Abstract
Bioactivation of 5-hydroxy-[carbonyl-14C]thalidomide, a known metabolite of thalidomide, by human artificial or native cytochrome P450 3A enzymes, and nonspecific binding in livers of mice was assessed using two-dimensional electrophoresis combined with accelerator mass spectrometry. The apparent major target proteins were liver microsomal cytochrome c oxidase subunit 6B1 and ATP synthase subunit α in mice containing humanized P450 3A genes or transplanted humanized liver. Liver cytosolic retinal dehydrogenase 1 and glutathione transferase A1 were targets in humanized mice with P450 3A and hepatocytes. 5-Hydroxythalidomide is bioactivated by human P450 3A enzymes and trapped with proteins nonspecifically in humanized mice.
Graphical Abstract
The metabolism of thalidomide is important for both teratogenicity and anticancer efficacy.1 Recently, a whole-embryo culture system from transchromosomic mice containing a human cytochrome P450 3A cluster2 (in which the endogenous mouse Cyp3a genes were deleted) showed limb abnormalities.3 It was demonstrated that human P450s 3A4 and 3A5 can also oxidize thalidomide to primary 5-hydroxy- and secondary dihydroxy metabolites4–7 in mice with highly “humanized” liver cells.8 This finding would suggest generation of a putative reactive arene oxide1 in the oxidation of 5-hydroxy thalidomide, trapped by GSH to give GSH adducts. Determining if covalent protein binding of thalidomideoccurs and whether the primary metabolites are bound to specific proteins9,10 or not can be important in evaluating toxic and pharmacological potential. Because the secondary oxidation of 5-hydoxythalidomide was noted to be faster than the primary thalidomide 5-hydoxylation mediated by recombinant human P450 3A4/5,7 the in vivo metabolic activation a primary metabolite of thalidomide was investigated after administration of radiolabeled compound to two humanized model mice in this study. One mouse had fully humanized CYP3A gene2 (designated P450 3A-human artificial chromosome (HAC)/Cyp3a knock-out (KO)) and the other was a mouse with highly humanized liver cells harboring cytochrome CYP3A5*1.8 Both humanized CYP3A-HAC/Cyp3a KO mice5 and humanized TK-NOG mice11 were previously confirmed to have adequate human P450 3A enzyme levels and activities. We report herein that analyses using two-dimensional electrophoresis with accelerator mass spectrometry revealed that in vivo bioactivated 5-hydroxythalidomide bound nonspecifically to a variety of microsomal and cytosolic proteins in livers in mice with humanized CYP3A gene or with transplanted humanized liver.
Mean concentrations of radiolabeled 5-hydroxythalidomide in plasma, livers, and fetal samples were 0.64 ± 0.25 µg equivalent/mL (mean ± SD, n=5), 1.4 ± 1.0 µg equivalent/g liver, and 0.06 ± 0.04 µg equivalent/g fetus, respectively, 1 h after i.v. administration in female mice containing humanized CYP3A gene. Similarly, mean concentrations of radiolabeled 5-hydroxyhtalidomide in plasma and livers were 0.50 ± 0.14 µg equivalent/mL (mean ± SD, n=5) and 0.43 ± 0.09 µg equivalent/g liver 1 h after i.v. administration in male mice with humanized liver, respectively: the mean plasma concentration of 5-hydroxythalidomide determined by LC-MS/MS was 5.5 ± 1.2 ng/mL, implying extensive metabolism of 5-hydroxythalidomide in humanized liver mice.
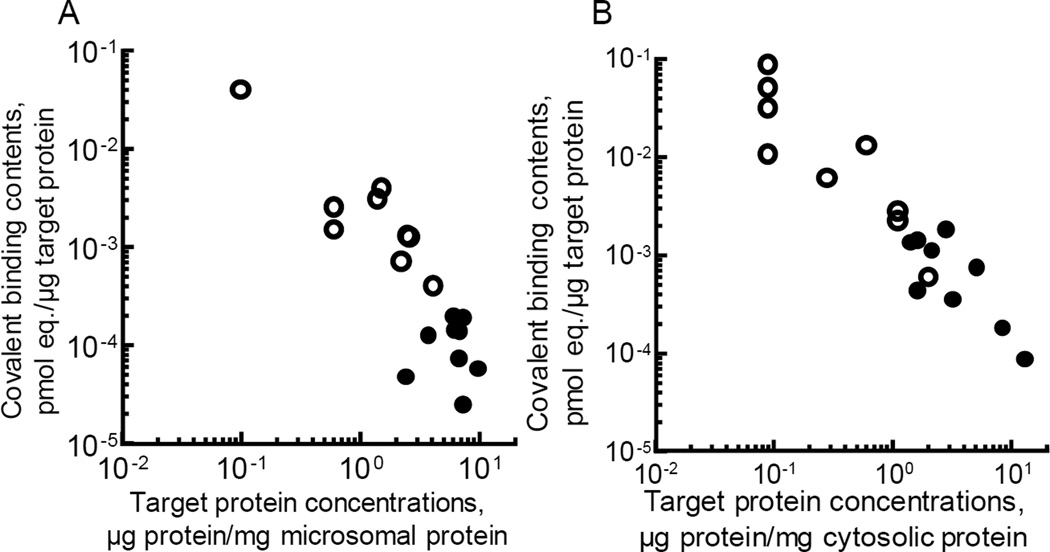
Profiles of liver microsomal and cytosolic fractions were investigated by two-dimensional electrophoresis in livers of mice containing humanized CYP3A gene or with transplanted humanized liver 1 h after the administration of radiolabeled 5-hydroxythalidomide. Protein samples (100 µg) were separated by isoelectric point (pH 3–10) and molecular weight (10–225 kDa); two-dimensional electrophoresis (20 cm × 20 cm) revealed a variety of liver microsomal (Supporting Information Figure S2) and cytosolic (Supporting Information Figure S3) proteins. The most abundant protein spots (in two-dimensional gels from livers of mice with humanized CYP3A genes and with transplanted humanized liver) were selected and analyzed for radioactivity incorporated from 5-hydroxythalidomide. Consequently, nine target protein spots related to 5-hydroxythalidomide exposure were chosen for both microsomal and cytosolic protein (Figure 1). Target protein contents and covalent binding contents of these analyte spots in the gels are summarized in Table S1 (Supporting Information). The apparently highest covalent binding content (in terms of pmol equivalent/µg target protein) was seen in liver microsomal cytochrome c oxidase subunit 6B1 (NCBI accession number gi:3023546) and ATP synthase subunit α(gi:416677) in mice with humanized CYP3A-HAC and with humanized liver mice (Table S1), respectively. On the other hand, retinal dehydrogenase 1 (gi:194375548) and GSH transferase (GST) A1 (gi:121730) showed the highest covalent binding content (in terms of pmol equivalent/µg target protein) in the liver cytosol fractions in mice with humanized CYP3A-HAC and with humanized liver mice, respectively, after two-dimensional electrophoresis and accelerator mass spectrometry (Table S2, Supporting Information). It is possible that every spot in the 2D-gels (20 cm × 20 cm) could potentially contain multiple proteins but that only the major one might have been detected under the current protein analysis system. Two-dimensional electrophoresis of pooled fetal homogenate samples (11.5 days), with one-tenth radioactivity of liver samples, was also carried out (Supporting Information Figure S4). However, the database software for peptide identification did not have sufficient mouse fetal protein information, and further analysis for protein identification and/or radioactivity determination was not possible. Rough linearity between covalent binding content and proposed target protein concentration was seen in the case of 5-hydroxythalidomide (Figure 1). The estimated covalent binding levels (pmol drug equivalent/µg target protein) indicate an inverse relationship with target protein concentration under the present conditions.
Figure 1.
In vivo protein binding profiles of liver microsomal (A) and cytosolic (B) fractions derived from metabolically activated 5-hydroxy-[14C]-thalidomide in mice with humanized CYP3A-HAC (open circles) and with humanized liver (closed circles) analyzed by two-dimensional electrophoresis and accelerator mass spectrometry.
The mechanism of action and teratogenicity of thalidomide still remains unclear, but a ubiquitously expressed ligase protein, cereblon, has been reportedly identified as a primary teratogenic target of thalidomide, lenalidomide, and pomalidomide, using modified chemical probes located at an aromatic position, in conjunction with ferric beads.12,13 Unlike some of the binding proteins for radiolabeled troglitazone and flutamide (reported previously),9,10 few proteins with high covalent binding content and high target protein concentration were observed (Figure 1). Under the electrophoretic conditions used here, cereblon would be expected to be found in an area defined by an isoelectric point of pH 4.5–5.5 and a molecular weight of 48–60 kDa (UniProt accession numbers, Q96SW2 and Q8C7D2); however, there were no large protein spots in this study. Selenium binding protein 1 (56 kDa, acetaminophen binding protein, gi: 16306550), suggested in previous studies with troglitazone and 5-n-butyl-pyrazolo[1,5-a]pyrimidine,9,10 was not detected in the case of 5-hydroxythalidomide. In the future, immunoprecipitation experiments with specific antibodies against cereblon or selenium binding protein 1 might be helpful in understanding the roles of these proteins in trapping activated 5-hydroxythalidomide or its further metabolites. Although GST A1 has been ranked as a minor target binding protein with trogltazone,9,10 GST A1 appeared to be one of more important enzymes trapping the activated 5-hydroxythalidomide in both humanized mouse models (Table S2), perhaps because of its abundance or its ability to bind hydrophobic chemicals (originally defined as a “ligandin”14). Another proteomics analysis has reported on thalidomide embryotoxicity using differentiation of human embryonic stem cells, and one novel possible mechanism involved inhibition of GST A1.15 Some of target liver proteins detected in this study—cytochrome c oxidase subunit 6B1, cytochrome b-c1 complex subunit 7, and ATP synthase subunit α (Table S1)—have been reported to be involved in protein-protein interaction networking16,17 and associated with Leigh syndrome caused by aspirin use, but only in children.18 Cytochrome c oxidase subunit 6A1 has been associated with Charcot-Marie-Tooth neuropathy disease, which causes nerve damage (mostly in the arms and legs).19
Apparently different target proteins for 5-hydroxythalidomide were seen in liver microsomal and cytosolic fractions in both female and male mice with humanized CYP3A-HAC and humanized liver in the present study. Intravenously administered 5-hydroxythalidomide was further bioactivated by human P450 3A enzymes in vivo and trapped with nonspecific proteins in humanized mouse models with P450 3A or hepatocytes. Aromatic hydroxylation of 5-hydroxythalidomide occurred in humanized mice models one hour after i.v. administration: however, this reactive secondary metabolite(s) is nonspecifically trapped. In the case of future treatments with GSH- depleting agents, abundant protein adducts might be analyzed with potential importance of the secondary metabolism of 5-hydroxythalidomide in thalidomide toxicity in pregnant mice. Thalidomide could exert its variety of pharmacological and toxic actions through multiple mechanisms including non-specific modification of many protein networks after bioactivation by human P450 enzymes.
Supplementary Material
Acknowledgments
Funding Sources
This work was supported in part by the Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research 26460206 (H.Y.), the JCIA LRI program, the MEXT (Ministry of Education, Science, Sports, and Culture of Japan)-Supported Program for the Strategic Research Foundation at Private Universities, 2013–2018 and National Institutes of Health grants R37 CA090426 and R01 GM118122 (F.P.G.).
The authors thank Drs. Takao Matsui, Norie Murayama, and Shotaro Uehara for their technical assistance.
ABBREVIATIONS
- CYP
P450 gene
- GST
GSH transferase
- HAC
human artificial chromosome
- HSVtk
herpes simplex virus type 1 thymidine kinase
- NOG mice
non-obese diabetes- severe combined immunodeficiency- interleukin-2 receptor gamma chain-deficient mice
Footnotes
ASSOCIATED CONTENT
Supporting Information. Experimental Procedures, Table S1 (microsomal proteins bound with radiolabeled 5-hydroxythalidomide in increasing order of spot ID numbers), Table S2 (cytosolic proteins bound with radiolabeled 5-hydroxythalidomide in increasing order of spot ID numbers), Figure S1 (chemical structure of 5-hydroxy-[carbonyl-14C]thalidomide), Figure S2 (two-dimensional electrophoretic profiles of liver microsomal protein fractions obtained from mice with humanized CYP3A-HAC and with humanized liver 1 h after intravenous administration of radiolabeled 5-hydroxythalidomide), Figure S3 (two-dimensional electrophoretic profiles of liver cytosolic protein fractions obtained from mice with humanized CYP3A-HAC (A) and with humanized liver 1 h after intravenous administration of radiolabeled 5-hydroxythalidomide), Figure S4 (two-dimensional electrophoresis of pooled fetal homogenates obtained from mice with humanized CYP3A-HAC, treated intravenously with 5-hydroxythalidomide). This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Gordon GB, Spielberg SP, Blake DA, Balasubramanian V. Thalidomide teratogenesis: evidence for a toxic arene oxide metabolite. Proc. Natl. Acad. Sci. U. S. A. 1981;78:2545–2548. doi: 10.1073/pnas.78.4.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kazuki Y, Kobayashi K, Aueviriyavit S, Oshima T, Kuroiwa Y, Tsukazaki Y, Senda N, Kawakami H, Ohtsuki S, Abe S, Takiguchi M, Hoshiya H, Kajitani N, Takehara S, Kubo K, Terasaki T, Chiba K, Tomizuka K, Oshimura M. Trans-chromosomic mice containing a human CYP3A cluster for prediction of xenobiotic metabolism in humans. Hum. Mol. Genet. 2013;22:578–592. doi: 10.1093/hmg/dds468. [DOI] [PubMed] [Google Scholar]
- 3.Kazuki Y, Akita M, Kobayashi K, Osaki M, Satoh D, Abe S, Takehara S, Kazuki K, Yamazaki H, Kamataki T, Oshimura M. Thalidomide-induced limb abnormalities in a humanized CYP3A mouse model. Sci. Rep. 2016;6:21419. doi: 10.1038/srep21419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamazaki H, Suemizu H, Igaya S, Shimizu M, Shibata M, Nakamura M, Chowdhury G, Guengerich FP. In vivo formation of a glutathione conjugate derived from thalidomide in humanized uPA-NOG mice. Chem. Res. Toxicol. 2011;24:287–289. doi: 10.1021/tx200005g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamazaki H, Suemizu H, Shimizu M, Igaya S, Shibata N, Nakamura N, Chowdhury G, Guengerich FP. In vivo formation of dihydroxylated and glutathione conjugate metabolites derived from thalidomide and 5-hydroxythalidomide in humanized TK-NOG mice. Chem. Res. Toxicol. 2012;25:274–276. doi: 10.1021/tx300009j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chowdhury G, Shibata N, Yamazaki H, Guengerich FP. Human cytochrome P450 oxidation of 5-hydroxythalidomide and pomalidomide, an amino analog of thalidomide. Chem. Res. Toxicol. 2014;27:147–156. doi: 10.1021/tx4004215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chowdhury G, Murayama N, Okada Y, Uno Y, Shimizu M, Shibata N, Guengerich FP, Yamazaki H. Human liver microsomal cytochrome P450 3A enzymes involved in thalidomide 5-hydroxylation and formation of a glutathione conjugate. Chem. Res. Toxicol. 2010;23:1018–1024. doi: 10.1021/tx900367p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishiyama S, Suemizu H, Shibata N, Guengerich FP, Yamazaki H. Simulation of human plasma concentrations of thalidomide and primary 5-hydroxylated metabolites explored with pharmacokinetic data in humanized TK-NOG mice. Chem. Res. Toxicol. 2015;28:2088–2090. doi: 10.1021/acs.chemrestox.5b00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamazaki H, Kuribayashi S, Inoue T, Tateno C, Nishikura Y, Oofusa K, Harada D, Naito S, Horie T, Ohta S. Approach for in vivo protein bindings of 5-n-butyl-pyrazolo[1,5-a]pyrimidine bioactivated in chimeric mice with humanized liver by two-dimensional electrophoresis with accelerator mass spectrometry. Chem. Res. Toxicol. 2010;23:152–158. doi: 10.1021/tx900323a. [DOI] [PubMed] [Google Scholar]
- 10.Yamazaki H, Kuribayashi S, Inoue T, Honda T, Tateno C, Oofusa K, Ninomiya S, Ikeda T, Izumi T, Horie T. Zone analysis by two-dimensional electrophoresis with accelerator mass spectrometry of in vivo protein bindings of idiosyncratic hepatotoxicants troglitazone and flutamide bioactivated in chimeric mice with humanized liver. Toxicol. Res. 2015;4:106–111. [Google Scholar]
- 11.Hasegawa M, Kawai K, Mitsui T, Taniguchi K, Monnai M, Wakui M, Ito M, Suematsu M, Peltz G, Nakamura M, Suemizu H. The reconstituted 'humanized liver' in TK-NOG mice is mature and functional. Biochem. Biophys. Res. Commun. 2011;405:405–410. doi: 10.1016/j.bbrc.2011.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y, Yamaguchi Y, Handa H. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327:1345–1350. doi: 10.1126/science.1177319. [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Girona A, Mendy D, Ito T, Miller K, Gandhi AK, Kang J, Karasawa S, Carmel G, Jackson P, Abbasian M, Mahmoudi A, Cathers B, Rychak E, Gaidarova S, Chen R, Schafer PH, Handa H, Daniel TO, Evans JF, Chopra R. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia. 2012;26:2326–2335. doi: 10.1038/leu.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Habig WH, Pabst MJ, Fleischner G, Gatmaitan Z, Arias IM, Jakoby WB. The identity of glutathione S-transferase B with ligandin, a major binding protein of liver. Proc. Natl. Acad. Sci. U. S. A. 1974;71:3879–3882. doi: 10.1073/pnas.71.10.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meganathan K, Jagtap S, Wagh V, Winkler J, Gaspar JA, Hildebrand D, Trusch M, Lehmann K, Hescheler J, Schluter H, Sachinidis A. Identification of thalidomide-specific transcriptomics and proteomics signatures during differentiation of human embryonic stem cells. PLoS One. 2012;7:e44228. doi: 10.1371/journal.pone.0044228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The UniProt Consortium. UniProt: a hub for protein information. Nucleic Acids Res. 2015;43:D204–D212. doi: 10.1093/nar/gku989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von MC. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Massa V, Fernandez-Vizarra E, Alshahwan S, Bakhsh E, Goffrini P, Ferrero I, Mereghetti P, D'Adamo P, Gasparini P, Zeviani M. Severe infantile encephalomyopathy caused by a mutation in COX6B1, a nucleus-encoded subunit of cytochrome c oxidase. Am. J. Hum. Genet. 2008;82:1281–1289. doi: 10.1016/j.ajhg.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamiya G, Makino S, Hayashi M, Abe A, Numakura C, Ueki M, Tanaka A, Ito C, Toshimori K, Ogawa N, Terashima T, Maegawa H, Yanagisawa D, Tooyama I, Tada M, Onodera O, Hayasaka K. A mutation of COX6A1 causes a recessive axonal or mixed form of Charcot-Marie-Tooth disease. Am. J Hum. Genet. 2014;95:294–300. doi: 10.1016/j.ajhg.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.