Abstract
We confirmed previous findings by a Japanese group that there is an accumulation of 7α-hydroxy-3-oxo-4-cholestenoic acid (7-Hoca) in human subdural hematomas. The accumulation correlated with the time from the bleeding to the sample collection. We present evidence that these accumulations are likely to be caused by the strong affinity of 7-Hoca to albumin and the marked difference between plasma and brain with respect to levels of albumin. In the circulation, 80–90% of 7-Hoca is bound to albumin with a ratio between the steroid acid and albumin of ∼1.4 ng/mg. In cerebrospinal fluid (CSF), the ratio between 7-Hoca and albumin is ∼30 ng/mg. When albumin or hemolyzed blood in a dialysis bag was exposed to CSF, there was a flux of 7-Hoca from CSF to the albumin. We suggest that the major explanation for accumulation of 7-Hoca in subdural hematoma is a flux from the brain into the hematoma due to the high affinity to albumin and the high capacity of 7-Hoca to pass biomembranes. We discuss the possibility that the markedly different ratios between 7-Hoca and albumin in circulation and brain can explain the flux of 7-Hoca from the brain into circulation against a concentration gradient.
Keywords: steroid acid, blood-brain barrier, subdural hematoma, subarachnoidal bleeding, brain cholesterol metabolism
7α-Hydroxy-3-oxo-4-cholestenoic acid (7-Hoca) is a cholesterol metabolite that occurs naturally in human blood (1). It is mainly produced from 7α-hydroxy-4-cholestene-3-one in extracerebral tissues and from 27-hydroxycholesterol3 in the brain (3). There is a net uptake from the circulation by the liver (4) where it is metabolized into bile acids, mainly chenodeoxycholic acid (5).
Meaney et al. (3) showed that the conversion of 27-hydroxycholesterol into 7-Hoca is an important mechanism to eliminate the former oxysterol from brain. In view of the toxic properties of 27-hydroxycholesterol (6), the conversion may be regarded as a detoxification. Based on the difference in 7-Hoca levels between internal jugular vein and brachial artery in healthy volunteers, the flux from the brain into the circulation was calculated to be ∼2 mg/24 h (3).
Early reports could not detect any significant levels of 7-Hoca in cerebrospinal fluid (CSF) (7, 8). In 2010, Ogundare et al. (9) used LC/MSn to reveal that CSF contains ∼7 ng/ml of 7-Hoca. By using a new simplified analytical method utilizing GC/MS and deuterium-labeled 7-Hoca as internal standard, a higher average level of 15 ng/ml was reported (10). No difference between controls, Alzheimer’s patients, and patients with vascular dementia could be observed regarding 7-Hoca levels in CSF. However, patients with blood-brain barrier (BBB) disruption showed significantly higher levels indicating that 7-Hoca can be used as a marker for BBB dysfunction (10).
Nagata et al. (8) reported high levels of 7-Hoca in chronic subdural hematoma (CSH) that were around five times higher than the levels in plasma. Such high levels were not observed in patients with subdural hygromas lacking capsular membrane (8) but were seen in CSF immediately following subarachnoid hemorrhage (7). The same group also reported some increase of cholic acid and chenodeoxycholic acid in CSH compared with plasma (5). The reason behind accumulation of 7-Hoca and bile acids in CSH remains unclear.
In the current work, we modified our simplified method to detect 7-Hoca in CSF to apply on hematoma samples. We confirmed the finding by the Japanese group that 7-Hoca accumulates in subdural hematomas and found that the accumulation correlates with the time duration from the bleeding to the collection of the hematoma sample. We also designed two in vitro models to investigate the cause of 7-Hoca accumulation in CSH. Our findings suggest that the strong binding of 7-Hoca to albumin and the marked difference of 7-Hoca/albumin ratio between CSF and plasma plays an important role in the above accumulations.
MATERIALS AND METHODS
Assay of 7-Hoca and 3-oxo-cholesta-4,6-dienoic acid
D4-7-Hoca was synthesized as described previously (10) and used as internal standard. Hematoma, 0.5 ml, was added to a separation funnel along with 40 ng of the internal standard and extracted twice with 4.0 ml of chloroform. The solvent was evaporated under nitrogen before addition of 1.0 ml of chloroform. The latter mixture was subjected to solid phase extraction using an NH2 column (Bond Elut, Agilent Technology). A chloroform isopropanol mixture (2:1) was used to elute oxysterols and 2% acetic acid in ether to elute 7-Hoca. Methylation, derivatization, and GC/MS analysis was done as described previously (10). The ions at m/z 426 and m/z 430 were used for the tracing of 7-Hoca and D4-7-Hoca, respectively. The standard curve with a fixed amount of D4-7-Hoca and increasing amounts of unlabeled 7-Hoca could also be used for a quantitation of 3-oxo-cholesta-4,6-dienoic acid. In the latter quantitation, a factor of 2.5 was used to correct for the difference in intensity of the ion at m/z 426 between 7-Hoca and 3-oxo-cholesta-4,6-dienoic acid. The latter correction factor was obtained by mixing a fixed amount of 3-oxo cholesta-4,6-dienoic acid with a fixed amount of the internal standard and analysis by GC/MS.
Assay of 7-Hoca in CSF and in albumin solutions was assayed by GC/MS as described previously (10) without including a chromatographic step.
Albumin determination
Albumin was determined in hematoma material, blood, and CSF samples using Tina-quant Albumin Gen.2 kit (Roche).
Specimens from patients with cerebral hematoma
Hematoma and blood samples were collected from patients operated on to remove CSH. In most of the patients, the approximate time for the bleeding could be defined based on previous hospital records or reported head trauma. Head trauma that could not be defined to have occurred on a certain day was disregarded. Twenty-two patients were involved in the study after providing informed consent. All the investigations carried out on the patients were approved by the ethical committee. The hematomas were frozen at −70°C immediately after the collection and stored for a maximum of 2 months before analysis.
CSF samples from patients with intracerebral bleedings
We collected a few CSF samples containing blood from our routine laboratory at the division of clinical chemistry at the Karolinska University Hospital Huddinge (visual inspection only). This CSF corresponds to excess materials that were available 1 week after the diagnostic measurements had been performed. The identity of the patients was not revealed, but it was assumed that the blood originated from subarachnoid bleeding in most of these cases. No information was available concerning the time between the bleeding and the collection of the sample.
Incubation of albumin solution in CSF
A bovine albumin solution (Sigma) in normal saline was prepared to a final concentration of 40 mg/ml. Two milliliters of albumin solution was sealed in a dialysis membrane bag (Medicell International Ltd.) and incubated in 50 ml of a CSF pool with magnetic stirring at 37°C for 48 h.
Incubation of artificial hematoma material in CSF
Hemolyzed blood was prepared according to the method described by Moore et al. (11) by snap-freezing a few milliliters of a blood pool in liquid nitrogen. Two milliliters of the hemolyzed blood was sealed in a dialysis membrane and incubated in CSF in the same way described for the albumin solution. The pools of CSF and blood were obtained from anonymous donors (waste materials from the routine laboratory).
RESULTS AND DISCUSSION
Bile acids are known to be efficiently bound to albumin in the circulation (12). The hydrophobicity appears to be the governing factor in the interaction between albumin and bile acids. Given the structural similarity between 7-Hoca and bile acids, with the former possessing a C27 steroid side chain instead of a C24 steroid side chain, a firm binding of this compound to albumin could be expected in the circulation. Electrophoresis of hematomas followed by subsequent extraction of the different protein bands and analysis for 7-Hoca showed that 83% of this compound was located in the albumin band. Only traces of 7-Hoca was bound to hemoglobin, which together with albumin is the other dominating protein present in hematomas.
As shown in Table 1 we could confirm previous reports (5, 7, 8) that the levels of 7-Hoca in general are higher in subdural hematomas than in peripheral blood (P < 0.001). The absolute levels of 7-Hoca in peripheral blood varied between 16 and 72 ng/ml with a mean of 25 ng/ml, while the absolute levels of 7-Hoca in the hematomas varied between 69 and 259 ng/ml with a mean of 157 ng/ml. The degree of accumulation of 7-Hoca in the hematomas, about 6-fold, is thus similar to that reported by the Japanese group (5). The ratio between 7-Hoca and albumin in peripheral blood varied between 1 and 3.5 ng/mg with a mean of 1.4 ng/mg. In the hematomas, the 7-Hoca/albumin ratio varied between 3 and 13 ng/mg with a mean of 7.1 ng/mg.
TABLE 1.
Levels of 7-Hoca, albumin, and 7-Hoca/albumin ratio obtained from analysis of hematoma and peripheral blood samples collected from 20 patients with CSH
| Hematoma | Peripheral Blood | ||||||
| Time (days) | 7-Hoca (ng/ml) | Albumin (mg/ml) | 7-Hoca/Albumin (ng/mg) | 7-Hoca (ng/ml) | Albumin (mg/ml) | 7-Hoca/Albumin (ng/mg) | |
| Patient 1 | 51 | 181 | 20.6 | 8.8 | 27 | 18 | 1.5 |
| Patient 2 | 45 | 93 | 18 | 5.2 | 23 | 20.5 | 1.1 |
| Patient 3 | 196 | 21.4 | 9.2 | 22 | 21.2 | 1 | |
| Patient 4 | 15 | 69 | 10.1 | 6.8 | 32 | 19.4 | 1.6 |
| Patient 5 | 71 | 235 | 23.1 | 10.2 | 72 | 20.4 | 3.5 |
| Patient 6 | 143 | 27.6 | 5.2 | 23 | 23.8 | 1 | |
| Patient 7 | 84 | 27.9 | 3 | 16 | 18 | 0.9 | |
| Patient 8 | 33 | 104 | 33.9 | 3.1 | 21 | 22.1 | 1 |
| Patient 9 | 16 | 87 | 16.8 | 5.2 | 17 | 17.7 | 1 |
| Patient 10 | 123 | 244 | 30.7 | 7.9 | 15 | 17.7 | 0.9 |
| Patient 11 | 136 | 21.7 | 6.3 | 19 | 22.2 | 0.9 | |
| Patient 12 | 71 | 129 | 29.4 | 4.4 | 35 | 18 | 1.9 |
| Patient 13 | 69 | 259 | 20.5 | 12.7 | 23 | 20.5 | 1.1 |
| Patient 14 | 173 | 25.3 | 6.8 | 23 | 14.6 | 1.6 | |
| Patient 15 | 372 | 30.7 | 12.1 | 19 | 11.9 | 1.6 | |
| Patient 16 | 122 | 16.9 | 7.2 | 18 | 18.4 | 1 | |
| Patient 17 | 123 | 25.1 | 4.9 | 31 | 16.2 | 1.9 | |
| Patient 18 | 82 | 98 | 9.1 | 10.7 | 18 | 15.2 | 1.2 |
| Patient 19 | 74 | 117 | 20.4 | 5.7 | 24 | 18.7 | 1.3 |
| Patient 20 | 21 | 166 | 29.3 | 5.7 | 22 | 16.5 | 1.3 |
| Means | 157 | 22.9 | 7.1 | 25 | 18.6 | 1.4 | |
In 12 of the patients, the time between the symptoms of the bleeding and the collection of the hematoma sample was defined.
As expected, electrophoresis showed that albumin and hemoglobin were the dominating proteins in the hematomas. The trapping of these proteins in the hematoma is likely to be due to the presence of the encapsulating membranes that are able to prevent passage of high molecular compounds.
In the GC/MS analysis of most the hematoma samples, a peak appeared in the tracing of the ion at m/z 426 with a retention time longer than that of 7-Hoca (22.16 min vs. 21.74 min). The compound had the same retention time and the same mass spectrum as 3-oxo-cholesta-4,6-dienoic acid. This steroid is the dehydrated product of 7-Hoca. Because little or no deuterated 3-oxo-cholesta-4,6-dienoic acid appeared in our analyses, it is evident that the production of the dehydrated product had occurred in the hematoma and not in connection with extraction or chromatography of the samples.
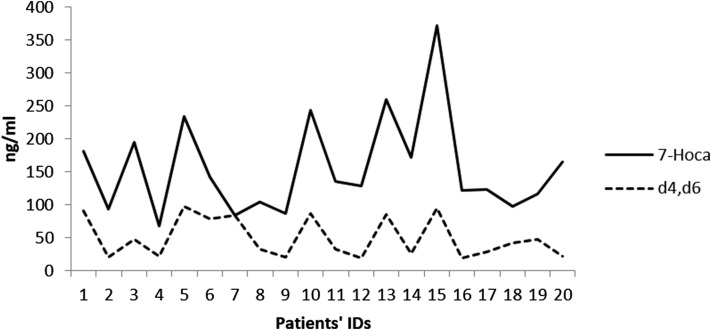
A quantitation was performed with use of the same standard curve as that used in the analysis of 7-Hoca. Figure 1 shows the levels of 3-oxo-cholesta-4,6-dienoic acid compared with the levels of 7-Hoca. There was a close relation between the two compounds in most of the samples, consistent with a possible precursor-product relationship.
Fig. 1.
Levels of 7-Hoca and 3-oxo-cholesta-4,6-dienoic acid in patients’ hematomas.
It should be pointed out that we could not find significant levels of 3-oxo-cholesta-4,6-dienoic acid in peripheral blood or in CSF.
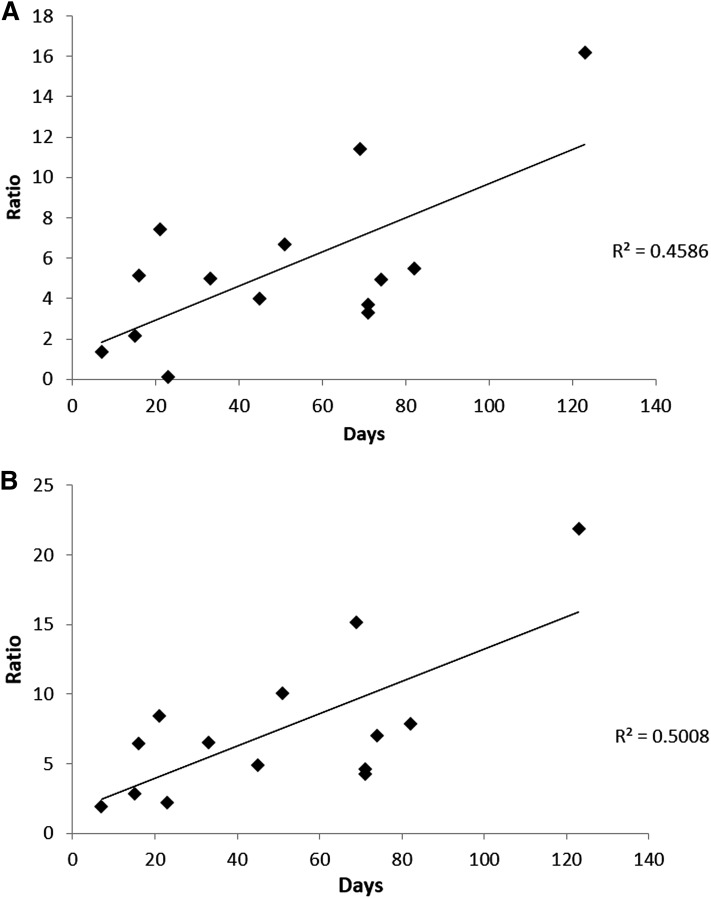
The accumulation of 7-Hoca and 3-oxo-cholesta-4,6-dienoic acid in the hematoma is likely to increase with time. In most of the patients, the time between the initial head trauma and the time for the collection of the hematoma sample could be defined (Table 1). As seen in Fig. 2A, there was a correlation between the ratio between 7-Hoca in the hematoma and in the circulation and the number of days passed from the initial head trauma (r2 = 0.46). Because 3-oxo-cholesta-4,6-dienoic acid is a product of 7-Hoca in the hematoma, it is suitable to compare the ratio between 7-Hoca + 3-oxo-cholesta-4,6-dienoic acid in the hematoma and 7-Hoca in the circulation with the duration of the time for development of the hematoma. As seen in Fig. 2B, there was an even better correlation in this case with r2 = 0.50. This correlation was significant according to a Spearman rank order test (P = 0.04).
Fig. 2.
Ratio between 7-Hoca in hematoma and in circulation in 14 patients with subdural hematoma compared with the time for the development of the hematoma (A) and ratio between 7-Hoca + 3-oxo-cholesta-4,6-dienoic acid in hematoma and 7-Hoca in circulation of the same patients compared with the time for development of the hematoma (B). Two of the patients belonged to another population than that shown in Table 1.
In order to test our hypothesis that there may be a flux of 7-Hoca from CSF across the membranes encapsulating the hematoma into the albumin in the hematoma, we exposed a pure albumin solution in a dialysis bag to CSF under the conditions described in Materials and Methods. The results are shown in Table 2. After 48 h incubation, the levels of 7-Hoca in the albumin solution increased from 0 ng/ml to 18 ng/ml. This increase was paralleled by a decrease in the level of 7-Hoca in the CSF pool. In the table, only one experiment is shown, but an additional experiment was performed with essentially the same results. Because the absolute concentration of 7-Hoca was twice that of the surrounding CSF, it is evident that it is not a mere equilibration process directing the flux of the steroid acid from CSF to the albumin solution. It seems likely that the high affinity of 7-Hoca to albumin is the driving force behind the flux.
TABLE 2.
Levels of 7-Hoca obtained from the experiment of incubation of albumin solution in CSF
| 7-Hoca | |
| (ng/ml) | |
| Albumin solution | 0 |
| Incubated albumin | 18 |
| CSF pool | 9 |
| CSF remnant | 8 |
Next, we repeated the above experiment, replacing the pure albumin solution with hemolyzed blood, mimicking the situation in a subdural hematoma. The results are shown in Table 3. Because of the osmotic conditions, the volume of the incubated blood sample expanded about 2-fold after the incubation. The amount of 7-Hoca increased, however, almost 3-fold as did the ratio between 7-Hoca and albumin. There was a corresponding decrease in the amount of 7-Hoca in the CSF pool. It is noteworthy that the ratio 7-Hoca/albumin in the incubated hemolyzed blood was of the same magnitude as the mean of the hematomas obtained from patients.
TABLE 3.
Results of the experiment of incubation of artificial hematoma in CSF
| Volume (ml) | 7-Hoca (ng/ml) | Total 7-Hoca (ng) | Albumin (mg/ml) | Total Albumin (mg) | 7-Hoca/Albumin (ng/mg) | |
| Hemolyzed blood | 2.0 | 81 | 163 | 19.7 | 39 | 4.1 |
| Incubated blood | 4.2 | 111 | 467 | 9.7 | 41 | 11.5 |
| CSF pool | 50 | 10 | 481 | 0.3 | 17 | 28.0 |
| CSF remnant | 38 | 8 | 310 | 0.4 | 20 | 19.9 |
Volumes were measured at the beginning of the experiment for hemolyzed blood and CSF pool and at the end of the experiment for the incubated blood and CSF remnant. Total amounts of 7-Hoca and albumin were calculated by multiplying the concentration by the volume. 7-Hoca/albumin ratio is calculated by dividing 7-Hoca concentration by albumin concentration.
Under the in vitro conditions used in the experiment shown in Table 3, volume changes occurred due to the osmotic conditions. Acute subdural hematomas that are not evacuated are frequently seen to expand during the first weeks after a bleeding as the hematoma gradually changes from a compact mass to a fluid CSH. The phase between days 4 and 21 after the bleeding can be referred to as subacute. Under in vivo conditions, volume changes due to the osmotic conditions are likely to be compensated for. Such compensatory mechanisms are, however, not likely to change the ratio between 7-Hoca and albumin.
In the Japanese study (7), it was observed that the absolute concentration of 7-Hoca in CSF increased to a level ∼5-fold higher than in plasma immediately after craniotomy due to aneurysmal subarachnoid bleeding. Continuous drainage from the prechiasmatic cistern was carried out for 10 days after the surgery. The levels of 7-Hoca decreased markedly during the first 24 h but were still increased 10 days after the surgery. Albumin was not measured in CSF from these patients. A subarachnoid bleeding represents a deposition of albumin in cerebral tissues directly exposing albumin to the brain surface. Given our finding of a rapid and efficient binding of 7-Hoca to albumin and a marked difference between CSF and blood with respect to the ratio between 7-Hoca and albumin, a flux of 7-Hoca from the brain into the albumin-enriched CSF could be expected.
We did not have access to CSF from patients with a fresh subarachnoid bleeding. We were, however, able to analyze blood-containing samples of CSF from anonymous patients, most probably cases with subarachnoid bleeding. It should be emphasized that according to the Japanese study, the levels of 7-Hoca in CSF after the subarachnoid bleeding decrease markedly during the first 24 h, and we had no information about the time span between the bleeding and the collection of the CSF sample. In one of the samples analyzed, we found a 9-fold increase in the CSF level of 7-Hoca (117 ng/ml) and a 3-fold increase in the level of albumin. The ratio between 7-Hoca and albumin was thus increased about 3-fold. Although analyses of more samples are needed for a firm conclusion, the result is consistent with the Japanese study and in accord with our hypothesis about the role of albumin binding.
An efficient “extraction” of 7-Hoca from the brain with subsequent trapping in the albumin present in CSF or in a subdural hematoma is consistent with our previous finding that 7-Hoca has a unique ability to pass biomembranes. When tested in a model for the BBB, 7-Hoca was found to pass the barrier considerably more efficiently than any of the other oxysterols tested (3).
In a previous study, we demonstrated a very high positive correlation between 7-Hoca and albumin in CSF from patients with a leaking BBB (10). Both the flux of albumin and the flux of 27-hydroxycholesterol across the barrier are known to increase in such patients (13). 27-Hydroxycholesterol is the precursor to 7-Hoca in the brain, and we suggested that increased substrate availability with increased formation of 7-Hoca may explain the high correlation between 7-Hoca and albumin (9). According to the results of the present work, an alternative hypothesis is that a primary flux of albumin across the disrupted barrier into the brain causes a flux of 7-Hoca from the brain to the binding site of albumin. The relative importance of the two alternative mechanisms is difficult to evaluate.
Our results are of interest in relation to the continuous flux of 7-Hoca from the brain into the circulation (3). Because the absolute levels of 7-Hoca are higher in plasma than in CSF, this flux occurs against a concentration gradient. It seems likely that the high affinity of 7-Hoca to albumin is important for this flux.
Acknowledgments
The skillful technical assistance of Jakob Lundin and Inger Moberg is gratefully acknowledged.
Footnotes
Abbreviations:
- 7-Hoca
- 7α-hydroxy-3-oxo-4-cholestenoic acid
- BBB
- blood-brain barrier
- CSF
- cerebrospinal fluid
- CSH
- chronic subdural hematoma
This work was supported by grants from Swedish Brain Power, Hjärnfonden, and Stiftelsen för Gamla Tjänarinnor (I.B.) and by National Institutes of Health Grant GM 062882 (I.P.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Axelson M., Mork B., and Sjovall J.. 1988. Occurrence of 3 beta-hydroxy-5-cholestenoic acid, 3 beta,7 alpha-dihydroxy-5-cholestenoic acid, and 7 alpha-hydroxy-3-oxo-4-cholestenoic acid as normal constituents in human blood. J. Lipid Res. 29: 629–641. [PubMed] [Google Scholar]
- 2.Fakheri R. J., and Javitt N. B.. 2012. 27-Hydroxycholesterol, does it exist? On the nomenclature and stereochemistry of 26-hydroxylated sterols. Steroids. 77: 575–577. [DOI] [PubMed] [Google Scholar]
- 3.Meaney S., Heverin M., Panzenboeck U., Ekstrom L., Axelsson M., Andersson U., Diczfalusy U., Pikuleva I., Wahren J., Sattler W., et al. 2007. Novel route for elimination of brain oxysterols across the blood-brain barrier: conversion into 7alpha-hydroxy-3-oxo-4-cholestenoic acid. J. Lipid Res. 48: 944–951. [DOI] [PubMed] [Google Scholar]
- 4.Lund E., Andersson O., Zhang J., Babiker A., Ahlborg G., Diczfalusy U., Einarsson K., Sjovall J., and Bjorkhem I.. 1996. Importance of a novel oxidative mechanism for elimination of intracellular cholesterol in humans. Arterioscler. Thromb. Vasc. Biol. 16: 208–212. [DOI] [PubMed] [Google Scholar]
- 5.Nagata K., Axelson M., Bjoerkhem I., Matsutani M., and Takakura K.. 1993. Significance of cholesterol metabolites in chronic subdural hematoma. In Recent Advances in Neurotraumatology. N. Nakamura, T. Hashimoto, and M. Yasue, editors. Springer, Tokyo. 49–52. [Google Scholar]
- 6.Heverin M., Maioli S., Pham T., Mateos L., Camporesi E., Ali Z., Winblad B., Cedazo-Minguez A., and Bjorkhem I.. 2015. 27-hydroxycholesterol mediates negative effects of dietary cholesterol on cognition in mice. Behav. Brain Res. 278: 356–359. [DOI] [PubMed] [Google Scholar]
- 7.Nagata K., Seyama Y., and Shimizu T.. 1995. Changes in the level of 7 alpha-hydroxy-3-oxo-4-cholestenoic acid in cerebrospinal fluid after subarachnoid hemorrhage. Neurol. Med. Chir. (Tokyo). 35: 294–297. [DOI] [PubMed] [Google Scholar]
- 8.Nagata K., Takakura K., Asano T., Seyama Y., Hirota H., Shigematsu N., Shima I., Kasama T., and Shimizu T.. 1992. Identification of 7 alpha-hydroxy-3-oxo-4-cholestenoic acid in chronic subdural hematoma. Biochim. Biophys. Acta. 1126: 229–236. [DOI] [PubMed] [Google Scholar]
- 9.Ogundare M., Theofilopoulos S., Lockhart A., Hall L. J., Arenas E., Sjovall J., Brenton A. G., Wang Y., and Griffiths W. J.. 2010. Cerebrospinal fluid steroidomics: are bioactive bile acids present in brain? J. Biol. Chem. 285: 4666–4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saeed A., Floris F., Andersson U., Pikuleva I., Lovgren-Sandblom A., Bjerke M., Paucar M., Wallin A., Svenningsson P., and Bjorkhem I.. 2014. 7alpha-hydroxy-3-oxo-4-cholestenoic acid in cerebrospinal fluid reflects the integrity of the blood-brain barrier. J. Lipid Res. 55: 313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore H. B., Moore E. E., Gonzalez E., Hansen K. C., Dzieciatkowska M., Chapman M. P., Sauaia A., West B., Banerjee A., and Silliman C. C.. 2015. Hemolysis exacerbates hyperfibrinolysis, whereas platelolysis shuts down fibrinolysis: evolving concepts of the spectrum of fibrinolysis in response to severe injury. Shock. 43: 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudman D., and Kendall F. E.. 1957. Bile acid content of human serum. II. The binding of cholanic acids by human plasma proteins. J. Clin. Invest. 36: 538–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leoni V., Masterman T., Patel P., Meaney S., Diczfalusy U., and Bjorkhem I.. 2003. Side chain oxidized oxysterols in cerebrospinal fluid and the integrity of blood-brain and blood-cerebrospinal fluid barriers. J. Lipid Res. 44: 793–799. [DOI] [PubMed] [Google Scholar]