Abstract
The five-year survival rate for patients with malignant glioma is less than 10%. Despite aggressive chemo/radiotherapy these tumors have remained resistant to almost every interventional strategy evaluated in patients. Resistance to these agents is attributed to extrinsic mechanisms such as the tumor microenvironment, poor drug penetration, and tumoral heterogeneity. In addition, genetic and molecular examination of these tumors has revealed defective apoptotic regulation, enhanced pro-survival autophagy signaling, and a propensity for necrosis that aids in the adaptation to environmental stress and resistance to treatment. The combination of extrinsic and intrinsic hallmarks in glioma contributes to the multifaceted resistance to traditional anti-tumor agents. Here we describe the biology of the disease relevant to therapeutic resistance, with a specific focus on molecular deregulation of cell death pathways. Emerging studies investigating the targeting of these pathways including BH3 mimetics and autophagy inhibitors are being evaluated in both the preclinical and clinical settings are discussed. This review highlights the pathways exploited by glioblastoma cells that drive their hallmark pro-survival predisposition and makes therapy development such a challenge.
Keywords: glioma, apoptosis, autophagy, necrosis, resistance
While primary malignant brain tumors are rare, the 5-year survival for these patients is dismal at approximately 10%. Utilizing the World Health Organization based classification, these tumors are categorized as low-grade (I-II) or high-grade malignant gliomas (III-IV)[1]. Specifically, grade IV glioma or glioblastoma (GBM) [2] are the most common and destructive form of malignant glioma. Headaches, seizures, cognitive and personality changes, gait imbalance, sensory loss, and incontinence are common symptoms and can be dependent on the location of the tumor. Symptomatic management for these patients consists mainly of steroids to relieve neurological symptoms associated with edema and anticonvulsants in patients with seizures. Current treatment strategies include surgical resection, radiation and chemotherapy for newly diagnosed GBM and bevacizumab and the Novo TTF device for recurrent GBM. However, despite rigorous molecular, preclinical, and clinical research these tumors remain a challenge to treat.
Despite aggressive surgical resection and concurrent chemo/radiotherapy 70% of patients diagnosed with GBM will succumb to the disease within two years. A better understanding of the molecular changes in these malignant cells in response to therapy is necessary to unveil how these malignant cells can survive intense chemo/radiation insult. Recent studies have identified numerous mechanisms by which cells “commit suicide” (Fig. 1) and a better understanding of the mechanisms of cell death exploited by different therapeutics is essential for the rational design of future therapeutic strategies. Here we discuss the current treatment strategies utilized to kill GBM cells, and various cell death mechanisms relevant to glioma destruction and resistance.
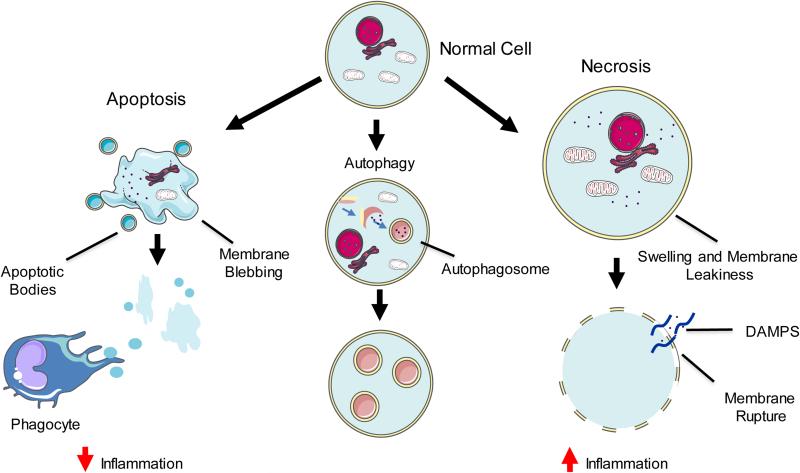
Fig. 1. Morphological classification of cell death.
Cell death has been traditionally characterized as: Apoptosis – cellular shrinkage, membrane blebbing, chromatin condensation, and nuclear fragmentation. Small apoptotic bodies are digested by phagocytic immune cells, reducing potential inflammation. Autophagic cell death – increased sequestration of cytoplasmic components by double membrane vesicles (autophagosomes) resulting in an extensive autophagic vacuolization of the cytoplasm. Necrosis – swelling of cytoplasm and organelles, an electron lucent cytoplasm, and loss of plasma membrane integrity. Release of damage-associated molecular pattern molecules (DAMPs) leads to high levels of inflammation.
Apoptosis
Apoptosis is perhaps the most well studied form of cellular demise consisting of an energy-dependent cascade of molecular signaling typically involving the cysteine-dependent aspartate-directed proteases called caspases. Caspase-dependent apoptosis is subcategorized as extrinsic or intrinsic, but both signaling cascades converge on similar downstream execution caspases (caspase-3 and -7). Extrinsic apoptosis is induced through death receptor signaling and intrinsic apoptosis is initiated through upstream stress signaling resulting in mitochondrial outer membrane permeabilization (MOMP), allowing for the release of pro-apoptotic proteins from the intermembrane space and subsequent cleavage and activation of executioner caspases. Typically, the induction of MOMP is dependent on BAX or BAK, which when activated can homo-oligomerize and insert into the outer mitochondrial membrane [3]. BAX and BAK are tightly regulated through the binding and inhibition of anti-apoptotic Bcl-2 proteins (Bcl-2, Mcl-1, and Bcl-xL). A further layer of control is regulated by pro-apoptotic BH3-only Bcl-2 proteins that can activate BAX/BAK directly or indirectly through inhibition of anti-apoptotic Bcl-2 family proteins [4]. While there are numerous BH3-only Bcl-2 proteins, BAD, BID, BIM, NOXA, and PUMA are some of the best studied.
There has been a considerable appreciation for the apoptosis resistant nature of GBM (Fig. 2). The most well elucidated aberrant apoptotic pathway in GBM is the deregulation of Bcl-2 family proteins. The expression level of BH3-only proteins has been observed to inversely correlate with overall survival in GBM patients [5]. The Bcl-2 family rheostat was shown to be further shifted towards an anti-apoptotic state in patients with recurrent GBM, as these tumors displayed increased protein levels of anti-apoptotic Bcl-2, Bcl-xL, and Mcl-1 with a concurrent decrease in the important apoptotic protein BAX [6]. Deregulation of the Bcl-2 rheostat has also been associated with cancer stem cell resistance to apoptosis. Specifically, Bcl-2 has been observed to be elevated in GBM stem cells [7]. In addition, GBM cells with the common EGFRvIII variant have been shown to confer resistance to chemotherapy through elevation of the anti-apoptotic Bcl-xL [8]. Interestingly, both activated EGFR and EGFRvIII have been observed to influence resistance to apoptosis through the direct binding and inhibition of PUMA [9].
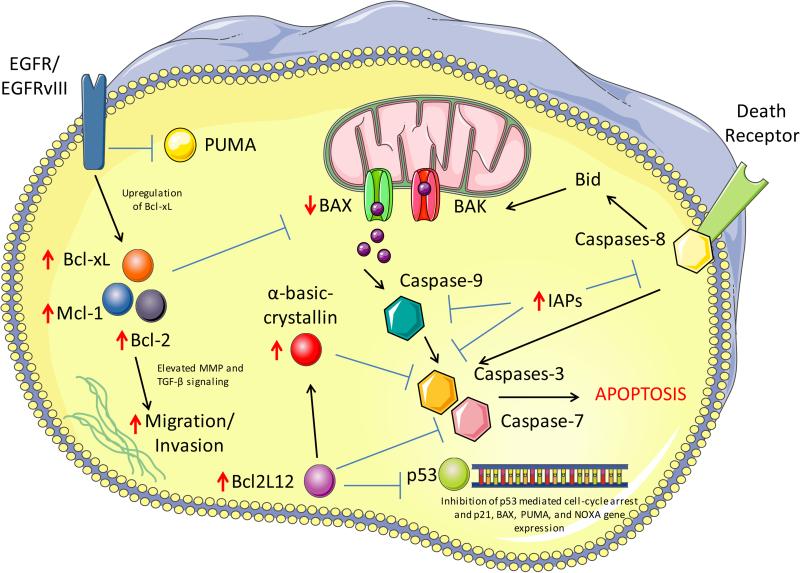
Fig. 2. Defective apoptosis signaling in GBM.
GBM cells utilize numerous mechanisms to resist DNA damage induced cell death. GBM cells have increased levels of anti-apoptotic proteins Bcl-2, Bcl-xL, and Mcl-1 (indicated by upward red arrow) as well as a decrease in the apoptotic protein BAX (indicated by downward red arrow) leading to a predisposed anti-apoptotic state. EGFR and EGFRvIII signaling further contribute towards apoptosis resistance through direct inhibition of the BH3-only protein PUMA and activation of EGFRvIII specifically can lead to elevated Bcl-xL. Bcl2L12 (also frequently elevated in GBM) binds and inhibits the cell cycle and pro-apoptotic gene transcription of p53 as well as inhibiting executioner caspases directly (caspase-7) and indirectly (caspase-3) through elevation of α-basic-crystallin. Several inhibitors of apoptosis family proteins are also frequently elevated in GBM, which inhibit caspase activation at numerous levels.
In a recent study, Stegh et al. identified and characterized the non-canonical Bcl-2 family protein Bcl-2-like-12 (Bcl2L12), which was observed to be overexpressed almost universally in GBM [10]. Surprisingly, Bcl2L12 did not act upstream of MOMP with no effect on cytochrome c release and subsequent apoptosome formation, but acted directly on the activation of caspase-3 and -7 through distinct mechanisms [10, 11]. In addition to its function in the inhibition of post-mitochondrial caspase activation, Bcl2L12 was observed to bind to p53 and inhibit the induction of cell cycle arrest and proapoptotic gene transcription including p21, BAX, PUMA, and NOXA [12]. Although small molecule inhibition of Bcl-2 family proteins has been investigated in numerous cancer types, the use in GBM has been relatively unexplored. The Bcl-2 inhibitors HA14-1 and ABT-737 have been shown to promote apoptosis in GBM cells, particularly in combination with other apoptotic-inducing agents such as chemo/radiotherapy [13, 14]. Gossypol is currently the only Bcl-2 targeting drug that has been evaluated clinically in GBM. Gossypol, which has also been observed to cause damage to the plasma and mitochondrial membranes, is able to bind to the BH3 pocket of both Bcl-2 and Bcl-xL [15]. Two clinical trials evaluating tumor response rates and dosage/toxicity in combination with the standard of care in GBM have completed, but results of these trails have yet to be published (NCT00540722 and NCT00390403).
Inhibitor of apoptosis family proteins (IAPs), which can bind to and inhibit numerous caspases, have also been observed to be deregulated through elevated expression in GBM [16]. One the most well characterized IAPs in GBM, survivin, is inversely correlated with overall survival and is associated with reduced apoptotic capacity [17]. Inhibition of IAP proteins has been an effective strategy to overcome apoptotic resistance in response to a variety of therapeutic strategies including growth factor inhibitors [18], radiotherapy [19], Apo2L/TRAIL [20], and chemotherapy with Temozolomide (TMZ) [21]. Additional mutations and deregulation of other apoptotic mediators such as p53 and death receptor signaling have also been explored and are garnering interest as barriers to effective apoptotic-inducing agents in GBM.
DNA damage induced apoptosis is thought to be the major mechanism of cell death after radiation and chemotherapy. Radiotherapy is typically given over the course of multiple fractions for several weeks culminating in about a 60 Gy total dose and remains a standard adjuvant post resection. Identification of agents that function as radiosensitizers to improve patient response to radiotherapy is an active area of research [22]. TMZ and the biodegradable polymers containing carmustine are currently the only two chemotherapeutic agents approved by the US Food and Drug Administration (FDA) for first-line therapy for GBM [23]. A landmark randomized phase III trial revealed that concomitant and adjuvant TMZ with radiation to result in a median survival of 14.6 months compared to 12.1 months for radiotherapy alone [24]. TMZ, a DNA alkylating agent, induces numerous DNA adducts, with 06-methylguanine regarded as the most cytotoxic. If not repaired by 06-methylguanine-DNA methyltransferase (MGMT), futile repair cycles by the mismatch repair system will eventually lead to the stalling of the replication fork, subsequent double-stranded DNA breaks, and induction of apoptosis [25]. The expression of MGMT has been shown to be a significant prognostic factor for TMZ response [26], and its protein expression has been shown to be associated with resistance to TMZ in numerous primary GBM cultures in vitro and in vivo. Additional MGMT-independent resistance mechanisms are also thought to play a significant role in GBM recurrence after chemo/radiotherapy and are currently being investigated. To date, no other chemotherapies are approved for newly diagnosed or recurrent GBM, but there are a number of open Phase III clinical trials. A phase II/III trial for newly diagnosed GBM patients with TMZ and the PARP inhibitor Veliparib is ongoing (NCT02152982). PARP inhibitors (also thought to induce apoptosis) have demonstrated strong anti-tumor efficacy against GBM in preclinical studies via their ability to inhibit DNA damage repair [27-30]. While apoptosis inducing chemotoxic agents hold promise, the eventual appearance of resistance has led to disappointing results, underscoring the urgent need to understand tumor escape mechanisms.
Autophagy
Autophagy is a form of self-cannibalism recycling process characterized by a coordinated multi-step process in which cytoplasmic components are sequestered in a double membrane vesicle and eventually fused with the lysosome (Fig. 3). In normal healthy tissues, autophagy is responsible for the degradation and recycling of damaged organelles and misfolded proteins, but it can be deregulated in autoimmune disorders, neurodegenerative diseases, and cancer [31]. Reducing the accumulation of damaged misfolded proteins/organelles can limit genomic instability and inflammation, pathways linked to tumor initiation. A putative tumor suppressor role of autophagy has been suggested as mutations in several autophagy genes have been associated with numerous cancers [32]. The role of autophagy in the development of cancer and response to chemotherapy has been highly debated and the current hypothesis is that autophagy may suppress tumor initiation, but promote established tumor progression and resistance to therapeutics [32, 33]. Although some chemotherapeutics have been shown to induce autophagic cell death, autophagy signaling has been primarily observed to provide a pro-survival response to both intrinsic and environmental stress. Robust autophagy signaling is considered a significant obstacle in overcoming resistance to numerous therapeutic agents, which has led to the use of autophagy inhibitors in combination with numerous treatment strategies. In particular, PI3K and mTOR inhibition using Rapamycin and other small molecule inhibitors has been shown to induce robust autophagy signaling [34]. Knockdown of essential autophagy genes and/or pharmacologic inhibitors have been shown to enhance the efficacy of PI3K-mTOR inhibitors, as well as other autophagy inducing treatments such as growth factor inhibitors, histone deacetylase inhibitors, proteasome inhibitors, and interferon-β treatment suggesting its role in cellular defense against these agents (Table 1) (Fig. 3) [34-38]. Autophagy has also been described as a mechanism of resistance to TMZ-induced cell death and recent studies have pointed towards up-regulation of the vesicle associated membrane protein 8 (VAMP8) as a potential component in inducing autophagy and TMZ resistance [39]. A novel TMZ analog NEO212, which induces ER stress and blocks autophagy apart from DNA alkylation was recently tested in preclinical studies and found to be promising [40].
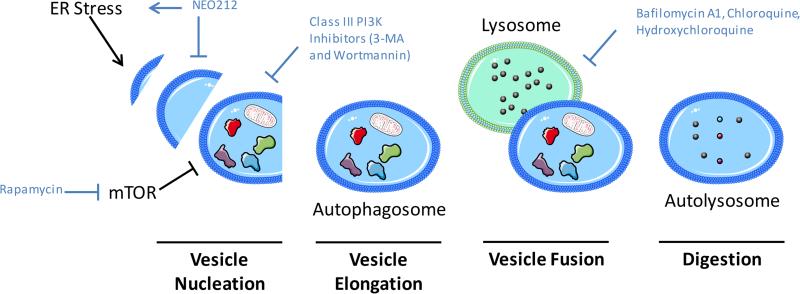
Fig. 3. Autophagy signaling mediates resistance to therapeutic agents.
Knockdown of essential autophagy genes and/or pharmacologic inhibitors have been shown to enhance the efficacy of anti-cancer agents. NEO212 induces ER stress and inhibits autophagy. Rapamycin strongly inhibits mTOR a negative regulator of autophagy, while Class III PI3K inhibitors prevent PI3K mediated autophagy induction. Bafilomycin inhibits lysosome and autophagosome fusion while Chloroquine and Hydroxychloroquine alter endosomal pH interfering with end stage autophagy.
Table 1.
Preclinical studies to overcome autophagy mediated resistance to chemotherapy.
| Drug | Function | Findings | Reference |
|---|---|---|---|
| ZD6474 | Small molecule inhibitor of receptor tyrosine kinases (VEGFR, EGFR, and RET). | Co-treatment of GBM xenografts with ZD6474 and chloroquine inhibited autophagic resistance and induced tumor cell death. | [35] |
| NVP-BEZ235 | mTOR and PI3K inihibitor | NVP-BEZ235 synergized with chloroquine to inhibit autophagic resistance and induce cell death in GBM xenografts | [34] |
| Vorinostat | Histone deacetylase inhibitor | Combination of Vorinostat treatment with ATG7 siRNA resulted in increased apoptotic cell death of GBM cells in vitro | [36] |
| Interferon Beta (IFN-β) | Anti-proliferative and immunomodulatory cytokine | Inhibition of ERK 1/2 activation inhibited autophagic resistance to IFN-β mediated cell death in GBM cells | [37] |
GBM is characterized by hypervascular proliferation and angiogenesis accompanied by increased production of Vascular Endothelial Growth Factor (VEGF). Bevacizumab, a humanized monoclonal antibody against VEGF, has been tested against GBM and gained FDA approval for the treatment of recurrent GBM in 2009 [41]. Optimism from its clinical use coupled with the accelerated FDA approval of bevacizumab for recurrent GBM led to the assessment of bevacizumab for patients with newly diagnosed GBM. Two similar randomized phase III clinical trials evaluating bevacizumab in combination with TMZ and radiotherapy were conducted and both of these studies reported a benefit in progression free survival, but did not see a significant increase in overall survival (OS) [42, 43]. While bevacizumab was found to be safe and tolerable for up front treatment of GBM, the OS did not support the addition of bevacizumab as a standard of care for first-line treatment. Thus, the development of acquired resistance and tumor regrowth following treatment has been a significant impediment. Among other mechanisms of compensation for reduced tumor angiogenesis, autophagy in glioma is elevated in areas of hypoxia and poor perfusion [44]. It has been hypothesized that autophagy induction could be a mechanism of resistance to bevacizumab, as increased hypoxia and the autophagy-mediating BNIP3 were observed in clinical samples from patients who acquired resistance to bevacizumab treatment compared to patient matched pretreatment samples [44]. In preclinical models, the inhibition of autophagy in combination with bevacizumab increased in vivo efficacy in GBM xenograft models [44]. These studies provide preliminary evidence for pro-survival autophagy signaling as a mechanism of resistance to anti-angiogenic therapy in GBM and support further clinical studies combining autophagy inhibitors with anti-angiogenic therapeutics.
The accumulation of preclinical studies describing the influence of autophagy signaling in acquired resistance to chemo/radiotherapy has led to evaluation of autophagy inhibitors in the clinic. Chloroquine, which has been utilized in the clinic for the treatment of malaria and autoimmune disorders for a number of years, is a lysosomotropic agent that alters endosomal pH and interferes with end stage autophagy. Interestingly, CQ co-treatment or ATG7 knockdown in preclinical models treated with bevacizumab has also been shown to result in increased in vivo efficacy in GBM xenograft models. A small randomized double-blinded placebo controlled study evaluating CQ in combination with conventional chemo/radiotherapy observed a median survival of 24 months compared to 11 months in placebo controls, but because of the small sample size the study was not powered sufficiently and failed to reach statistical significance [45]. A recent phase I/II clinical trial evaluated the combination of Hydroxychloroquine (differs from CQ by additional hydroxyl group) (HCQ), radiation, and TMZ in patients with newly diagnosed GBM [46]. Although inhibition of autophagy was achievable using high doses of HCQ, dose-limiting toxicity in combination with TMZ and radiotherapy prevented a conclusive evaluation for the potential use of autophagy inhibition with HCQ as an adjuvant treatment for GBM patients. Although initial preclinical and clinical data using CQ and HCQ have been encouraging, these inhibitors lack specificity and are limited by toxicity in combination with chemotherapeutic drugs. As a result, novel drug identification and development is needed to evaluate the clinical impact of autophagy inhibition with chemo/radiotherapy.
Interestingly, while efforts to block autophagy have successfully been used to sensitize tumors to chemo/radiotherapy, the induction of autophagy has also been exploited to synergize with alternative therapies. Recent reports have shown synergistic interactions between oncolytic adenoviruses and other agents that induce autophagy [47]. Exploitation of TMZ-induced autophagy to aid virus replication and overall anti-tumor efficacy is currently being evaluated in patients (NCT01956734).
Necrosis/Necroptosis
Traditionally necrosis has been thought of as an accidental and passive form of cell death, but accumulating evidence suggests this process is regulated. Morphological characteristics of necrotic cell death include swelling of cytoplasmic organelles, an electron-lucent cytoplasm, and loss of plasma membrane integrity. Typical mediators of necrotic cell death poly-(ADP-ribose) polymerase (PARP) over-activation, Ca2+ influx, and reactive oxygen species (ROS) result in ATP depletion and failure to maintain plasma membrane integrity [48]. Well recognized for its resistance to apoptosis, GBM has been shown to have a propensity for tumoral necrosis as well. An intriguing connection between apoptotic and necrotic cell death in GBM has been proposed to involve the novel GBM oncoprotein Bcl2L12. This protein has been hypothesized to be a significant driver of apoptosis resistance in GBM through the inhibition of caspase-3 and -7 activation and p53 function as described earlier. The substantial resistance to apoptosis caused by Bcl2L12 and elevation of other necrotic cell death mediators such as cathepsins, have been proposed to be causal factors for the widespread necrosis found in these tumors [49-51]. The idea of programmed necrosis has been hypothesized and debated for many years, but recently came to the forefront with the seminal study by Degterev et al. in which they coined the term “necroptosis” to describe a specific form of non-apoptotic programmed necrotic cell death [52]. Necroptosis is a caspase-independent type of cell death mediated by high levels of ROS induced by activity of the receptor interacting protein 1 kinase (RIP1). RIP1 is a serine/threonine kinase containing an essential death domain and is pivotal for necroptosis, but not required for NF-kB activation and apoptotic signaling [46]. Apart from high levels of Bcl2L12, low levels of caspase-8 have also been associated with necroptotic cell death in glioma treated with a synthetic alkylphospholipid analog [53]. Future studies will be necessary to evaluate the use of necroptotic-inducing agents as a strategy to treat GBM tumors that possess resistance to traditional apoptosis-inducing therapies.
Necrotic cell death is often immunogenic and it is associated with the release of damage associated molecular patterns (DAMPs) that can trigger inflammation and activate anti-tumor immune responses [54]. Immune cell dictated cell death is frequently associated with necrosis or necroptotic cell death and several ongoing studies are currently evaluating immunotherapies for GBM. For example, Northwest Bio therapeutics is currently sponsoring a phase III trial for newly diagnosed GBM evaluating the dendritic cell vaccine DCVax® (NCT00045968). The combination of the anti-PD1 antibody Nivolumab and the CTLA-4 antibody Ipilimumab, is also currently in phase III clinical trials for recurrent GBM (NCT02017717). A variety of other targeted chemotherapeutic drugs and immunotherapies, such as oncolytic viruses are also being investigated for efficacy against brain tumors [55]. While the putative activation of inflammation could reverse the immunosuppressive nature of GBM and potentially synergize with necroptotic-inducing agents through modulation of tumor microenvironment, this has not been explored for GBM in detail. Given the inherent resistance of glioma cells to apoptotic-inducing mechanisms of cell death, a more comprehensive understanding of necrotic/necroptotic cell death involvement in glioma can be exploited for future therapeutic development.
Alternative forms of cell death
Numerous alternative mechanisms of cell death including entosis, mitotic catastrophe, anoikis have been recently identified, but their role in GBM progression and/or response to therapy is not known. The NovoTTF-100A System is a device that utilizes alternating electric fields to induce catastrophic, anti-mitotic effects on dividing cells. A phase III study evaluating safety and efficacy of Novo TTF in recurrent GBM patients revealed comparable efficacy and activity of this device with chemotherapy leading to its FDA approval for recurrent GBM [56]. It is interesting to note that no chemotherapy has currently been shown to be an effective treatment for recurrent GBM, and the device was approved for lack of toxicity and side effects frequently associated with chemotoxic agents. Ongoing studies of Novo TTF are designed to test efficacy of this device in newly diagnosed GBM (NCT00916409) and confirm efficacy in recurrent patients (NCT01756729).
While not technically its own type of cell death, lysosomal membrane permeabilization (LMP) has been shown to have a significant influence on cell fate. Lysosomes contain various hydrolytic enzymes including proteases, lipases, and nucleases which has led to the term “suicide bags,” describing the serious damage inflicted by the release of these enzymes into the cytosol [57]. LMP allows for the release of cathepsins and other proteases into the cytosol causing toxic digestion of vital intracellular proteins and the activation of caspases. The most well elucidated components of LMP leading to apoptosis are cathepsins B and D, which can cleave and activate BID, resulting in MOMP and subsequent intrinsic apoptotic signaling [58]. Recently, thymoquinone was shown to induce glioma cell death by blocking autophagy and inducing LMP [59]. On the other hand, large and uncontrolled LMP can led to necrotic cell death void of caspase activation providing a crossroads for cell fate determinations [60]. The significance of theses novel mechanisms of cell death is currently being uncovered, and further studies will be necessary to evaluate the translation of these findings into putative strategies for therapeutic development.
Conclusions
Detailed investigation of GBM has led to the identification of various genetic, molecular, and environmental alterations that contribute to the pathology of the disease and to the failure of traditional therapeutic strategies. Specifically, numerous areas of deregulation in various cell death pathways provides tumor cells avenues for escaping cell death when placed under stress from therapy or rapid tumor growth. Future translational studies taking into account the interactions between different molecular mechanisms of death will be pivotal in identifying successful therapeutic strategies for GBM.
Acknowledgments
The authors acknowledge Servier Medical Art Image Bank (http://www.servier.com/Powerpoint-image-bank) for the free image components used to create Figures 1-3 (http://creativecommons.org/licenses/by/3.0/legalcode).
Footnotes
Ethical Statement
This article does not contain any studies with human participants or animals performed by any of the authors. The authors declare that they have no conflict of interest.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang YG, Peng Y, Koussougbo KS. Necroptosis: a novel therapeutic target for glioblastoma. Med Hypotheses. 2011;76(3):350–2. doi: 10.1016/j.mehy.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 3.Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nature reviews Molecular cell biology. 2010;11(9):621–32. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 4.Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol Cell. 2010;37(3):299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cartron PF, Loussouarn D, Campone M, Martin SA, Vallette FM. Prognostic impact of the expression/phosphorylation of the BH3-only proteins of the BCL-2 family in glioblastoma multiforme. Cell Death Dis. 2012;3:e421. doi: 10.1038/cddis.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strik H, Deininger M, Streffer J, Grote E, Wickboldt J, Dichgans J, et al. BCL-2 family protein expression in initial and recurrent glioblastomas: modulation by radiochemotherapy. J Neurol Neurosurg Psychiatry. 1999;67(6):763–8. doi: 10.1136/jnnp.67.6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiu B, Wang Y, Tao J, Wang Y. Expression and correlation of Bcl-2 with pathological grades in human glioma stem cells. Oncol Rep. 2012;28(1):155–60. doi: 10.3892/or.2012.1800. [DOI] [PubMed] [Google Scholar]
- 8.Nagane M, Levitzki A, Gazit A, Cavenee WK, Huang HJ. Drug resistance of human glioblastoma cells conferred by a tumor-specific mutant epidermal growth factor receptor through modulation of Bcl-XL and caspase-3-like proteases. Proc Natl Acad Sci U S A. 1998;95(10):5724–9. doi: 10.1073/pnas.95.10.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu H, Cao X, Ali-Osman F, Keir S, Lo HW. EGFR and EGFRvIII interact with PUMA to inhibit mitochondrial translocalization of PUMA and PUMA-mediated apoptosis independent of EGFR kinase activity. Cancer Lett. 2010;294(1):101–10. doi: 10.1016/j.canlet.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stegh AH, Kim H, Bachoo RM, Forloney KL, Zhang J, Schulze H, et al. Bcl2L12 inhibits post-mitochondrial apoptosis signaling in glioblastoma. Genes Dev. 2007;21(1):98–111. doi: 10.1101/gad.1480007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stegh AH, Kesari S, Mahoney JE, Jenq HT, Forloney KL, Protopopov A, et al. Bcl2L12-mediated inhibition of effector caspase-3 and caspase-7 via distinct mechanisms in glioblastoma. Proc Natl Acad Sci U S A. 2008;105(31):10703–8. doi: 10.1073/pnas.0712034105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stegh AH, Brennan C, Mahoney JA, Forloney KL, Jenq HT, Luciano JP, et al. Glioma oncoprotein Bcl2L12 inhibits the p53 tumor suppressor. Genes Dev. 2010;24(19):2194–204. doi: 10.1101/gad.1924710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tagscherer KE, Fassl A, Campos B, Farhadi M, Kraemer A, Bock BC, et al. Apoptosis-based treatment of glioblastomas with ABT-737, a novel small molecule inhibitor of Bcl-2 family proteins. Oncogene. 2008;27(52):6646–56. doi: 10.1038/onc.2008.259. [DOI] [PubMed] [Google Scholar]
- 14.Manero F, Gautier F, Gallenne T, Cauquil N, Gree D, Cartron PF, et al. The small organic compound HA14-1 prevents Bcl-2 interaction with Bax to sensitize malignant glioma cells to induction of cell death. Cancer Res. 2006;66(5):2757–64. doi: 10.1158/0008-5472.CAN-05-2097. [DOI] [PubMed] [Google Scholar]
- 15.Dodou K, Anderson RJ, Small DA, Groundwater PW. Investigations on gossypol: past and present developments. Expert Opin Investig Drugs. 2005;14(11):1419–34. doi: 10.1517/13543784.14.11.1419. [DOI] [PubMed] [Google Scholar]
- 16.Wagenknecht B, Glaser T, Naumann U, Kugler S, Isenmann S, Bahr M, et al. Expression and biological activity of X-linked inhibitor of apoptosis (XIAP) in human malignant glioma. Cell Death Differ. 1999;6(4):370–6. doi: 10.1038/sj.cdd.4400503. [DOI] [PubMed] [Google Scholar]
- 17.Chakravarti A, Noll E, Black PM, Finkelstein DF, Finkelstein DM, Dyson NJ, et al. Quantitatively determined survivin expression levels are of prognostic value in human gliomas. J Clin Oncol. 2002;20(4):1063–8. doi: 10.1200/JCO.2002.20.4.1063. [DOI] [PubMed] [Google Scholar]
- 18.Ziegler DS, Wright RD, Kesari S, Lemieux ME, Tran MA, Jain M, et al. Resistance of human glioblastoma multiforme cells to growth factor inhibitors is overcome by blockade of inhibitor of apoptosis proteins. J Clin Invest. 2008;118(9):3109–22. doi: 10.1172/JCI34120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chakravarti A, Zhai GG, Zhang M, Malhotra R, Latham DE, Delaney MA, et al. Survivin enhances radiation resistance in primary human glioblastoma cells via caspase-independent mechanisms. Oncogene. 2004;23(45):7494–506. doi: 10.1038/sj.onc.1208049. [DOI] [PubMed] [Google Scholar]
- 20.Fulda S, Wick W, Weller M, Debatin KM. Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat Med. 2002;8(8):808–15. doi: 10.1038/nm735. [DOI] [PubMed] [Google Scholar]
- 21.Wagner L, Marschall V, Karl S, Cristofanon S, Zobel K, Deshayes K, et al. Smac mimetic sensitizes glioblastoma cells to Temozolomide-induced apoptosis in a RIP1- and NF-kappaB-dependent manner. Oncogene. 2013;32(8):988–97. doi: 10.1038/onc.2012.108. [DOI] [PubMed] [Google Scholar]
- 22.Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer. 2011;11(4):239–53. doi: 10.1038/nrc3007. [DOI] [PubMed] [Google Scholar]
- 23.Venur VA, Peereboom DM, Ahluwalia MS. Current medical treatment of glioblastoma. Cancer treatment and research. 2015;163:103–15. doi: 10.1007/978-3-319-12048-5_7. [DOI] [PubMed] [Google Scholar]
- 24.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 25.Kondo N, Takahashi A, Ono K, Ohnishi T. DNA damage induced by alkylating agents and repair pathways. Journal of nucleic acids. 2010;2010:543531. doi: 10.4061/2010/543531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weller M, Stupp R, Reifenberger G, Brandes AA, van den Bent MJ, Wick W, et al. MGMT promoter methylation in malignant gliomas: ready for personalized medicine? Nature reviews Neurology. 2010;6(1):39–51. doi: 10.1038/nrneurol.2009.197. [DOI] [PubMed] [Google Scholar]
- 27.Barazzuol L, Jena R, Burnet NG, Meira LB, Jeynes JC, Kirkby KJ, et al. Evaluation of poly (ADP-ribose) polymerase inhibitor ABT-888 combined with radiotherapy and temozolomide in glioblastoma. Radiation oncology. 2013;8:65. doi: 10.1186/1748-717X-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin F, de Gooijer MC, Roig EM, Buil LC, Christner SM, Beumer JH, et al. ABCB1, ABCG2, and PTEN determine the response of glioblastoma to temozolomide and ABT-888 therapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20(10):2703–13. doi: 10.1158/1078-0432.CCR-14-0084. [DOI] [PubMed] [Google Scholar]
- 29.Tentori L, Leonetti C, Scarsella M, D'Amati G, Vergati M, Portarena I, et al. Systemic administration of GPI 15427, a novel poly(ADP-ribose) polymerase-1 inhibitor, increases the antitumor activity of temozolomide against intracranial melanoma, glioma, lymphoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003;9(14):5370–9. [PubMed] [Google Scholar]
- 30.Donawho CK, Luo Y, Luo Y, Penning TD, Bauch JL, Bouska JJ, et al. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13(9):2728–37. doi: 10.1158/1078-0432.CCR-06-3039. [DOI] [PubMed] [Google Scholar]
- 31.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132(1):27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu EY, Ryan KM. Autophagy and cancer--issues we need to digest. J Cell Sci. 2012;125(Pt 10):2349–58. doi: 10.1242/jcs.093708. [DOI] [PubMed] [Google Scholar]
- 33.White E, DiPaola RS. The double-edged sword of autophagy modulation in cancer. Clin Cancer Res. 2009;15(17):5308–16. doi: 10.1158/1078-0432.CCR-07-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan QW, Cheng C, Hackett C, Feldman M, Houseman BT, Nicolaides T, et al. Akt and autophagy cooperate to promote survival of drug-resistant glioma. Sci Signal. 2010;3(147):ra81. doi: 10.1126/scisignal.2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen J, Zheng H, Ruan J, Fang W, Li A, Tian G, et al. Autophagy inhibition induces enhanced proapoptotic effects of ZD6474 in glioblastoma. Br J Cancer. 2013;109(1):164–71. doi: 10.1038/bjc.2013.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gammoh N, Lam D, Puente C, Ganley I, Marks PA, Jiang X. Role of autophagy in histone deacetylase inhibitor-induced apoptotic and nonapoptotic cell death. Proc Natl Acad Sci U S A. 2012;109(17):6561–5. doi: 10.1073/pnas.1204429109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Zhu H, Zeng X, Fan J, Qian X, Wang S, et al. Suppression of autophagy enhanced growth inhibition and apoptosis of interferon-beta in human glioma cells. Mol Neurobiol. 2013;47(3):1000–10. doi: 10.1007/s12035-013-8403-0. [DOI] [PubMed] [Google Scholar]
- 38.Ge PF, Zhang JZ, Wang XF, Meng FK, Li WC, Luan YX, et al. Inhibition of autophagy induced by proteasome inhibition increases cell death in human SHG-44 glioma cells. Acta Pharmacol Sin. 2009;30(7):1046–52. doi: 10.1038/aps.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y, Meng D, Wang H, Sun R, Wang D, Wang S, et al. VAMP8 facilitates cellular proliferation and temozolomide resistance in human glioma cells. Neuro Oncol. 2014 doi: 10.1093/neuonc/nou219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cho HY, Wang W, Jhaveri N, Lee DJ, Sharma N, Dubeau L, et al. NEO212, temozolomide conjugated to perillyl alcohol, is a novel drug for effective treatment of a broad range of temozolomide-resistant gliomas. Mol Cancer Ther. 2014;13(8):2004–17. doi: 10.1158/1535-7163.MCT-13-0964. [DOI] [PubMed] [Google Scholar]
- 41.Curry RC, Dahiya S, Alva Venur V, Raizer JJ, Ahluwalia MS. Bevacizumab in high-grade gliomas: past, present, and future. Expert review of anticancer therapy. 2015;15(4):387–97. doi: 10.1586/14737140.2015.1028376. [DOI] [PubMed] [Google Scholar]
- 42.Gilbert MR, Sulman EP, Mehta MP. Bevacizumab for newly diagnosed glioblastoma. The New England journal of medicine. 2014;370(21):2048–9. doi: 10.1056/NEJMc1403303. [DOI] [PubMed] [Google Scholar]
- 43.Chinot OL, Wick W, Cloughesy T. Bevacizumab for newly diagnosed glioblastoma. The New England journal of medicine. 2014;370(21):2049. doi: 10.1056/NEJMc1403303. [DOI] [PubMed] [Google Scholar]
- 44.Hu YL, DeLay M, Jahangiri A, Molinaro AM, Rose SD, Carbonell WS, et al. Hypoxia-induced autophagy promotes tumor cell survival and adaptation to antiangiogenic treatment in glioblastoma. Cancer Res. 2012;72(7):1773–83. doi: 10.1158/0008-5472.CAN-11-3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Golden EB, Cho HY, Jahanian A, Hofman FM, Louie SG, Schonthal AH, et al. Chloroquine enhances temozolomide cytotoxicity in malignant gliomas by blocking autophagy. Neurosurg Focus. 2014;37(6):E12. doi: 10.3171/2014.9.FOCUS14504. [DOI] [PubMed] [Google Scholar]
- 46.Rosenfeld MR, Ye X, Supko JG, Desideri S, Grossman SA, Brem S, et al. A phase I/II trial of hydroxychloroquine in conjunction with radiation therapy and concurrent and adjuvant temozolomide in patients with newly diagnosed glioblastoma multiforme. Autophagy. 2014;10(8):1359–68. doi: 10.4161/auto.28984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alonso MM, Jiang H, Yokoyama T, Xu J, Bekele NB, Lang FF, et al. Delta-24-RGD in combination with RAD001 induces enhanced anti-glioma effect via autophagic cell death. Mol Ther. 2008;16(3):487–93. doi: 10.1038/sj.mt.6300400. [DOI] [PubMed] [Google Scholar]
- 48.Zong WX, Thompson CB. Necrotic death as a cell fate. Genes & development. 2006;20(1):1–15. doi: 10.1101/gad.1376506. [DOI] [PubMed] [Google Scholar]
- 49.Stegh AH, Chin L, Louis DN, DePinho RA. What drives intense apoptosis resistance and propensity for necrosis in glioblastoma? A role for Bcl2L12 as a multifunctional cell death regulator. Cell Cycle. 2008;7(18):2833–9. doi: 10.4161/cc.7.18.6759. [DOI] [PubMed] [Google Scholar]
- 50.Flannery T, McQuaid S, McGoohan C, McConnell RS, McGregor G, Mirakhur M, et al. Cathepsin S expression: An independent prognostic factor in glioblastoma tumours--A pilot study. International journal of cancer Journal international du cancer. 2006;119(4):854–60. doi: 10.1002/ijc.21911. [DOI] [PubMed] [Google Scholar]
- 51.Keerthivasan S, Keerthivasan G, Mittal S, Chauhan SS. Transcriptional upregulation of human cathepsin L by VEGF in glioblastoma cells. Gene. 2007;399(2):129–36. doi: 10.1016/j.gene.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 52.Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT, Liu B, et al. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012;45(6):487–98. doi: 10.1111/j.1365-2184.2012.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Melo-Lima S, Celeste Lopes M, Mollinedo F. Necroptosis is associated with low procaspase-8 and active RIPK1 and -3 in human glioma cells. Oncoscience. 2014;1(10):649–64. doi: 10.18632/oncoscience.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kang TB, Yang SH, Toth B, Kovalenko A, Wallach D. Activation of the NLRP3 inflammasome by proteins that signal for necroptosis. Methods Enzymol. 2014;545:67–81. doi: 10.1016/B978-0-12-801430-1.00003-2. [DOI] [PubMed] [Google Scholar]
- 55.Reardon DA, Wucherpfennig KW, Freeman G, Wu CJ, Chiocca EA, Wen PY, et al. An update on vaccine therapy and other immunotherapeutic approaches for glioblastoma. Expert review of vaccines. 2013;12(6):597–615. doi: 10.1586/erv.13.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stupp R, Wong ET, Kanner AA, Steinberg D, Engelhard H, Heidecke V, et al. NovoTTF-100A versus physician's choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. European journal of cancer. 2012;48(14):2192–202. doi: 10.1016/j.ejca.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 57.Turk B, Turk V. Lysosomes as “suicide bags” in cell death: myth or reality? J Biol Chem. 2009;284(33):21783–7. doi: 10.1074/jbc.R109.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blomgran R, Zheng L, Stendahl O. Cathepsin-cleaved Bid promotes apoptosis in human neutrophils via oxidative stress-induced lysosomal membrane permeabilization. J Leukoc Biol. 2007;81(5):1213–23. doi: 10.1189/jlb.0506359. [DOI] [PubMed] [Google Scholar]
- 59.Racoma IO, Meisen WH, Wang QE, Kaur B, Wani AA. Thymoquinone inhibits autophagy and induces cathepsin-mediated, caspase-independent cell death in glioblastoma cells. PLoS One. 2013;8(9):e72882. doi: 10.1371/journal.pone.0072882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boya P, Kroemer G. Lysosomal membrane permeabilization in cell death. Oncogene. 2008;27(50):6434–51. doi: 10.1038/onc.2008.310. [DOI] [PubMed] [Google Scholar]