Abstract
Asymptomatic ebolavirus infection could greatly influence transmission dynamics, but there is little consensus on how frequently it occurs or even if it exists. This paper summarises the available evidence on seroprevalence of Ebola, Sudan and Bundibugyo virus IgG in people without known ebolavirus disease. Through systematic review, we identified 51 studies with seroprevalence results in sera collected from 1961 to 2016. We tabulated findings by study population, contact, assay, antigen and positivity threshold used, and present seroprevalence point estimates and 95% confidence intervals. We classified sampled populations in three groups: those with household or known case-contact; those living in outbreak or epidemic areas but without reported case-contact; and those living in areas with no recorded cases of ebolavirus disease. We performed meta-analysis only in the known case-contact group since this is the only group with comparable exposures between studies. Eight contact studies fitted our inclusion criteria, giving an overall estimate of seroprevalence in contacts with no reported symptoms of 3.3% (95% CI 2.4–4.4, P<0.001), but with substantial heterogeneity.
Subject terms: Viral infection, Diagnostic markers
Introduction
Knowing if ebolavirus infection manifests asymptomatically is critical to understanding its spread and to estimating the role herd immunity could have in reducing transmission. Investigating unrecognised infections could also help in the development and targeting of vaccines. However, despite a surprisingly large number of investigations into the seroprevalence of ebolavirus IgG since the first outbreak in Yambuku, Zaire (now Democratic Republic of Congo)1–51, consensus on results has proved elusive. The main reasons for this are the range of findings, positive results in unexpected locations, and a lack of confidence in immunofluorescence antibody (IFA) tests used in early studies.
Concerns about IFA specificity stem largely from studies showing positive results in populations expected to be negative, although the most frequently cited—in 200 Panamanian Indians with no known exposure—found only one Ebola virus IgG positive on a high cut-off giving a specificity of 99.5%4. Unexpected seropositivity has also been seen in African countries without reported cases of ebolavirus disease (EVD) such as the Central African Republic, Cameroon and Zimbabwe, only some of which can be attributed to using low test cut-offs. But, as some ELISA-based studies have produced similar findings37,38, these positive results may indicate zoonotic exposure with filoviruses or unrecognised human-to-human transmission rather than poor specificity.
‘Asymptomatic’ status can only be defined for a certain period, such as during an outbreak, though excluding mild symptoms is difficult. In outbreak areas asymptomatic subjects could have experienced unrecognised symptomatic EVD in the past so, even apart from problems with the test, ebolavirus antibody seropositivity does not necessarily mean asymptomatic infection.
We aimed to provide an up-to-date and easily accessible overview of serological findings to date, to help researchers contextualise studies prompted by the 2014–16 West Africa epidemic. The most comprehensive review of ebolavirus serology—Kuhn’s Filoviruses: A Compendium of 40 years of Epidemiological, Clinical and Laboratory Studies52—covers work to 2008. In addition to reviewing this key reference, we carried out a systematic review of serosurveys in people without symptoms of EVD up to July 2016.
Results
Characterisation of seroprevalence surveys of IgG antibodies to ebolavirus
We identified 51 studies covering 84 sample populations reported to have had no symptoms of EVD during the outbreak period, or to have come from populations with no known outbreaks. In total these studies investigated the presence of ebolavirus IgG in 44,147 subjects using samples collected since 1961.
Thirteen studies reported 16 study populations involving 2,664 participants with household or known case-contact5–7,9,12,36,41,42,45,47,49–51. Eleven studies reported 17 study populations covering 5,327 participants living in outbreak areas but without reported case-contact5–7,9,14,33,39,40,42,43,46. The remaining studies reported on 51 groups involving 36,156 subjects from general populations, often in settings ecologically similar to ebolavirus outbreak areas but without known cases of EVD1–3,5,8,10,11,13,15–35,37,38,44–46,48,51.
Table 1 (available online only) gives a detailed breakdown of the study populations, test methods and results.
Table 1. Findings of seroprevalence studies investigating presence of ebolavirus immunoglobulin G antibodies in ‘asymptomatic’ populations, 1961–2016.
| Location | Year sera collected | Population type (as described in paper) | Group | No. of samples | # IgG +ve | Species % +ve | Assay type | Cut-off (as described in paper | Antigen & validation information | Notes |
|---|---|---|---|---|---|---|---|---|---|---|
| A=populations with household contact or known case contact |
||||||||||
| B=populations without known contact but in outbreak areas or villages with cases |
||||||||||
| C=general population—no known outbreak exposure or contact |
||||||||||
|
Abbreviations: CDC (US): Centers for Disease Control and Prevention, Atlanta, USA; PHE: Public Health England; EBOV: Zaire ebolavirus; SUDV: Sudan ebolavirus; BDBV: Bundibugyo ebolavirus; TAFV: Tai Forest Fever Virus; MARV: Marburg Fever Virus; CCHFV: Crimean Congo Haemorrhagic Fever Virus; RVFV: Rift Valley Fever Virus; LASV: Lassa Fever Virus; IFA: immunofluorescence assays; ELISA: Enzyme-linked immunosorbent assay; WB: Western Blot; OD: optical density; SD: standard deviation |
||||||||||
|
Assay technique notation |
||||||||||
| IFA (w): Wulff & Lange62 |
||||||||||
| IFA( j): Johnson63 |
||||||||||
| IFA (g): Gardner64 |
||||||||||
| IFA (b) Baskirtsev65 |
||||||||||
| Double IFA: Emmerich66 |
||||||||||
| IFA (?) ELISA (?): technique not referenced |
||||||||||
| ELISA (k): Ksiazek67 |
||||||||||
| ELISA (s): Schoepp68 |
||||||||||
| ELISA (v): Viral Haemorrhagic Fever Consortium (SL)69 |
||||||||||
| ELISA (n): Nicklasson70 |
||||||||||
| ELISA(r): Rezapkin71 |
||||||||||
| ELISA(PHE): Lambe54 |
||||||||||
| ELISA(Alpha): ADI75 | ||||||||||
| Assab, Awash, Blue Nile, Illubor, & Ogaden regions, Ethiopia1 | 1961/62 | Asymptomatic individuals from area not affected by ongoing Yellow Fever epidemic | C | 178 | 42 | EBOV 24% | IFA(w) | ≥1:16 | Antigens: Polyvalent ELM (Ebola-Lassa-Marburg viruses) & monovalent EBOV (Mayinga): all sera reacting with ELM also reacted with monovalent EBOV antigen but not with monovalent Marburg and Lassa. | Samples from symptomatic Yellow Fever-negative individuals also tested: 12% positive. |
| N.W. Zaire (now Democratic Republic of Congo DRC)2 | 1972–78 | General population from area west of Yambuku (site of 1st recorded outbreak in 1976) | C | 251 | 4326 | EBOV 17.1%10.4% | IFA(w) | ≥1:16≥1:64 | Antigen: EBOV (May. 80826) | |
| Harbel, Bong town, Yekepa, Liberia3 | 1973 | Staff & family members of rubber and mining companies | C | 592 | 83 | Ebolavirus 14.0% | ELISA/WB | Not stated | Antigen not stated | Sera taken in 1973 and investigated for Lassa and ebolavirus in ~1986. Ebola statistic reported without further information. |
| Northern Rhodesia (now Zimbabwe)4 | 1975 | ‘Control group’ | C | 243 | 20 | EBOV 0.8%0.0% | IFA(w) | ≥1:8≥1:64 | Antigen not specified in report: Kuhn52 notes antigen as EBOV | Areas sampled had no known outbreak |
| Yambuku, Zaire (DRC)5 | 1976 | Asymptomatic contacts of cases | A | 404 | 10 | EBOV 2.5% | IFA(w) | ≥1:64 | Antigen: EBOVValidation: Repeat testing of the 4 antibody positive with no known contact gave the same results. 32/33 (97%) positive samples (including samples from cases) confirmed positive by CDC (US)4 (p141) | |
| Residents from villages with cases but no known contact | B | 448 | 4 | EBOV 0.9 | ||||||
| Residents from 4 neighbouring villages with no cases | C | 442 | 5 | EBOV 1.1% | ||||||
| Maridi, Sudan (now South Sudan)6 | 1976 | Close family contacts | A | 93 | 13 | SUDV 14.0% | IFA(w) | ≥1:8 | Antigen: SUDVValidation: 42 of 48 clinically diagnosed survivors from Nzara (87%) were considered positive using the same IFA protocol. Several samples from Nzara were retested and confirmed positive by CDC (US) using the same protocol. | 9 antibody positive family contacts had symptoms and have been excluded from these figures. Not clear if all subjects in this group were interviewed about symptoms. |
| Asymptomatic Maridi schoolboys with no known contact | B | 29 | 3 | SUDV 10.3% | ||||||
| Asymptomatic hospital staff with probable/possible contact | A | 64 | 7 | SUDV 10.9% | 4 nurses; 1 cleaner, 1 toilet cleaner, 1 water carrier were positive | |||||
| Nzara, Sudan (now South Sudan)6 | 1976 | Asymptomatic cotton factory workers (site of index case but reportedly no direct contact) | B | 109 | 7 | SUDV 6.4% | IFA(w) | ≥1:8 | Among factory workers titre range was 1:16–1:32 | |
| Close family contacts of clinically diagnosed cases | A | 78 | 1 | SUDV 1.3% | Only 6/31 (19.4%) of clinically diagnosed subjects were antibody positive, and none had levels>1:32 | |||||
| San Blas Islands, Panama4 | 1977 | San Blas Indians | C | 200 | 1 | EBOV 0.5% | IFA(w) | ≥1:64 | Antigen: not specified in report: Kuhn52 records antigen as EBOV | Areas sampled had no known outbreak. Method of selection not known |
| Tandala, Zaire (DRC)7 | 1977–78 | Missionaries and ‘a few’ hospital staff with case contact (1977) | A | 50 | 0 | EBOV 0% | IFA(w) | ≥1:16 | Antigen: EBOV | One doctor, who tested positive in 1977 and 1978 and had history of severe illness after attending the autopsy of a haemorrhagic fever victim in 1972, is excluded. Some individuals gave samples in both the 1977 and 1978 groups |
| Hospital staff with case contact (1978) | A | 71 | 0 | EBOV 0% | ||||||
| Residents of villages with confirmed /suspect cases | B | 346 | 21 | EBOV 6.1% | ||||||
| Residents of other villages in same area | B | 750 | 58 | EBOV 7.7% | ||||||
| Liberia 8 | 1978-79 | Random rural general population in multiple counties | C | 433 | 26 | Ebolavirus 6.0% | IFA(w) | ≥1:16 | Antigens: unspecified ebolavirus, Marburg & Lassa viruses. No sera positive for ebolavirus was positive for MARV or LASV. | No known outbreak. Titre range: 1:16 to 1:1024 |
| Nzara/Yambio, (South) Sudan9 | 1979 | Asymptomatic adult family members of cases with known physical contact | A | 38 | 12 | Ebolavirus 32% | IFA(w) | ≥1:16 | Antigen: unspecified | |
| Asymptomatic adult family members of cases who denied physical contact | B | 23 | 3 | Ebolavirus 13% | ||||||
| Adults from families without known cases in same area | B | 45 | 8 | Ebolavirus 18% | Unknown if these people were exposed in 1976 outbreak, which could explain the high prevalence | |||||
| Bangassou, Central African Republic (CAR)10 | 1979 | General population in forest and semi-forest zones | C | 499 | 103 | Ebolavirus 2.0%0.6% | IFA(w) | ≥1:16≥1:64 | Antigen: Polyvalent of unspecified ebolavirus, MARV & LASV, followed by monovalent test for positive samples. Positive sera sent to CDC (US) for repeat testing; results not reported. | Areas sampled had no known outbreak |
| Moloundou, Lolodorf Bipindi, Lomie, Yaounde & Pete, Cameroon11 | 1980 | General population in five regions (forest, pre Sahelian savannah and the capital) and different ethnic groups | C | 1517 | 147 | Ebolavirus 9.7% | IFA(w) | ≥1:16 | Antigens: unspecified ebolavirus provided by CDC (US) | No known outbreak. Positives in all areas, range 3%-23%. Highest in Pygmies and rain forest farmers. 6% in the capital, Yaoundé. Report to OCEAC in the same year gave positivity of 6.2% (51/821) in Moloundou, compared to 13.2% for the same location in this study, and 29% (20/70) in Mbatika, but positivity threshold used is not reported.63 |
| Lugulu, western Kenya12 | 1980 | Family and close neighbours of an IFA confirmed case (asymptomatic?) | A | 84 | 4 | Ebolavirus 4.8% | IFA (?) | ≥1:16 | Antigens: monospecific, triple (unspecified ebolavirus, MARV, LASV) and poly-antigen (CCHFV, RVFV, ebolavirus, LASV, MARV).Validation: sera examined at National Institute of Virology, Johannesburg and CDC(US): labs used different thresholds, so positive confirmed only where both found ≥1:16 | Area in Western Kenya, close to Nzoia. Samples collected during investigation of 2 MARV suspect cases who were later shown to be ebolavirus positive. |
| Kenya13 | 1980 | Different studies in 5 regions of Kenya:—Lodwar, Laisamis, Masia, Malindi/Kilifi- Nzoia | C | 1058841 | 189 | EBOV/SUDV 1.7%1.1% | IFA(w) | ≥1:16 | Antigens: inactivated unspecified ebolavirus, MARV, CCHFV, RVFV, & LASV; positives tested against EBOV(May) & SUDV (Boniface & Maleo). Authors report ‘most’ of the Nzoia samples were only tested against EBOV(May) | No known outbreak but Nzoia cohort reported to include suspected cases and their contacts. Highest prevalence: Lodwar 7.8% (north-west Kenya). Note referenced paper includes some sera reported on in other papers.72 |
| Haute Ogooue, Gabon14 | 1980 | General population in outbreak area but no known contact | B | 253 | 16851218 | EBOV 6.3%3.2%SUDV 2.0%0.4%EBOV/SUDV 8.3%3.1% | IFA(w) | ≥1:16≥1:64≥1:16≥1:64≥1:16≥1:64 | Antigens: inactivated polyvalent unspecified ebolavirus/LASV MARV: positives tested against EBOV(802850) & SUDV(802681). | Samples from the Occupational Health Services, plus 28 women & their newborns. One sample was positive ≥1:64 on both EBOV & SUDV |
| Northern Rhodesia (now Zimbabwe)15 | 1980 | Asymptomatic schoolboys (8-10y): no known outbreak | C | 486 | 94 | EBOV 1.9%0.8% | IFA(g) | ≥1:8≥1:128 | Antigen: polyvalent CCHFV, RVFV, LASV, MARV, unspecified ebolavirus slides provided by CDC (US): positives tested against individual antigens (EBOV & SUDV)Validation: 4 of 5 positive samples sent to CDC (US) were ≥1:128 in repeat IFA testing. | None were positive for SUDV. |
| Pool, Congo-Brazzaville (now Republic of Congo)16 | 1981 | Children from 20 villages aged 3-15 years and unvaccinated for smallpox | C | 790 | 119 | Ebolavirus 15.0% | IFA(?) | Not stated | Antigen: polyvalent CCHFV, RVFV, LASV, MARV, unspecified ebolavirus; also tested against monovalent antigens | Pool region is on the border with DRC. Areas sampled had no known outbreak but populations were selected for close contact with animals |
| Grand Bassa, Liberia17 | 1981-82 | Individuals asymptomatic for EVD consisting of 106 epilepsy patients; 87 healthy relatives of these patients; 32 unrelated geographically matched controls. | C | 225 | 26429 | EBOV 11.6%SUDV 1.8%Overall 12.9% | IFA(w) | unclear | Antigen: polyvalent CCHFV, RVFV, LASV, MARV, EBOV (May), SUDV(Bon) (known as CRE2LM); positives retested against individual antigens.Validation: Difficulties with non-specific binding led researchers to replicate and use blinded observers to read results. Only samples with unequivocally positive results by 2 observers were considered positive. | No known outbreak. Similar proportion positive in each participant group. 38% epilepsy patients had a febrile illness 1-4 weeks before onset of epilepsy, but no significant difference in seroprevalence with & without febrile history. Paper says 30 positives in total but one counted twice (positive for EBOV and SUDV). Titres ranged from 32-128 for EBOV; 6/29 had antibodies to more than 1 virus. |
| Sud-Ubangi sub-region, DRC (includes Tandala)18 | 1981-85 | Age/sex matched controls from the same villages as reported cases | C | 137 | 2 | Ebolavirus 1.5% | IFA(w) | ≥1:64 | Antigen: polyvalent CRE2LM; positives tested against unspecified ebolavirus-specific antigens. | In addition 188 contacts of possible and probable cases were tested; 28 were positive at ≥1:64 but all had had symptoms fitting the definition of a possible or clinical case. It is not clear how many of the other contacts had symptoms. |
| Northeastern, southeastern & western Gabon19 | 1981-1997 | Six rural communities (Makokou, Doussala, Doussieousou, Matadi-Ngoussa, Moukoro, Latoursville): sera gathered during onchocerciasis research | C | 1147 | 14 | EBOV 1.2% | ELISA (k) | Mean +3SD of OD of negative controls | Antigen: EBOVValidation: In 2003, 6 of original 14 positives were re-bled (others unavailable): 2 were still positive. 14 controls (relatives and ‘cohorts’) were unreactive | 6 seropositives were from north-eastern Gabon where outbreaks had occurred; 8 were from western communities more than 500km from known epidemics. Authors also investigate and correlate animal with human outbreaks. Conclude that less virulent strains of EBOV affected western areas. |
| Madina-Ula, Guinea20 | 1982-83 | Healthy adults sampled during an outbreak of an unknown disease | C | 138 | 1142 | EBOV 7.8%2.9%2.2% | ELISA(r)ELISA(r)IFA(b)ELISA(r)IFA (b) | ≥1:8≥1:512≥1:16≥1:512≥1:64 | Antigen: EBOV | Areas sampled had no known outbreak |
| Benin21 | 1983 | General population, non-outbreak country | C | 603 | 2 | EBOV or SUDV? 0.3% | IFA (?) | ≥1:64 | Antigen: EBOV, SUDV | Unpublished data cited by Gonzalez et al (2005): no further information, not specified which ebolavirus antigen samples were reactive to. |
| Ethiopia, Awash valley1 | 1983 | Unexposed children | C | 250 | 0 | EBOV 0.0% | IFA(w) | ≥1:16 | Antigens: Polyvalent ELM (Ebola-Lassa-Marburg viruses) & monovalent EBOV (May) | Areas sampled had no known outbreak |
| Karamoja, Uganda22 | 1984 | ‘Healthy’ adults 20-40y recruited during visits to a health centre, excluding any with current or recent fever | C | 132 | 44 | EBOV 3.0%SUDV 3.0% | IFA(w) | unclear | Antigen: polyvalent CCHFV, RVFV, LASV, MARV, EBOV (May), SUDV(Bon) (CRE2LM); positives retested against individual antigens. | No known outbreak. Not clear if some samples had antibodies to both EBOV & SUDV. Not specified how many>1:64. All samples<1: 128. |
| Mobai, Sierra Leone & unspecified location Sudan23 | <1984 | No information on population type: general population assumed from description- Mobai, Sierra Leone- Sudan | C | 556284 | 101010 | EBOV (a) 1.8%(b) 1.8%(a) 0.35(b) 0 | (a) ELISA +ve/IFA+ve (b) ELISA +ve/IFA −ve or unclear | ELISA: +ve within 2SD of +ve ref. sera; −ve within 1SD of negative ref seraIFA≥1:100 | Antigen: EBOVValidation: All? samples were tested using both assays | Year of sample collection is not recorded. Paper reports a high level of cross reactivity with MARV, lasting a number a number of years after infection. |
| Haute Ogooue, Gabon24 | 1985 | Inhabitants of Ambinda village | C | 213 | 20621227 | EBOV 9.4%1.4%SUDV 0.9%0.5%Overall 10.3%3.3% | IFA (j) | ≥1:16≥1:64≥1:16≥1:64≥1:16≥1:64 | Antigen: polyvalent CCHFV (IB-AR-10200 Nigeria), RVFV (ZH-501 Egypt), LASV (Josiah), MARV (Musoki), EBOV (May), SUDV(Bon) (CRE2LM); positives retested against individual antigens. | No known outbreak |
| Nola,Ikaumba, Bozo, Bangassou, Mbre & Birao, Central African Republic 25 | 1984-85 | General population from 5 ecological regions including one close to Zaire/DRC outbreak area | C | 836 | 15263 | EBOV 18.2%SUDV 7.5% | IFA(j) | ≥1:16 | Antigens: EBOV (May), SUDV (Bon), MARV (Mus) | No known outbreak but Zemio which borders DRC accounted for 43% of EBOV positives |
| Nola,Ikaumba, Bozo, Bangassou, Mbre & Birao, Central African Republic 26 | 1984-85 | Asymptomatic general population from 5 ecological distinct zones selected on accessibility: additional villages wer chosen where multiple ethnic groups coexisted. | C | 4295*4078* | 681209853259914335 | EBOV15.9%5.1%SUDV 19.8%6.4%Overall 21.3%8.2% | IFA (j) | ≥1:16≥1:128≥1:16≥1:128≥1:16≥1:128 | Antigens: polyvalent EBOV (May), SUDV (Bon), MARV (Mus), LASV (Jos), CCHFV (10200), RVFV(ZH501); positives (≥1:16) retested against monovalent antigen.Validation: 185 samples were reanalysed by ELISA in 1996: results confirmed original analysis.21 | Study linked to one above in CAR25 but using different set of sera sampled in the same period.* Two different denominators are cited: 4296 people are reported for all titre levels; 4078 people are reported in the table describing only samples showing titres≥1:128.Highest prevalence in woods and forest regions. 86% of titres≥1:64 but reached as high as 1:2048 |
| Nkongsamba, Cameroon27 | 1985 | Randomly selected urban general population (15-44 years) | C | 375 | 75 | Ebolavirus 1.9%1.3% | IFA (?) | ≥1:16≥1:64 | Antigens: polyvalent unspecified ebolavirus, CCHRV, RVFV, MARV) | These samples were included in the following multi-country study which used a different threshold.28 One sample was positive for both ebolavirus and RVFV |
| Central Africa28 (now Middle Africa) | 1985-87 | Randomly selected sera collected in:Cameroon (Mora, Maroua, Nkongsamba)Central African Republic (Bangui)Chad (N’djamena)Republic of Congo (Pointe Noire, Brazzaville)Equatorial Guinea (Bioco Island, Nsork)Gabon (Libreville, Port-Gentil, Ogooue-Ivindo, Haut Ogooue, Ngounie) | C | 11523273347286881841 | 8910733451111259 | EBOV/SUDVλ7.7%32.7%3.6%7.0%16.1%14.0% | IFA (w) | ≥1:16 | Antigens: polyvalent EBOV (May), SUDV (Bon), MARV (Mus), LASV (Jos), CCHFV (10200), RVFV(ZH501); positives (≥1:16) retested against monovalent antigen. | Areas sampled had no known outbreak |
| Chobe, Northern Botswana29 | 1984-86 | 1984: 52 asymptomatic villagers)1985: 25 villagers with non-specific or ictero-haemorrhagic symptoms1986: 77 asymptomatic villagers | C | 154 | 0 | EBOV/SUDV 0% | IFA (J) | ≥1:16 | Antigens: polyvalent CCHFV, RVFV, LASV, MARV, EBOV (May), SUDV(Bon): positives re-tested against monovalent antigens. | Areas sampled had no known outbreak. Unable to separate results for the symptomatic group. Only reaction found was against RVFV. Testing performed in Paris. |
| Lobaye, Central African Republic30 | 1987 | Asymptomatic general population, Lobaye district: Pygmy hunter-gathers | C | 127 | 31 | EBOV/SUDV 24.4% | IFA (J) | ≥1:128 | Antigens: polyvalent EBOV (May), SUDV (Bon), MARV (Mus), LASV (Jos), CCHFV (10200), RVFV(ZH501); positives (≥1:16) retested against monovalent antigen, considered reactive if≥1:128.Validation: 296 samples from this study and 185 samples from the CAR 1984-85 study above were re-analysed in 1996 using ELISA (≥1:400 & sum of 4 ODs≥1.000). 6.2% were Ebola IgG positive (30/481) compared to 6.4% in these samples previously by IFA.21,26 | Area with no known outbreak.Of the positives, 45 reacted to both EBOV & SUDV: it is not possible to identify how this splits between the groups. |
| Asymptomatic general population Lobaye district: Mozombo/Mbati subsistence farmers | C | 300 | 42 | EBOV/SUDV 14.4% | ||||||
| Nigeria31 | 1988 | Asymptomatic general population in different locations | C | 1677 | 3022 | SUDV 1.8%EBOV/SUDV 1.3% | IFA(w) | ≥1:10 | Antigens: polyvalent CCHFV, RVFV, LASV, MARV, EBOV (May), SUDV(Bon): positives with titre≥1:10 retested against monovalent antigens. Known positive/negative controls used | Areas sampled had no known outbreak. All positive samples came from savannah areas (Benue/Gongola)Of the positives, none reacted to EBOV alone. |
| Antanarivo, Mandoto, andasibe, Tsiroanomandidy & Ampijoroa, Madagascar32 | 1989 | Asymptomatic adults from 5 different areas (urban & rural, cattle-lands, forested) | C | 381 | 17 | EBOV 4.5% | IFA (j) | ≥1:16 | Antigens: polyvalent CCHFV, RVFV, LASV, MARV, EBOV (May), SUDV(Bon): positives retested against monovalent antigens | Areas sampled had no known outbreak. Range of titres: 1:16 to 1:512; highest prevalence in the capital Antanarivo 13.3%. |
| United States33 | 1990 | CDC (US) employees with current or previous occupational exposure to monkeys. None ill. | B | 550 | 42 | EBOV/SUDV/RESTV/MARV 7.6% | IFA (?) | ≥1:16 | Antigens: EBOV, SUDV, Reston ebolavirus (RESTV), MARV.Validation: confirmed by western Blot | This paper summarises 2 others 73,74Results are for positivity to at least one of the four antigens, which include Marburg. |
| Adult primary care outpatients in US | C | 449 | 12 | 2.7% | ||||||
| Germany34 | 1991 | Various groups of healthy individuals, blood donors and routine diagnostic samples, plus 56 individuals who had had contact with Marburg patients in 1972. | C | 1288 | 1144 | EBOV 0.85%RESTV 3.4% | ELISAIFA or WB | ELISA: 1:100IFA: 1:40WB: +ve if stained≥2 viral proteins) | Antigens: EBOV (May), RESTV, Marv(Mus)Validation: Considered positive if ELISA confirmed by IFA or WB.Confirmation: ELISA vs IFA 75%; ELISA vs Western Blot 77%. | Authors state that the sample groups showed no significant differences in the prevalence of antibody against the 3 filoviruses and so they treated as one group for analysis and only overall results reported.WB results: ‘most’ sera reacted with the NP protein, ‘less’ with VP40, VP35 & VP30, and ‘few’ with VP24. None reacted to GP or L proteins. |
| Kikwit, DRC35 | 1995 | Four forest site populations near Kikwit town, site of outbreak | B | 230 | 5 | EBOV 2.2% | ELISA (k) | ≥1:400 & OD sum≥1.25 | Antigen: unspecified but Kuhn52 reports EBOV; sera tested in CDC (US) Special Pathogens Lab. | Differentiation between forest and city workers was difficult: publicity brought people out of their areas: self identified occupations. 95% of participants including all 9 positives said they knew someone with Ebola. |
| City workers, Kikwit | B | 184 | 4 | EBOV 2.2% | ||||||
| Asymptomatic volunteers from unaffected villages near Kikwit | C | 161 | 15 | EBOV 9.3% | 5/15 positives knew someone who had had Ebola | |||||
| Kikwit, DRC36 | 1995 | Household contacts aged 3 m-58y | A | 101 | 4 | Ebolavirus 4.0% | ELISA (k) | ≥1:400 & OD sum≥1.25 | Antigen: unspecified but probably EBOV; sera tested in CDC (US) Special Pathogens Lab. | Paper cites 5 positive sera but 1 miscarried 3 days before giving her positive specimen so fits case definition for Ebola. One of the remaining 4 may have acquired Ebola by sexual transmission from a convalescent. Out of 81 sero-negative household contacts, 15 had episodes of illness fitting case definition at some point during follow-up. |
| Central African Republic 37 | 1992-97 | Pygmy general population: southern regions of CAR (Lobaye, Belemboke) | C | 684 | 48 | EBOV 7.0% | ELISA (n) | ≥1: 400 | Antigens: EBOV, MARV, RVFV, LASV, Yellow fever (YF) Hantaviruses (Seoul, Puumala and Thottapalayam)Validation: 244 sera taken in Lobaye in 1995 (11.6% ELISA positive to EBOV) were retested with IFA to EBOV (May) & SUDV (Bon): 34% were positive. | Prevalence of EBOV seropositivity varied between 2% and 13% in different participant groups. |
| Bantu villagers: southern region of CAR (Lobaye, Belemboke, Nola, Bangassou) | C | 860 | 44 | EBOV 5.1% | ||||||
| Central African Republic38 | 1992-95 | Pygmy subgroup (Lobaye, Belemboke: all sites no known outbreaks) | C | 683 | 48 | EBOV 7.0% | ELISA (k) | Mean+2SD of negative controls ≥1:400 & OD sum of 4 dilutions>1.0 | Antigens: EBOV (May) Marv(Mus); tests performed by Institut Pasteur, Bangui.Validation: 14 positive & 54 negative samples sent to CDC (US) to be tested against strain antigens: all results confirmed. | Primary or secondary forest areas with some agricultural activities |
| Non-pygmy subgroup (Lobaye, Belemboke, Bangassou, Nola: all sites no known outbreaks) | C | 648 | 23 | EBOV 3.5% | ||||||
| Ogooue Ivindo, Gabon39 | 1995-96 | Residents of 3 encampments (Andock, Minkebe, Mekoua) in the area where the epidemic occurred (including some contacts) | B | 236 | 23 | EBOV/SUDV/RESTV 9.7% | ELISA (k) | mean+3SD of negative controls | Antigens: EBOV, SUDV, RESTV with known positive/negative controls | 1 positive serum from a survivor excluded from encampment group; unclear how many known case contacts are included in this group. |
| Residents of 3 outbreak villages (Mayibout 1 & 2, Mvadi) where cases were reported during the outbreak | B | 205 | 34 | EBOV/SUDV/RESTV 16.6% | ||||||
| Kikwit40 | 1995 | Healthcare workers in outbreak area (70% hospital; 30% health centre) who did not have known EVD | B | 400 | 8 | EBOV 2.0% | ELISA (k) | Sum of adjusted OD >1.25 | Antigen: EBOV | The 8 positives were from a group of 12 samples which were ‘borderline positive’ on 1st test. Only 4 of these samples were retested: all were negative and have been excluded.129 of the 402 subjects reported being ill during Ebola period. Two with fever and haemorrhage (tested EBOV negative) have been excluded. |
| Gabon 41 | 1996 | Selected asymptomatic family members directly exposed to body fluids during outbreaks in 1996 | A | 24 | 11 | EBOV 45.9% | ELISA (k) | Mean adjusted OD for 10 control samples | Antigen: EBOVValidation: confirmed with western blot on NP and VP40 proteins | Subjects were asymptomatic throughout and were sampled several times. 1st samples showed no antibodies suggesting no prior immunity; IgG appeared 15-18 days after first possible exposure.Paper also describes results of viral RNA detection after 2 rounds of RT-PCR, finding positive results in 7/11 antibody-positive individuals tested and 0/13 antibody-negative individuals. |
| Nouna River, Ogooue-Ivindo, Gabon42 | 1996 | Residents in gold-mining villages with contact exposure in 1995 epidemic | A | 56 | 12 | EBOV 21.4% | ELISA (?) | OD>mean +2SD of 3 known negative controls | Antigen: ebolavirus Gabon 95-39/3 (Centre International de Recherches Medicales de Franceville) | All subjects reported fever and diarrhoea at least once in 1-year period of study, but not haemorrhagic symptoms. IgG positive titre range (OD 310-2,666).Age, sex, ethnic group not associated with seropositivity. Non- significant difference in seropositivity in people on site during 1995 epidemic (8.2%) and not on site (3.7%), among those with no reported contact |
| Residents in same villages without contact exposure | B | 180 | 12 | EBOV 6.7% | ||||||
| Upper Ivindo River, Ogooue-Ivindo, Gabon43 | 1997 | Individuals from 8 permanent villages in outbreak-prone region (4 survivors excluded) | B | 975 | 10 | EBOV 1.0% | ELISA (k) | Mean OD negative controls +3SD | Antigen: EBOV, performed in National Institute for Communicable Diseases South Africa.Validation: All positives plus a random selection of 28 negatives were retested with same protocol in CDC (US)—all were confirmed with response mainly directed to NP, VP40, VP35 and sGP viral proteins. | Serosurvey done in 1997; questionnaires done in 1999 on 10 positives: only 1 had contact, none were ill. |
| Belarus & Ukraine44 | 1997 | ‘Foreign visitors’ mostly from Africa: unclear if any had history of EVD symptoms | C | 562 | 30 | EBOV 5.3% | IFA (w) | Not specified | Antigens: EBOV (May), MARV (Voege) LASV (Jos) | Authors suggest positive results among foreign visitors reflect historic infection/ recovered cases, and unexpected results reflect cross-reactivity with infections such as malaria, HIV and influenza. Other observers suggest the results are just as likely to be artifact.63 |
| Belarus/Ukraine residents ‘at risk of HIV’ | C | 506 | 20 | EBOV 4.0% | ||||||
| Blood donors from the Blood Transfusion Institute, MoH Belarus & workers at the Belorussian Scientific Research Institute of Epidemiology & Microbiology | C | 131 | 21 | EBOV 16.0% | ||||||
| Watsa region, DRC45 | 2002 | Efe tribe pygmies exposed to a possible case at some time in their lives in household, occupation or funeral setting; no history of haemorrhagic fever symptoms | A | 38 | 4 | EBOV10.5% | ELISA (k) | 2×mean +3SD of negative controls value | Antigen: EBOVODs were expressed as percent positivity of a confirmed EBOV-positive sample; negative controls were from 60 South African subjects ‘almost certain’ to be seronegative. | A total of 300 people were sampled from 39 communities. 137 who reported experiencing haemorrhagic fever symptoms sometime in their life are excluded from this summary. 22% of those reporting symptoms were IgG positive. |
| Efe pygmies no reported exposure to possible cases; no history of haemorrhagic fever symptoms | C | 125 | 22 | EBOV 17.6% | ||||||
| Gabon46 | 2005-08 | Random sample of asymptomatic people aged >16 years without exposure, over all 9 provinces of Gabon | C | 4349 | 667 | EBOV 15.3% | ELISA (k) & WB | Cut-off based on negative controls from a French population | Antigen: EBOV Validation: Random sample of 138 positives were tested by western blot in 2008 and all were positive to at least one EBOV antigen.53 | Gabon experienced 7 outbreaks between 1994 & 2002 affecting >20 villages and towns; in total there were 208 cases and 151 deaths. |
| Random sample of asymptomatic children from 6 villages in outbreak-prone province (Ogooue-Ivindo) | B | 362 | 47 | EBOV 12.9% | ||||||
| Bundibugyo, Uganda47 | 2007 | Adult contacts of survivors >18 y. Samples taken ~29 months after outbreak | A | 210 | 2 | EBOV/SUDV/TAFV/BDBV 1.0% | ELISA (s) | Mean OD of negative controls plus 3SD | Antigens: EBOV, SUDV, Tai Forest ebolavirus (TAFV), Bundibugyo ebolavirus (BDBV), MARV(Mus)Validation: 28/36 confirmed cases tested positive to BDBV, 20/36 to EBOV, 10/36 to SUDV, 12/36 to TAFV & 29/39 to any of the 4 strains. | 15/223 contacts positive but 13 were symptomatic at the time of the outbreak, therefore only the 2 asymptomatic contacts are included in the table. |
| Republic of Congo48 | 2011 | Healthy blood donors 18-65y, no known case exposure | C | 809 | 20 | EBOV 2.5% | Double IFA | Reciprocal endpoint titres ≥20 | Antigens: EBOV (ATCC 1978), MARV (Popp 1967)Authors state: ‘double IFA’ technique has higher specificity than ‘regular’ IFA because only antibodies that detect filoviral antigens in co-localisation with a monoclonal antibody are considered. | Seropositivity ranged from 1.6–4% depending on city/rural location; 4% in Pointe Noire. |
| Liberia [PREVAIL]49 | 2015 | Close contacts of cases. NB 126 of the contacts were sexual partners of survivors after discharge. | A | 760 | 98 | EBOV 12.9% | ELISA (Alpha) | unspecified | Antigen: EBOV | Preliminary results. Study excluded from meta-analysis of known case contact group (A) because unclear what proportion of participants were symptomatic. |
| Kono, Sierra Leone50 | 2015-16 | Asymptomatic close contacts of cases aged≥4 years who had been resident in quarantined houses during the period of active Ebola transmission | A | 185 s | 12 | EBOV 6.4% | ELISA (Alpha) | 4.7 U/ml | Antigen: EBOV GPValidation: 29/30 PCR-confirmed EVD survivors, and 3/132 community controls were positive: 96.7% sensitivity, 97.7% specificity | 2 other positives had fever. Not clear if negatives were asked about symptoms |
| Individuals from 3 villages without reported cases | C | 132 | 3 | 2.3 | ||||||
| Western Area, Sierra Leone51 | 2015 | Household contacts of cases, asymptomatic at the time EVD was in the household | A | 388 | 10 | EBOV 2.6% | ELISA (PHE) | Mean OD of negative controls+fixed OD measure (0.1) | Antigen: EBOV GP. ‘Positive’ only if repeat test was positiveValidation: 93/97 PCR-confirmed EVD survivors and 0/339 community controls were positive: sensitivity 95.9% (95%CI 89.9–98.9%); specificity 100% (95%CI 98.9–100%) | Tests were done on oral fluid. |
| Individuals from 3 villages in Western Area without reported EVD cases | C | 339 | 0 | EBOV 0% |
Overall estimates of ebolavirus seroprevalence in asymptomatic individuals
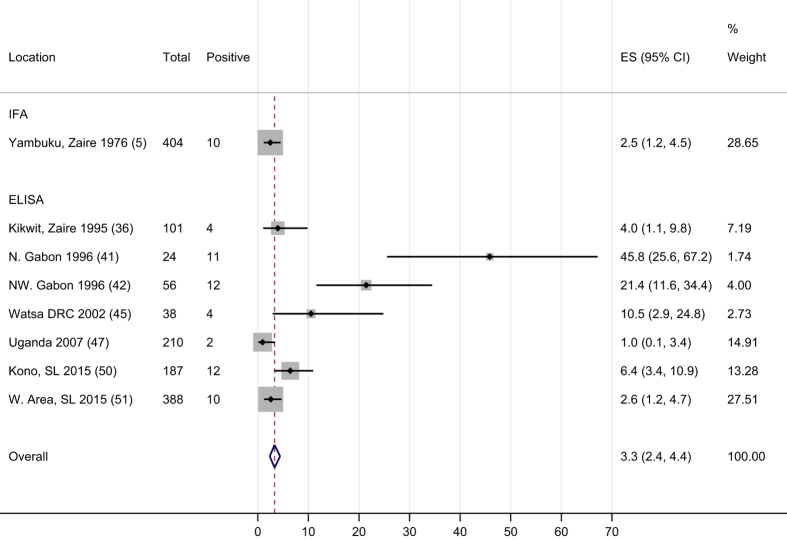
Only the group with known case-contact had exposures that are comparable across studies and are therefore appropriate to combine by meta-analysis. In this group eight study populations fulfilled the inclusion criteria of testing by ELISA or using a IFA cut-off ≥1:64 (ref. 5,36,41,42,47,49–51). Pooling these results gave an overall estimate of seroprevalence in asymptomatic people with known case-contact of 3.3% (95% CI 2.4–4.4, P<0.001), but with substantial heterogeneity due to three small studies with higher estimates.
In the other two categories—participants living in outbreak areas but without reported case-contact exposure and general populations in areas without known cases of EVD—exposure was either not well characterised or not well known. Even where EVD cases had not been reported, zoonotic exposure or different forms of disease manifestation could not be ruled out. The highly heterogeneous nature of these study populations makes any single summary estimate inappropriate. In outbreak areas estimates ranged from 0.9 to 17%, and in general populations described as unexposed estimates ranged from 0 to 24%.
Evidence of assay validation
Few teams reported any validation of the assays used. Some studies repeated analyses with the same technique, usually in a US or European laboratory, but only seven of the 51 studies reported validation work through a different diagnostic platform. Of these, two retested a proportion of IFA positives against ELISA, finding close to 100% consensus26,30. Three tested ELISA against western blot of which two found 100% specificity38,46,53; the third did not report results41. Another found 77 and 75% specificity for ELISA against western Blot and IFA respectively34, and a further study confirmed IFA results by western blot but did not report results33.
Two studies in Sierra Leone included field testing of ELISA assays in PCR-confirmed positive samples from EVD survivors and community controls with no known exposure to EVD cases from the research area. One, using a novel IgG-capture ELISA54, found 95.9% (95%CI 89.9–98.9%) sensitivity and 100% specificity (95%CI 98.9–100%) using oral fluid samples from 97 survivors and 339 community controls51. The other, using the commercially available ALPHA Diagnostics assay, selected a cut-off that gave 96.7% sensitivity and 97.7% specificity in serum samples from 30 survivors and 132 community controls50.
Discussion
We identified 51 studies covering 84 sample populations of 44,147 subjects reported to have had no symptoms of EVD during the outbreak period or to come from populations with no known outbreaks. Most data originated from Western and Middle Africa, and were collected during epidemiological investigations around outbreaks, or in serosurveys in countries without outbreaks but with similar ecology and animal hosts, which aimed to map the geographical extent of the virus. Some studies reported retrospective analysis of samples collected for other reasons prior to the first known outbreak in 1976.
An important finding of our review is the extreme heterogeneity of the studied populations and the lack of clarity in describing their exposure levels. We found that while some studies characterised their sample population clearly by level of contact and presence of symptoms, in many the level of contact/exposure was less clear, and some did not separate results for symptomatic and asymptomatic subjects. This makes comparison of results difficult, and combining results from the majority of the studies impossible. It may also explain the wide variation of findings which have perplexed investigators over time.
Many studies also employed very different cut-offs to define seropositivity meaning a simple review of results can be misleading. For our analysis, we excluded any study that used a cut off below≥1:64 for the studies using IFA, based on the advice in the literature, but there is no definitive evidence that this is an appropriate threshold. The cause of low IFA titre and whether it reflects false positives, or waning antibody response resulting from historical infection which may or may not have been symptomatic, has been frequently discussed. Recently 10 of 12 survivors from Yambuku were reported to have varying degrees of EBOV GP and NP reactivity by ELISA, 40 years after the outbreak55. Other studies have shown positive ELISA results in survivors up to 11 years after infection, but neither reported IFA results for comparison56.
There is no international reference measurement procedure for ebolavirus antibodies and the World Health Organisation has acknowledged the urgent need for one. Interestingly, given the scepticism often expressed regarding the specificity of IFA techniques in ebolavirus serology, a WHO collaborative study undertaken in 2015 to identify an interim reference standard found IFA no less specific or sensitive than the other methods employed, but only a few samples were tested57.
There are several limitations to the work presented here. The full information necessary for precision or clear interpretation was often not available. To pursue as high quality research as possible, we have focussed on publications that have undergone peer review and did not search grey literature. With the exception of Kuhn et al.52, which has been the standard reference on filovirus seroprevalence surveys to date, we did not search books. In addition to the limitations of the studies themselves noted above and in Table 1, we also note that the distinction of symptomatic and asymptomatic in the papers relied on self-reported health status, which may not be reliable.
To conclude, we present here a comprehensive updated review of seroprevalence surveys for ebolavirus infection in order to better understand the variation in rates found. We highlight the urgent need for validated standardised assays and for detailed characterisation of study population exposures to enable more generalizable estimates of the extent of asymptomatic ebolavirus infection to be made.
Methods
Search strategy and systematic review
A systematic search was done in PubMed to identify peer-reviewed papers presenting original data on ebolavirus infection seroprevalence using the following search string:
ebola AND (asymptom* OR antibod* OR IgG OR immun* OR ELISA OR serol*) NOT vacc* NOT immuniz* AND (Humans[Mesh])
No limitations were placed on language or location of study. Reference lists of the most comprehensive review to date52 and other papers were also reviewed. Although the focus of interest was data on subjects reported not to have symptoms at the time of an outbreak, we included papers reporting seroprevalence in all populations apart from those with diagnosed EVD in the initial review to ensure relevant studies were not missed.
The search produced 355 citations which were reviewed by title and abstract. Inclusion criteria were: investigation of any African species of ebolavirus immunoglobulin G (ie. not Reston) in individuals without ebolavirus symptoms or in general population groups, with information on denominators and seropositivity and description of those tested. The same search but limited to 2008 to 2016 was rerun on Web of Science; references prior to 2008 were checked against Kuhn et al’s list52. Four additional citations were found on Web of Science but none were retained for detailed reading. Six citations for papers not already included were identified from reference lists and retained for detailed reading.
Total citations: 365 of which 297 (81%) discarded for the following reasons:
Detailed immunology or genetics with no relevant data collection for seroprevalence
Description of acute phase diagnosis and/or investigation of convalescent subjects
Epidemiology and/or treatment of symptomatic confirmed cases without investigation of non-case populations
Investigations on sample populations without identifiable non-symptomatic individuals
Studies examining immune response related to vaccination trials
Review/comment articles without original data
Modelling papers without original data
Preliminary or duplicate reports of the same research study/data.
Sixty-eight papers were read in detail after which a further 20 were discarded for the reasons above. Data extracted from the remaining 48 papers included date of sera collection, composition of study population(s) in terms of exposure, location, selection process and any other defining characteristics, assay type, technique and antigens used, positivity threshold, number of participants per population type, number/proportion of IgG positive individuals, and any information on repeatability or test validity. All selected papers were scrutinised by both authors independently and results discussed and reconciled.
The last search was made on 31 July 2016. Two presentations from the 2016 Conference on Retroviruses and Opportunistic Infections (CROI, Feb. 2016) and one from the 8th International Symposium on Filoviruses (Sept. 2016) describing findings from the 2014–2016 outbreak were also included. A paper reporting one of the CROI presentations has subsequently been published (Nov 2016) and is referenced.
Categorisation of exposure
Many of the studies reported results on sub-populations with different exposures. To reduce heterogeneity for analysis we categorised these sub-populations under three broad headings according to the extent of exposure: household or known case-contact; living in outbreak areas but without reported case-contact; and subjects drawn from general populations in locations without known EVD. Where study populations were reported to include symptomatic cases and gave enough information to identify these cases, we removed them and recalculated results.
We excluded one study of PCR negative ‘suspects’ with close, no or unknown contact exposure due to lack of information on symptom status58. In two other studies, sub-groups were not included in the table because they were reported to include symptomatic cases but gave insufficient information to allow recalculation of the seroprevalence estimate excluding those with symptoms1,18.
Interpretation of seropositivity
We have recorded seropositivity results by antigen species where reported; where results were not reported by species, we record positivity to ‘ebolaviruses’. ‘Overall’ positivity is noted where it was reported or where it was possible to rule out double-counting.
To expose the problem of the different positivity thresholds used, we have recorded all studies and their reported cut-off in Table 1. Study characteristics and results have also been formatted as a machine-readable open access dataset (Data Citation 1).
Data visualisation
To summarise the data visually and present 95% confidence intervals, we created Forest plots for each of the three exposure categories (Figs 1,2,3) which allow results to be compared in the different contact groups. To address the problem of varying thresholds, we included only those IFA studies that reported results according to the 1:64 titre cut-off cited as more stringent by WHO and others5,18,21,59, or which reported enough detail for this threshold to be applied. For ELISA studies, the range of methods used to define positivity was too wide to assign a common threshold so all have been included in the Forest plots, with their method of defining the cut-off detailed in Table 1 (available online only).
Figure 1. Forest plot and meta-analysis of seroprevalence of ebolavirus IgG among contacts of EVD cases reported to be asymptomatic during the outbreak period.
Further details of each included study are given in Table 1. Legend: Ref: reference number; IFA: Immunofluorescence Assay; ELISA: Enzyme-linked immunosorbent assay; ES: Estimated proportion; N, NW: North, Northwestern; SL: Sierra Leone; W. Area: Western Area Province. Note: Zaire now Democratic Republic of Congo; Rhodesia now Zimbabwe.
Figure 2. Forest plot of seroprevalence of ebolavirus IgG in individuals reported to be asymptomatic during the outbreak period, recruited in areas with known EVD cases, excluding direct contacts of EVD cases.
Further details of each included study are given in Table 1. Legend: Ref: reference number; ES: Estimated proportion; IFA: Immunofluorescence Assay ELISA: Enzyme-linked immunosorbent assay; DRC: Democratic Republic of Congo; N, NE: North, Northeastern.
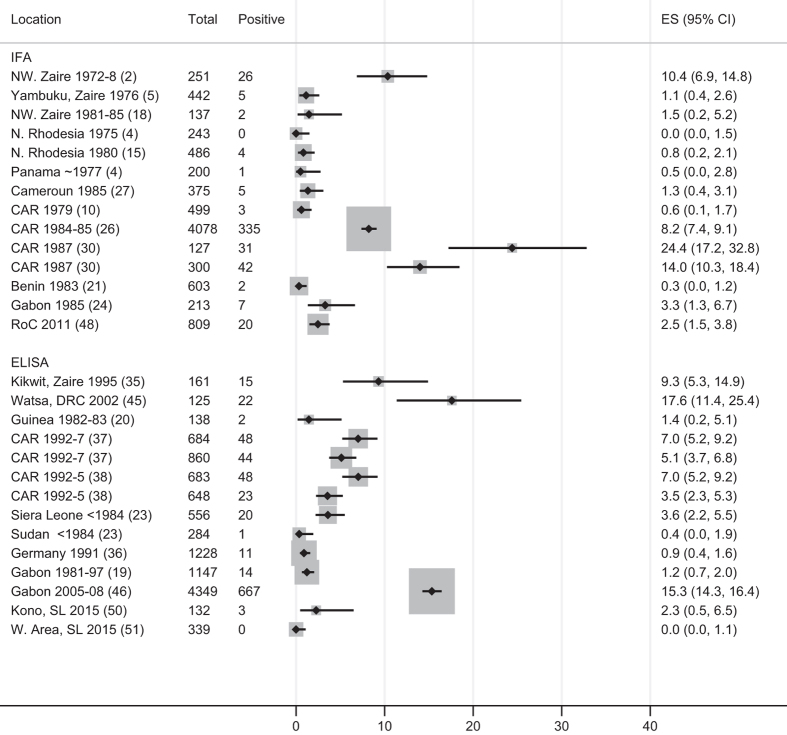
Figure 3. Forest plot of seroprevalence of ebolavirus IgG in general populations living in areas without reported EVD cases.
Further details of each included study are given in Table 1. Legend: Ref: reference number; IFA: Immunofluorescence Assay; ELISA: Enzyme-linked immunosorbent assay; ES: Estimated proportion; IFA: Immunofluorescence Assay; ELISA: Enzyme-linked immunosorbent assay; DRC: Democratic Republic of Congo; RoC: Republic of Congo; CAR: Central African Republic; N, NW: North, Northwestern. Note: Zaire now Democratic Republic of Congo; Rhodesia now Zimbabwe.
Statistical analyses
We performed a meta-analysis using the Freeman Tukey arcsine square root transformation method and ‘fixed effects’ (weighted average) inverse variance (metaprop, STATA60) on the eight study populations with known-case contact. We chose a ‘fixed effects’ (weighted average) model as contact should give similar risks in different contexts, and because random effects models give too much weight to small studies61. We present an pooled summary estimate for the group with known contact exposure (Fig. 1). We do not show summary estimates for the groups covering subjects living in outbreak areas but without reported case-contact, or drawn from general populations in locations without known EVD (Figs 2 and 3) as these populations are likely to have very different exposure levels so an overall summary estimate of prevalence would be meaningless.
Additional information
How to cite this article: Bower, H. & Glynn, J. R. A systematic review and meta-analysis of seroprevalence surveys of ebolavirus infection. Sci. Data 4:160133 doi: 10.1038/sdata.2016.133 (2017).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
We acknowledge with thanks the support of the Wellcome Trust’s Enhancing Research Activity in Epidemic Situations (ERAES) programme (Grant No. ER1502).
Footnotes
H.B. and J.R.G. declare they have no competing financial interests nor conflicts of interest. Both authors have had had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Data Citations
- Bower H, Glynn J. R. 2016. Dryad Digital Repository. http://dx.doi.org/10.5061/dryad.gn95r
References
- Tignor G. H., Casals J. & Shope R. E. The yellow fever epidemic in Ethiopia, 1961-1962: retrospective serological evidence for concomitant Ebola or Ebola-like virus infection. Transactions of the Royal Society of Tropical Medicine and Hygiene 87, 162 (1993). [DOI] [PubMed] [Google Scholar]
- van der Groen G. & Pattyn S. R. Measurement of Antibodies to Ebola Virus in Human-Sera from Nw-Zaire. Annales de la Societe belge de medecine tropicale 59, 87–92 (1979). [PubMed] [Google Scholar]
- Neppert J., Gohring S., Schneider W. & Wernet P. No Evidence of Lav Infection in the Republic-of-Liberia, West-Africa, in the Year 1973. Blut 53, 115–117 (1986). [DOI] [PubMed] [Google Scholar]
- van der Groen G., Johnson K., Webb F., Wulff H, Lange J. in Ebola Virus Haemorrhagic Fever (ed. Pattyn S. R. 141–142 (Elsevier/North-Holland Biomedical Press, 1978). [Google Scholar]
- The International Commission. Ebola Haemorrhagic Fever in Zaire. Bulletin WHO (1976).
- WHO International Study Team. Ebola haemorrhagic fever in Sudan, 1976. Report of a WHO/International Study Team. Bulletin of the World Health Organization 56, 247–270 (1978). [PMC free article] [PubMed] [Google Scholar]
- Heymann D. L. et al. Ebola Hemorrhagic Fever: Tandala, Zaire, 1977–1978. Journal of Infectious Diseases 142, 372–376 (1980). [DOI] [PubMed] [Google Scholar]
- Knobloch J., Albiez E. J. & Schmitz H. A serological survey on viral haemorrhagic fevers in Liberia. Annales de l'Institut Pasteur/Virologie 133, 125–128 (1982). [Google Scholar]
- Baron R. C., McCormick J. B. & Zubeir O. A. Ebola virus disease in southern Sudan: hospital dissemination and intrafamilial spread. Bull World Health Organ 61, 997–1003 (1983). [PMC free article] [PubMed] [Google Scholar]
- Saluzzo J. F., Gonzalez J. P., Herve J. P., Georges A. J. & Johnson K. M. Preliminary note on the presence of antibodies to Ebola virus in the human population in the eastern part of the Central African Republic. Bulletin de la Societe de pathologie exotique et de ses filiales 73, 238–241 (1980). [PubMed] [Google Scholar]
- Bouree P. & Bergmann J.-F. Ebola Virus Infection in Man: A Serological and Epidemiological Survey in the Cameroons. The American Journal of Tropical Medicine and Hygiene 32, 1465–1466 (1983). [DOI] [PubMed] [Google Scholar]
- Smith D. H. et al. Marburg-Virus Disease in Kenya. The Lancet 319, 816–820 (1982). [DOI] [PubMed] [Google Scholar]
- Johnson B. K. et al. Antibodies against haemorrhagic fever viruses in Kenya populations. Transactions of the Royal Society of Tropical Medicine and Hygiene 77, 731–733 (1983). [DOI] [PubMed] [Google Scholar]
- Ivanoff B. et al. Haemorrhagic fever in Gabon. I. Incidence of Lassa, Ebola and Marburg viruses in Haut-Ogooue. Transactions of the Royal Society of Tropical Medicine and Hygiene 76, 719–720 (1982). [DOI] [PubMed] [Google Scholar]
- Blackburn N. K., Searle L. & Taylor P. Viral haemorrhagic fever antibodies in Zimbabwe schoolchildren. Transactions of the Royal Society of Tropical Medicine and Hygiene 76, 803–805 (1982). [DOI] [PubMed] [Google Scholar]
- Talani P. K., Konongo J. D., Gromyko A. I., Nanga-Maniane, Yala F. & Bodzongo D. Prevelence des anticorps anti-fievres hamorragiques d'origine virale dans la region du Pool (Congo-Brazzaville) Médecine d'Afrique Noire 46 (1999).
- Van der Waals F. W., Pomeroy K. L., Goudsmit J., Asher D. M. & Gajdusek D. C. Hemorrhagic fever virus infections in an isolated rainforest area of central Liberia. Limitations of the indirect immunofluorescence slide test for antibody screening in Africa. Tropical and geographical medicine 38, 209–214 (1986). [PubMed] [Google Scholar]
- Jezek Z., Szczeniowski M. Y., Muyembe-Tamfum J. J., McCormick J. B. & Heymann D. L. Ebola between Outbreaks: Intensified Ebola Hemorrhagic Fever Surveillance in the Democratic Republic of the Congo, 1981–1985. Journal of Infectious Diseases 179, S60–S64 (1999). [DOI] [PubMed] [Google Scholar]
- Lahm S. A., Kombila M., Swanepoel R. & Barnes R. F. Morbidity and mortality of wild animals in relation to outbreaks of Ebola haemorrhagic fever in Gabon, 1994-2003. Transactions of the Royal Society of Tropical Medicine and Hygiene 101, 64–78 (2007). [DOI] [PubMed] [Google Scholar]
- Boiro I. et al. Clinico-epidemiologic and laboratory research on hemorrhagic fevers in Guinea. Bulletin de la Societe de pathologie exotique et de ses filiales 80, 607–612 (1987). [PubMed] [Google Scholar]
- Gonzalez J. P. Ebola Virus Circulation in Africa: a balance between clinical expression and epidemiological silence. Epidemiologie 98, 210–217 (2005). [PubMed] [Google Scholar]
- Rodhain F. et al. Arbovirus infections and viral haemorrhagic fevers in Uganda: a serological survey in Karamoja district, 1984. Transactions of the Royal Society of Tropical Medicine and Hygiene 83, 851–854 (1989). [DOI] [PubMed] [Google Scholar]
- Slenczka W., Rietschel M., Hoffmann C. & W S. Seroepidemiologische Untersuchungen über das Vorkommen von Antikörpern gegen Marburg und Ebola Virus in Afrika. Mitt. Österr. Ges. Tropical Med. Parasitol 6, 53–60 (1984). [Google Scholar]
- Meunier D. M. Y., Dupont A., Madelon M. C., Gonzalez J. P. & Ivanoff B. Surveillance sérologique des fièvres hémorragiques virales dans le Haut-Ogooue (Gabon). Annales de l'Institut Pasteur / Virologie 138, 229–235 (1987). [Google Scholar]
- Meunier D. M. et al. Current serologic data on viral hemorrhagic fevers in the Central African Republic. Bulletin de la Societe de pathologie exotique et de ses filiales 80, 51–61 (1987). [PubMed] [Google Scholar]
- Johnson E. D., Gonzalez J. P. & Georges A. Haemorrhagic fever virus activity in equatorial Africa: distribution and prevalence of filovirus reactive antibody in the Central African Republic. Transactions of the Royal Society of Tropical Medicine and Hygiene 87, 530–535 (1993). [DOI] [PubMed] [Google Scholar]
- Paix M. A. et al. Serological study of the virus responsible for hemorrhagic fever in an urban population of Cameroon. Bulletin de la Societe de pathologie exotique et de ses filiales 81, 679–682 (1988). [PubMed] [Google Scholar]
- Gonzalez J. P. et al. Antibody prevalence against haemorrhagic fever viruses in randomized representative Central African populations. Research in virology 140, 319–331 (1989). [DOI] [PubMed] [Google Scholar]
- Tessier S. F., Rollin P. E. & Sureau P. Viral haemorrhagic fever survey in Chobe (Botswana). Transactions of the Royal Society of Tropical Medicine and Hygiene 81, 699–700 (1987). [DOI] [PubMed] [Google Scholar]
- Johnson E. D., Gonzalez J. P. & Georges A. Filovirus activity among selected ethnic groups inhabiting the tropical forest of equatorial Africa. Transactions of the Royal Society of Tropical Medicine and Hygiene 87, 536–538 (1993). [DOI] [PubMed] [Google Scholar]
- Tomori O., Fabiyi A., Sorungbe A., Smith A. & McCormick J. B. Viral hemorrhagic fever antibodies in Nigerian populations. Am. J. Trop. Med. Hyg. 38, 407–410 (1988). [DOI] [PubMed] [Google Scholar]
- Mathiot C. C., Fontenille D., Georges A. J. & Coulanges P. Antibodies to haemorrhagic fever viruses in Madagascar populations. Transactions of the Royal Society of Tropical Medicine and Hygiene 83, 407–409 (1989). [DOI] [PubMed] [Google Scholar]
- US Centers for Disease Control. Update: filovirus infection associated with contact with nonhuman primates or their tissues. MMWR. Morbidity and mortality weekly report 39, 404–405 (1990). [PubMed] [Google Scholar]
- Becker S., Feldmann H., Will C. & Slenczka W. Evidence for Occurrence of Filovirus Antibodies in Humans and Imported Monkeys—Do Subclinical Filovirus Infections Occur Worldwide. Medical microbiology and immunology 181, 43–55 (1992). [DOI] [PubMed] [Google Scholar]
- Busico K. M. et al. Prevalence of IgG Antibodies to Ebola Virus in Individuals during an Ebola Outbreak, Democratic Republic of the Congo, 1995. Journal of Infectious Diseases 179, S102–S107 (1999). [DOI] [PubMed] [Google Scholar]
- Rowe A. K. et al. Clinical, virologic, and immunologic follow-up of convalescent Ebola hemorrhagic fever patients and their household contacts, Kikwit, Democratic Republic of the Congo. Journal of Infectious Diseases 179, S28–S35 (1999). [DOI] [PubMed] [Google Scholar]
- Nakounne E., Selekon B. & Morvan J. Microbiological surveillance: viral hemorrhagic fever in Central African Republic: current serological data in man. Bulletin de la Societe de pathologie exotique (1990) 93, 340–347 (2000). [PubMed] [Google Scholar]
- Gonzalez J. P., Nakoune E., Slenczka W., Vidal P. & Morvan J. M. Ebola and Marburg virus antibody prevalence in selected populations of the Central African Republic. Microbes and Infection 2, 39–44 (2000). [DOI] [PubMed] [Google Scholar]
- Georges A. J. et al. Ebola hemorrhagic fever outbreaks in Gabon, 1994-1997: epidemiologic and health control issues. The Journal of infectious diseases 179(Suppl 1): S65–S75 (1999). [DOI] [PubMed] [Google Scholar]
- Tomori O. et al. Serologic Survey among Hospital and Health Center Workers during the Ebola Hemorrhagic Fever Outbreak in Kikwit, Democratic Republic of the Congo, 1995. Journal of Infectious Diseases 179, S98–S101 (1999). [DOI] [PubMed] [Google Scholar]
- Leroy E. M. et al. Human asymptomatic Ebola infection and strong inflammatory response. The Lancet 355, 2210–2215 (2000). [DOI] [PubMed] [Google Scholar]
- Bertherat E., Renaut A., Nabias R., Dubreuil G. & Georges-Courbot M. C. Leptospirosis and Ebola virus infection in five gold-panning villages in northeastern Gabon. The American Journal of Tropical Medicine and Hygiene 60, 610–615 (1999). [DOI] [PubMed] [Google Scholar]
- Heffernan R. T. et al. Low seroprevalence of IgG antibodies to Ebola virus in an epidemic zone: Ogooue-Ivindo region, Northeastern Gabon, 1997. The Journal of infectious diseases 191, 964–968 (2005). [DOI] [PubMed] [Google Scholar]
- Vladyko A. S. et al. False-positive reactions in laboratory diagnosis of Lassa, Marburg, and Ebola viral hemorrhagic fevers and AIDS. Russian Progress in Virology 2, 25–30 (1997). [PubMed] [Google Scholar]
- Mulangu S. et al. High prevalence of IgG antibodies to Ebola virus in the Efe pygmy population in the Watsa region, Democratic Republic of the Congo. Bmc Infectious Diseases 16, 263 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkoghe D. et al. Risk factors for Zaire ebolavirus--specific IgG in rural Gabonese populations. The Journal of infectious diseases 204(Suppl 3): S768–S775 (2011). [DOI] [PubMed] [Google Scholar]
- Clark D. V. et al. Long-term sequelae after Ebola virus disease in Bundibugyo, Uganda: a retrospective cohort study. The Lancet Infectious Diseases 15, 905–912 (2015). [DOI] [PubMed] [Google Scholar]
- Moyen N. et al. Risk Factors Associated with Ebola and Marburg Viruses Seroprevalence in Blood Donors in the Republic of Congo. PLoS neglected tropical diseases 9, e0003833 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallah M. & PREVAIL III Research Team. A cohort study of survivors of Ebola Virus Infection in Liberia. Conference on Retroviruses and Opportunistic Infections (CROI). At: http://www.croiwebcasts.org/console/player/29569?mediaType=slideVideo& (2016).
- Richardson E. T. et al. Minimally Symptomatic Infection in an Ebola 'Hotspot': A Cross-Sectional Serosurvey. PLoS neglected tropical diseases 10, e0005087 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn J. B. et al. Asymptomatic infection and unrecognised Ebola Virus Disease, Sierra Leone (Filovirus 2016 Symposium, Antwerp, Belgium, 2016).
- Kuhn J. H. & Calisher C. H. Filoviruses: a compendium of 40 years of epidemiological, clinical and laboratory studies. (Springer Science and Business Media, 2008). [PubMed] [Google Scholar]
- Becquart P. et al. High Prevalence of Both Humoral and Cellular Immunity to Zaire ebolavirus among Rural Populations in Gabon. PLoS ONE 5, e9126 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe T. et al. Detection of Vaccine-Induced Antibodies to Ebola Virus in Oral Fluid. Open forum infectious diseases 3, ofw031 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remoin A. et al. Persistent Immune Response in Ebola Survivors from Yambuku Outbreak 40 years after Infection (Filovirus 2016 Symposium, Antwerp, Belgium, 2016).
- Wauquier N., Becquart P., Gasquet C. & Leroy E. M. Immunoglobulin G in Ebola outbreak survivors, Gabon. Emerg. Infect. Dis. 15, 1136–1137 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Expert committee on Biological Standardisation. WHO collaborative study to assess the suitability of an interim standard for antibodies to Ebola virus. Report No. WHO/BS/2015.2280 post-ECBS, (World Health Organisation (2015).
- de La Vega M. A. et al. Ebola viral load at diagnosis associates with patient outcome and outbreak evolution. The Journal of clinical investigation 125, 4421–4428 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattyn S. R. Ebola Virus Haemorrhagic Fever: Proceedings of an International Colloquium on Ebola Virus Infection and Other Haemorrhagic Fevers held in Antwerp, Belgium, 6-8 December, 1977. (Elsevier/North-Holland Biomedical Press, 1978). [Google Scholar]
- Nyaga V. N., Arbyn M. & Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Archives of Public Health 72, 39 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. & Green S. (eds) in The Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0) (The Cochrane Collaboration).
- Wulff H. & Lange J. Indirect Immunoflouresce for the diagnosis of Lassa Fever Infection. Bulletin of the World Health Organization 52, 429–436 (1975). [PMC free article] [PubMed] [Google Scholar]
- Johnson K. M., Elliott L. H. & Heymann D. L. Preparation of polyvalent viral immunofluorescent intracellular antigens and use in human serosurveys. Journal of clinical microbiology 14, 527–529 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner P. S. & McQuillin J. Rapid virus diagnosis, application of immunoflourescence. (Butterworth & Co, 1974). [Google Scholar]
- Bashkirtsev V. N., Tkachenko E. A., Dzagurova T. K. & Ryl'tseva E. V. Isolation of strains of the virus of hemorrhagic fever with renal syndrome in cell culture. Voprosy virusologii 29, 497–502 (1984). [PubMed] [Google Scholar]
- Emmerich P. et al. Reverse ELISA for IgG and IgM antibodies to detect Lassa virus infections in Africa. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology 37, 277–281 (2006). [DOI] [PubMed] [Google Scholar]
- Ksiazek T. G., West C. P., Rollin P. E., Jahrling P. B. & Peters C. J. ELISA for the Detection of Antibodies to Ebola Viruses. Journal of Infectious Diseases 179, S192–S198 (1999). [DOI] [PubMed] [Google Scholar]
- Schoepp R. J., Rossi C. A., Khan S. H., Goba A. & Fair J. N. Undiagnosed acute viral febrile illnesses, Sierra Leone. Emerg Infect Dis 20, 1176–1182 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisen M. L. et al. Multiple circulating infections can mimic the early stages of viral hemorrhagic fevers and possible human exposure to filoviruses in Sierra Leone prior to the 2014 outbreak. Viral immunology 28, 19–31 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niklasson B., Peters C. J., Grandien M. & Wood O. Detection of human immunoglobulins G and M antibodies to Rift Valley fever virus by enzyme-linked immunosorbent assay. Journal of clinical microbiology 19, 225–229 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezapkin G. V., Tkachenko E. A., Ivanov A. P., Bashkirtsev V. N. & Dzagurova T. K. Determination of arenavirus antigens and antibodies by solid-phase radioimmunological analysis. Voprosy virusologii 459–462 (1981). [PubMed] [Google Scholar]
- Johnson B. K. et al. Viral haemorrhagic fever surveillance in Kenya, 1980-1981. Tropical and geographical medicine 35, 43–47 (1983). [PubMed] [Google Scholar]
- US Centers for Disease Control. Update: evidence of filovirus infection in an animal caretaker in a research/service facility. MMWR. Morbidity and mortality weekly report 39, 296–297 (1990). [PubMed] [Google Scholar]
- US Centers for Disease Control. Update: filovirus infections among persons with occupational exposure to nonhuman primates. MMWR. Morbidity and mortality weekly report 39: 266–267; 273 (1990). [PubMed] [Google Scholar]
- Alpha Diagnostic International, I. Zaire-Ebola virus nucleoprotein (EBOV-NP) IgG (AE-320520-1) kit and IgM (AE-320530-1) ELISA kit product descriptions. http://www.4adi.com/ (2016). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Bower H, Glynn J. R. 2016. Dryad Digital Repository. http://dx.doi.org/10.5061/dryad.gn95r