Abstract
OBJECTIVE
To evaluate the association between performance on a single chair stand and moderate-to-severe exertional dyspnea. Prior work has shown that the most important factor when performing a chair stand is proximal muscle function of the lower extremities.
DESIGN
Cross-sectional.
SETTING
Cardiovascular Health Study.
PARTICIPANTS
Community-dwelling, aged ≥65 (N=4413).
MEASUREMENTS
Single chair stand (poor vs. normal performance, defined by inability vs. ability to rise without arm use, respectively), moderate-to-severe exertional dyspnea (American Thoracic Society grade ≥2), and the following covariates: age, sex, ethnicity, obesity, smoking, frailty status (Fried-defined non-frail, pre-frail, and frail), high cardiopulmonary risk (composite of cardiopulmonary disease and diabetes), spirometric impairment, arthritis, depression, stroke, and kidney disease.
RESULTS
Mean age was 72.6; 2518 (57.1%) were female, 199 (4.5%) were non-white, 788 (17.9%) were obese, and 2410 (54.6%) had a smoking history. Poor performance on the single chair stand was established in 369 (8.4%) and moderate-to-severe exertional dyspnea in 773 (17.5%). Pre-frail and frail status were established in 2210 (50.1%) and 360 (8.2%), respectively, arthritis in 2241 (51.4%), high cardiopulmonary risk in 2469 (55.9%), spirometric impairment in 1076 (24.4%), kidney disease in 111 (2.5%), depression in 107 (2.4%), and stroke in 93 (2.1%). In multivariable regression models, relative to normal performance, having poor performance on the single chair stand was associated with moderate-to-severe exertional dyspnea, yielding unadjusted and adjusted odds ratios (95% confidence interval) of 3.48 (2.78, 4.36) and 1.85 (1.41, 2.41), respectively.
CONCLUSION
Poor performance on a single chair stand increased the likelihood of moderate-to-severe exertional dyspnea by an adjusted 85%, relative to normal performance. These results suggest that reduced proximal muscle function of the lower extremities is associated with moderate-to-severe exertional dyspnea, even after adjusting for multiple confounders.
Keywords: chair stand, dyspnea, spirometry
INTRODUCTION
Among older persons, dyspnea is prevalent and associated with adverse outcomes.1–8 Prior work has shown, for example, that dyspnea is reported in a quarter to one-third of older persons and occurs most often on exertion.1–5 Adverse outcomes associated with dyspnea include exercise intolerance, physical disability, and increased mortality.1–8
Prior work also suggests that a large proportion of older persons who report dyspnea do not have a cardiopulmonary impairment.9,10 In two separate studies involving dyspneic older persons, less than one-third had a spirometric impairment (airflow-obstruction or restrictive-pattern) and less than one-fifth had a cardiac impairment (systolic or diastolic).9,10 Moreover, physiologic measures such as the forced expiratory volume in 1-second (FEV1) and the left ventricular ejection fraction cannot by themselves fully explain the experience of dyspnea, including in patients who have chronic obstructive pulmonary disease (COPD) and heart failure, respectively.11 Hence, it is important to consider additional mechanisms when evaluating dyspnea in older persons, particularly given the age-related increase in multimorbidity.8,9
Frailty is a prevalent geriatric syndrome that is commonly characterized by low physical activity, reduced grip strength, slow gait speed, exhaustion, or unintentional weight loss.12 It has been previously shown that older persons who have one or more of these frailty features have a significantly greater frequency of respiratory symptoms, including dyspnea, as compared with those who have no frailty features (p<.001).13 Because frailty features may also represent a phenotypic expression of sarcopenia (loss of skeletal muscle mass and function),12–14 we postulate that weakness of the proximal muscles of ambulation may be an important contributor to exertional dyspnea in older persons. The physiologic rationale is that proximal weakness of the muscles of ambulation may lead to an early exercise-induced lactic acidosis, thereby increasing CO2 flux (bicarbonate buffering of lactate), ventilatory requirements, and, ultimately, exertional dyspnea.1,7
Using data on 4,413 participants aged ≥65 from the Cardiovascular Health Study,15,16 we have therefore evaluated the cross-sectional association between lower extremity proximal muscle function and exertional dyspnea. In particular, we evaluated: 1) lower extremity function that is largely dependent on proximal muscle strength vis-à-vis a single chair stand;14,17–20 2) exertional dyspnea at a moderate–to-severe level, as defined by an American Thoracic Society grade of ≥2;1,21 and 3) potential confounders, including cardiopulmonary disease and related risk factors, spirometric impairment, Fried-defined frailty features, arthritis, and depression (among others).12–14 We hypothesized that poor performance on a single chair stand (inability to rise without arm use) would be significantly associated with exertional dyspnea. If our results support our hypothesis, this would suggest that reductions in lower extremity proximal muscle function may be associated with exertional dyspnea.
METHODS
Study Population
The Cardiovascular Health Study (CHS) is a population-based study of persons aged ≥65, identified from a random sample of Medicare eligibility lists in four U.S. communities and included whites and African Americans — assembled between 1988–1993 (N=5,888).15,16 For the current study, our analytical sample was comprised of 4,413 CHS participants who at the baseline visit had completed an ATS dyspnea questionnaire and at least two ATS-acceptable spirometric maneuvers (described below).21–24
The institutional review boards from the Veterans Affairs Connecticut Healthcare System and Yale University approved the study, granting exemption from participant consent because it involved de-identified data that were publicly available.
Baseline Demographic and Clinical Characteristics
These included age, sex, ethnicity/race, body mass index (BMI, kilograms/meters2), smoking history, medical conditions, high cardiopulmonary risk, and frailty features. Medical conditions were ascertained by self-report, except depression which was established by a Center for Epidemiologic Studies Depression Scale (CES-D) ≥16.25 High cardiopulmonary risk was defined by the presence of any of the following medical conditions: hypertension, diabetes mellitus, coronary artery disease, heart failure, chronic obstructive pulmonary disease (COPD), or asthma. Using procedures published by Fried and colleagues,12 the following five component frailty features were evaluated: 1) low physical activity—lowest quintile of kilocalories/week (sex-adjusted), 2) reduced grip strength—lowest quintile of the average of three dynamometer readings (sex- and BMI-adjusted), 3) slow gait speed—slowest quintile during a timed 15-feet walk (sex- and height-adjusted), 4) exhaustion—two questions from CES-D; and 5) unintentional weight loss—at least 10 pounds in the prior year. Based on the Fried phenotype, a three-level frailty status was established, as follows: 1) non-frail, as having none of the component frailty features, 2) pre-frail, as having 1 or 2 of the component frailty features, and 3) frail, as having at least 3 of the component frailty features.12
Moderate-to-Severe Dyspnea
Dyspnea was evaluated by the ATS Adult Dyspnea Questionnaire, graded in severity according to five questions that described everyday experiences—the more severe the dyspnea, the higher the ATS grade (range 0–5).21 Using this questionnaire, exertional dyspnea was classified as moderate-to-severe based on ATS grade of at least 2, which included a Yes response to the following question: “Do you have to walk slower than people of your age on the level because of breathlessness?”21
The rationale for establishing moderate-to-severe exertional dyspnea at a minimum ATS grade 2 is that this level of dyspnea includes a comparison to a reference group of the same age, occurs at a low exercise workload, and is associated with health outcomes.21,26 In contrast, an ATS dyspnea grade 1 defines mild severity—“I get short of breath when hurrying on the level or walking up a slight hill.”21,26
Spirometric Impairment
Participants underwent spirometry using contemporary ATS protocols.22 The measured values of interest included the ratio of forced expiratory volume in 1-second to forced vital capacity (FEV1/FVC) and FVC alone. The FEV1/FVC was calculated from the largest set of FEV1 and FVC values that were recorded in any of the spirometric maneuvers meeting ATS-acceptability criteria.22–24
To establish age-appropriate comparisons between measured and predicted values for FEV1/FVC and FVC, we used reference equations from the Global Lung Initiative (GLI).27 The use of GLI equations is ideal for aging populations, because they rigorously account for age-related changes in lung function.27 Using the GLI equations, Z-scores for FEV1/FVC and FVC were calculated for each participant, with a Z-score of −1.64 defining the lower limit of normal (LLN) as the 5th percentile of distribution.27–29 Participants were classified as having 1) normal spirometry, if FEV1/FVC and FVC were both ≥LLN, 2) airflow-obstruction, if FEV1/FVC <LLN, or 3) restrictive-pattern, if FEV1/FVC ≥LLN but FVC <LLN.24,28,29
Single Chair Stand
Participants were asked to stand up from a seated position in a chair without using their arms (single chair stand).15,16 The performance on the single chair stand was classified as poor if participants were “unable to rise without arm use” or as normal if “able to rise without arm use.” Of the 4,413 participants who completed the ATS dyspnea questionnaire and at least two ATS-acceptable spirometric maneuvers at the baseline visit, 4,368 (99.0%) also performed the single chair stand.
Statistical Analysis
The baseline characteristics, including demographic and clinical features, frailty (five component measures and three level status), spirometric impairment (airflow-obstruction and restrictive-pattern), moderate-to-severe exertional dyspnea, and single chair stand performance, were summarized using means and standard deviations, or counts and percentages.
Using unadjusted and frailty-adjusted logistic regression models, odds ratio with 95% confidence intervals (95%CIs) were calculated as measures of the association between poor performance on the single chair stand and moderate-to-severe exertional dyspnea. The frailty covariates included the five component frailty measures and frailty status, expressed as a two-level variable (non-frail and pre-frail or frail) and as a nominal three-level variable (non-frail, pre-frail, and frail). The rationale for these frailty-adjusted analyses is that, because it is a potential phenotype of sarcopenia, frailty is likely to be a prominent confounder of the association between poor performance on the single chair stand and exertional dyspnea.
In addition, using multivariable logistic regression models, adjusted odds ratios with 95%CIs were calculated as measures of the association between poor performance on the single chair stand and moderate-to-severe exertional dyspnea. Covariates included age, female sex, non-white race, smoking status, obesity (BMI ≥30), high cardiopulmonary risk, three-level frailty status, spirometric impairment, depression, arthritis, kidney disease, and stroke. The covariates that had p values >.20 were not included in the final adjusted model.
Potential effect modification of the association between poor performance on the single chair stand and moderate-to-severe exertional dyspnea was also assessed in the multivariable logistic regression models. In this analysis, interactions were evaluated that involved “crossing” the potential effect modifier with the poor single chair stand performance indicator variable. The effect modifiers of interest included the earlier described covariates. In tests of potential effect modification, p values for interaction terms were adjusted for the multiplicity of comparisons. Model fit was assessed using residual analysis, influence diagnostics, and goodness-of-fit statistics.
Upon investigating the quantity, pattern, and nature of missing values in the analytical sample, multiple imputation was performed to reduce the probability that missing data introduced bias to the inferential analyses. SAS® v9.4 (SAS Institute; Cary, NC) was used for all analyses, with p<0.05 (two-sided) interpreted as connoting statistical significance. For the interaction tests, the level of significance was adjusted as discussed earlier.
RESULTS
Table 1 reports the baseline characteristics of the analytical sample (N=4413). The mean age was 72.6; 2518 (57.1%) were female, 199 (4.5%) were non-white, and the mean BMI was 26.3 kg/m2. Obesity (BMI ≥30 kg/m2) was established in 788 (17.9%), and a smoking history (former and current smokers) was reported by 2410 (54.6%). Arthritis was the most prevalent medical condition, reported by 2241 (51.4%), while 2469 (55.9%) had at least one condition that established a high cardiopulmonary risk (hypertension, diabetes mellitus, coronary artery disease, heart failure, COPD, or asthma). Of the component frailty features, the three most prevalent were slow gait speed (29.4%), reduced grip strength (23.6%), and low physical activity (20.9%). Based on the Fried phenotype, pre-frail and frail status was established in 2210 (50.1%) and 360 (8.2%), respectively. A spirometric impairment (airflow-obstruction and restrictive-pattern) was established in 1076 (24.4%), and moderate-to-severe exertional dyspnea was reported by 773 (17.5%). Among the 4368 participants who underwent the single chair stand, 369 (8.4%) were classified as having poor performance (unable to rise without arm use).
Table 1.
Baseline characteristics (N=4413)
| Characteristic | Na | Mean (± SD) or No. (%) |
|---|---|---|
| Age (years) | 4413 | 72.6 (± 5.3) |
| 65–70 | 1896 (43.0) | |
| 71–80 | 2103 (47.6) | |
| 81+ | 414 (9.4) | |
| Females | 2518 (57.1) | |
| Non-white | 199 (4.5) | |
| BMI (kg/m2) | 4407 | 26.3 (± 3.9) |
| Obesity (BMI ≥30) | 788 (17.9) | |
| Smoking status | ||
| Never | 4411 | 2001 (45.4) |
| Former | 1895 (43.0) | |
| Current | 515 (11.7) | |
| Medical conditions | ||
| Arthritis | 4363 | 2241 (51.4) |
| High blood pressure | 4371 | 1529 (35.0) |
| Coronary artery diseaseb | 4156 | 831 (20.0) |
| Diabetes mellitus | 4387 | 416 (9.5) |
| COPDc | 4388 | 409 (9.3) |
| Asthma | 4400 | 285 (6.5) |
| Depressiond | 4408 | 107 (2.4) |
| Heart failure | 4250 | 118 (2.8) |
| Kidney disease | 4385 | 111 (2.5) |
| Stroke | 4382 | 93 (2.1) |
| High cardiopulmonary riske | 4413 | 2469 (55.9) |
| Frailty featuresf | ||
| Low physical activity | 4412 | 923 (20.9) |
| Reduced grip strength | 4104 | 969 (23.6) |
| Slow gait speed | 4367 | 1282 (29.4) |
| Exhaustion | 4404 | 741 (16.8) |
| Unintentional weight loss | 4134 | 217 (5.2) |
| Frailty statusg | ||
| Pre-frail | 4413 | 2210 (50.1) |
| Frail | 360 (8.2) | |
| Spirometric category, No. (%)h | ||
| Normal pulmonary function | 4413 | 3337 (75.6) |
| Restrictive-pattern | 267 (6.1) | |
| Airflow limitation | 809 (18.3) | |
| Moderate-to-severe dyspneai | 773 (17.5) | |
| Single chair standsj | ||
| Normal performance | 4368 | 3999 (91.6) |
| Poor performance | 369 (8.4) | |
Abbreviation: ATS, American Thoracic Society; CES-D, Center for Epidemiologic Studies Depression Scale; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1-second; FVC, forced vital capacity; GLI, Global Lung Initiative; LLN, lower limit of normal (5th percentile distribution of GLI-derived Z-scores); SD, standard deviation.
Varies due to missing data.
Included angina pectoris and myocardial infarction.
Included emphysema and chronic bronchitis.
Based on a CES-D score of ≥16.
Defined by the presence of any of the following: high blood pressure, diabetes mellitus, coronary artery disease, heart failure, COPD, or asthma.
Using procedures published by Fried and colleagues, the following five component frailty features were evaluated: 1) low physical activity—lowest quintile of kilocal/week (sex-adjusted), 2) reduced grip strength—lowest quintile of the average of three dynamometer readings (sex- and BMI-adjusted), 3) slow gait speed—slowest quintile during a timed 15-feet walk (sex- and height-adjusted), 4) exhaustion—two questions from CES-D; and 5) unintentional weight loss—at least 10 pounds in the prior year.
Pre-frail status was established by having 1 or 2 component frailty features, while frail status was established by having at least 3 component frailty features.
Normal defined by an FEV1/FVC ≥ LLN and FVC ≥ LLN; airflow-obstruction defined by FEV1/FVC < LLN; restrictive-pattern defined by FEV1/FVC ≥ LLN and FVC < LLN.
Classified by the ATS questionnaire (ATS-DLD-78-A), as ATS grade 2–5.
Performance was classified as normal if able to rise without arm use, or as poor if unable to rise without arm use.
Table 2 reports odds ratios (95%CI) for moderate-to-severe exertional dyspnea according to performance on the single chair stand, using unadjusted and frailty-adjusted logistic regression models (N=4413). Relative to normal performance, having a poor performance on the single chair stand was associated with more than a 3-fold greater odds of having moderate-to-severe exertional dyspnea, yielding an unadjusted odds ratio of 3.48 (2.78, 4.36). In the frailty-adjusted models, the association between poor performance on the single chair stand and moderate-to-severe exertional dyspnea was most attenuated by the nominal three-level frailty variable, yielding an adjusted odds ratio of 2.26 (1.40, 3.65). In contrast, the attenuation of the association between poor performance on the single chair stand and moderate-to-severe exertional dyspnea was more modest when the covariates included the component frailty-features (singly), e.g., slow gait speed, low physical activity, and exhaustion yielded adjusted odds ratios of 2.82 (1.74, 4.55), 2.90 (1.76, 4.76), and 2.94 (2.33, 3.72), respectively.
Table 2.
Odds ratio for moderate-to-severe exertional dyspnea according to performance on the single chair stand, using unadjusted and frailty-adjusted logistic regression models (N = 4413)a
| Single chair stand | Moderate-to-Severe Exertional Dyspneab |
|---|---|
| Odds Ratio (95% confidence interval) Poor vs. Normal Performance on Single Chair Standc | |
| Poor performancec | |
| Model 1: unadjusted | 3.48 (2.78, 4.36) |
| Model 2: adjusted for component frailty featuresd | |
| Model 2a: low physical activity | 2.90 (1.76, 4.76) |
| Model 2b: reduced grip strength | 3.15 (2.06, 4.80) |
| Model 2c: slow gait speed | 2.82 (1.74, 4.55) |
| Model 2d: exhaustion | 2.94 (2.33, 3.72) |
| Model 2e: unintentional weight loss | 3.31 (1.97, 5.56) |
| Model 3: adjusted for frailty status | |
| Model 3a: two-level frailty statuse | 2.69 (1.90, 3.80) |
| Model 3b: three-level frailty statusf | 2.26 (1.40, 3.65) |
Missing values provided by multiple imputation.
American Thoracic Society grade 2–5.
Performance was classified as normal if able to rise without arm use, or poor if unable to rise without arm use.
Using procedures published by Fried and colleagues, the following five component frailty features were evaluated: 1) low physical activity—lowest quintile of kilocal/week (sex-adjusted), 2) reduced grip strength—lowest quintile of the average of three dynamometer readings (sex- and BMI-adjusted), 3) slow gait speed—slowest quintile during a timed 15-feet walk (sex- and height-adjusted), 4) exhaustion—two questions from CES-D; and 5) unintentional weight loss—at least 10 pounds in the prior year. Based on the Fried phenotype, non-frail indicated having none of the component frailty features, pre-frail as having 1 or 2 of the component frailty features, and frail as having at least 3 of the component frailty features.
Participants who are frail or pre-frail are compared to participants who are non-frail in a single comparison.
Nominal variable including non-frail, pre-frail, and frail status; participants who are frail and participants who are pre-frail are compared separately to a reference group of those who are non-frail.
Table 3 reports adjusted odds ratios (95%CI) for moderate-to-severe exertional dyspnea according to performance on the single chair stand, using a multivariable logistic regression model (N=4413). Relative to normal performance, having poor performance on the single chair stand was significantly associated with moderate-to-severe exertional dyspnea, yielding an adjusted odds ratio (adjOR) of 1.85 (1.41, 2.41). Significant associations were also found for the covariates of age, obesity, high cardiopulmonary risk, frailty, spirometric impairment, depression, and arthritis. None of these covariates, however, yielded statistically significant effect modification of the association between poor performance on the single chair stand and moderate-to-severe exertional dyspnea (p values were not significant when adjusted for the multiplicity of comparisons).
Table 3.
Adjusted odds ratios for moderate-to-severe exertional dyspnea according to performance on the single chair stand, using multivariable logistic regression models (N = 4413)a
| Explanatory Variable | Moderate-to-Severe Exertional Dyspneab |
|---|---|
| Adjusted Odds Ratio (95% Confidence Interval)c Poor vs. Normal Performance on Single Chair Standd | |
| Poor performance on the single chair standd | 1.85 (1.41, 2.41) |
| Age (each additional year) | 1.03 (1.01, 1.04) |
| Female | 1.14 (0.95, 1.38) |
| Obesity (BMI ≥30) | 1.88 (1.53, 2.31) |
| Smoking status | |
| Ever versus no smoking | 1.18 (0.97, 1.43) |
| Current versus no smoking | 1.29 (0.97, 1.71) |
| High cardiopulmonary riske | 2.86 (2.35, 3.49) |
| Frailty statusf | |
| Pre-frail versus non-frail | 2.30 (1.87, 2.84) |
| Frail versus non-frail | 3.24 (2.36, 4.45) |
| Spirometric impairmentg | |
| Airflow-obstruction versus normal spirometry | 2.37 (1.93, 2.91) |
| Restrictive-pattern versus normal spirometry | 2.02 (1.48, 2.75) |
| Depressionh | 2.10 (1.35, 3.28) |
| Arthritis | 1.56 (1.30, 1.87) |
| Stroke | 1.43 (0.86, 2.37) |
Abbreviation: ATS, American Thoracic Society; CES-D, Center for Epidemiologic Studies Depression Scale; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1-second; FVC, forced vital capacity; GLI, Global Lung Initiative; LLN, lower limit of normal (5th percentile distribution of GLI-derived Z-scores); NA, not applicable.
Missing values provided by multiple imputation.
ATS grade 2–5.
The covariates of non-white race and kidney disease had p values >0.20 and were not included in the final adjusted model.
Performance was classified as normal if able to rise without arm use, or as poor if unable to rise without arm use.
Defined by the presence of any of the following: high blood pressure, diabetes mellitus, coronary artery disease, heart failure, COPD, or asthma.
Three-level nominal variable including non-frail, pre-frail, and frail status, wherein non-frail indicates having none of the component frailty features, pre-frail as having 1 or 2 component frailty features, and frail as having at least 3 component frailty features. The component frailty features included: 1) low physical activity—lowest quintile of kilocal/week (sex-adjusted), 2) reduced grip strength—lowest quintile of the average of three dynamometer readings (sex- and BMI-adjusted), 3) slow gait speed—slowest quintile during a timed 15-feet walk (sex- and height-adjusted), 4) exhaustion—two questions from CES-D; and 5) unintentional weight loss—at least 10 pounds in the prior year.
Normal defined by an FEV1/FVC ≥ LLN and FVC ≥ LLN; airflow-obstruction defined by FEV1/FVC < LLN; restrictive-pattern defined by FEV1/FVC ≥ LLN and FVC < LLN.
Based on a CES-D score of ≥16.
DISCUSSION
In a sample of community-dwelling older persons, we found that poor performance on a single chair stand (unable to rise without arm use) increased the likelihood of having moderate-to-severe exertional dyspnea by an adjusted 85%, as compared with normal performance (able to rise without arm use) (see Table 3). Because it is the most important factor when performing a chair stand,17–20 our results suggest that reduced proximal muscle function of the lower extremities is associated with moderate-to-severe exertional dyspnea in older persons, even after adjusting for multiple potential confounders.
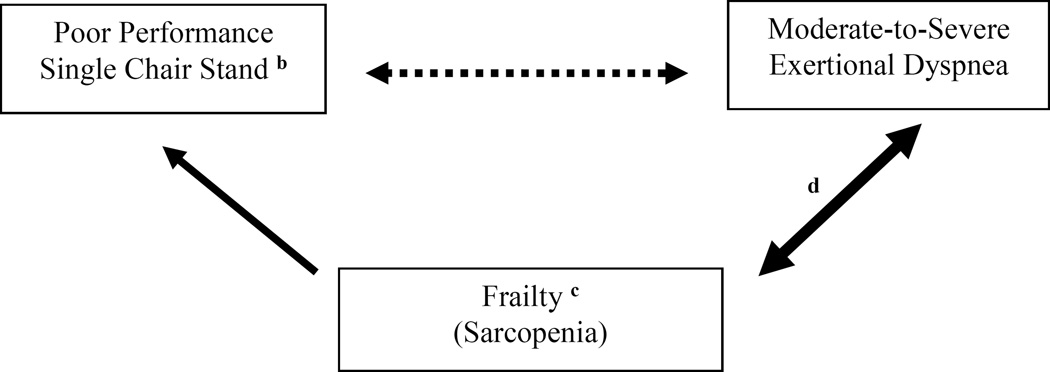
We also found that poor performance on a single chair stand and frailty status (three-level nominal variable) appeared to compete in accounting for the variability in the outcome of moderate-to-severe exertional dyspnea—in particular, the unadjusted odds ratio of 3.48 (2.78, 4.36) decreased to a frailty-adjusted odds ratio of 2.26 (1.40, 3.65) (see Table 2). Moreover, in the multivariable model (see Table 3), having a frail status (3 or more frailty measures) was more strongly associated with moderate-to-severe exertional dyspnea than having a pre-frail status (1 or 2 frailty measures), yielding adjusted odds ratios of 3.24 (2.36, 4.45) and 2.30 (1.87, 2.84), respectively. Based on these results and those of prior studies,8,12–14,17–20 we suggest a conceptual model in Figure 1,30 summarizing the proposed associations of poor performance on a single chair stand and frailty with the outcome of moderate-to-severe exertional dyspnea.
Figure 1.
Conceptual model summarizing the proposed associations of poor performance on a single chair stand and frailty with the outcome of moderate-to-severe exertional dyspneaa
aThe solid arrows indicate previously established longitudinal associations of frail status (≥3 frailty measures) with reduced mobility and spirometric impairment.12–14 The dotted arrow indicates an association that weakens when frail status is a covariate (see Table 2). The bidirectional arrows indicate that moderate-to-severe exertional dyspnea is itself associated with physical inactivity,8 which is a frailty component feature and a risk factor for sarcopenia.12,14
bProximal muscle function of the lower extremities is the most important factor when performing a chair stand.17–20
cThe phenotype of sarcopenia may include frailty, as defined by the Fried criteria.12,14
dIn the multivariable regresion model (see Table 3), having a frail status (≥3 frailty measures) was more strongly associated with moderate-to-severe exertional dyspnea than pre-frail status (1 or 2 frailty measures)—adjusted odds ratios of 3.24 (2.36, 4.45) and 2.30 (1.87, 2.84), respectively. This suggests a stronger association between frailty and exertional dyspnea, as compared with frailty and poor performance on the single chair stand
As discussed earlier, frailty may represent a phenotype of sarcopenia.12,14 However, the Fried-defined frailty phenotype may be limited as a proxy measure of sarcopenia, because it additionally reflects psychological and social factors.14 Accordingly, future work should evaluate whether objectively measured sarcopenia, defined by both a decrease in muscle mass (dual energy X-ray absorptiometry) and a decrease in the proximal muscle strength of the lower extremities (isokinetic dynamometer), underlies the association between poor performance on a single chair stand and exertional dyspnea, as proposed in Figure 1. Furthermore, because age-related sarcopenia can occur systemically, respiratory muscle weakness, defined by a decrease in the maximal inspiratory pressure, may coexist with weakness of the proximal muscles of the lower extremities and, as a result, additionally increase the likelihood of exertional dyspnea.8,13 Elucidation of the role of sarcopenia regarding the muscles of ambulation and respiration may inform novel therapeutic interventions among older persons who have exertional dyspnea.14
A prevalent and potentially modifiable factor that can lead to poor performance on a single chair stand relates to deconditioning from a low level of physical activity.14 Low physical activity when defined by the Fried criteria12 occurred in 20.9% of our analytical sample (see Table 1). As with the three-level frailty variable, but at a more modest level, we found that poor performance on a single chair stand and low physical activity appeared to compete in accounting for the variability in the outcome of moderate-to-severe exertional dyspnea—in particular, the unadjusted odds ratio of 3.48 (2.78, 4.36) decreased to a low physical activity adjusted odds ratio of 2.90 (1.76, 4.76) (see Table 2). Deconditioning from a sedentary status may exacerbate sarcopenia of the proximal muscles of the lower extremities,14 thus leading to poor performance on a single chair stand and, in turn, an increase in exertional dyspnea.1,7 These relationships, however, are likely to be complex (bidirectional), as prior work has also shown that moderate-to-severe exertional dyspnea is itself cross-sectionally associated with physical inactivity.8
We acknowledge two additional potential limitations to our study. First, although the current study has shown that the association between poor performance on a single chair stand and moderate-to-severe exertional dyspnea remained statistically significant even after adjusting for multiple potential confounders, our list of covariates was not exhaustive. For example, chair stands may be affected by other musculoskeletal impairments, as well as sensory impairments. These include impaired function of the lower back, hip, knees, and feet; reductions in lower extremity proprioception, peripheral tactile sensitivity, and balance; and symptom-limiting pain.14,20,31,32 Moreover, exertional dyspnea may be affected by reductions in cardiac function (ejection fraction), thoracic kyphoscoliosis, anxiety, and pain.1,6,7,33,34 Second, our analyses were cross-sectional and cannot infer cause-and-effect. Future work should therefore evaluate the longitudinal association of baseline performance on the chair stand with the development of exertional dyspnea, including a broader array of covariates. Furthermore, because poor performance on chair stands and dyspnea share similar outcomes,1–8,35 future work should evaluate whether their combined occurrence has a multiplicative effect on mobility limitations.
In conclusion, among community-dwelling older persons, poor performance on a single chair stand significantly increased the likelihood of having moderate-to-severe exertional dyspnea by an adjusted 85%, as compared with normal performance. These results suggest that reduced proximal muscle function of the lower extremities is associated with exertional dyspnea in older persons, even after adjusting for multiple confounders. Because they are potentially modifiable, future work should evaluate the factors that underlie the association between reduced proximal muscle function of the lower extremities and exertional dyspnea in older persons.
Acknowledgments
Funding source: The study was conducted at the Yale Claude D. Pepper Older Americans Independence Center (P30AG021342). Dr. Vaz Fragoso is currently a recipient of a Veterans Affairs Merit Award.
Sponsor’s Role: The investigators retained full independence in the conduct of this research.
Appendix
| Financial/Personal Conflicts | Vaz Fragoso | Araujo | Leo-Summers | Van Ness |
|---|---|---|---|---|
| No | No | No | No | |
| Employment/Affiliation | X | X | X | X |
| Grants/Funds | X | X | X | X |
| Honoraria | X | X | X | X |
| Speaker Forum | X | X | X | X |
| Consultant | X | X | X | X |
| Stocks | X | X | X | X |
| Royalties | X | X | X | X |
| Expert Testimony | X | X | X | X |
| Board Member | X | X | X | X |
| Patents | X | X | X | X |
| Personal Relationship | X | X | X | X |
Footnotes
The authors report no conflicts of interest.
Author Contributions: Dr. Vaz Fragoso had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors made substantial contributions to study concept and design, to data acquisition, analysis and interpretation, and to drafting the submitted article.
REFERENCES
- 1.Parshall MB, Schwartzstein RM, Adams L, et al. American Thoracic Society Statement Update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185:435–452. doi: 10.1164/rccm.201111-2042ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kroenke K, Arrington ME, Mangelsdorff AD. The prevalence of symptoms in medical outpatients and the adequacy of therapy. Arch Intern Med. 1990;150:1685–1689. doi: 10.1001/archinte.150.8.1685. [DOI] [PubMed] [Google Scholar]
- 3.Enright P, Kronmal RA, Higgins MW, et al. Prevalence and correlates of respiratory symptoms and disease in the elderly. Chest. 1994;106:827–834. doi: 10.1378/chest.106.3.827. [DOI] [PubMed] [Google Scholar]
- 4.Huijnen B, van der Horst F, van Amelsvoort L, et al. Dyspnea in elderly family practice patients. Occurrence, severity, quality of life and mortality over an 8-year period. Family Practice. 2006;23:34–39. doi: 10.1093/fampra/cmi064. [DOI] [PubMed] [Google Scholar]
- 5.Tessier JF, Nejjari C, Letteneur L, et al. Dyspnea and 8-year mortality among elderly men and women: the PAQUID cohort study. Eur J Epidemiol. 2001;17:223–229. doi: 10.1023/a:1017977715073. [DOI] [PubMed] [Google Scholar]
- 6.Abidov A, Rozanski A, Hachamovitch R, et al. Prognostic significance of dyspnea in patients referred for cardiac stress testing. N Engl J Med. 2005;353:1889–1898. doi: 10.1056/NEJMoa042741. [DOI] [PubMed] [Google Scholar]
- 7.Weisman IM, Beck KC, Casaburi R, et al. American Thoracic Society/American College of Chest Physicians (ATS/ACCP) Statement on Cardiopulmonary Exercise Testing. Am J Respir Crit Care Med. 2003;167:211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 8.Vaz Fragoso CA, Beavers DP, Hankinson JL, et al. Respiratory impairment and dyspnea and their associations with physical inactivity and mobility in sedentary community-dwelling older persons. J Am Geriatr Soc. 2014;62:622–628. doi: 10.1111/jgs.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcus BS, McAvay G, Gill TM, Vaz Fragoso CA. J Am Geriatr Soc. 2014. Respiratory symptoms, spirometric respiratory impairment, and respiratory disease in middle- and older-aged persons. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pedersen F, Raymond I, Mehlsen J, et al. Prevalence of diastolic dysfunction as a possible cause of dyspnea in the elderly. Am J Med. 2005;118:25–31. doi: 10.1016/j.amjmed.2004.07.048. [DOI] [PubMed] [Google Scholar]
- 11.Karapolat H, Eyigor S, Atasever A, et al. Effect of dyspnea and clinical variables on the quality of life and functional capacity in patients with chronic obstructive pulmonary disease and congestive heart failure. Chin Med J. 2008;121(7):592–596. [PubMed] [Google Scholar]
- 12.Fried L, Tangen M, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol Med Sci. 2001;56A:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 13.Vaz Fragoso CA, Enright PL, McAvay G, et al. Frailty and respiratory impairment in older persons. Am J Med. 2012;125:79–86. doi: 10.1016/j.amjmed.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis. Report of the European working group on sarcopenia in older people. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 16.Cardiovascular Health Study (CHS) [Accessed July 24, 2014]; Available at: https://chs-nhlbi.org/CHSOverview. [Google Scholar]
- 17.Csuka M, McCarty DJ. Simple method for measurement of lower extremity muscle strength. Am J Med. 1985;78:77–81. doi: 10.1016/0002-9343(85)90465-6. [DOI] [PubMed] [Google Scholar]
- 18.Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exercise Sport. 1999;70:113–119. doi: 10.1080/02701367.1999.10608028. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal S, Kiely PDW. Two simple, reliable and valid tests of proximal muscle function, and their application to the management of idiopathic inflammatory myositis. Rheumatology. 2006;45(7):874–879. doi: 10.1093/rheumatology/kel017. [DOI] [PubMed] [Google Scholar]
- 20.Lord SR, Murray SM, Chapman K, et al. Sit-to-stand performance depends on sensation, speed, balance, and psychological status in addition to strength in older people. J Gerontol A Biol Sci Med Sci. 2002;57(8):M539–M543. doi: 10.1093/gerona/57.8.m539. [DOI] [PubMed] [Google Scholar]
- 21.American Thoracic Society. Recommended respiratory disease questionnaires for use with adults and children in epidemiological research. Am Rev Respir Dis. 1978;6(2):7–23. [Google Scholar]
- 22.American Thoracic Society. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis. 1991;144:1202–1218. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 23.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 24.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 25.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D depression symptoms index. J Aging Health. 1993;5:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 26.Hajiro T, Nishimura K, Tsukino M, et al. A comparison of the level of dyspnea vs disease severity in indicating the health-related quality of life of patients with COPD. Chest. 1999;116(6):1632–1637. doi: 10.1378/chest.116.6.1632. [DOI] [PubMed] [Google Scholar]
- 27.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95 year age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stanojevic S, Wade A, Stocks J, et al. Reference ranges for spirometry across all ages. Am J Respir Crit Care Med. 2008;177:253–260. doi: 10.1164/rccm.200708-1248OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaz Fragoso CA, Gill T. Respiratory impairment and the aging lung: A novel paradigm for assessing pulmonary function. J Gerontol A Biol Sci Med Sci. 2012;67A:264–275. doi: 10.1093/gerona/glr198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kraemer HC, Stice E, Kazdin A, et al. How do risk factors work together? mediators, moderators, and independent, overlapping, and proxy risk factors. Am J Psychiatry. 2001;158:848–856. doi: 10.1176/appi.ajp.158.6.848. [DOI] [PubMed] [Google Scholar]
- 31.Leveille SG, Wee CC, Iezzoni LI. Trends in obesity and arthritis among baby boomers and their predecessors, 1971–2002. Am J Public Health. 2005;95(9):1607–1613. doi: 10.2105/AJPH.2004.060418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Covinsky KE, Lindquist K, Dunlop DD, Yelin E. Pain, functional limitations, and aging. J Am Geriatr Soc. 2009;57:1556–1561. doi: 10.1111/j.1532-5415.2009.02388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanaka K, Akechi T, Okuyama T, et al. Factors correlated with dyspnea in advanced lung cancer patients: organic causes and what else? J Pain Symptom Manage. 2002;23:490–500. doi: 10.1016/s0885-3924(02)00400-1. [DOI] [PubMed] [Google Scholar]
- 34.Di Bari M, Chiarlone M, Matteuzzi D, et al. Thoracic kyphosis and ventilatory dysfunction in unselected older persons: an epidemiological study in Dicomano, Italy. J Am Geriatr Soc. 2004;52:909–915. doi: 10.1111/j.1532-5415.2004.52257.x. [DOI] [PubMed] [Google Scholar]
- 35.Cesari M, Kritchevsky SB, Newman AB, et al. Added value of physical performance measures in predicting adverse health-related events: results from the Health, Aging, and Body Composition Study. J Am Geriatr Soc. 2009;57(2):251–259. doi: 10.1111/j.1532-5415.2008.02126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]