Abstract
Acute kidney injury (AKI) caused by ischemia reperfusion is a major clinical problem in both native and transplanted kidneys. We previously showed that deficiency of Nrf2, a potent bZIP transcription factor that binds to the antioxidant response element, enhances susceptibility to experimental ischemic AKI. Here we further explored the role of Nrf2 in AKI by amplifying Nrf2 activation in vivo and in vitro with the synthetic triterpenoid CDDO-imidazolide. Mice treated with CDDO-imidazolide and undergoing experimental bilateral ischemic AKI had improved survival and renal function. Treated mice had improved renal histology with a decrease in tubular injury, as well as a decrease in pro-inflammatory cytokine and chemokine production compared to vehicle-treated mice. In an exploration of protective mechanisms, we found an up-regulation of Nrf2 target antioxidant genes in CDDO-imidazolide treated mouse kidneys. Furthermore, Nrf2 deficient mice treated with CDDO-imidazolide had no significant improvement in mortality, renal function or histology, pro-inflammatory cytokine gene expression, and no significant increase in antioxidant gene expression. In vitro studies demonstrated that the renal epithelial cells were likely an important target of CDDO-imidazolide. Thus, activation of Nrf2 signaling with CDDO-imidazolide confers protection from AKI, and presents a new therapeutic opportunity for this common and serious condition.
Introduction
Ischemia–reperfusion injury (IRI) is a major cause of acute kidney injury (AKI) in native kidneys, affecting approximately 1000 people per million and conferring a high mortality rate of up to 50% (1). Renal IRI is also associated with an increased risk of death in transplant patients, and is the primary cause of delayed graft function (DGF) in renal allograft recipients from deceased donors (2, 3). Despite these risks, there is currently no specific therapy to treat AKI, and finding an effective treatment remains an urgent priority. Oxidative stress is a major known contributor to the pathogenesis of renal IRI, responsible for direct and progressive cell damage (4, 5); therefore, factors that control this pathway may be attractive drug targets.
Nuclear factor-erythroid 2 p45-related factor 2 (Nrf2), a member of the Cap’n’collar (CNC)-bZIP transcription factor family, is responsible for regulating the cell’s antioxidant response via the antioxidant response element (ARE), and is thought to be involved in repair and recovery from AKI. Nrf2 and its downstream target genes were found to be upregulated in the kidney tissue of wild type mice following induction of renal ischemia and reperfusion (6). Our group showed that deficiency of Nrf2 caused worsened ischemic and nephrotoxic AKI in mice (7). Based on these studies, we hypothesized that pharmacological upregulation of Nrf2 would confer protection against AKI.
Among the known activators of Nrf2 are a class of synthetic triterpenoids, including CDDO (2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid), and its methyl (CDDO-Me, bardoxolone methyl) and imidazolide (CDDO-Im) derivatives. These compounds have been shown to enhance Nrf2-mediated antioxidant activity in a number of diseases, including chronic kidney disease (8). In this study, we have pharmacologically up-regulated expression and activity of Nrf2 beyond normal cellular levels and evaluated in vivo and in vitro effects on experimental AKI.
Results
CDDO-Im treatment improved mouse survival after AKI
To determine the effect of increased Nrf2 activation on injury response in the kidney, wild type mice were administered either CDDO-Im (30 µmol/kg in 200 µl volume) or vehicle by oral gavage 24 h before, 3 h before, and 24 h after undergoing thirty-minute bilateral kidney ischemia. While fewer than 50% of vehicle treated mice survived up to 72 h (6 out of 11), only one CDDO-Im treated mouse died by 72 h, indicating a significant survival advantage conferred by Nrf2 activation (P<0.05; Figure 1A).
Figure 1.
Effect of CDDO-Im on mouse survival and renal function after ischemia reperfusion injury. Nrf2+/+ mice were administered either CDDO-Im (30 µmol/kg in 200 µl volume) or vehicle at -24 h, -3 h, and at 24 h after undergoing 30 min bilateral kidney ischemia followed by reperfusion for 72 h. (A) Mice receiving CDDO-Im showed significantly improved survival after kidney IRI when compared with mice receiving vehicle (P<0.05 by log-rank test, n=10 in CDDO-Im, n=11 in vehicle). (B) Serum creatinine concentration was measured as a marker of renal function at 0, 24, 48 and 72 h post ischemia. Mice receiving CDDO-Im showed significantly less increase in serum creatinine after kidney IRI when compared with mice receiving vehicle. Data presented are the mean ± SEM from 11–13 mice per group (*P<0.05 by log-rank test).
CDDO-Im treatment improved renal function after AKI
Wild type mice received either CDDO-Im (30 µmol/kg) or vehicle 24 h before, 3 h before, and 24 h after undergoing thirty-minute bilateral kidney ischemia, and observed up to 72 h. Serum creatinine concentration was measured as a marker of renal function at 0 (baseline), 24, 48, and 72 h IRI. While all mice exhibited an increase in serum creatinine, mice receiving CDDO-Im showed significantly better post-injury creatinine levels than those receiving vehicle (P<0.05; Figure 1B).
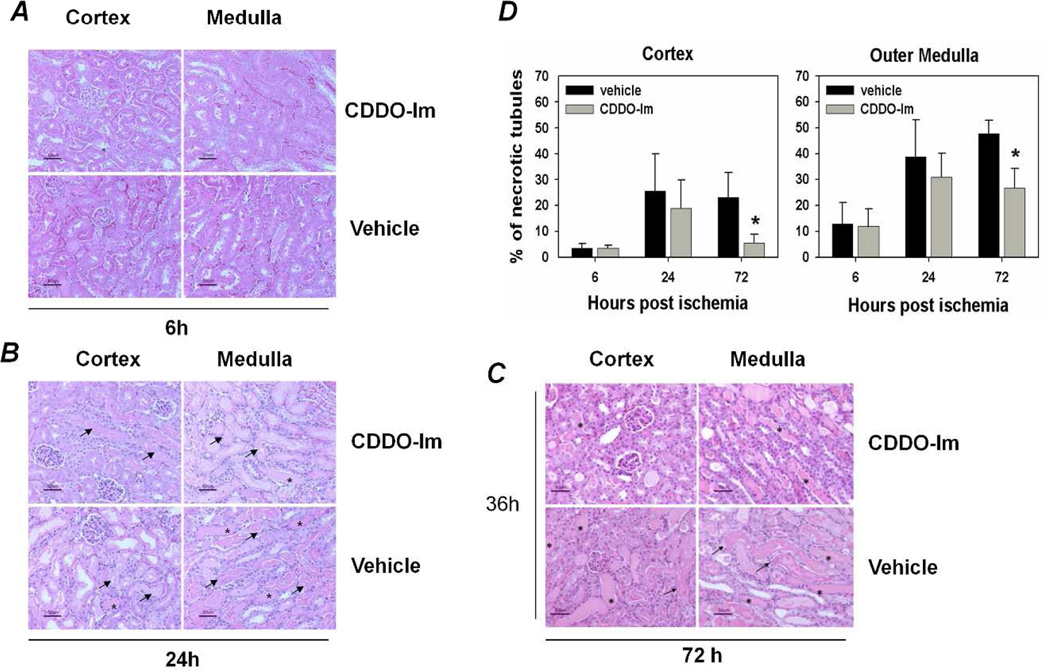
CDDO-Im treatment improved kidney histology after AKI
Kidneys from CDDO-Im treated and vehicle treated WT mice were harvested at 6 h, 24 h, and 72 h after ischemia, and were processed for histological examination with hematoxylin and eosin stain. To determine kidney tubular injury and regeneration, samples were scored by a renal pathologist blinded to the experimental groups, and the percentage of necrotic or regenerating tubules among the total tubules was averaged in each of 10 high power fields. After kidney ischemia, all mice had acute tubular necrosis in the kidney. At early time points (6 h and 24 h), CDDO-Im treated mice showed a trend toward decreased tubular injury. However, at 72 h after injury, mice that received CDDO-Im showed significantly less tubular necrosis when compared with mice receiving vehicle (P<0.05; Figure 2).
Figure 2.
Effect of CDDO-Im treatment on kidney tubular injury and regeneration after ischemia reperfusion injury. Nrf2+/+ mice were administered either CDDO-Im (30 µmol/kg in 200 µl volume) or vehicle at -24 h, -3 h, and at 24 h after undergoing 30 min bilateral kidney ischemia followed by reperfusion for 72 h. Harvested kidneys were stained with H&E, and kidney tubular injury and regeneration was scored under light microscope by assessing the average percentage of necrotic or regenerating tubules among the total tubules in each of 10 high power fields. After kidney ischemia, all mice had acute tubular necrosis (arrows) in the kidney. However, mice receiving CDDO-Im had significantly less tubular necrosis when compared with those receiving vehicle. Photomicrographs of representative sections (magnification: 400×) from 6h (A), 24 h (B) and 72 h (C) IRI groups show necrotized tubules (arrows) and cast formation (asterisks). (D) Tubular injury scores. Data presented are the mean ± SEM from 5–7 mice per group in two independent experiments (*P<0.05 by log-rank test).
CDDO-Im treatment decreased production of pro-inflammatory cytokines induced by AKI
To examine proinflammatory molecules generated by ischemia–reperfusion injury, early protein levels of IL-6, G-CSF, MCP-1, and KC were measured in mouse kidneys by multiplex cytokine assay (Bio-Rad Laboratories, Hercules, CA). Kidneys from CDDO-Im treated and vehicle treated WT mice were harvested at 6 h and 24 h after ischemia. IL-6, G-CSF, and KC were all found to be significantly decreased at 6 h after injury (P<0.05); however, CDDO-Im conferred no preferential decrease at 24 h (Figure 3).
Figure 3.
Effect of CDDO-Im treatment on the expression of inflammatory cytokines. Nrf2+/+ mice were administered either CDDO-Im (30 µmol/kg in 200 µl volume) or vehicle at -24 h, -3 h, and at 24 h after undergoing 30 min bilateral kidney ischemia followed by reperfusion for 6 h or 24 h. Harvested kidneys were analyzed for levels of inflammatory cytokines G-CSF, IL-6, KC, and MCP-1 at 6 h and 24 h. CDDO-IM significantly decreased levels of inflammatory cytokines G-CSF, IL-6, and KC at 6 h after injury when compared to vehicle. Data presented are the mean fold change ± SEM from 5 mice per group (*P<0.05 by log-rank test).
CDDO-Im treatment upregulated Nrf2 target gene activation
To explore the mechanism of CDDO-Im’s protective effect, kidneys from CDDO-Im treated and vehicle treated WT mice were harvested at 6 h, 24 h, and 72 h after ischemia, and were analyzed by quantitative real-time PCR for expression of Nrf2 target genes. At 72 h after injury, mice receiving CDDO-Im showed upregulation of all tested target genes, with a significant increase in Gclc, Nqo1 and Ho-1 over vehicle treated mice (P<0.05, Figure 4). Cytoprotective gene expression was not observed at 6 h or at 24 h.
Figure 4.
Effect of CDDO-Im treatment on Nrf2 target gene activation. Nrf2+/+ mice were administered either CDDO-Im (30 µmol/kg in 200 µl volume) or vehicle at -24 h, -3 h, and at 24 h after undergoing 30 min bilateral kidney ischemia followed by reperfusion for 6 h, 24 h, or 72 h. RNA was isolated from kidneys and expression of Nrf2 target genes, Ho1, Nqo1, Gclc and Gpx2 at 6 h, 24 h, and 72 h were determined by qPCR using TaqMan real time probes. At 72 h after injury, CDDO-Im significantly elevated the expression levels of Gclc, Ho-1, and Nqo1 (gray bar) when compared to vehicle (black bar). Gene expression did not increase significantly at 6h or at 24 h. Data presented are the mean fold change ± SEM from 4–6 mice per group (*P<0.05 by log-rank test).
Delayed CDDO-Im treatment did not confer protection against AKI
To determine whether Nrf2 activation would be protective when initiated after the onset of injury instead of before, we delayed treatment of CDDO-Im. Mice received either CDDO-Im (30 µmol/kg) or vehicle at 3 h before and 24 h after undergoing thirty-minute bilateral kidney ischemia, and observed up to 72 h. Delayed CDDO-Im treatment did not improve the outcome of renal IRI when compared to vehicle treatment, as evidenced by no significant difference in mortality, renal function, renal histology, or inflammatory cytokine levels.
CDDO-Im conferred protection against hypoxia in mouse renal epithelial cells
To evaluate if CDDO-Im worked through the renal epithelial cell, primary kidney epithelial cells were cultured from C57BL/6 mice, and treated with either CDDO-Im or DMSO. Cells were glucose- and serum-starved, and exposed to either room air (RA, middle panel, Figure 5A) or hypoxia (bottom panel, Figure 5A) for 12 h, then allowed to recover in RA for 6 h; cells cultured in normal medium and exposed to room air were used as controls (top panel, Figure 5A). DCFDA staining showed less ROS generation in all CDDO-Im treated groups, including glucose/serum starvation as well as starvation accompanied by hypoxia (Figure 5A & 5B). To explore the mechanism of ROS protection, cells treated with either CDDO-Im or DMSO for 6 h or 12 h were then analyzed for the mRNA expression of Nrf2 target genes, , Ho-1, Nqo1 and Gpx2. CDDO-Im caused a significant upregulation of target genes when compared to DMSO (Figure 5C). This effect was also observed at the protein level. Cells were treated with CDDO-Im or DMSO for 6 h, total cell lysates were prepared, and subjected to western blot analysis using anti-Nrf2, anti-Ho-1, and anti-Nqo1 antibodies. CDDO-Im increased Nrf2, Ho-1 and Nqo1 protein levels over when compared to cells treated with DMSO (Figure 5D).
Figure 5.
Effect of CDDO-Im treatment on mouse kidney epithelial cells. Primary kidney epithelial cells were cultured from Nrf2+/+ and Nrf2−/− mice, and treated with either CDDO-Im (25 nM) or DMSO. (A) Cells were placed in medium without glucose and serum, and exposed to either room air (RA) or hypoxia (1% O2, 5% CO2, 94% nitrogen) for 12 h. The cultures were replenished with standard medium and allowed to recover in room air with 5% CO2 for 6 h. DCFDA staining was used to measure ROS generation. CDDO-Im treatment conferred protection against glucose and serum starvation, as well as starvation accompanied by hypoxia, as evidenced by less ROS generation when compared to DMSO treated cells. As anticipated, cells cultured in standard medium showed no DCFDA staining. (B) DCF positive cells were counted (5 fields per culture) and average ± SEM were given. †P ≤ 0.05, room air vs hypoxia; *P ≤ 0.05, vehicle vs CDDO-Im treated cultures. (C) Cells were treated with either CDDO-Im (gray bars) or DMSO (black bars) for 6 h or 12 h, and were analyzed for mRNA expression of Nrf2 target genes Ho-1, Nqo1, Gclc, and Gpx2,. CDDO-Im caused a significant upregulation of target genes when compared to DMSO. Data presented are the mean fold change ± SEM (*P<0.05 by log-rank test). †P ≤ 0.05, room air vs hypoxia; *P ≤ 0.05, vehicle vs CDDO-Im treated cultures. (D) Cells were treated with either CDDO-Im or DMSO for 6 h, and were analyzed Nrf2, Ho-1, and Nqo1 protein expression by Western blot analysis. CDDO-Im increased cellular expression of Nrf2, Ho-1, and Nqo1 compared to DMSO. Band intensities were quantified using ImageJ software and normalized with that of β-actin. Data represented as the average intensity ratios ± SEM of three samples. †P ≤ 0.05, room air vs hypoxia; *P ≤ 0.05, vehicle vs CDDO-Im treated cultures.
CDDO does not confer protection against AKI in the absence of Nrf2
To confirm that CDDO-Im acts primarily through the Nrf2 pathway, we examined the results of CDDO-Im treatment on mice deficient in Nrf2. Nrf2−/− mice, aged-matched littermates to wild type mice used in previous experiments, were administered either CDDO-Im (30 µmol/kg in 200 µl volume) or vehicle at -24 h, -3 h, and at 24 h after undergoing 30 min bilateral kidney ischemia, and observed up to 72 h. CDDO-Im did not confer improvement in survival or renal function in Nrf2−/− mice (Figure 6A, 6B). Kidneys from CDDO-Im treated and vehicle treated Nrf2−/− mice were harvested at 72 h after ischemia, and were scored for tissue injury as described previously. Tubular necrosis was not ameliorated in CDDO-Im treated mice (Figure 6C). Harvested kidneys were also analyzed by quantitative real-time PCR for expression of inflammatory cytokine genes and Nrf2 target genes. CDDO-Im did not decrease production of inflammatory cytokines when compared with mice receiving vehicle, and also failed to upregulate Nrf2 target genes in Nrf2−/− mice, suggesting that CDDO-Im cytoprotection acts directly through Nrf2 (Figure 6D).
Figure 6.
CDDO-Im did not confer protection against renal ischemia reperfusion injury in Nrf2−/− mice. Nrf2−/− mice were generated as previously described. Mice were administered either CDDO-Im (30 µmol/kg in 200 µl volume) or vehicle at -24 h, -3 h, and at 24 h after undergoing 30 min bilateral kidney ischemia followed by reperfusion for 72 h. (A–D) Mice receiving CDDO-Im showed no improvement in survival, renal function, renal injury score, Nrf2 target gene expression, or inflammatory cytokine profile at 72 h after kidney IRI when compared with mice receiving vehicle. Data presented are the mean fold change ± SEM from 4–8 mice per group.
Discussion
Oxidative stress, caused by the excessive generation of reactive oxygen and nitrogen species (ROS, RNS), plays an important part in the development of ischemic renal injury. Ischemia/reperfusion-induced radicals directly cause cell death by lipid peroxidation, DNA breakdown, and protein damage, as well as act as signal transducers and gene regulators, which in turn have both negative, as well as protective, consequences (4, 5, 9, 10). Furthermore, oxidative stress is strongly involved in the progression to fibrosis, which can turn an acute ischemic event into chronic kidney disease (11). Previous pharmacological studies have focused on preventing ROS generation and supplying or boosting individual cellular antioxidants and ROS scavengers (12); however, these methods have been limited by various complicating factors, including dosage and in vivo stability, and better targets remain to be investigated. With the recent emergence of research on Nrf2, a master regulator of cellular antioxidant response, we can begin to explore a more comprehensive drug-based intervention based on a single target pathway.
2-Cyano-3,12-dioxoolean-1,9-dien-28-oic acid (CDDO), a synthetic oleanane triterpenoid, was first developed as a chemopreventive agent, though it has since been shown to act through a variety of mechanisms (13). CDDO and its analogues, including CDDO-Im and CDDO-Me, are capable of suppressing the de novo synthesis of the inflammatory enzymes iNOS and COX-2; also, while they can induce apoptosis and inhibit proliferation of malignant and premalignant cells at high doses, subapoptotic doses of CDDO promote phagocytic activity and granulocytic-monocytic differentiation (14). More recently, CDDO-Im was found to be a potent activator of the Nrf2 pathway (15), and has been used to promote antioxidant activity in a number of disease models, including nephrotoxicity, hepatotoxicity, stroke, hyperoxic acute lung injury, emphysema, and sepsis (16–21).
In the present study, we have found that CDDO-Im confers protection against AKI, improving mortality, renal function, and renal histology. This was accompanied by a decrease in production of IL-6, G-CSF, and KC, which are modifiers of ischemic AKI. It is likely that these effects were due to the induction of cytoprotective genes, including GCLC, HO-1, and NQO1. To determine if renal tubular epithelial cells (RTEC) were directly targeted by CDDO-Im, we studied primary RTEC cultures under hypoxia and starvation conditions, and found that ROS production was attenuated by CDDO-Im. Upregulation of Nrf2 target genes by CDDO-Im was a likely mechanism of this effect. Although CDDO-Im-mediated effects appear to primarily operate through Nrf2, recent studies suggest that this compound also regulates cellular processes in an Nrf2-independent manner (22–25). Given that CDDO-Im did not protect against AKI in Nrf2 deficient mice nor upregulate target genes, we conclude that the mechanism of CDDO-Im action in this model is primarily through Nrf2.
In the present study, delayed treatment with CDDO-Im, begun immediately before injury instead of 24 h before, did not confer protection against AKI in the timeframe studied. This time dependency suggests that CDDO-Im’s protective effect may involve de novo protein synthesis; CDDO-Im boosts activity of Nrf2, which promotes increased expression of downstream antioxidant genes and leads to upregulation of cytoprotective proteins. Indeed, our data shows that CDDO-Im treatment begun 24 h before injury does not significantly upregulate Nrf2 targets until 72 h after initial injury. Alternatively, oral administration of CDDO-Im likely also contributed to its limited early effects, and while oral delivery is ideal for clinical situations, a more direct delivery (intraperitoneal or intravenous) may allow for a delayed administration while still conferring early injury improvement.
Recently, Pergola et. al. explored the mitigating effects of CDDO-Me (targeting Nrf2 but distinct from our compound) in patients with moderate to severe chronic kidney disease and type 2 diabetes. By oral treatment with CDDO-Me over several weeks, patients saw a large, steady, significant increase in eGFR from baseline. This change was accompanied by numerous markers of renal improvement, with decreased serum creatinine and BUN, increased creatinine clearance, and decreased CECs (markers of endothelial dysfunction and vascular injury) and inducible nitric oxide synthase expression, by day 56. Moreover, GFR remained elevated for several weeks after the discontinuation of CDDO-Me; this effect is most likely attributable to a decrease in inflammation and oxidative stress (8, 26). However, a followup of this trail was recently stopped due to adverse outcome in the long term treated CDDO-Me patients. Our mechanistic studies in mice were short term, in a frequently fatal disease, using a different Nrf2 activator. Our findings suggest that CDDO-Im intervention may be more useful as a prophylactic rather than a treatment intervention in AKI. A recent paper by Wu et al found that CDDO-Me was protective during ischemic AKI (27). Elegant localization of CDDO-ME induced genes was demonstrated. These results are consistent with our current data. However, there are certain differences as well. While Wu et al used a different analogue of triterpenoids (bardoxolone methyl), we used an imidazolide derivative. Wu et al used a unilateral ischemia model, while we used a bilateral model. Another difference was that we performed experiments using primary renal epithelial cells to examine Nrf2 target gene activation and cytoprotective effects of CDDO-Im on hypoxia-induced cellular stress. Furthermore, a unique feature of our paper was the use of Nrf2 deficient mice to prove that the CDDO-Im effects were through Nrf2.
Due to the multifactorial nature of AKI, it is unlikely that a single drug, or even a single mechanistic pathway, will confer complete protection (12, 28). CDDO-Im treatment produced significant improvements to renal function and architecture, and effectively increased survival; though it was unable to provide complete prevention, it more importantly highlights the Nrf2 as a promising target to improve outcomes in AKI.
Materials and Methods
Mice
Breeding pairs of Nrf2+/− (ICR/Sv129) mice were obtained from a colony at Tsukuba University and housed under specific pathogen-free conditions. Nrf2+/+ (wild type, WT) and Nrf2−/− mice were generated according to the breeding procedures previously described (29). All experimental protocols were performed in accordance with the institutional Animal Care and Use Committee guidelines.
Mouse model of AKI
An established mouse model of renal IRI was used. Animals were anesthetized with sodium pentobarbital at a dose of 75 mg/kg (I.P.). After an abdominal incision was made and the left and right renal pedicles were bluntly dissected, a non-traumatic microvascular clip was placed on each renal pedicle for 30 minutes. During the procedure, a total of 2 ml of warm saline (37–°C) was infused into the peritoneal cavity (1 ml at the beginning of ischemia and 1 ml at the beginning of reperfusion) to keep animals well hydrated. The mice were put on a heating pad (45°C) during the procedure until awake to keep their body temperatures constant at approximately 37°C (anal). As soon as the clips were removed, the wounds were sutured and the animals were allowed to recover.
Assessment of renal function
Blood samples were obtained from mice via tail bleeding prior to (0 h) and at 24 h, 48 h, and 72 h after kidney ischemia. Blood was centrifuged to collect serum, and serum creatinine was measured as a marker of renal function by Roche Cobas Mira Plus automated analyzer system (Roche Diagnostics, Indianapolis, IN) by using a Creatinine 557A kit (Sigma Disagnostics, St. Louis, MO).
Evaluation of kidney histology
Upon sacrifice at indicated time points, the kidneys were harvested and cut into longitudinal halves. One half of each kidney was snap-frozen with liquid nitrogen for molecular study, while the other half was fixed with 10% buffered formalin and embedded with paraffin for histological study. Tissue sections (5µm) were stained with hematoxylin and eosin (H&E). A renal pathologist at Johns Hopkins (LR) who was blind to the experimental groups scored the percentage of necrotic tubules out of total tubules in each of at least 10 high-power fields in the cortex or medulla, and the average percentage of tubular necrosis in all fields was presented as the renal tubular injury score of each mouse.
Administration of pharmacologic inhibitors
The synthetic triterpenoid CDDO-Im, which is known to disrupt the cytosolic Keap1:Nrf2 interaction, was synthesized as described previously (30)(31). CDDO-Im was prepared freshly just before administration by dissolving the frozen powder in PBS containing 10% DMSO and 10% Cremophor-EL. The suspension was then briefly sonicated to generate a uniform suspension. To assess the effects of CDDO-Im on kidney ischemia reperfusion injury, mice received CDDO-Im by gastric gavage (30 µmol/kg body weight) in 200 µL volume at 24 h or 3 h prior to, or 24 h after ischemia. In a different set of experiments, mice received delayed CDDO-Im treatment, at 3 h prior to or 24 h after ischemia, by gastric gavage (30 µmol/kg body weight) in 200 µL volume. Control mice received the same volume of vehicle. The dose of CDDO-Im (30 µmol/kg) for this study was based on pharmacodynamics studies showing maximum induction of Nrf2 target genes.
Cytokine analysis
To examine pro-inflammatory cytokines generated by ischemia–reperfusion injury, protein levels of G-CSF, IL-6, KC, and MCP-1 were measured in mouse kidneys by Bio-Plex multiple cytokine array (Bio-Rad Laboratories Inc., Hercules, CA, USA). Briefly, a portion of snap frozen tissue was homogenized in a cell-lysis buffer, and the homogenates were centrifuged at 12,000 rpm for 15 min at 4°C. Each sample was first incubated with a mixture of all types of micro-beads for 90 min followed by incubation with biotinylated detection antibodies for 30 min and finally with a streptavidin-coupled phycoerythrin for 10 min at room temperature. Finally, the samples were subjected to a flow cytometric system. All acquired data was analyzed using Bio-Plex Manager 3.0 software (Bio-Rad). Total protein concentration of each sample was determined using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL, USA), and used to normalize the measured cytokine levels.
Real time PCR Analysis
Total RNA (2 µg) from mouse kidney tissues or renal epithelial cells was reverse transcribed using superscript™ first strand cDNA synthesis system (Invitrogen, Carlsbad, CA, USA), and real time RT-PCR reactions were performed using TaqMan® gene expression assays for Nqo1, Gclc, Ho-1,and Gpx2 (Applied Biosystems). β-actin was used as internal control genes The absolute expression values for each gene were normalized to that of β-actin and the relative value for the vehicle control group was set as one.
Primary renal epithelial cell culture
Kidney tissue from WT mice was gently minced in dispase II solution (Roche Applied Science, Indianapolis, IN, USA) and incubated at 37°C for 45 minutes. The tissue/cell suspension was passed through 100 and 40 µm cell strainers and centrifuged at 130 g for 5 min at 4°C. The cells were suspended in 25 mM hydroxyethyl piperazine ethanesulfonic acid (HEPES) buffered DMEM and incubated in mouse IgG coated plates for 2h at 37°C. After incubation, the non-adherent cells were resuspended in DMEM supplemented with 10% FBS, 25 mM HEPES, 10 ng/ml keratinocyte growth factor and antibiotics cultured on petridishes coated with fibronectin and collagen. The cell culture was grown for 5 days with the medium replenishment every 2 days.
Statistics
Data are presented as mean ± standard error of the mean (SEM), and are compared by an unpaired, two-tailed student t test for a single comparison between two groups, or by a post-hoc ANOVA test for multiple comparisons. Cumulative survival was analyzed by logrank test (Kaplan-Meier). Statistical significance of difference was defined as a p-value less than 0.05.
Acknowledgments
We thank Masayuki Yamamoto, Japan, for providing Nrf2 knockout mice used in our studies. This work was funded and supported by the National Institutes of Health Grants NIH RO1DK084445 and RO1HL066109.
Footnotes
Disclosure statement: The authors have nothing to disclose.
Author contributions: M.L., N.M.R., E.M.H., S.P.R., and H.R. designed research; M.L., N.M.R., E.M.H., and H.R.P. performed research; L.R. scored histological samples; T.W.K. and M.B.S. contributed CDDO-Im; S. N. helped with revisions, M.L., N.M.R., E.M.H., H.R.P., and H.R. analyzed data; and E.M.H., S.P.R., and H.R. wrote the paper.
References
- 1.Ali T, Khan I, Simpson W, et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol. 2007;18:1292–1298. doi: 10.1681/ASN.2006070756. [DOI] [PubMed] [Google Scholar]
- 2.Ojo AO, Wolfe RA, Held PJ, et al. Delayed graft function: risk factors and implications for renal allograft survival. Transplantation. 1997;63:968–974. doi: 10.1097/00007890-199704150-00011. [DOI] [PubMed] [Google Scholar]
- 3.Mehrotra A, Rose C, Pannu N, et al. Incidence and consequences of acute kidney injury in kidney transplant recipients. Am J Kidney Dis. 2012;59:558–565. doi: 10.1053/j.ajkd.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 4.Baker GL, Corry RJ, Autor AP. Oxygen free-radical induced damage in kidneys subjected to warm ischemia and reperfusion - protective effect of superoxide-dismutase. Ann Surg. 1985;202:628–641. doi: 10.1097/00000658-198511000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nath KA, Norby SM. Reactive oxygen species and acute renal failure. Am J Med. 2000;109:665–678. doi: 10.1016/s0002-9343(00)00612-4. [DOI] [PubMed] [Google Scholar]
- 6.Leonard MO, Kieran NE, Howell K, et al. Reoxygenation-specific activation of the antioxidant transcription factor Nrf2 mediates cytoprotective gene expression in ischemia-reperfusion injury. FASEB J. 2006;20:2624–2626. doi: 10.1096/fj.06-5097fje. [DOI] [PubMed] [Google Scholar]
- 7.Liu M, Grigoryev DN, Crow MT, et al. Transcription factor Nrf2 is protective during ischemic and nephrotoxic acute kidney injury in mice. Kidney Int. 2009;76:277–285. doi: 10.1038/ki.2009.157. [DOI] [PubMed] [Google Scholar]
- 8.Pergola PE, Raskin P, Toto RD, et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011;365:327–336. doi: 10.1056/NEJMoa1105351. [DOI] [PubMed] [Google Scholar]
- 9.Walker LM, York JL, Imam SZ, et al. Oxidative stress and reactive nitrogen species generation during renal ischemia. Toxicol Sci. 2001;63:143–148. doi: 10.1093/toxsci/63.1.143. [DOI] [PubMed] [Google Scholar]
- 10.Sen C, Packer L. Antioxidant and redox regulation of gene transcription. The FASEB Journal. 1996;10:709–720. doi: 10.1096/fasebj.10.7.8635688. [DOI] [PubMed] [Google Scholar]
- 11.Small DM, Coombes JS, Bennett N, et al. Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology. 2012;17:311–321. doi: 10.1111/j.1440-1797.2012.01572.x. [DOI] [PubMed] [Google Scholar]
- 12.Chatterjee PK. Novel pharmacological approaches to the treatment of renal ischemia-reperfusion injury: a comprehensive review. Naunyn Schmiedebergs Arch Pharmacol. 2007;376:1–43. doi: 10.1007/s00210-007-0183-5. [DOI] [PubMed] [Google Scholar]
- 13.Suh N, Wang Y, Honda T, et al. A novel synthetic oleanane triterpenoid, 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, with potent differentiating, antiproliferative, and anti-inflammatory activity. Cancer Research. 1999;59:336–341. [PubMed] [Google Scholar]
- 14.Koschmieder S, D'Alo F, Radomska H, et al. CDDO induces granulocytic differentiation of myeloid leukemic blasts through translational up-regulation of p42 CCAAT enhancer binding protein alpha. Blood. 2007;110:3695–3705. doi: 10.1182/blood-2006-11-058941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liby K, Hock T, Yore MM, et al. The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE signaling. Cancer Research. 2005;65:4789–4798. doi: 10.1158/0008-5472.CAN-04-4539. [DOI] [PubMed] [Google Scholar]
- 16.Aleksunes LM, Goedken MJ, Rockwell CE, et al. Transcriptional regulation of renal cytoprotective genes by Nrf2 and its potential use as a therapeutic target to mitigate cisplatin-induced nephrotoxicity. J Pharmacol Exp Ther. 2010;335:2–12. doi: 10.1124/jpet.110.170084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reisman SA, Buckley DB, Tanaka Y, et al. CDDO-Im protects from acetaminophen hepatotoxicity through induction of Nrf2-dependent genes. Toxicology and Applied Pharmacology. 2009;236:109–114. doi: 10.1016/j.taap.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang F, Wang S, Zhang M, et al. Pharmacological induction of heme oxygenase-1 by a triterpenoid protects neurons against ischemic injury. Stroke. 2012;43:1390–1397. doi: 10.1161/STROKEAHA.111.647420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reddy NM, Suryanaraya V, Yates MS, et al. The triterpenoid CDDO-imidazolide confers potent protection against hyperoxic acute lung injury in mice. Am J Respir Crit Care Med. 2009;180:867–874. doi: 10.1164/rccm.200905-0670OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sussan TE, Rangasamy T, Blake DJ, et al. Targeting Nrf2 with the triterpenoid CDDO-imidazolide attenuates cigarette smoke-induced emphysema and cardiac dysfunction in mice. Proc Natl Acad Sci. 2009;106:250–255. doi: 10.1073/pnas.0804333106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thimmulappa RK, Scollick C, Traore K, et al. Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-Imidazolide. Biochem Biophys Res Commun. 2006;351:883–889. doi: 10.1016/j.bbrc.2006.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yates MS, Tran QT, Dolan PM, et al. Genetic versus chemoprotective activation of Nrf2 signaling: overlapping yet distinct gene expression profiles between Keap1 knockout and triterpenoid-treated mice. Carcinogenesis. 2009;30:1024–1031. doi: 10.1093/carcin/bgp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yates MS, Kwak M-K, Egner PA, et al. Potent protection against aflatoxin-induced tumorigenesis through induction of Nrf2-regulated pathways by the triterpenoid 1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole. Cancer Research. 2006;66:2488–2494. doi: 10.1158/0008-5472.CAN-05-3823. [DOI] [PubMed] [Google Scholar]
- 24.Yore MM, Kettenbach AN, Sporn MB, et al. Proteomic analysis shows synthetic oleanane triterpenoid binds to mTOR. PLoS ONE. 2011;6:e22862. doi: 10.1371/journal.pone.0022862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osburn WO, Yates MS, Dolan PD, et al. Genetic or pharmacologic amplification of Nrf2 signaling inhibits acute inflammatory liver injury in mice. Toxicol Sci. 2008;104:218–227. doi: 10.1093/toxsci/kfn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pergola PE, Krauth M, Huff JW, et al. Effect of bardoxolone methyl on kidney function in patients with T2D and Stage 3b-4 CKD. Am J Nephrol. 2011;33:469–476. doi: 10.1159/000327599. [DOI] [PubMed] [Google Scholar]
- 27.Wu QQ, Wang Y, Senitko M, et al. Bardoxolone methyl (BARD) ameliorates ischemic AKI and increases expression of protective genes Nrf2, PPARgamma, and HO-1. Am J Physiol Renal Physiol. 2011;300:F1180–F1192. doi: 10.1152/ajprenal.00353.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly KJ, Molitoris BA. Acute renal failure in the new millennium: time to consider combination therapy. Semin Nephrol. 2000;20:4–19. [PubMed] [Google Scholar]
- 29.Itoh K, Chiba T, Takahashi S, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 30.Liby K, Hock T, Yore MM, et al. The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE signaling. Cancer Res. 2005;65:4789–4798. doi: 10.1158/0008-5472.CAN-04-4539. [DOI] [PubMed] [Google Scholar]
- 31.Yates MS, Tauchi M, Katsuoka F, et al. Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of Nrf2-regulated genes. Mol Cancer Ther. 2007;6:154–162. doi: 10.1158/1535-7163.MCT-06-0516. [DOI] [PubMed] [Google Scholar]