Abstract
Objective
To determine the risk of gestational diabetes (GDM) and insulin resistance (IR) in obesity defined by body mass index (BMI), waist-to-hip ratio (WHR) or both combined.
Methods
Secondary analysis of a randomized multicenter trial of antioxidant supplementation versus placebo in nulliparous low-risk women to prevent pregnancy associated hypertension. Women between 9–16 weeks with data for WHR and BMI were analyzed for GDM (n=2300). Those with fasting glucose and insulin between 22–26 weeks (n=717) were analyzed for IR by homeostasis model assessment of insulin resistance (HOMA-IR; normal≤75thpercentile). WHR and BMI were categorized as normal (WHR<0.80; BMI<25kg/m2); overweight (WHR:0.8–0.84; BMI:25–29.9kg/m2); and obese (WHR≥0.85; BMI≥30kg/m2). ROC curves and logistic regression models were used.
Results
Compared with normal, the risks of GDM or IR were higher in obese by BMI or WHR. The subgroup with obesity by WHR but not by BMI had no increased risk of GDM. BMI was a better predictor of IR (AUC-0.71(BMI), 0.65(WHR), p=0.03) but similar to WHR for GDM (AUC-0.68(BMI), 0.63(WHR), p=0.18.
Conclusion
Increased WHR and BMI in early pregnancy are associated with IR and GDM. BMI is a better predictor of IR compared with WHR. Adding WHR to BMI does not improve its ability to detect GDM or IR.
Trial Registration number
Keywords: Maternal BMI, Waist-to-Hip ratio, gestational diabetes, Insulin resistance
Introduction
Obesity is a major epidemic in the United States, affecting almost one-third of all individuals, and its incidence during pregnancy has doubled over the past 2 decades1. Various indices have been developed to characterize obesity and assess its association with adverse health outcomes. The most commonly studied index, body mass index (BMI) [body weight (kilograms)/height2 (meter2)] is a measure of total body fat. An elevated BMI has been associated with increased risk of coronary artery disease, hypertension and type II diabetes in the general population2. However, BMI values may have different connotations in individuals with diverse ethnic background, short/tall stature, or varied muscle mass, and does not reflect the regional distribution of fat in the body, i.e. subcutaneous versus visceral/central2. Such differentiation is important, as subcutaneous fat has fundamentally different metabolic properties than visceral fat.
Visceral fat secretes inflammatory and thrombogenic factors, and inhibits adiponectin production, thus leading to impaired glucose metabolism, diabetes mellitus, hypertension, metabolic syndrome and other cardiovascular diseases3. Therefore, measures of central/abdominal obesity such as waist circumference (WC) and waist to hip ratio (WHR) have been compared to BMI for their association with adverse cardiovascular and metabolic consequences. Larger WC is predictive of type 2 diabetes, dyslipidemia, hypertension and coronary artery disease4. In non-pregnant females, WHR of >0.85 had a stronger association with type 2 diabetes compared with WC or BMI5.
During pregnancy, high BMI has been associated with adverse maternal and neonatal outcomes, and is a known risk factor for gestational diabetes6, 7 and insulin resistance8. However, the association between WHR and gestational diabetes and insulin resistance has not been systematically evaluated. The correlation amongst these obesity indices is varied (0.88, 0.34, and 0.44 for BMI-WC, BMI-WHR, WC-WHR, respectively)9, which suggests that they may provide different information and thus may not be interchangeable. Accordingly, our objective was to determine the risk of GDM and IR in obesity defined by BMI, WHR or both combined and to test the strength of association of WHR versus BMI with subsequent development of GDM or IR.
Materials and Methods
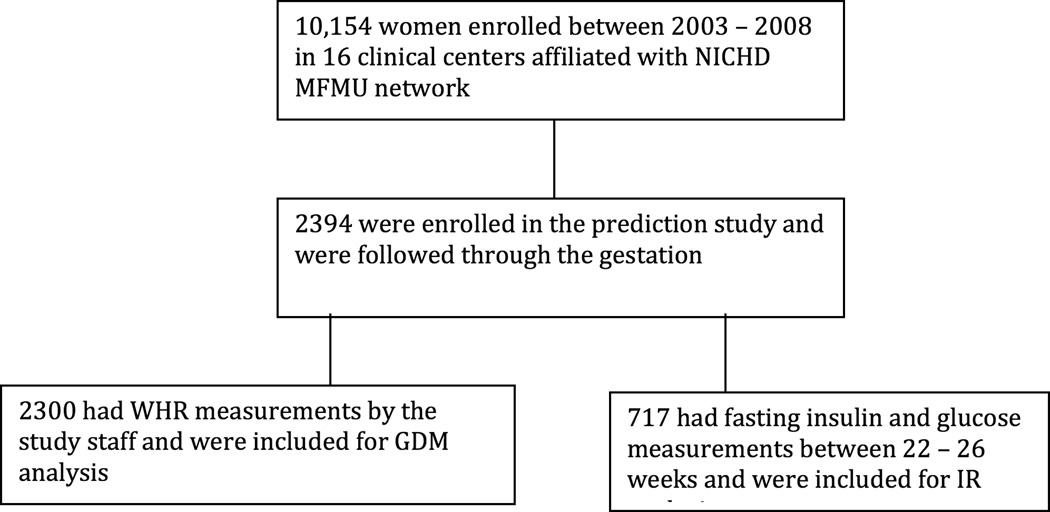
This is a secondary analysis of a randomized multicenter trial of Vitamin C and E supplementation for prevention of pregnancy associated hypertension. The eligibility criteria for enrollment in the trial were gestational age 9 – 16 weeks with singleton pregnancy in nulliparous women with no history of pre-gestational hypertension, proteinuria, diabetes or other medical problems, substance abuse, fetal abnormalities, uterine bleeding or in-vitro fertilization. A total of 10,154 women were enrolled from 16 clinical centers affiliated with Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal Fetal Medicine Units (MFMU) Network between 2003 and 2008. Of all the patients enrolled in the randomized control trial, 2394 women participated in the “prediction study”, and were followed through the pregnancy. A prospective cohort study had been designed to complement the randomized controlled trial and a sub-population of subjects participated in this “prediction study”. These women were nulliparous, underwent original randomization between 9–12 weeks and had additional procedures such as blood testing and uterine artery doppler. This was designed to test for various biochemical and biophysical markers for their ability to predict preeclampsia in these women. Of these, 2300 had data for WHR, BMI at enrollment and were analyzed for development of GDM. A subgroup of women who had fasting insulin and glucose levels drawn between 22 – 26 weeks (n=717) were analyzed for development of insulin resistance. Outline of study participants analyzed is shown in figure 1. There was no statistical difference between vitamin treatment groups for development of GDM or IR, so the groups were combined. Full details of the study design and technique of data collection have been described previously10. The Institutional Review Boards of each clinical site and the data-coordinating center approved the study.
Figure 1.
Study participants analyzed for Gestational Diabetes Mellitus and Insulin Resistance
The waist and hip measurements were standardized and the study personnel were trained to perform the measurements while patient was standing erect, at a horizontal plane, at the level of umbilicus at the end of normal expiration for waist and at the site of maximum extension of the buttocks for hip measurement.
Gestational diabetes was diagnosed at 26 weeks, per the guidelines of each clinical center. Insulin resistance was estimated by using the previously validated surrogate marker of Homeostatic model assessment of insulin resistance (HOMA-IR)11 as defined below.
The measurements were categorized as normal range (WHR <0.80, BMI <25 kg/m2); overweight (WHR 0.80 – 0.84, BMI 25 – 29.9 kg/m2); and obese (WHR ≥0.85, BMI≥30 kg/m2) based on the WHO criteria2. Since HOMA-IR values were not normally distributed, we used percentiles to define elevated HOMA-IR, which was dichotomized into normal (≤75th percentile) versus abnormal (>75 percentile).
The primary outcome of the study was the risk of GDM and insulin resistance in patients classified as normal, overweight and obese by either WHR criteria, BMI criteria or both combined. The secondary outcome was the association of early pregnancy WHR versus BMI with subsequent GDM and insulin resistance.
The Wilcoxon rank sum test was used to analyze continuous variables and chi-square test was used for categorical variables. Receiver operating characteristics curves and logistic regression models adjusting for maternal demographics including maternal age, education, race, weeks of gestation at enrollment, alcohol and smoking status, were used to evaluate the association of WHR and BMI with GDM and HOMA-IR. Statistical analysis was conducted with SAS software (SAS Institute, Cary, NC). A nominal two-sided P value less than 0.05 was considered to indicate statistical significance and no adjustments were made for multiple comparisons.
Results
Baseline maternal demographic characteristics for the 2300 women are described in Table 1. The demographics of the subset of 717 patients were analyzed and were similar to the rest of the participants in terms of pre-pregnancy weight and BMI at enrollment and smoking status (data not shown).
Table 1.
Demographic and baseline characteristics
| Waist hip ratio ≥ 0.80 |
Waist hip ratio < 0.80 |
P-value | BMI ≥ 25 | BMI < 25 | P-value | |
|---|---|---|---|---|---|---|
| N=1656 | N=644 | N=1104 | N=1196 | |||
| Maternal age (years) | 23.3 ± 4.7 | 23.4 ± 4.9 | 0.99 | 23.2 ± 4.7 | 23.5 ± 4.8 | 0.08 |
| Gestational age at enrollment (weeks) |
11.4 ± 1.1 | 11.4 ± 1.1 | 0.21 | 11.3 ± 1.1 | 11.5 ± 1.0 | 0.003 |
| Race/Ethnicity | ||||||
| Non-Hispanic white | 754 (45.5) | 354 (55.0) | <0.001 | 286 (25.9) | 644 (53.9) | <0.001 |
| African American | 340 (20.5) | 215 (33.3) | 337 (30.5) | 218 (18.2) | ||
| Hispanic | 529 (31.9) | 63 (9.8) | 464 (42.0) | 306 (25.6) | ||
| Other | 33 (2.0) | 12 (1.9) | 17 (1.5) | 28 (2.3) | ||
| Smoking | 287(17.3) | 92 (14.3) | 0.08 | 204 (18.5) | 175 (14.6) | 0.01 |
| Education >=12 years |
1218 (73.6) | 549 (85.3) | <0.001 | 834 (75.5) | 933 (78.0) | 0.16 |
Data are presented as mean ± standard deviation or n (%).
3.5% (80/2300) of the participants developed GDM and 25% (179/717) developed insulin resistance during pregnancy. Mean WHR and BMI of the patients who developed GDM in the study were significantly higher than those who did not develop GDM (0.88 +/− 0.07 versus 0.84 +/− 0.08 for WHR, p<0.0001 and 30.85 +/− 8.3 versus 26.06 +/− 6.12 Kg/m2 for BMI, p<0.0001, respectively). Mean WHR and BMI were also significantly higher for those who developed IR compared with normal (0.86 ± 0.08 versus 0.83 ± 0.07, p=0.0001 for WHR and 30.33 ± 8.15 versus 24.94 ± 5.19, p<0.0001 for BMI, respectively.
Women who were overweight or obese by BMI definition (BMI≥25) regardless of their WHR had higher odds of GDM and IR compared to normals (Table 2). Those who were obese by BMI ≥30 had adjusted odd ratios of 4.75 (95% CI 2.65–8.52) and 5.9 (95% CI 3.79–9.19) for GDM and IR, respectively. Those who were obese by WHR regardless of their BMI had increased odds of GDM and IR, (aOR 2.65, 95% CI 1.34–5.25) and (aOR 2.63, 95% CI 1.68–4.13), respectively. However, overweight by WHR was not significantly different from those with normal by WHR for either GDM or IR (Table 2).
Table 2.
Incidence and adjusted odds ratio for development of gestational diabetes mellitus and insulin resistance in normal, overweight and obese women defined by WHR versus BMI
| Gestational diabetes mellitus | Insulin resistance | |||||||
|---|---|---|---|---|---|---|---|---|
| WHR | BMI | WHR | BMI | |||||
| N/Total (%) |
aOR (95% CI) |
N/Total (%) |
aOR (95% CI) |
N/Total (%) |
aOR (95% CI) |
N/Total (%) |
aOR (95% CI) |
|
| Normal | 11/644 (1.7) |
1.0 | 19/1196 (1.6) |
1.0 | 36/229 (15.7) |
1.0 | 51/382 (13.4) |
1.0 |
|
Over- weight |
17/591 (2.9) |
1.64 (0.76,3.54) |
26/601 (4.3) |
2.72 (1.49, 4.98) |
38/205 (18.5) |
1.14 (0.69, 1.91) |
50/172 (29.1) |
2.57 (1.63, 4.05) |
|
Obese |
52/1065 (4.9) |
2.65 (1.34, 5.25) |
35/503 (7.0) |
4.75 (2.65, 8.52) |
105/283 (37.1) |
2.63 (1.68, 4.12) |
78/163 (47.9) |
5.9 (3.79, 9.19) |
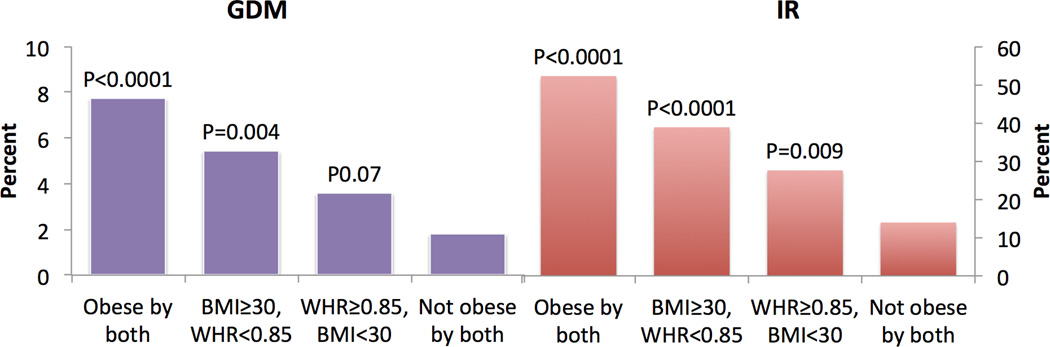
Additional regression analyses were done where BMI and WHR were combined, to ascertain their significance when either one or both were abnormal (BMI≥30 and or WHR≥0.85) compared to when both were in non-obese range (BMI<30 and WHR<0.85) in the study population. In this analysis, the aOR in those who were obese by both criteria compared to non-obese by both was 4.28 (95% CI 2.28–8.02) for GDM and 6.03 (95% CI 3.70–9.83) for IR respectively. Rates of GDM and IR were also highest in the group that was obese by both criteria (Figure 2).
Figure 2.
Percentage with GDM and IR by obesity category when BMI and WHR were combined. P-values presented pertain to adjusted odds ratios for GDM and IR from multivariable logistic regression analysis. The obesity category is compared with the reference category of non-obese (WHR<0.85 and BMI <30).
Those patients who had obesity by BMI only and had non-obese range WHR also had higher aOR of GDM in comparison with non obese by both criteria, (aOR 3.38, 1.48–7.70). Conversely, those who had obesity by WHR only but with non-obese range BMI were not significantly different than those who were non-obese by both criteria, (aOR 1.79, 0.96–3.31). For IR, obesity by WHR, or BMI, or both showed a significant increase in aOR compared to non-obese by both (Figure 2).
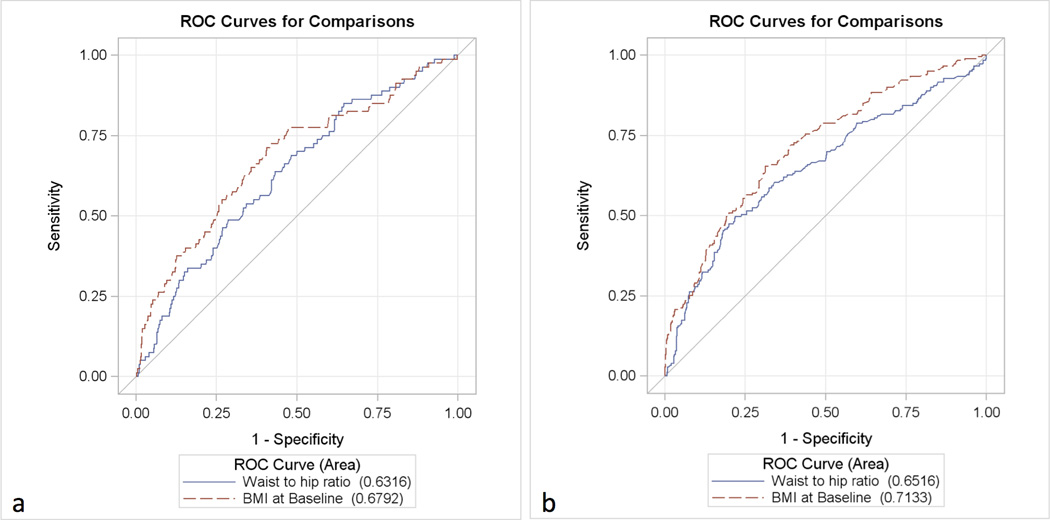
ROC curves analysis showed that BMI and WHR were not significantly different in prediction of GDM (AUC 0.68 and 0.63 for BMI and WHR respectively, p=0.18) (Figure 3-a). However, BMI was a stronger predictor of an abnormal HOMA-IR (AUC 0.71 and 0.65 for BMI and WHR respectively p=0.03) (Figure 3-b).
Figure 3.
Receiver operating characteristics curves showing the association of WHR versus BMI with GDM (a) and HOMA-IR (b).
We also performed an ethnicity-stratified analysis. GDM developed in 7.8% of Hispanics, 5.1% of African Americans and 8.7% of whites with BMI≥30 compared with 2.6%, 0.9% and 1.2% of Hispanics, African Americans, and Whites with normal BMI, respectively. There were significant odds of developing GDM with BMI≥30 in Hispanics (aOR 3.1, 95% CI 1.1–8.6) and whites (aOR 7.7, 95% CI 3.2–18.4) compared with normal BMI. In African Americans, the odds of developing GDM were statistically significant only with WHR≥0.85 (aOR 5.0, 95% CI 1.03–24.3) compared with normal WHR and not BMI ≥30 compared with normal BMI. The trend of developing IR with increasing BMI was statistically significant in all races but with increasing WHR only in Whites (Table 3).
Table 3.
Obesity and development of GDM and Insulin Resistance by Ethnicity. Adjusted odds ratios (aOR) and 95% CI are presented for patients categorized as obese by WHR or BMI compared with normal WHR (<0.80) and BMI (< 25), respectively.
| Gestational diabetes mellitus | Insulin resistance | |||||||
|---|---|---|---|---|---|---|---|---|
| WHR≥0.85 | BMI≥30 | WHR≥0.85 | BMI≥30 | |||||
| N/Total (%) |
aOR (95% CI) |
N/Total (%) |
aOR (95% CI) |
N/Total (%) |
aOR (95% CI) |
N/Total (%) |
aOR (95% CI) |
|
|
Hispanic |
23/423 (5.44) |
3.22 (0.42,24.71) |
8/103 (7.77) |
3.09 (1.11,8.62) |
30/67 (44.78) |
2.22 (0.68,7.30) |
10/19 (52.63) |
3.52 (1.10, 11.31) |
|
African American |
12/212 (5.66) |
5.0 (1.03, 24.32) |
10/198 (5.05) |
4.77 (0.95,23.87) |
23/51 (45.10) |
1.90 (0.72,5.01) |
26/45 (57.78) |
6.18 (2.33, 16.45) |
|
Whites |
17/412 (4.13) |
1.82 (0.76,4.33) |
17/195 (8.72) |
7.71 (3.23, 18.37) |
49/160 (30.63) |
2.7 (1.50, 4.87) |
40/97 (41.24) |
6.04 (3.36, 10.86) |
Discussion
In this secondary analysis, we found that BMI ≥30 kg/m2 and WHR ≥0.85 during early pregnancy are significant risk factors for development of GDM and insulin resistance.
Our findings are consistent with previous studies confirming the association of BMI6, 11–14 and high WHR15 in early pregnancy with development of gestational diabetes.
When we compared these indices with each other, BMI was a stronger predictor of IR but similar to WHR for GDM. This finding implies that development of IR during pregnancy may be a function of total body fat and less dependent on the central fat distribution, which is in contrast to the various cardiovascular and metabolic consequences of the latter outside of pregnancy16, 17.
It is known that WHR has a weak correlation with BMI (r=0.4)18, 19. However we found that WHR was still comparable to BMI for its association with diabetes. Therefore, it could be hypothesized that combining the two as a collective risk assessment tool may improve their ability to predict GDM and IR. We found that those who were obese by both criteria (BMI≥30/WHR≥0.85) represented the highest risk of GDM and IR compared to non obese by both. However, overall, using both obesity measures together gave similar adjusted odds ratios to using BMI as the only measure of obesity. Thus, BMI should remain as the standard clinical guide to establish the risk of GDM and IR during pregnancy.
It is important to note that the ability of various obesity indicators to predict diabetes varies by ethnicity, likely due to differences in percentage of total body fat and body fat distribution20 – 23. In our ethnicity stratified analysis, we noted that in Hispanics and whites, BMI was associated with increased odds of developing GDM, while in African Americans, WHR was associated with GDM. This may suggest a higher role of central adiposity in development of GDM in African Americans that the total body fat alone. BMI ≥30 was associated with increased odds of developing insulin resistance in all races. In whites only, WHR≥0.85 was also associated with insulin resistance. The lack of significance in some categories may be due to small numbers and the wide confidence intervals suggest that validation in other cohorts would be desirable.
Our findings imply that being a strong predictor of GDM and IR, elevated BMI should continue to be a significant risk factor requiring early screening for effective management of pregnancy. In African Americans however, WHR can also be used as an independent risk factor for development of GDM.
Strengths of this study are that it includes data collected by trained staff prospectively on a large number of women. However, our study was limited by the lack of a more precise gestational age range when BMI and WHR were calculated. It ranged from 9 – 16 weeks, and there may be concerns that as the pregnancy progresses, these indices may be influenced by gestational weight gain in lean tissues, thus limiting their use in pregnancy. An alternative would be to use pre-pregnancy BMI or WHR as an indicator of obesity in pregnancy, but that calculation may be frequently self-reported and inaccurate. In addition, due to the waist measurement taken at the level of the umbilicus, the measurements may be confounded in those in whom the umbilicus descended below the waist along with the panniculus.
In conclusion, BMI and WHR are significant risk factors for development of gestational diabetes and insulin resistance. This association varies among different ethnicities. Combining these 2 indicators does not significantly improve the detection of GDM or IR in the general population compared to using only BMI as a measure of obesity status. Thus, WHR should not replace BMI as a predictor of GDM and IR during pregnancy
Acknowledgments
The project described was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) [HD34208, HD27869, HD40485, HD40560, HD40544, HD34116, HD40512, HD21410, HD40545, HD40500, HD27915, HD34136, HD27860, HD53118, HD53097, HD27917, and HD36801]; the National Heart, Lung, and Blood Institute; and the National Center for Research Resources [M01 RR00080, UL1 RR024153, UL1 RR024989] and its contents do not necessarily represent the official view of NICHD, NHLBI, NCRR or NIH.
The authors thank the following Network members who participated in protocol development and coordination between clinical research centers (Sabine Bousleiman, R.N.C., M.S.N. and Margaret Cotroneo, R.N.), protocol/data management and statistical analysis (Elizabeth Thom, Ph.D. and Rebecca G. Clifton, Ph.D.), and protocol development and oversight (George Saade, M.D., Gail D. Pearson, M.D., Sc.D. and Catherine Y. Spong, M.D.).
In addition to the authors, other members of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network are as follows:
University of Texas Medical Branch, Galveston, TX – G. Saade, J. Moss, B. Stratton, G. Hankins, J. Brandon, C. Nelson-Becker, G. Olson, L. Pacheco
University of Pittsburgh, Pittsburgh, PA – S. Caritis, T. Kamon (deceased), M. Cotroneo, D. Fischer
University of Utah, Salt Lake City, UT – P. Reed, S. Quinn (LDS Hospital), V. Morby (McKay-Dee Hospital), F. Porter (LDS Hospital), R. Silver, J. Miller (Utah Valley Regional Medical Center), K. Hill
University of Alabama at Birmingham, Birmingham, AL – D.J. Rouse, A. Northen, P. Files, J. Grant, M. Wallace, K. Bailey
Columbia University, New York, NY – S. Bousleiman, R. Alcon, K. Saravia, F. Loffredo, A. Bayless (Christiana), C. Perez (St. Peter's University Hospital), M. Lake (St. Peter's University Hospital), M. Talucci
University of North Carolina at Chapel Hill, Chapel Hill, NC – K. Boggess, K. Dorman, J. Mitchell, K. Clark, S. Timlin
Case Western Reserve University-MetroHealth Medical Center, Cleveland, OH – B. Mercer, J. Bailit, C. Milluzzi, W. Dalton, C. Brezine, D. Bazzo
University of Texas Southwestern Medical Center, Dallas, TX – KJ. Sheffield, L. Moseley, M. Santillan, K. Buentipo, J. Price, L. S. Hermann, C. Melton, Y. Gloria-McCutchen, B. Espino
Northwestern University, Chicago, IL – M. Dinsmoor (NorthShore University HealthSystem), T. Matson-Manning, G. Mallett
University of Texas Health Science Center at Houston-Children’s Memorial Hermann Hospital, Houston, TX – S. Blackwell, K. Cannon, S. Lege-Humbert, Z. Spears
Brown University, Providence, RI – M. Carpenter, J. Tillinghast, M. Seebeck
The Ohio State University, Columbus, OH – P. Samuels, J. Iams, F. Johnson, S. Fyffe, C. Latimer, S. Frantz, S. Wylie
Drexel University, Philadelphia, PA – M. Talucci, M. Hoffman (Christiana), J. Benson (Christiana), Z. Reid, C. Tocci
Wake Forest University Health Sciences, Winston-Salem, NC – M. Harper, P. Meis, M. Swain
Oregon Health & Science University, Portland, OR – W. Smith, L. Davis, E. Lairson, S. Butcher, S. Maxwell, D. Fisher
University of Texas Medical Branch, Galveston, TX – J. Moss, B. Stratton, G. Hankins, J. Brandon, C. Nelson-Becker, G. Olson, L. Pacheco
Wayne State University, Detroit, MI – G. Norman, S. Blackwell, P. Lockhart, D. Driscoll, M. Dombrowski
The George Washington University Biostatistics Center, Washington, DC – E. Thom, R. Clifton, T. Boekhoudt, L. Leuchtenburg
National Heart, Lung, and Blood Institute, Bethesda, MD – G. Pearson, V. Pemberton, J. Cutler, W. Barouch
Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD – C. Spong, S. Tolivaisa
MFMU Steering Committee Chair (University of Texas Medical Center, Galveston, TX) – G.D. Anderson, M.D.
Source of the work or study: Secondary analysis of Vitamin C and E supplementation in nulliparous low risk women for prevention of pregnancy associated hypertension- A randomized multicenter clinical trial from the Eunice Kennedy Shriver Maternal-Fetal Medicine Unit Network.
Abbreviations
- aOR
Adjusted Odds Ratio
- AUC
Area Under the Curve
- BMI
Body Mass Index
- CI
Confidence Interval
- GDM
Gestational Diabetes Mellitus
- HOMA-IR
Homeostasis Model Assessment of Insulin Resistance
- IR
Insulin Resistance
- ROC
Receiver Operating Characteristics
- WC
Waist Circumference
- WHR
Waist-to-Hip ratio
Footnotes
Disclosure: None
References
- 1.Wendland EM, Duncan BB, Mengue SS, Nucci LB, Schmidt MI. Waist circumference in the prediction of obesity-related adverse pregnancy outcomes. Cadernos de saude publica / Ministerio da Saude, Fundacao Oswaldo Cruz, Escola Nacional de Saude Publica. 2007;23(2):391–398. doi: 10.1590/s0102-311x2007000200015. [DOI] [PubMed] [Google Scholar]
- 2.WHO. World Health Organization. WHO technical report series: 894; 2000. Obesity- Preventing and Managing the global epidemic: Report of a WHO consultation on Obesity. [PubMed] [Google Scholar]
- 3.Carey DG, Jenkins AB, Campbell LV, Freund J, Chisholm DJ. Abdominal fat and insulin resistance in normal and overweight women: Direct measurements reveal a strong relationship in subjects at both low and high risk of NIDDM. Diabetes. 1996;45(5):633–638. doi: 10.2337/diab.45.5.633. [DOI] [PubMed] [Google Scholar]
- 4.Han TS, van Leer EM, Seidell JC, Lean ME. Waist circumference action levels in the identification of cardiovascular risk factors: prevalence study in a random sample. BMJ. 1995;311(7017):1401–1405. doi: 10.1136/bmj.311.7017.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalton M, Cameron AJ, Zimmet PZ, Shaw JE, Jolley D, Dunstan DW, et al. Waist circumference, waist-hip ratio and body mass index and their correlation with cardiovascular disease risk factors in Australian adults. Journal of internal medicine. 2003;254(6):555–563. doi: 10.1111/j.1365-2796.2003.01229.x. [DOI] [PubMed] [Google Scholar]
- 6.Makgoba M, Savvidou MD, Steer PJ. An analysis of the interrelationship between maternal age, body mass index and racial origin in the development of gestational diabetes mellitus. BJOG : an international journal of obstetrics and gynaecology. 2012;119(3):276–282. doi: 10.1111/j.1471-0528.2011.03156.x. [DOI] [PubMed] [Google Scholar]
- 7.Dennedy MC, Avalos G, O'Reilly MW, O'Sullivan EP, Gaffney G, Dunne F. ATLANTIC-DIP: Raised Maternal Body Mass Index (BMI) Adversely Affects Maternal and Fetal Outcomes in Glucose-Tolerant Women according to International Association of Diabetes and Pregnancy Study Groups (IADPSG) Criteria. J Clin Endocrinol Metab. 2012 Apr;97(4):E608–E6122012. doi: 10.1210/jc.2011-2674. [DOI] [PubMed] [Google Scholar]
- 8.Hauth JC, Clifton RG, Roberts JM, Myatt L, Spong CY, Leveno KJ, et al. Maternal insulin resistance and preeclampsia. American journal of obstetrics and gynecology. 2011;204(4):327, e1–e6. doi: 10.1016/j.ajog.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vazquez G, Duval S, Jacobs DR, Jr, Silventoinen K. Comparison of body mass index, waist circumference, and waist/hip ratio in predicting incident diabetes: a meta-analysis. Epidemiologic reviews. 2007;29:115–128. doi: 10.1093/epirev/mxm008. [DOI] [PubMed] [Google Scholar]
- 10.Roberts JM, Myatt L, Spong CY, Thom EA, Hauth JC, Leveno KJ, et al. Vitamins C and E to prevent complications of pregnancy-associated hypertension. The New England journal of medicine. 2010;362(14):1282–1291. doi: 10.1056/NEJMoa0908056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 12.Baeten JM, Bukusi EA, Lambe M. Pregnancy complications and outcomes among overweight and obese nulliparous women. American journal of public health. 2001;91(3):436–440. doi: 10.2105/ajph.91.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu GC, Rouse DJ, DuBard M, Cliver S, Kimberlin D, Hauth JC. The effect of the increasing prevalence of maternal obesity on perinatal morbidity. American journal of obstetrics and gynecology. 2001;185(4):845–849. doi: 10.1067/mob.2001.117351. [DOI] [PubMed] [Google Scholar]
- 14.Nucci LB, Schmidt MI, Duncan BB, Fuchs SC, Fleck ET, Santos Britto MM. Nutritional status of pregnant women: prevalence and associated pregnancy outcomes. Revista de saude publica. 2001;35(6):502–507. doi: 10.1590/s0034-89102001000600002. [DOI] [PubMed] [Google Scholar]
- 15.Branchtein L, Schmidt MI, Mengue SS, Reichelt AJ, Matos MC, Duncan BB. Waist circumference and waist-to-hip ratio are related to gestational glucose tolerance. Diabetes care. 1997;20(4):509–511. doi: 10.2337/diacare.20.4.509. [DOI] [PubMed] [Google Scholar]
- 16.Bray GA, Jablonski KA, Fujimoto WY, Barrett-Connor E, Haffner S, Hanson RL, et al. Relation of central adiposity and body mass index to the development of diabetes in the Diabetes Prevention Program. The American journal of clinical nutrition. 2008;87(5):1212–1218. doi: 10.1093/ajcn/87.5.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pascot A, Despres JP, Lemieux I, Almeras N, Bergeron J, Nadeau A, et al. Deterioration of the metabolic risk profile in women. Respective contributions of impaired glucose tolerance and visceral fat accumulation. Diabetes care. 2001;24(5):902–908. doi: 10.2337/diacare.24.5.902. [DOI] [PubMed] [Google Scholar]
- 18.Wei M, Gaskill SP, Haffner SM, Stern MP. Waist circumference as the best predictor of noninsulin dependent diabetes mellitus (NIDDM) compared to body mass index, waist/hip ratio and other anthropometric measurements in Mexican Americans--a 7-year prospective study. Obesity research. 1997;5(1):16–23. doi: 10.1002/j.1550-8528.1997.tb00278.x. [DOI] [PubMed] [Google Scholar]
- 19.Ohlson LO, Larsson B, Svardsudd K, Welin L, Eriksson H, Wilhelmsen L, et al. The influence of body fat distribution on the incidence of diabetes mellitus. 13.5 years of follow-up of the participants in the study of men born in 1913. Diabetes. 1985;34(10):1055–1058. doi: 10.2337/diab.34.10.1055. [DOI] [PubMed] [Google Scholar]
- 20.Resnick HE, Valsania P, Halter JB, Lin X. Differential effects of BMI on diabetes risk among black and white Americans. Diabetes care. 1998;21(11):1828–1835. doi: 10.2337/diacare.21.11.1828. [DOI] [PubMed] [Google Scholar]
- 21.Nakagami T, Qiao Q, Carstensen B, Nhr-Hansen C, Hu G, Tuomilehto J, et al. Age, body mass index and Type 2 diabetes-associations modified by ethnicity. Diabetologia. 2003;46(8):1063–1070. doi: 10.1007/s00125-003-1158-9. [DOI] [PubMed] [Google Scholar]
- 22.Abate N, Chandalia M. The impact of ethnicity on type 2 diabetes. Journal of diabetes and its complications. 2003;17(1):39–58. doi: 10.1016/s1056-8727(02)00190-3. [DOI] [PubMed] [Google Scholar]
- 23.Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes care. 2000;23(4):465–471. doi: 10.2337/diacare.23.4.465. [DOI] [PubMed] [Google Scholar]