plaque rupture, is thought to trigger the consequent events, such as platelet activation (6), while LysoPS might be derived from phosphatidylserine, which is exposed to the surface of the cell membrane during platelet activation (17) or cell apoptosis (18), proposed to be involved in the pathogenesis of ACS (19, 20). Therefore, the blood samples from culprit coronary arteries should theoretically reflect the origins of LysoPA and LysoPS to a greater degree than peripheral arteries, which is the main theme to be pursued in this study.
METHODS
Samples from patients who had undergone coronary angiography
The samples obtained from subjects who had undergone coronary angiography have been previously described (10). Briefly, 52 consecutive patients with ACS who underwent an emergency PCI and thrombectomy at Juntendo University Hospital between January and December 2009 were enrolled according to previously reported entry and exclusion criteria. The ethics review committee at Juntendo University Hospital approved the study, all the participants signed informed consent forms, and the study was registered in the UMIN protocol registration system (#UMIN000002103). This study was also approved by the institutional review boards of both the University of Tokyo and Juntendo University School of Medicine.
All the patients had received standard medication for ACS consisting of aspirin (162 mg) and clopidogrel (300 mg), and heparin (100 IU/kg) was administered before blood sampling. The coronary blood samples were collected from a culprit coronary artery through a thrombectomy catheter, and the peripheral blood samples were collected from an arterial sheath; the collected samples were transferred to glass vacutainer tubes with or without EDTA to obtain plasma and serum samples, respectively. The anticoagulated samples were centrifuged at 2,500 g for 30 min at 4°C to obtain the plasma samples. Whole blood samples collected without EDTA-2Na were left to clot, and the serum was then separated by centrifugation at 2,500 g for 30 min at 4°C. Both the plasma and serum samples were stored at −80°C, and freeze-thaw treatment was limited to once only before the measurement of the glycero-LPLs and their related enzymes or lipids.
Measurement of LPL species using LC-MS/MS
Quantification of the glycero-LPLs was performed as previously described (21). Briefly, the plasma samples were mixed with a 10-fold volume of methanol and an internal standard and then sonicated. After centrifugation at 21,500 g, the resulting supernatant was recovered and used for the LC-MS analysis. Then, 20 μl of methanol extract was separated using a Nanospace LC (Shiseido) equipped with a C18 CAPCELL PAK ACR column (1.5 × 250 mm; Shiseido) using a gradient of solvent A (5 mM ammonium formate in water) and solvent B [5 mM ammonium formate in 95% (v/v) acetonitrile]. Elution was sequentially ionized using an ESI probe, and the parent ion (m/z 380.2) and the fragment ion (m/z 264.2) were monitored in the positive mode using a Quantum Ultra triple quadrupole mass spectrometer (Thermo Fisher Scientific). For each glycerol-LPL class, 12 acyl chains (14:0, 16:0, 16:1, 18:0, 18:1, 18:2, 18:3, 20:3, 20:4, 20:5, 22:5, and 22:6) were monitored.
Measurement of serum ATX, ATX isoforms, and PS-PLA1 levels
The ATX, ATX isoforms, and PS-PLA1 levels in the serum were determined using a two-site immunoenzymetric assay with an ATX assay reagent equipped with the TOSOH AIA system (TOSOH, Tokyo, Japan) (22–24).
Measurement of plasma choline levels
The plasma choline levels were measured using an enzymatic method. Briefly, choline was oxidized with choline oxidase, and the produced H2O2 was determined using a colorimetric assay using TOOS and 4-aminoantipyline as substrates. The detailed procedure used for this assay will be published elsewhere.
Statistical analysis
All the data were statistically analyzed using SPSS (Chicago, IL). The results are expressed as the mean ± SD. The values obtained from two groups were compared using the paired Wilcoxon test, and correlations were sought using the Spearman correlation test, because normality or equality of variance had been rejected using the Kolmogorov-Smirnov test or the Levene test for most of the parameters or analyses. The independent effects of the glycero-LPLs and the total ATX level on LysoPA were evaluated using a stepwise multiple regression analysis. P values less than 0.05 were regarded as statistically significant in all the analyses.
RESULTS
Plasma LysoPA levels were not different, while plasma LysoPC, choline, and serum ATX levels were low in culprit coronary arteries, compared with peripheral arteries
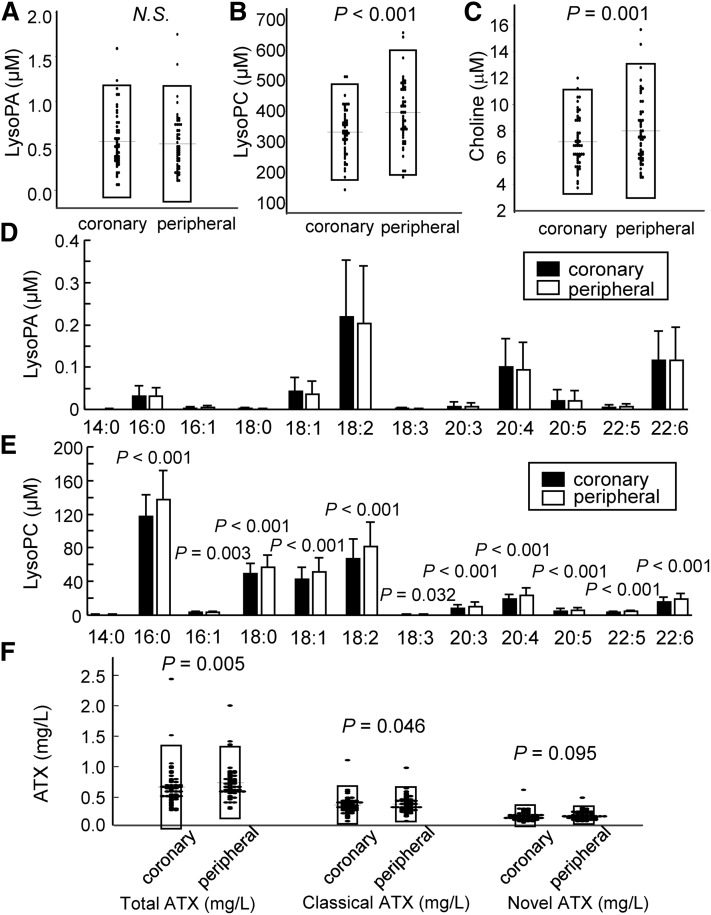
First, we compared the levels of LysoPA and its related molecules between culprit coronary arteries and peripheral arteries. When the glycero-LPL levels were measured using an LC-MS/MS method, the plasma total LysoPA level was not different between the two samples (Fig. 1A). Contrary to LysoPA, the plasma levels of total LysoPC (a precursor of LysoPA) and choline (produced when LysoPC is hydrolyzed into LysoPA by ATX) were lower in culprit coronary arteries (Fig. 1B, C). Regarding the molecular species, the pattern of LysoPA species was similar to that observed previously (11), with 18:2 LysoPA, 20:4 LysoPA, and 22:6 LysoPA being especially high in ACS (Fig. 1D). No differences between the two samples were seen for any of the LysoPA species, while all of the LysoPC species, except for 14:0 LysoPC, were lower in the culprit coronary arteries (Fig. 1D, E). We also measured the serum levels of total ATX and found that the total ATX level was significantly lower in the culprit coronary arteries (Fig. 1F). Because five alternative splicing isoforms of ATX have been identified as ATXα, ATXβ, ATXγ, ATXδ, and ATXε and the expression patterns of each isoform in several tissues differ somewhat, we also measured the classical ATX (ATXα, ATXβ, and ATXγ) and novel ATX (ATXδ and ATXε) levels using enzyme immunoassays that we recently developed (24); as a result, we observed that, in addition to the total ATX level, the classical ATX level was also significantly lower and the novel ATX level tended to be lower in the culprit coronary arteries (Fig. 1F).
Fig. 1.
Plasma LysoPA, its related lipids, and ATX levels in culprit coronary arteries and peripheral arteries in ACS. The levels of LysoPA, its related lipids, and ATX were measured in samples collected simultaneously from a culprit coronary artery and a peripheral artery in each of the subjects with ACS (n = 52). Plasma total LysoPA levels (A), plasma total LysoPC levels (B), plasma choline levels (C), plasma concentrations of LysoPA species (D), plasma concentrations of LysoPC species (E), and serum total ATX, classical ATX, and novel ATX levels (F).
Plasma LysoPE and LysoPI levels were lower in culprit coronary arteries than in peripheral arteries
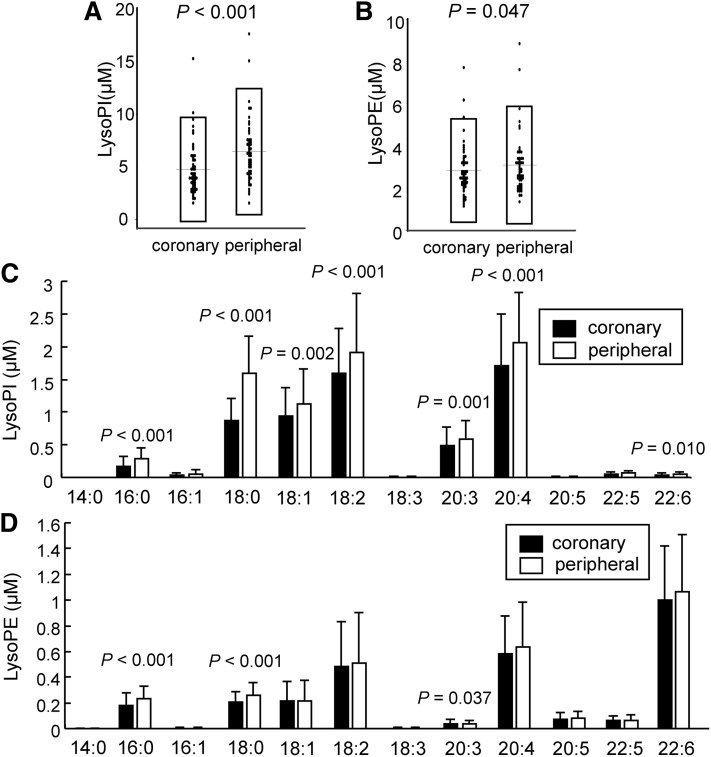
Next, we compared the plasma concentrations of glycero-LPLs other than LysoPA and LysoPC between the culprit coronary arteries and the peripheral arteries. As shown in Fig. 2A, B, the plasma LysoPI and LysoPE levels were lower in the culprit coronary arteries, while the plasma LysoPS and lysophosphatidylglycerol (LysoPG) levels were not different (data not shown). Regarding molecular species, LysoPI species, except for 14:0, 16:1, 18:3, 20:5, and 22:5 LysoPI, were lower in the culprit coronary arteries (Fig. 2C), while 16:0, 18:0, and 20:4 LysoPE were lower in culprit coronary arteries (Fig. 2D). None of the molecular species of LysoPG differed between the two samples (data not shown).
Fig. 2.
Plasma concentrations of minor lysophospholipids in culprit coronary arteries and peripheral arteries in ACS. The levels of minor lysophospholipids were measured in the same plasma samples analyzed in Fig. 1. Plasma total LysoPI levels (A), plasma total LysoPE levels (B), plasma concentrations of LysoPI species (C), and plasma concentrations of LysoPE species (D).
Differences in significant explanatory factors for LysoPA species between culprit coronary arteries and peripheral arteries
Because some glycero-LPLs and ATX levels differed between the culprit coronary arteries and the peripheral arteries, we next compared the correlations between 18:1, 18:2, 20:4, and 22:6 LysoPA, which are characteristic LysoPA species for ACS, and their corresponding glycero-LPLs or total ATX levels in each sample.
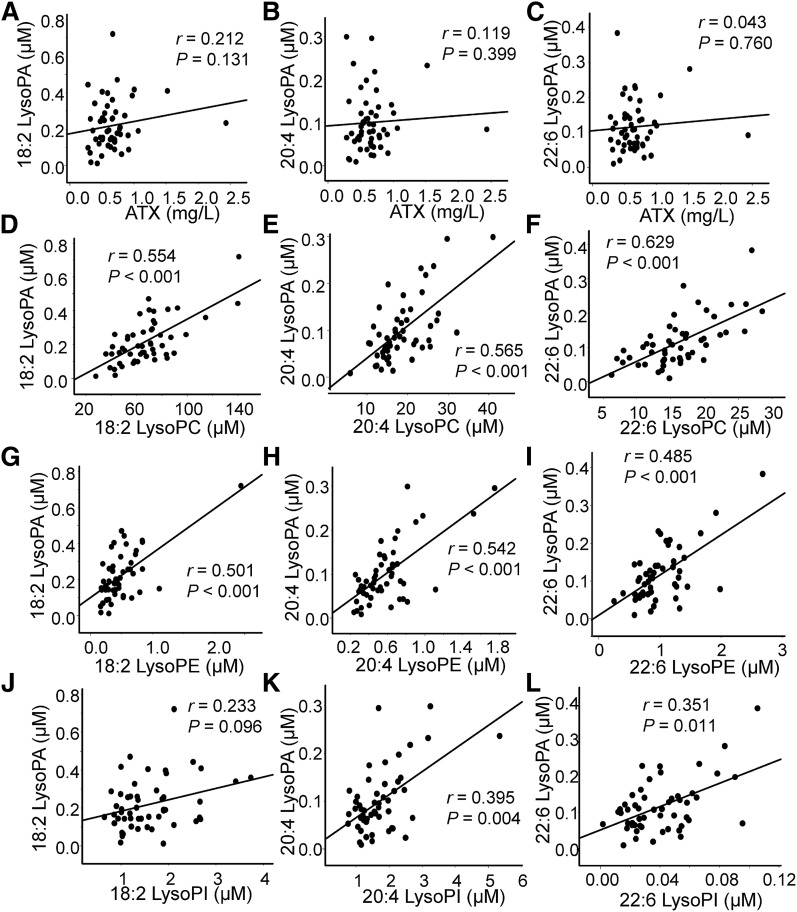
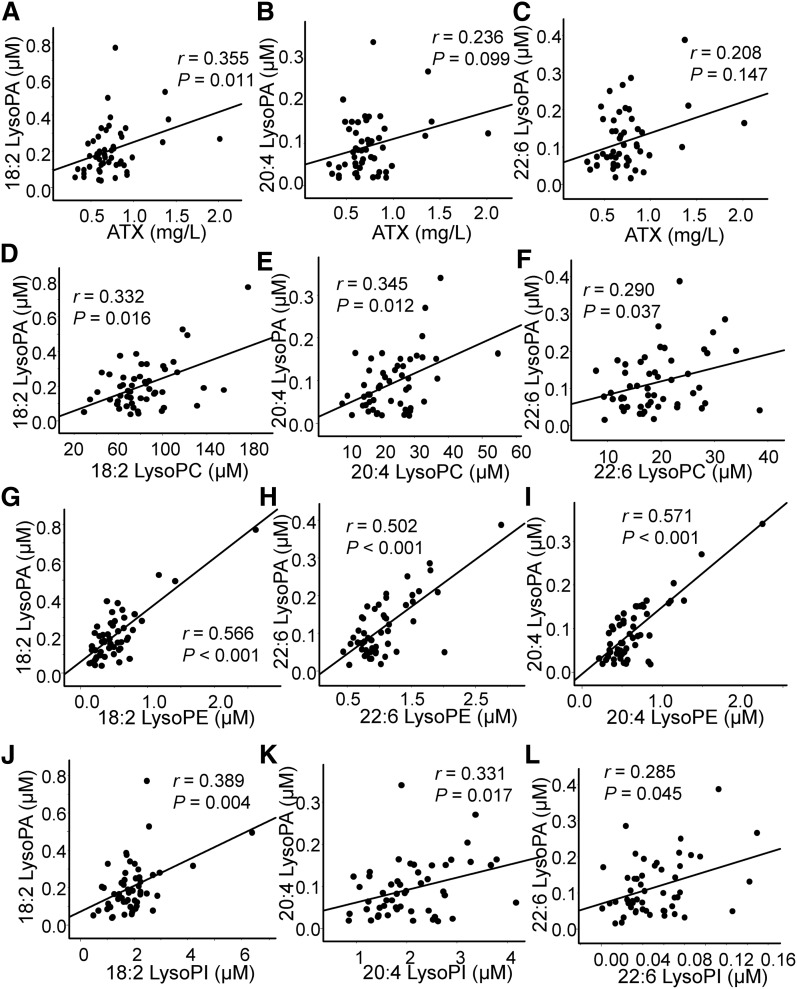
In the plasma samples collected from culprit coronary arteries, no significant correlation was observed between any of the LysoPA species and ATX, while a rather strong correlation was observed between LysoPA and the corresponding LysoPC, LysoPI, and LysoPE (Fig. 3; supplemental Fig. S1). Unlike the plasma samples from culprit coronary arteries, plasma from peripheral arteries exhibited a significant correlation between 18:2 LysoPA and total ATX and a marginally significant correlation between 20:4 LysoPA and total ATX. Regarding the correlation between LysoPA and the corresponding LysoPC, LysoPI, and LysoPE, although significant correlations were observed, the correlation between LysoPA and the corresponding LysoPC was rather weak compared with that in the culprit coronary arteries (Fig. 4; supplemental Fig. S2). Some LysoPG species were also positively correlated with LysoPA (supplemental Fig. S3); however, LysoPS did not have a significant positive correlation with LysoPA (data not shown).
Fig. 3.
Correlation between LysoPA and its related molecules in culprit coronary arteries. These panels show the correlations between LysoPA species and the total ATX levels (A–C) and the corresponding LysoPC levels (D–F), LysoPE levels (G–I), and LysoPI levels (J–L).
Fig. 4.
Correlation between LysoPA and its related molecules in peripheral arteries. These panels show the correlations between LysoPA species and the total ATX levels (A–C) and the corresponding LysoPC levels (D–F), LysoPE levels (G–I), and LysoPI levels (J–L).
Furthermore, when we investigated explanatory factors for LysoPA using multiple regression analyses with the glycero-LPLs and total ATX levels as candidate factors, LysoPC and LysoPE, but not ATX, were selected as significant explanatory factors for LysoPA in culprit coronary arteries, while LysoPE and ATX were selected as significant explanatory factors for 18:1, 18:2, and 20:4 LysoPA in peripheral arteries (Table 1; supplemental Table S1). The explanatory factors for 22:6 LysoPA were LysoPC and LysoPE in culprit coronary arteries, while LysoPE (but not ATX) was an explanatory factor in peripheral arteries. These results also suggest that of the LysoPA species that are elevated in ACS, at least 18:1, 18:2, and 20:4 LysoPA might be derived from the presence of increased substrates for ATX.
TABLE 1.
Multiple regression analyses for plasma LPA species in culprit coronary arteries and peripheral arteries
| B | 95% CI | Standardized β | P | |
| 18.2 LysoPA in culprit coronary arteries | ||||
| 18:2 LysoPC | 0.003 | (0.001–0.004) | 0.457 | <0.001 |
| 18:2 LPE | 0.127 | (0.024–0.230) | 0.332 | 0.016 |
| 18.2 LysoPA in peripheral arteries | ||||
| 18:2 LysoPE | 0.273 | (0.219–0.327) | 0.776 | <0.001 |
| ATX | 0.146 | (0.074–0.219) | 0.311 | <0.001 |
| 20:4 LysoPA in culprit coronary arteries | ||||
| 20:4 LysoPE | 0.104 | (0.050–0.159) | 0.450 | <0.001 |
| 20:4 LysoPC | 0.004 | (0.002–0.007) | 0.385 | 0.002 |
| 20:4 LysoPA in peripheral arteries | ||||
| 20:4 LysoPE | 0.151 | (0.119–0.183) | 0.779 | <0.001 |
| ATX | 0.051 | (0.014–0.088) | 0.226 | 0.008 |
Multiple regression analyses for plasma 18:2 LPA and 20:4 LPA in culprit coronary arteries and peripheral arteries. The LPLs of the corresponding molecular species and ATX were utilized as possible explanatory factors. B represents the unstandardized coefficients.
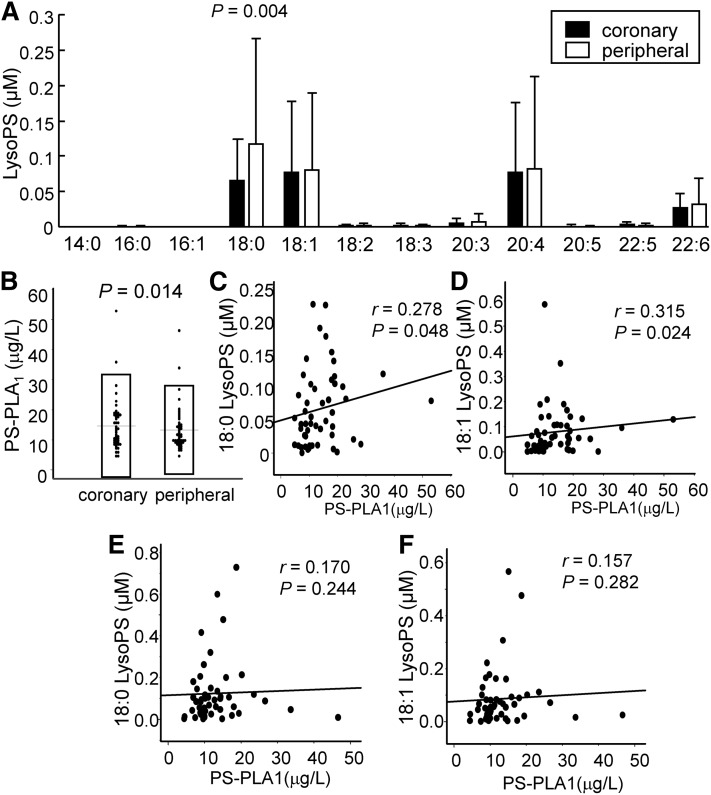
Plasma LysoPS levels had a positive correlation with PS-PLA1 in culprit coronary arteries, but not in peripheral arteries
Because LysoPS is increased in ACS (11) and is emerging as an important lysophospholipid in the pathogenesis of ACS, in addition to LysoPA (13), we also investigated the source of the elevated LysoPS levels in ACS in the present study.
As shown in Fig. 5A, only the 18:0 LysoPS level was lower in the culprit coronary arteries than in the peripheral arteries, while the total LysoPS levels were not different. In contrast, the PS-PLA1 levels were significantly higher in the culprit coronary arteries (Fig. 5B). Regarding the correlation between PS-PLA1 and LysoPS, both the 18:0 and 18:1 LysoPS levels were significantly correlated with PS-PLA1 only in the plasma samples collected from culprit coronary arteries, and not in the samples from peripheral arteries (Fig. 5C–F).
Fig. 5.
Plasma LysoPS and PS-PLA1 levels in culprit coronary arteries and peripheral arteries in ACS. We measured the plasma LysoPS and serum PS-PLA1 levels in samples collected simultaneously from culprit coronary arteries and peripheral arteries in subjects with ACS (n = 52). Plasma concentrations of LysoPS species (A), serum PS-PLA1 levels (B), correlation between 18:0 LysoPS or 18:1 LysoPS and PS-PLA1 levels in culprit coronary arteries (C, D), and correlation between 18:0 LysoPS or 18:1 LysoPS and PS-PLA1 levels in peripheral arteries (E, F).
DISCUSSION
Many basic studies have proposed that LysoPA is involved in the pathogenesis of ACS, resulting in its consideration as a possible pharmacological target (25, 26). Regarding clinical studies, however, only a few studies have examined the involvement and origins of LysoPA in ACS. Moreover, some glycero-LPLs, especially LysoPS, are emerging as important lipid mediators in various diseases, including ACS (27, 28). Recently, we reported that the levels of specific LysoPA species, as well as some corresponding glycero-LPLs, were elevated in ACS (11). Because LysoPA had only a weak correlation with ATX and rather strong correlations with the corresponding species of glycero-LPLs, we speculated that the elevated LysoPA levels observed in ACS were determined by increased levels as glycero-LPLs, which can function as substrates for ATX. In this study, we aimed to demonstrate this hypothesis using plasma samples collected simultaneously from culprit coronary arteries and peripheral arteries.
First, we compared the concentrations of glycero-LPLs, ATX, and ATX isoforms between culprit coronary arteries and peripheral arteries and found that the LysoPA levels determined using LC-MS/MS were not significantly different (Fig. 1A); meanwhile, the plasma LysoPA level was significantly higher in culprit coronary arteries when measured using an enzymatic method, as reported previously (10). The reason for this discrepancy might be the characteristics of the assays; although the concentrations of molecular species can be obtained using an LC-MS/MS assay, the percent coefficient of variations of the total LPLs as determined using an LC-MS/MS assay (which calculates the concentrations of the total LPLs as the sum of each LPL species) is somehow higher than those measured using an enzymatic assay and an autoanalyzer. Regarding LysoPA species, we confirmed that 18:2, 20:4, and 22:6 LysoPA are the main LysoPA species found in ACS, but no differences were observed between culprit coronary arteries and peripheral arteries. Although only a few reports have compared lipid concentrations between coronary and peripheral arteries, the fact that several glycero-LPLs were lower in the culprit artery (Fig. 2) strongly suggests that the characteristics of glycero-LPLs differ between these two types of samples. Actually, although the LysoPA levels were not different, the total ATX and classical ATX (producing enzymes for LysoPA) levels, the LysoPC, LysoPE, and LysoPI (possible substrates for ATX) levels, and the choline (a byproduct generated during the production of LysoPA from LysoPC with ATX) level were significantly lower in samples obtained from culprit coronary arteries (Figs. 1, 2).
Next, we investigated the correlation between LysoPA and ATX in the two types of samples. Concordant with a previous study demonstrating that 18:2 LysoPA is most strongly correlated with ATX in peripheral arteries (11), only the plasma 18:2 LysoPA level was significantly correlated with ATX in peripheral arteries (Fig. 4 A–C, supplemental Fig. S2A). Interestingly, in culprit coronary arteries, none of the plasma LysoPA species levels were correlated with the total ATX level (Fig. 3 A–C). In a previous article (11), we observed the existence of a strong correlation between LysoPA species and corresponding glycero-LPLs, especially LysoPC, LysoPE, and LysoPI. We were able to reproduce these close correlations again in both types of plasma samples measured in the present study (Figs. 3, 4), while the correlation between LysoPA and LysoPC seemed stronger in the culprit coronary arteries. Moreover, multiple regression analyses demonstrated that only LysoPC and LysoPE were selected as significant explanatory factors for the LysoPA level in culprit coronary arteries, while ATX and LysoPE were selected as significant explanatory factors in peripheral arteries. Regarding 22:6 LysoPA, ATX was not selected as an explanatory factor even in peripheral artery samples. In a previous study (11), 22:6 LysoPA exhibited a rather curious homeostasis, compared with other LysoPA species: plasma 22:6 LysoPA had the weakest correlation with ATX, and 22:6 LysoPG was selected as a negative explanatory factor for 22:6 LysoPA. In the present independent study, these characteristics for 22:6 LysoPA were reproduced (supplemental Table S1). Considering that palmitoyl-docosahexaenoyl (16:0-22:6)-phosphatidylcholine is extremely abundant in murine cardiac tissue (29), the 22:6 species of phospholipids might be characteristic of cardiac tissue and might be eluted into the blood when cardiac tissue is damaged, such as is the case in ACS. Further studies are needed to elucidate the dynamism and physiological roles of the 22:6 species of phospholipids in ACS. At the least, the present results suggest that LysoPC and LysoPE might be involved in the elevated concentration of LysoPA observed in ACS.
Considering LysoPC and LysoPE, it was noted that their concentrations were much higher than that of LysoPA. Regarding the metabolism of LysoPA in the circulation, LysoPA is produced by the LysoPLD activity of ATX from its substrates, such as LysoPC and LysoPE, and undergoes rapid degradation by lipid phosphate phosphatases (LPPs) (30). In the steady state, considering a strong correlation between the LysoPLD activity (31) and the LysoPA level and half reduction of the plasma LysoPA level observed in ATX heterozygote mice (32), ATX activity might determine the plasma LysoPA level. On the other hand, in the ACS subjects, the elevation of substrates, such as LysoPC and LysoPE (11), without elevation of ATX, might theoretically increase the plasma LysoPA levels. The decreased activity of LPPs, however, is another possible mechanism for the elevation of LysoPA in ACS; actually a genome-wide association analysis identified LPP3 as a candidate gene for coronary artery diseases (33) and basic studies have also supported the association between LPPs and atherosclerosis (34, 35).
In general, the origins of LysoPC and LysoPE have not yet been adequately established, although several possible pathways have been reported: 1) LCAT and oxidation of LDL produce especially saturated LysoPC (36, 37); 2) hepatocytes secrete especially unsaturated LysoPC and LysoPE (38, 39); and 3) platelet activation and deletion of the LDL receptor also increased LysoPC and LysoPE (40, 41). Regarding the pathogenesis of ACS, along with oxidation of LDL and activation of platelets, the elution from damaged heart tissue might also be involved. Because the components of cardiac tissue are eluted into the blood during ACS and because LysoPC and LysoPE reportedly exist in the heart (42), LysoPC and LysoPE might be eluted directly from damaged tissue. Further study is needed to elucidate the origins of LysoPC and LysoPE in the pathogenesis of ACS.
The major limitation of this study is that the samples were not always collected immediately after the occurrence of ACS. It seems reasonable to assume that elevated LysoPA levels during ACS might be diluted over time, because the blood would be circulated throughout the entire body. Theoretically, however, plasma samples collected from culprit coronary arteries should reflect changes in lipid mediators and their related proteins to a greater degree than samples collected from peripheral arteries. In addition, in this study, the blood samples were collected after the subjects received the infusion of heparin as a standard therapy for ACS. Although the effects of heparin on the plasma levels of lysophospholipids have not been demonstrated, we observed that the injection of heparin increased the plasma concentrations of LysoPA, LysoPG, and LysoPI among lysophospholipids in mice (data not shown). Although we think it is reasonable to compare both samples because all the subjects received heparin in a similar way, we cannot deny the possibility that these lysophospholipids (especially LysoPI) can be differently modulated by heparin between these two samples. Another limitation of this study is that because this is an observational study, we cannot demonstrate how LysoPA is generated or how LysoPA is involved in the pathogenesis of ACS. Actually, several other factors thought to be involved in regulating the production of LysoPA were not investigated in the present study, including production resulting from platelet activation (43, 44) and LDL oxidation (16), as well as degradation by LPPs (45, 46). Among these factors, in the present study, we could not especially rule out the involvement of the LysoPA-producing pathway from phosphatidic acids in the culprit coronary artery. Actually, secretory phospholipase A2 and phosphatidic acid-selective phospholipase A1 are reportedly involved in the production of extracellular LysoPA (47), although the latter is subjected to binding to the cell membrane after secretion. At least, however, we can safely conclude that the LysoPA level is determined by its substrates, rather than ATX, in culprit coronary arteries, which is not the case in peripheral arteries.
Along with LysoPA, we also investigated differences in the culprit coronary artery and peripheral artery levels of LysoPS and PS-PLA1, which is one of the enzymes that produces LysoPS (48), as well as the correlation between LysoPS and PS-PLA1. In the present study, only 18:0 LysoPS was lower and PS-PLA1 was higher in culprit coronary arteries. Although the source of PS-PLA1 in human subjects and the physiological difference between 18:0 LysoPS and 18:1 LysoPS have not yet been established, the present observations suggest the possible involvement of LysoPS and PS-PLA1 in ACS. Regarding the correlation with PS-PLA1, both 18:0 LysoPS and 18:1 LysoPS were significantly correlated with PS-PLA1 only in culprit coronary arteries (Fig. 5C–F). Although these correlations might suggest that LysoPS was somehow produced by PS-PLA1 during the pathogenesis of ACS, PS-PLA1 is believed to prefer to cleave the sn-1 position of phosphatidylserine, which is thought to produce unsaturated LysoPS (48). Contrary to this characteristic of PS-PLA1, the correlations between PS-PLA1 and 18:0 LysoPS or 18:1 LysoPS seemed similar in this study. Because the 18:0 LysoPS levels were strongly correlated with the 18:1 LysoPS levels (r = 0.822, P < 0.001 in the culprit coronary artery; r = 0.912, P < 0.001 in the peripheral artery; supplemental Fig. S4), it can safely be assumed that mechanisms other than PS-PLA1 might be involved in the generation of LysoPS in ACS.
The clinical significance of the present study depends on the physiological properties of LysoPA and LysoPS in the pathogenesis of ACS. As described in the Introduction section, because LysoPA has been proposed to play harmful roles in the pathogenesis of ACS, the increase in LysoPA in patients with ACS might accelerate pathologic conditions. There remains, however, the possibility that long unsaturated LysoPA molecules, especially 22:6 LysoPA, might protect against ACS, because the activity of LysoPA as an agonist toward each LysoPA receptor, especially LysoPA receptor 3, depends on the LysoPA molecular species (47, 49). Regarding LysoPS, because LysoPS reportedly suppresses inflammation, as mentioned in the Introduction section, the elevation of LysoPS in patients with ACS might be a compensatory reaction, although the physiological properties of LysoPS remain to be fully elucidated. Further studies are needed to elucidate the roles of the molecular species of LysoPA, LysoPS, and other glycero-LPLs in the pathogenesis of ACS.
In summary, the origins of LysoPA and LysoPS might differ between culprit coronary arteries and peripheral arteries, and substrates for ATX, such as LysoPC and LysoPE, might be important for the generation of LysoPA in ACS.
Supplementary Material
Footnotes
Abbreviations:
- ACS
- acute coronary syndrome
- ATX
- autotaxin
- glycero-LPL
- glycero-lysophospholipid
- LPP
- lipid phosphate phosphatase
- LysoPA
- lysophosphatidic acid
- LysoPC
- lysophosphatidylcholine
- LysoPE
- lysophosphatidylethanolamine
- LysoPG
- lysophosphatidylglycerol
- LysoPI
- lysophosphatidylinositol
- LysoPS
- lysophosphatidylserine
- PS-PLA1
- phosphatidylserine-specific phospholipase A1
This work was supported by CREST from JST/AMED, a Grant-in-Aid for Scientific Research on Innovative Areas from the Japan Society for the Promotion of Science 15H05906 (Y.Y.), Japan Society for the Promotion of Science Grant 16H06236 (M.K.), and Banyu Life Science Foundation International (M.K.).
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Teo S. T., Yung Y. C., Herr D. R., and Chun J.. 2009. Lysophosphatidic acid in vascular development and disease. IUBMB Life. 61: 791–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rizza C., Leitinger N., Yue J., Fischer D. J., Wang D. A., Shih P. T., Lee H., Tigyi G., and Berliner J. A.. 1999. Lysophosphatidic acid as a regulator of endothelial/leukocyte interaction. Lab. Invest. 79: 1227–1235. [PubMed] [Google Scholar]
- 3.Lin C. I., Chen C. N., Lin P. W., Chang K. J., Hsieh F. J., and Lee H.. 2007. Lysophosphatidic acid regulates inflammation-related genes in human endothelial cells through LPA1 and LPA3. Biochem. Biophys. Res. Commun. 363: 1001–1008. [DOI] [PubMed] [Google Scholar]
- 4.Tigyi G. 2001. Physiological responses to lysophosphatidic acid and related glycero-phospholipids. Prostaglandins Other Lipid Mediat. 64: 47–62. [DOI] [PubMed] [Google Scholar]
- 5.Siess W. 2002. Athero- and thrombogenic actions of lysophosphatidic acid and sphingosine-1-phosphate. Biochim. Biophys. Acta. 1582: 204–215. [DOI] [PubMed] [Google Scholar]
- 6.Siess W., and Tigyi G.. 2004. Thrombogenic and atherogenic activities of lysophosphatidic acid. J. Cell. Biochem. 92: 1086–1094. [DOI] [PubMed] [Google Scholar]
- 7.Pamuklar Z., Lee J. S., Cheng H. Y., Panchatcharam M., Steinhubl S., Morris A. J., Charnigo R., and Smyth S. S.. 2008. Individual heterogeneity in platelet response to lysophosphatidic acid: evidence for a novel inhibitory pathway. Arterioscler. Thromb. Vasc. Biol. 28: 555–561. [DOI] [PubMed] [Google Scholar]
- 8.Kishimoto T., Matsuoka T., Imamura S., and Mizuno K.. 2003. A novel colorimetric assay for the determination of lysophosphatidic acid in plasma using an enzymatic cycling method. Clin. Chim. Acta. 333: 59–67. [DOI] [PubMed] [Google Scholar]
- 9.Dohi T., Miyauchi K., Ohkawa R., Nakamura K., Kishimoto T., Miyazaki T., Nishino A., Nakajima N., Yaginuma K., Tamura H., et al. 2012. Increased circulating plasma lysophosphatidic acid in patients with acute coronary syndrome. Clin. Chim. Acta. 413: 207–212. [DOI] [PubMed] [Google Scholar]
- 10.Dohi T., Miyauchi K., Ohkawa R., Nakamura K., Kurano M., Kishimoto T., Yanagisawa N., Ogita M., Miyazaki T., Nishino A., et al. 2013. Increased lysophosphatidic acid levels in culprit coronary arteries of patients with acute coronary syndrome. Atherosclerosis. 229: 192–197. [DOI] [PubMed] [Google Scholar]
- 11.Kurano M., Suzuki A., Inoue A., Tokuhara Y., Kano K., Matsumoto H., Igarashi K., Ohkawa R., Nakamura K., Dohi T., et al. 2015. Possible involvement of minor lysophospholipids in the increase in plasma lysophosphatidic Acid in acute coronary syndrome. Arterioscler. Thromb. Vasc. Biol. 35: 463–470. [DOI] [PubMed] [Google Scholar]
- 12.Kurano M., Dohi T., Nojiri T., Kobayashi T., Hirowatari Y., Inoue A., Kano K., Matsumoto H., Igarashi K., Nishikawa M., et al. 2015. Blood levels of serotonin are specifically correlated with plasma lysophosphatidylserine among the glycero-lysophospholipids. BBA Clin. 4: 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishikawa M., Kurano M., Ikeda H., Aoki J., and Yatomi Y.. 2015. Lysophosphatidylserine has bilateral effects on macrophages in the pathogenesis of atherosclerosis. J. Atheroscler. Thromb. 22: 518–526. [DOI] [PubMed] [Google Scholar]
- 14.Bellini F., and Bruni A.. 1993. Role of a serum phospholipase A1 in the phosphatidylserine-induced T cell inhibition. FEBS Lett. 316: 1–4. [DOI] [PubMed] [Google Scholar]
- 15.Barnes M. J., Li C. M., Xu Y., An J., Huang Y., and Cyster J. G.. 2015. The lysophosphatidylserine receptor GPR174 constrains regulatory T cell development and function. J. Exp. Med. 212: 1011–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siess W., Zangl K. J., Essler M., Bauer M., Brandl R., Corrinth C., Bittman R., Tigyi G., and Aepfelbacher M.. 1999. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc. Natl. Acad. Sci. USA. 96: 6931–6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan X., Shi J., Fu Y., Gao C., Yang X., Li J., Wang W., Hou J., Li H., and Zhou J.. 2013. Role of erythrocytes and platelets in the hypercoagulable status in polycythemia vera through phosphatidylserine exposure and microparticle generation. Thromb. Haemost. 109: 1025–1032. [DOI] [PubMed] [Google Scholar]
- 18.Nagata S., Suzuki J., Segawa K., and Fujii T.. 2016. Exposure of phosphatidylserine on the cell surface. Cell Death Differ. 23: 952–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blanco-Colio L. M., Martin-Ventura J. L., de Teresa E., Farsang C., Gaw A., Gensini G., Leiter L. A., Langer A., Martineau P., Hernandez G., et al. 2007. Increased soluble Fas plasma levels in subjects at high cardiovascular risk: Atorvastatin on Inflammatory Markers (AIM) study, a substudy of ACTFAST. Arterioscler. Thromb. Vasc. Biol. 27: 168–174. [DOI] [PubMed] [Google Scholar]
- 20.Niessner A., Hohensinner P. J., Rychli K., Neuhold S., Zorn G., Richter B., Hulsmann M., Berger R., Mortl D., Huber K., et al. 2009. Prognostic value of apoptosis markers in advanced heart failure patients. Eur. Heart J. 30: 789–796. [DOI] [PubMed] [Google Scholar]
- 21.Okudaira M., Inoue A., Shuto A., Nakanaga K., Kano K., Makide K., Saigusa D., Tomioka Y., and Aoki J.. 2014. Separation and quantification of 2-acyl-1-lysophospholipids and 1-acyl-2-lysophospholipids in biological samples by LC-MS/MS. J. Lipid Res. 55: 2178–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura K., Igarashi K., Ide K., Ohkawa R., Okubo S., Yokota H., Masuda A., Oshima N., Takeuchi T., Nangaku M., et al. 2008. Validation of an autotaxin enzyme immunoassay in human serum samples and its application to hypoalbuminemia differentiation. Clin. Chim. Acta. 388: 51–58. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura K., Igarashi K., Ohkawa R., Saiki N., Nagasaki M., Uno K., Hayashi N., Sawada T., Syukuya K., Yokota H., et al. 2010. A novel enzyme immunoassay for the determination of phosphatidylserine-specific phospholipase A(1) in human serum samples. Clin. Chim. Acta. 411: 1090–1094. [DOI] [PubMed] [Google Scholar]
- 24.Tokuhara Y., Kurano M., Shimamoto S., Igarashi K., Nojiri T., Kobayashi T., Masuda A., Ikeda H., Nagamatsu T., Fujii T., et al. 2015. A new enzyme immunoassay for the quantitative determination of classical autotaxins (ATXα, ATXβ, and ATXγ) and novel autotaxins (ATXδ and ATXε). PLoS One. 10: e0130074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spector A. A. 2003. Plaque rupture, lysophosphatidic acid, and thrombosis. Circulation. 108: 641–643. [DOI] [PubMed] [Google Scholar]
- 26.Smyth S. S., Cheng H. Y., Miriyala S., Panchatcharam M., and Morris A. J.. 2008. Roles of lysophosphatidic acid in cardiovascular physiology and disease. Biochim. Biophys. Acta. 1781: 563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makide K., Kitamura H., Sato Y., Okutani M., and Aoki J.. 2009. Emerging lysophospholipid mediators, lysophosphatidylserine, lysophosphatidylthreonine, lysophosphatidylethanolamine and lysophosphatidylglycerol. Prostaglandins Other Lipid Mediat. 89: 135–139. [DOI] [PubMed] [Google Scholar]
- 28.Makide K., Uwamizu A., Shinjo Y., Ishiguro J., Okutani M., Inoue A., and Aoki J.. 2014. Novel lysophosphoplipid receptors: their structure and function. J. Lipid Res. 55: 1986–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harayama T., Eto M., Shindou H., Kita Y., Otsubo E., Hishikawa D., Ishii S., Sakimura K., Mishina M., and Shimizu T.. 2014. Lysophospholipid acyltransferases mediate phosphatidylcholine diversification to achieve the physical properties required in vivo. Cell Metab. 20: 295–305. [DOI] [PubMed] [Google Scholar]
- 30.Samadi N., Bekele R., Capatos D., Venkatraman G., Sariahmetoglu M., and Brindley D. N.. 2011. Regulation of lysophosphatidate signaling by autotaxin and lipid phosphate phosphatases with respect to tumor progression, angiogenesis, metastasis and chemo-resistance. Biochimie. 93: 61–70. [DOI] [PubMed] [Google Scholar]
- 31.Hosogaya S., Yatomi Y., Nakamura K., Ohkawa R., Okubo S., Yokota H., Ohta M., Yamazaki H., Koike T., and Ozaki Y.. 2008. Measurement of plasma lysophosphatidic acid concentration in healthy subjects: strong correlation with lysophospholipase D activity. Ann. Clin. Biochem. 45: 364–368. [DOI] [PubMed] [Google Scholar]
- 32.van Meeteren L. A., Ruurs P., Stortelers C., Bouwman P., van Rooijen M. A., Pradere J. P., Pettit T. R., Wakelam M. J., Saulnier-Blache J. S., Mummery C. L., et al. 2006. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol. Cell. Biol. 26: 5015–5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erbilgin A., Civelek M., Romanoski C. E., Pan C., Hagopian R., Berliner J. A., and Lusis A. J.. 2013. Identification of CAD candidate genes in GWAS loci and their expression in vascular cells. J. Lipid Res. 54: 1894–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panchatcharam M., Miriyala S., Salous A., Wheeler J., Dong A., Mueller P., Sunkara M., Escalante-Alcalde D., Morris A. J., and Smyth S. S.. 2013. Lipid phosphate phosphatase 3 negatively regulates smooth muscle cell phenotypic modulation to limit intimal hyperplasia. Arterioscler. Thromb. Vasc. Biol. 33: 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smyth S. S., Mueller P., Yang F., Brandon J. A., and Morris A. J.. 2014. Arguing the case for the autotaxin-lysophosphatidic acid-lipid phosphate phosphatase 3-signaling nexus in the development and complications of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 34: 479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glomset J. A. 1968. The plasma lecithins:cholesterol acyltransferase reaction. J. Lipid Res. 9: 155–167. [PubMed] [Google Scholar]
- 37.Murugesan G., and Fox P. L.. 1996. Role of lysophosphatidylcholine in the inhibition of endothelial cell motility by oxidized low density lipoprotein. J. Clin. Invest. 97: 2736–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sekas G., Patton G. M., Lincoln E. C., and Robins S. J.. 1985. Origin of plasma lysophosphatidylcholine: evidence for direct hepatic secretion in the rat. J. Lab. Clin. Med. 105: 190–194. [PubMed] [Google Scholar]
- 39.Robinson B. S., Baisted D. J., and Vance D. E.. 1989. Comparison of albumin-mediated release of lysophosphatidylcholine and lysophosphatidylethanolamine from cultured rat hepatocytes. Biochem. J. 264: 125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yokoyama K., Shimizu F., and Setaka M.. 2000. Simultaneous separation of lysophospholipids from the total lipid fraction of crude biological samples using two-dimensional thin-layer chromatography. J. Lipid Res. 41: 142–147. [PubMed] [Google Scholar]
- 41.Ou Z. J., Li L., Liao X. L., Wang Y. M., Hu X. X., Zhang Q. L., Wang Z. P., Yu H., Zhang X., Hu P., et al. 2012. Apolipoprotein A-I mimetic peptide inhibits atherosclerosis by altering plasma metabolites in hypercholesterolemia. Am. J. Physiol. Endocrinol. Metab. 303: E683–E694. [DOI] [PubMed] [Google Scholar]
- 42.Kim J., Lampe J. W., Yin T., Shinozaki K., and Becker L. B.. 2015. Phospholipid alterations in the brain and heart in a rat model of asphyxia-induced cardiac arrest and cardiopulmonary bypass resuscitation. Mol. Cell. Biochem. 408: 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aoki J., Taira A., Takanezawa Y., Kishi Y., Hama K., Kishimoto T., Mizuno K., Saku K., Taguchi R., and Arai H.. 2002. Serum lysophosphatidic acid is produced through diverse phospholipase pathways. J. Biol. Chem. 277: 48737–48744. [DOI] [PubMed] [Google Scholar]
- 44.Pamuklar Z., Federico L., Liu S., Umezu-Goto M., Dong A., Panchatcharam M., Fulkerson Z., Berdyshev E., Natarajan V., Fang X., et al. 2009. Autotaxin/lysopholipase D and lysophosphatidic acid regulate murine hemostasis and thrombosis. J. Biol. Chem. 284: 7385–7394. [Erratum. 2009. J. Biol. Chem. 284: 21100.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sciorra V. A., and Morris A. J.. 2002. Roles for lipid phosphate phosphatases in regulation of cellular signaling. Biochim. Biophys. Acta. 1582: 45–51. [DOI] [PubMed] [Google Scholar]
- 46.Ren H., Panchatcharam M., Mueller P., Escalante-Alcalde D., Morris A. J., and Smyth S. S.. 2013. Lipid phosphate phosphatase (LPP3) and vascular development. Biochim. Biophys. Acta. 1831: 126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sonoda H., Aoki J., Hiramatsu T., Ishida M., Bandoh K., Nagai Y., Taguchi R., Inoue K., and Arai H.. 2002. A novel phosphatidic acid-selective phospholipase A1 that produces lysophosphatidic acid. J. Biol. Chem. 277: 34254–34263. [DOI] [PubMed] [Google Scholar]
- 48.Aoki J., Nagai Y., Hosono H., Inoue K., and Arai H.. 2002. Structure and function of phosphatidylserine-specific phospholipase A1. Biochim. Biophys. Acta. 1582: 26–32. [DOI] [PubMed] [Google Scholar]
- 49.Bandoh K., Aoki J., Hosono H., Kobayashi S., Kobayashi T., Murakami-Murofushi K., Tsujimoto M., Arai H., and Inoue K.. 1999. Molecular cloning and characterization of a novel human G-protein-coupled receptor, EDG7, for lysophosphatidic acid. J. Biol. Chem. 274: 27776–27785. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.