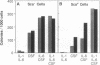Abstract
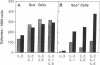
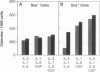
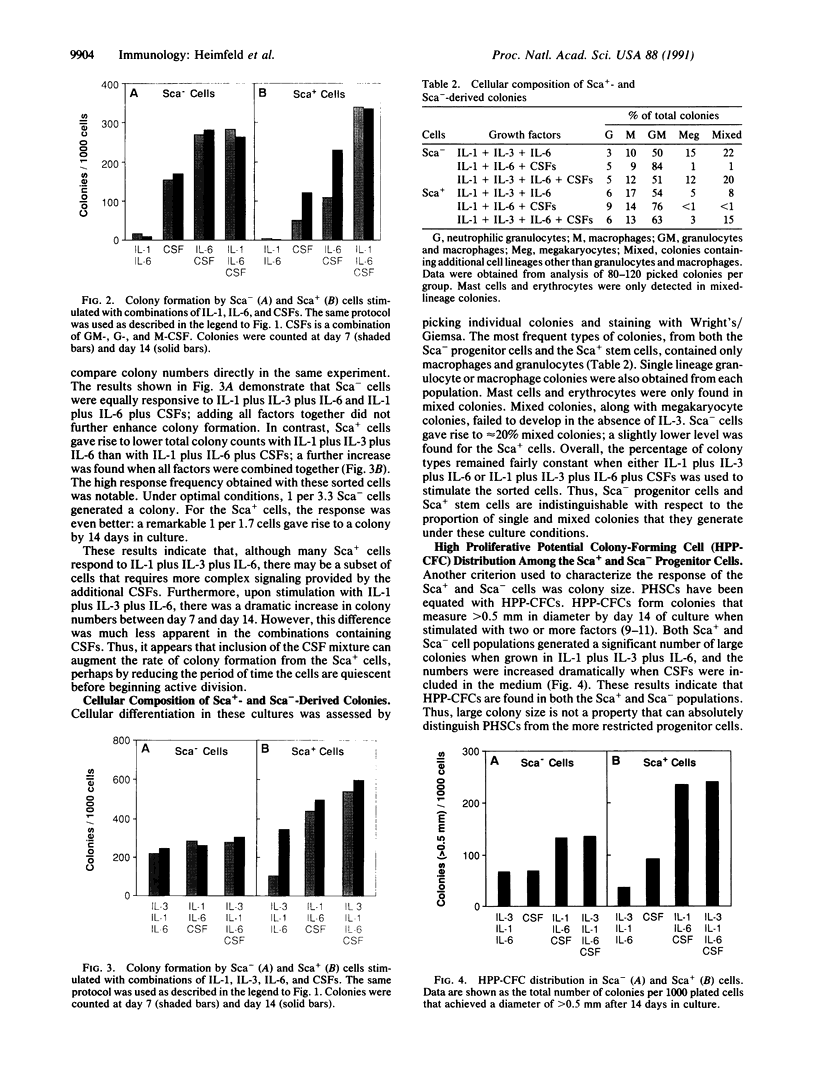
Pluripotential stem cells (Thylo Lin- Sca+; referred to as Sca+) and primitive myeloerythroid progenitor cells (Thylo Lin- Sca-; referred to as Sca-), defined by their in vivo repopulating properties, have been purified from mouse bone marrow. In this study, the growth factor requirements of these two subsets were compared in colony-forming assays. Sca- progenitor cells grew well in interleukin (IL) 3 alone and showed maximum growth when two factors, IL-3 plus IL-1 or IL-3 plus IL-6, were combined. In contrast, Sca+ stem cells were generally not responsive to any single factor tested. Some colony formation was found when IL-3 was paired with either IL-1 or IL-6, and this was significantly enhanced as additional factors were included. A remarkable frequency of as much as 1 colony per 1.7 input Sca+ cells was achieved when IL-1, IL-3, IL-6, and colony-stimulating factors were used together. These differences in factor requirements presumably reflect the need for multiple factor signaling in the more primitive stem cell population. In most other aspects of colony formation, Sca+ and Sca- cells were very similar. They generated colonies that had equivalent distributions in size and cellular composition. One notable difference was found in the kinetics of their response. Whereas nearly all Sca- cells formed colonies within 7 days, a significant fraction of Sca+ cells delayed colony formation for greater than 1 week. During this quiescent period, cell survival was absolutely dependent on the presence of factors in the medium.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartelmez S. H., Bradley T. R., Bertoncello I., Mochizuki D. Y., Tushinski R. J., Stanley E. R., Hapel A. J., Young I. G., Kriegler A. B., Hodgson G. S. Interleukin 1 plus interleukin 3 plus colony-stimulating factor 1 are essential for clonal proliferation of primitive myeloid bone marrow cells. Exp Hematol. 1989 Mar;17(3):240–245. [PubMed] [Google Scholar]
- Bertoncello I., Hodgson G. S., Bradley T. R. Multiparameter analysis of transplantable hemopoietic stem cells. II. Stem cells of long-term bone marrow-reconstituted recipients. Exp Hematol. 1988 May;16(4):245–249. [PubMed] [Google Scholar]
- Bertoncello I., Hodgson G. S., Bradley T. R. Multiparameter analysis of transplantable hemopoietic stem cells: I. The separation and enrichment of stem cells homing to marrow and spleen on the basis of rhodamine-123 fluorescence. Exp Hematol. 1985 Nov;13(10):999–1006. [PubMed] [Google Scholar]
- Bradley T. R., Hodgson G. S. Detection of primitive macrophage progenitor cells in mouse bone marrow. Blood. 1979 Dec;54(6):1446–1450. [PubMed] [Google Scholar]
- Chiu C. P., Moulds C., Coffman R. L., Rennick D., Lee F. Multiple biological activities are expressed by a mouse interleukin 6 cDNA clone isolated from bone marrow stromal cells. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7099–7103. doi: 10.1073/pnas.85.19.7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter T. M., Heyworth C. M., Spooncer E., Ponting I. L. The role of growth factors in self-renewal and differentiation of haemopoietic stem cells. Philos Trans R Soc Lond B Biol Sci. 1990 Mar 12;327(1239):85–98. doi: 10.1098/rstb.1990.0045. [DOI] [PubMed] [Google Scholar]
- Ikebuchi K., Clark S. C., Ihle J. N., Souza L. M., Ogawa M. Granulocyte colony-stimulating factor enhances interleukin 3-dependent proliferation of multipotential hemopoietic progenitors. Proc Natl Acad Sci U S A. 1988 May;85(10):3445–3449. doi: 10.1073/pnas.85.10.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikebuchi K., Wong G. G., Clark S. C., Ihle J. N., Hirai Y., Ogawa M. Interleukin 6 enhancement of interleukin 3-dependent proliferation of multipotential hemopoietic progenitors. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9035–9039. doi: 10.1073/pnas.84.24.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iscove N. N., Shaw A. R., Keller G. Net increase of pluripotential hematopoietic precursors in suspension culture in response to IL-1 and IL-3. J Immunol. 1989 Apr 1;142(7):2332–2337. [PubMed] [Google Scholar]
- Korn A. P., Henkelman R. M., Ottensmeyer F. P., Till J. E. Investigations of a stochastic model of haemopoiesis. Exp Hematol. 1973;1(6):362–375. [PubMed] [Google Scholar]
- Leary A. G., Ikebuchi K., Hirai Y., Wong G. G., Yang Y. C., Clark S. C., Ogawa M. Synergism between interleukin-6 and interleukin-3 in supporting proliferation of human hematopoietic stem cells: comparison with interleukin-1 alpha. Blood. 1988 Jun;71(6):1759–1763. [PubMed] [Google Scholar]
- McNiece I. K., Bradley T. R., Kriegler A. B., Hodgson G. S. Subpopulations of mouse bone marrow high-proliferative-potential colony-forming cells. Exp Hematol. 1986 Oct;14(9):856–860. [PubMed] [Google Scholar]
- McNiece I. K., Stewart F. M., Deacon D. M., Quesenberry P. J. Synergistic interactions between hematopoietic growth factors as detected by in vitro mouse bone marrow colony formation. Exp Hematol. 1988 Jun;16(5):383–388. [PubMed] [Google Scholar]
- Mulder A. H., Visser J. W. Separation and functional analysis of bone marrow cells separated by rhodamine-123 fluorescence. Exp Hematol. 1987 Jan;15(1):99–104. [PubMed] [Google Scholar]
- Muller-Sieburg C. E., Whitlock C. A., Weissman I. L. Isolation of two early B lymphocyte progenitors from mouse marrow: a committed pre-pre-B cell and a clonogenic Thy-1-lo hematopoietic stem cell. Cell. 1986 Feb 28;44(4):653–662. doi: 10.1016/0092-8674(86)90274-6. [DOI] [PubMed] [Google Scholar]
- Nakahata T., Gross A. J., Ogawa M. A stochastic model of self-renewal and commitment to differentiation of the primitive hemopoietic stem cells in culture. J Cell Physiol. 1982 Dec;113(3):455–458. doi: 10.1002/jcp.1041130314. [DOI] [PubMed] [Google Scholar]
- Ploemacher R. E., Brons N. H. Cells with marrow and spleen repopulating ability and forming spleen colonies on day 16, 12, and 8 are sequentially ordered on the basis of increasing rhodamine 123 retention. J Cell Physiol. 1988 Sep;136(3):531–536. doi: 10.1002/jcp.1041360320. [DOI] [PubMed] [Google Scholar]
- Rennick D., Yang G., Gemmell L., Lee F. Control of hemopoiesis by a bone marrow stromal cell clone: lipopolysaccharide- and interleukin-1-inducible production of colony-stimulating factors. Blood. 1987 Feb;69(2):682–691. [PubMed] [Google Scholar]
- Smith L. G., Weissman I. L., Heimfeld S. Clonal analysis of hematopoietic stem-cell differentiation in vivo. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2788–2792. doi: 10.1073/pnas.88.7.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangrude G. J., Heimfeld S., Weissman I. L. Purification and characterization of mouse hematopoietic stem cells. Science. 1988 Jul 1;241(4861):58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Spangrude G. J., Johnson G. R. Resting and activated subsets of mouse multipotent hematopoietic stem cells. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7433–7437. doi: 10.1073/pnas.87.19.7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. R., Bartocci A., Patinkin D., Rosendaal M., Bradley T. R. Regulation of very primitive, multipotent, hemopoietic cells by hemopoietin-1. Cell. 1986 Jun 6;45(5):667–674. doi: 10.1016/0092-8674(86)90781-6. [DOI] [PubMed] [Google Scholar]
- Till J. E., McCulloch E. A. Hemopoietic stem cell differentiation. Biochim Biophys Acta. 1980 Nov 26;605(4):431–459. doi: 10.1016/0304-419x(80)90009-8. [DOI] [PubMed] [Google Scholar]
- Weilbaecher K., Weissman I., Blume K., Heimfeld S. Culture of phenotypically defined hematopoietic stem cells and other progenitors at limiting dilution on Dexter monolayers. Blood. 1991 Aug 15;78(4):945–952. [PubMed] [Google Scholar]
- Zhou Y. Q., Stanley E. R., Clark S. C., Hatzfeld J. A., Levesque J. P., Federici C., Watt S. M., Hatzfeld A. Interleukin-3 and interleukin-1 alpha allow earlier bone marrow progenitors to respond to human colony-stimulating factor 1. Blood. 1988 Dec;72(6):1870–1874. [PubMed] [Google Scholar]
- Zsebo K. M., Williams D. A., Geissler E. N., Broudy V. C., Martin F. H., Atkins H. L., Hsu R. Y., Birkett N. C., Okino K. H., Murdock D. C. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990 Oct 5;63(1):213–224. doi: 10.1016/0092-8674(90)90302-u. [DOI] [PubMed] [Google Scholar]