Abstract
Background/Aims
Irritable bowel syndrome (IBS) is a heterogeneous condition with a number of pathophysiological mechanisms that appear to contribute to symptom chronicity. One of these is altered pain sensitivity.
Methods
Women between ages 18–45 were recruited through the community. Of those enrolled, 56 had IBS and 36 were healthy control (HC) women. Participants completed questionnaires, kept a four-week symptom diary and had a 12-hour Holter placed to assess night-time heart rate variability including high frequency power (HF), low frequency power (LF), and total power (TP). At mid-follicular phase approximately 80% of women completed a thermal pain sensitivity test with conditioned pain modulation and visceral pain sensitivity using a water load symptom provocation (WLSP) test.
Results
As expected, daily abdominal pain was significantly higher in the IBS compared to HC group. There were no differences between the bowel pattern subgroups (IBS-diarrhea [IBS-D], IBS-constipation plus mixed [IBS-CM]). Thermal pain sensitivity did not differ between the IBS and HC groups, but was significantly higher in the IBS-CM group than the IBS-D group. In the WLSP test, the IBS group experienced significantly more symptom distress than HCs and the IBS-CM group was higher than the IBS-D group. Heart rate variability indicators did not differ between the groups or IBS subgroups. Daily abdominal pain was positively correlated with LF and TP in the IBS group.
Conclusions
Despite similar levels of abdominal pain in IBS, the IBS-CM group demonstrated greater sensitivity to both thermal and visceral testing procedures.
Keywords: Autonomic nervous system (ANS), conditioned pain modulation (CPM), heart rate variability (HRV), irritable bowel syndrome (IBS), water load symptom provocation (WLSP) test
INTRODUCTION
Irritable bowel syndrome (IBS) is a common functional gastrointestinal (GI) disorder with a disproportionate number of women compared to men seeking health care services.(1) The criteria for IBS includes abdominal pain and extremes of stool types (e.g., constipation, diarrhea). While the pathophysiology remains to be fully elucidated, there is agreement that IBS needs to be viewed within a biopsychosocial framework.(2–4) This framework identifies several areas to consider when trying to understand the heterogeneity of this IBS: early life events/exposures, genetics, psychosocial factors, and physiology. With regard to the latter, alterations in both peripheral and central mechanisms related to pain sensitivity, inflammatory response, and stress reactivity may contribute to the pathophysiology of IBS. In addition, the autonomic nervous system (ANS), which connects the brain to the gut has shown to be dysregulated in some patients with IBS when compared to healthy controls.(5) But whether this dysregulation is related to heightened pain sensitivity or stool pattern alterations remains to be determined.
Increased pain sensitivity in IBS may be due to several mechanisms including afferent pain signaling and alterations in spinal and central processing of pain sensory information. Descending inhibitory pain modulation may be dysfunctional in subgroups of IBS patients.(6–8) The ability to inhibit sensory input can be measured by conditioned pain modulation (CPM) testing.(9–11) CPM efficiency is determined by the degree to which the perception of a painful stimulus is decreased after a second painful stimulus is applied.(7) A decrease in the ability to reduce pain sensitivity in the presence of a second test stimulus is referred to as decreased CPM efficiency. Two studies found that some women with IBS have reduced CPM efficiency.(9, 10) In a prior study we found that 5 out of 20 women with IBS had reduced CPM efficiency as compared to none of the age-matched controls.(7) This relatively small sample of women precluded examination of bowel pattern subgroups or sex differences.
Non-nutrient volume ingestion (e.g., water load symptom provocation [WLSP]) is one mechanism to assess upper GI visceral sensitivity. It is a relatively simple, non-invasive technique, in which the individual ingest in a blind fashion as much fluid as they can tolerated over a 5-minute period then symptoms are assessed over a 30-minute post ingestion time. This approach has been used to study patients with dyspepsia and children with abdominal pain.(12) Others have shown that when esophageal and gastric barostat are employed individuals with IBS show reduced threshold for discomfort when compared to controls.(13, 14) To our knowledge WLSP has not been used to assess bowel pattern subgroup differences or to test the relationship of gastric volume tolerance to CPM in adults.
There is some evidence that ANS balance influences the endogenous inhibitory pain network in individuals with chronic pain conditions.(15–17) One non-invasive approach to assessing ANS activity is heart rate variability (HRV). HRV is a measure of the heart period variability based on within-subject statistical or time-series summarization of sequences of measured normal R-R intervals.(18) Spectral analysis measures high frequency power (HF, parasympathetic), low frequency power (LF, sympathetic and parasympathetic) and total power. In IBS where there is some evidence that autonomic dysregulation is present, however, the relationship to pain sensitivity measures remains unexplored.
In this study we chose to include only women for several reasons: women seek health care services more often for symptoms; women report more pain-related syndromes; and by studying only one gender within a specific age range, we could ‘control’ for reproductive hormone status. Our purpose was first to compare daily reports of GI symptoms, thermal pain sensitivity, CPM efficiency, and upper GI visceral pain sensitivity using the WLSP test in women with IBS to healthy control women (HCs). Second, we tested whether these results differed by bowel pattern subgroups. Third, we tested the relationship of visceral pain sensitivity, thermal pain sensitivity and CPM with HRV in IBS and HC groups.
Based on the literature we hypothesized first that the IBS group and subgroups will report significantly higher thermal pain sensitivity, lower CPM efficiency and higher upper GI visceral pain sensitivity compared to HCs. Second, based on our prior work(19–21) we hypothesized that the IBS-CM group will have reduced HF power compared to IBS-D. Third, we hypothesized that decreased HF will be related to higher reports of daily abdominal pain, higher thermal pain sensitivity, lower CPM efficiency and higher visceral pain sensitivity.
MATERIALS AND METHODS
Participants and setting
Women with IBS and HCs were recruited through community advertisements. Screening for eligibility was initially done over the telephone. The women had to be 18 to 45 years old. To be eligible, women in the IBS group had to have a prior medical diagnosis of IBS, currently meet the Rome III criteria for IBS, and have abdominal pain or discomfort at least two days a week for 2 weeks. HCs had to have no history of functional GI disorders or other moderate to very severe diseases/disorders. Women in either group were excluded if they 1) had a history of co-existing GI pathology, surgery, renal or reproductive pathology, or severe cardiovascular disease. They were also excluded if they were currently taking select medications (e.g., antibiotics, anticholinergics, hypnotics, narcotics, enema preparation). This study was approved and reviewed annually by the University of Washington’s institutional review board.
Procedures
At the initial visit to the research office, women gave written informed consent, returned completed questionnaires and were oriented to the study. At the end of the visit electrodes were placed to record their electrocardiography (ECG) for approximately 12 hours. Starting with their next menses the women completed a daily diary each evening (e.g., symptoms, stool type, medications) for one menstrual cycle (approximately 28 days for those using contraceptives). The testing session was conducted in the ANS laboratory on one day (i.e., follicular phase days 5 to 10). Participants fasted after midnight except for small sips of water, then came to the ANS laboratory after they woke up to complete the testing session.
Measures
GI and psychological distress symptoms
The Rome III Diagnostic Questionnaire for Adult Functional GI Disorders was used to assess the Rome III criteria for IBS and to determine the stool pattern subgroups.(22) The clinical criteria for IBS is recurrent abdominal pain or discomfort at least 3 days per month in the last 3 months associated with two of three criteria: pain/discomfort improves with bowel movement, onset is associated with a change in stool frequency, or a change in stool form (appearance). Abdominal pain and discomfort were rated by how often they occurred in the last 3 months (never [0] to every day [6]) while change in stool frequency and appearance were rated from never or rarely (0) to always (4). The participant had to have met this criteria in the last 3 months and have had the symptoms for at least 6 months prior to diagnosis. The recommended research criteria was used, which includes pain/discomfort frequency of at least 2 days a week for 2 weeks.
In both our prior studies of HRV during the night(18, 21) and in preliminary analysis for the current study, HRV was similar in the IBS-C and IBS-M groups. Based on this, and other evidence that IBS-C and IBS-M are similar in certain respects such as response to Tegaserod,(23) these two groups were combined into a single group (IBS-CM) for the analyses presented in this report.
Daily GI diary symptoms included abdominal pain or discomfort, abdominal pain/discomfort after eating, diarrhea, constipation, and bloating. Daily psychological distress symptoms included feeling stressed, anxiety, and depression (feeling sad or blue). These symptoms were rated each evening based on the highest severity rating for each symptom over the past 24 hours as 0 (not present), 1 (mild), 2 (moderate), 3 (severe), or 4 (very severe). Each symptom was summarized as the percent of days with moderate to very severe symptom severity.
The Threshold Phase included
Thermal Pain Sensitivity and CPM efficiency testing were done using the Pain & Sensory Evaluation System (Pathway model ATS, Israel). It was used to generate a noxious heat stimulus via a thermode attached to the dominant forearm, during each of four experimental phases with a 5-minute break after each phase. In the first two phases, each heat stimulus trial proceeded as follows: A 2-second baseline at 32°C (89.6°F), a 2 second ramp-up, a 7 second hold at the specified temperature, and a 2 second decline to baseline. At 6 seconds into the 7-second hold, the participant verbally rated her pain intensity on a Verbal Numeric Rating Scale ranging from 0 (no pain) to 10 (worst pain imaginable). The Familiarization Phase included two heat stimulus trials at 43°C and 44°C (109.4° F and 111.2° F). The Threshold phase included 3 to 9 heat stimulus trials at 45°C to 48°C [113.4° to 118.4° F], presented in sets of 3. The goal of this phase was to determine the temperature at which the participant rated pain as a 6. Once this ‘pain-6’ temperature was determined, it was verified by a final heat stimulus trial at that temperature.
Next, in the Unconditioned Test Stimulus phase, the thermode was set at the “pain-6” temperature for 30 seconds. The participant rated her pain from the test stimulus at 10, 20 and 30 seconds. In the last, Conditioning Stimulus phase, the participant placed her non-dominant hand in a hot water bath maintained at 46.5°C (115.7°F) keeping the water up to the wrist and their fingers apart for one minute. The participant rated her pain from hand in the water bath at 10, 20 and 30 seconds. After 30 seconds, while the non-dominant hand remained in the warm bath. The thermode on the dominant hand was increased to pain-6 temperature for 30 seconds. The participant rated her pain from the thermode stimulus at 40, 50 and 60 seconds. The CPM efficiency score was calculated as the average of the three thermal pain ratings from the Unconditioned Test Stimulus phase minus the average of the three thermal pain ratings from the Conditioning Stimulus phase. Higher positive values indicate a greater CPM efficiency.(7) The pain ratings for the range of different temperatures from all 4 phases were to determine pain rating at 47°C for the analyses of thermal pain sensitivity.
Visceral pain sensitivity testing
A water load symptom provocation (WLSP) test was used to test for visceral pain. Participants were encouraged to drink as much non-carbonated water as they could from a cup. The cup was continuously refilled by the research staff to obscure the amount that was consumed. A second liter bottle was used before the first liter one was empty. The bottles were kept in dark neoprene bags. The participant stopped when they felt completely full or reached a limit of intolerability with a non-informed ceiling of 5 minutes. They rated four symptoms (pain/discomfort, fullness, nausea, and bloating) on a 10-cm VAS scale before drinking (−1) and at 5, 10, 20, and 30 minutes after they started drinking.
Heart Rate Variability (HRV)
Information about cardiac rhythm dynamics was measured by ambulatory ECG and recorded on a 3-channel digital Holter ECG (Quinton-Burdick, Bothell, WA). Flash cards were processed through Vision Premier Holter software system by a trained operator who over-scored any aberrant beats. Spectral HRV measures, using fast Fourier transform in 5-minute blocks, were computed via the Vision Premier system software by estimating the power or variance of the beat-to-beat fluctuations in the heart period that fall into slow-medium (LF: f = 0.04 – 0.15 Hz) and fast rhythm patterns (HF: f = 0.15 – 0.40 Hz).(24) The mean interval was calculated as the simple arithmetical average of R-R intervals measured in msec. The time between 02:00 and 06:00 was used for the night-time recording.
Data Analyses
Chi-square tests and analysis of variance (ANOVA) were used to compare demographic characteristics between IBS and HC women and across bowel pattern subgroups. Since many of the variables of interest have skewed, non-normal distributions, rank-based non-parametric analyses were used to compute p-values for evaluating statistical significance. For group comparisons (i.e., HC vs. IBS, HC vs. IBS-CM, HC vs. IBS-D, and IBS-CM vs. IBS-D), group means and standard deviations are presented as descriptive statistics. Mann-Whitney tests were used to test the significance of group differences for diary-based GI and psychological distress symptoms, thermal pain sensitivity, CPM efficiency, visceral pain sensitivity, and HRV. The HRV measures are correlated with age and BMI and thus these variables were controlled for as potential confounders. Group comparisons for the HRV measures were done using analysis of covariance (ANCOVA) based on rank transforms; that is the outcome is rank of the HRV variable and the covariates are rank of age and rank of BMI.(25) These models were fit using the GENLIN function in SPSS 19.0, so that robust estimates of standard errors for parameter estimates could be used. Rank-based partial correlations were used to test the association between HRV measures and the pain measures, while controlling for age and BMI. This was done by computing the partial correlation between the rank of each HRV measure and the rank of each pain or psychological distress symptom, controlling for the rank of age and the rank of BMI. These analyses were done separately within the HC and IBS groups.
Additional analyses were conducted to determine whether the results change when controlling for psychological distress as well as age and BMI. Data analysis was conducted with IBM SPSS Statistics for Windows, version 19.0 (SPSS, Inc., Armonk, NY: IBM Corp, USA). A p value ≤ .05 was considered statistically significant, and tables include p-values whenever the p-values is less than .20. No adjustments were made for multiple comparisons so results should be interpreted with this in mind.
RESULTS
Characteristics of participants
Demographic characteristics did not differ between the 54 IBS and 37 HC participants. The mean (sd) age was 28.4 (6.7) years in the IBS group and 28.6 (6.8) years in the HC group. The sample was primarily White (92% IBS, 88% HC), single (70% IBS, 81% HC) with 60% of IBS and 59% of HC group earning an annual income of less than $50,000. Approximately 72% of women in both groups had a college degree. Among the 54 women in the IBS group, 25 were classified as IBS-CM (12 in IBS-C, 13 in IBS-M) and 29 as IBS-D. Mean BMI did not differ between groups (IBS, 24.2 [5.1]; HC, 23.7 [4.0]). All women completed the overnight 12 hour Holter recording, 78% completed the pain testing while 82% returned their completed diaries. Participants in the IBS group had been diagnosed with IBS for a mean (sd) of 8.0 (1.4) years and reported IBS symptom for 11.8 (2.6) years.
Eighty-six women were consented. Of these two IBS participant dropped out before providing any of the physiological data. One HC was an extreme outlier and excluded. She reported extremely high pain at the baseline on the WLSP test, and she had moderate or worse abdominal pain on 17% of diary days. One HC participant took off the Holter at bedtime and hence there is no night time HRV available, but WLSP and CPM data are available. Two IBS subjects were taking TCAs and hence were excluded from the HRV analyses, but were included in the CPM and WLSP analyses. Five HCs and nine IBS subjects provided HRV data but dropped out before the CPM and WLSP test procedures. Thus 37 HCs and 56 IBS subjects provided at least one of the measures of interest (CPM, WLSP or HRV). The analyses of HRV included 36 HCs and 54 IBS subjects. The analyses of CPM and WLSP included 31 HCs and 56 IBS subjects.
Comparison of symptoms by groups and subgroups
In the diary, as expected, women with IBS reported more days with moderate to very severe abdominal pain, pain after eating, constipation, diarrhea, and bloating, as compared to HCs (Table 1). A few women in the HC group reported moderate to very severe abdominal pain but only during menses. With the exception of constipation and diarrhea, there were no differences in other GI symptoms (bloating, intestinal gas, nausea) in the IBS bowel pattern subgroup (IBS-CM vs. IBS-D). Psychological distress measures were significantly higher in the IBS group than in HC. There were no significant IBS bowel pattern subgroup differences in psychological distress.
Table 1.
Percent of days with moderate to severe GI symptoms and psychological distress in women with IBS compared to HC
| HC vs IBS | IBS Subgroups | |||||||
|---|---|---|---|---|---|---|---|---|
| HC n = 29 |
IBS n = 47 |
pa | IBS-CM n = 22 |
IBS-D n = 25 |
pb | pc | pd | |
| Percent of days with moderate to very severe GI distress | ||||||||
| Abdominal pain/ discomfort |
1.3 (2.7) | 40.2 (27.0) | <.001 | 40.6 (24.5) | 39.8 (29.5) | <.001 | <.001 | NS |
| Abdominal pain/ discomfort after eating |
0.4 (1.5) | 26.6 (21.7) | <.001 | 28.7 (22.9) | 24.8 (20.9) | <.001 | <.001 | NS |
| Constipation | 0.2 (0.9) | 19.1 (23.6) | <.001 | 26.8 (25.0) | 12.3 (20.5) | <.001 | <.001 | .022 |
| Diarrhea | 0.5 (1.2) | 20.2 (22.7) | <.001 | 12.5 (13.7) | 26.9 (26.8) | <.001 | <.001 | .075 |
| Bloating | 2.9 (4.7) | 31.4 (29.4) | <.001 | 30.0 (26.5) | 32.7 (32.2) | <.001 | <.001 | NS |
| Percent of days with moderate to very severe psychological distress | ||||||||
| Stressed | 10.0 (21.7) | 32.1 (26.5) | <.001 | 30.9 (24.5) | 33.1 (28.5) | <.001 | <.001 | NS |
| Anxiety | 6.1 (13.6) | 29.4 (27.5) | <.001 | 26.9 (19.0) | 31.5 (33.5) | <.001 | <.001 | NS |
| Depression | 5.6 (16.8) | 14.8 (24.4) | .005 | 8.7 (10.8) | 20.2 (31.3) | .020 | .012 | NS |
Note: HC = healthy controls. IBS = irritable bowel syndrome. IBS-CM = Constipation- and Mixed-predominant irritable bowel syndrome (IBS) group. IBS-D = Diarrhea-predominant IBS group.
pa = 2-group comparison IBS vs. HC using Mann-Whitney test; pb = IBS-CM vs. HC; pc = IBS-D vs. HC; pd = IBS-CM vs. IBS-D. NS = non-significant (p > .20).
Comparison of pain sensitivity tests by groups and subgroups
As shown in Table 2, there were no significant IBS vs. HC group differences in pain reported at thermal settings of 45°C to 48°C or in the CPM efficiency score. However, statistically significant differences were found between IBS-CM and IBS-D at temperatures of 46°C to 48°C, with IBS-CM participants reporting higher pain sensitivity. There was also a trend for the IBS-CM group to have a lower CPM efficiency than the IBS-D group (p = .096).
Table 2.
Comparison of thermal pain sensitivity, conditioned pain modulation, and upper GI visceral sensitivity in women with IBS compared to HC
| HC vs IBS | IBS Subgroups | |||||||
|---|---|---|---|---|---|---|---|---|
| HC n = 31 |
IBS n = 46 |
pa | IBS-CM n = 22 |
IBS-D n = 24 |
pb | pc | p d | |
| Thermal pain sensitivity | ||||||||
| 45 °C | 3.8 (2.4) | 3.5 (2.0) | NS | 4.2 (2.1) | 2.9 (1.6) | NS | .165 | .171 |
| 46 °C | 4.2 (2.7) | 4.3 (2.3) | NS | 5.3 (2.3) | 3.3 (1.7) | .121 | NS | .005 |
| 47 °C | 5.3 (2.5) | 5.4 (2.4) | NS | 6.4 (2.3) | 4.5 (2.2) | .141 | NS | .011 |
| 48 °C | 6.3 (2.4) | 6.7 (2.2) | NS | 7.5 (2.1) | 5.9 (2.0) | .079 | NS | .011 |
| CPM efficiency score | 1.1 (1.3) | 0.9 (1.1) | NS | .8 | 1.1 (0.9) | NS | NS | .096 |
| Visceral Pain Sensitivity | ||||||||
| Pain/discomfort | 15.4 (17.5) | 26.9 (16.0) | .001 | 31.9 (16.9) | 22.3 (14.4) | .001 | .016 | .039 |
| Bloating | 20.6 (21.7) | 34.6 (20.3) | .003 | 38.9 (20.9) | 30.8 (19.0) | .003 | .039 | .187 |
| Fullness | 35.3 (19.9) | 44.6 (17.4) | .050 | 50.0 (16.7) | 39.7 (16.8) | .006 | NS | .014 |
| Nausea | 12.5 (19.1) | 22.9 (20.3) | .003 | 24.2 (20.7) | 21.7 (20.3) | .005 | .024 | NS |
Note: IBS = irritable bowel syndrome. HC = healthy controls. IBS-CM = Constipation- and Mixed-predominant irritable bowel syndrome (IBS) group. IBS-D = Diarrhea-predominant IBS group. CPM = conditioned pain modulation.
P values were determined using Mann–Whitney test.
pa= 2 group comparison of IBS vs. HC; pb = IBS-CM vs. HC; pc = IBS-D vs. HC; pd = IBS-CM vs. IBS-D. NS = non-significant (p > .20).
We further explored this difference by conducting a post-hoc analyses that dichotomized CPM efficiency as less than 1 (‘poor’ CPM) or equal to or greater than 1 (‘good’ CPM). In the IBS-CM group almost three-fourths (72%) had a ‘poor’ CPM response while in the IBS-D group 33% had ‘poor’ CPM response. Unexpectedly, 45% of the HCs had ‘poor’ CPM effeciency.
Using the WLSP test there was no significant group difference in the mean (sd) amount of water consumed during the 5-minute ingestion phase of the WLSP: HC, 962 (302) ml; IBS, 994 (331) ml. Compared to HCs, the IBS group reported significantly more pain, bloating, fullness, and nausea during the post-ingestion phase (Table 2). The IBS-CM subgroup reported significantly higher pain than HCs in the first 10 minutes (Figure 1). IBS-CM also showed higher GI symptoms than IBS-D subgroup but only reached significance for fullness and a trend for pain/discomfort.
Figure 1.
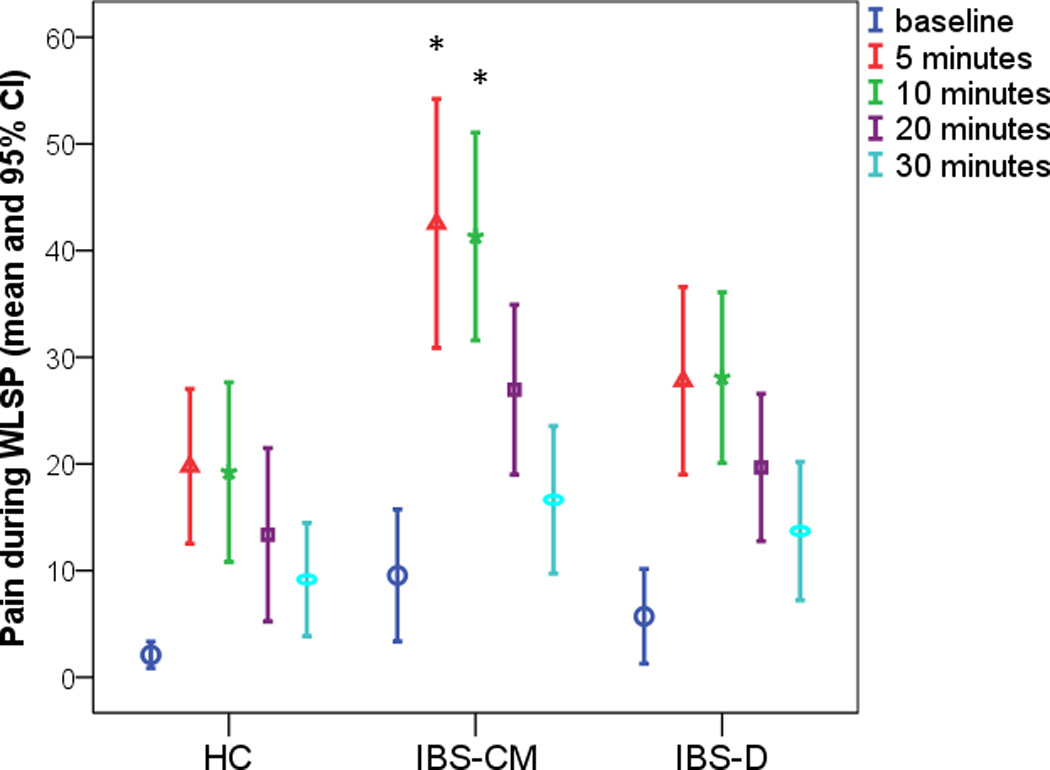
Report of pain rating in response to Water Load Symptom Provocation [WLSP] test for different time points. Baseline is just prior to water ingestion. HC = Healthy controls. IBS-CM = Constipation- and Mixed-irritable bowel syndrome (IBS) group. IBS-D = Diarrhea-predominant IBS group.
*p-value < .05 for the comparison (IBS-CM. versus HC).
Based on the results in Table 2 showing IBS versus HC differences in visceral pain sensitivity but not thermal pain sensitivity, we computed the Spearman rank correlation of these two pain sensitivity measures with abdominal pain from the diary in the IBS group. The correlation of mean percent of days with moderate to very severe abdominal pain with WLSP was .37 (p = .012). The correlation with pain at 47°C was smaller, namely .20 (p = .18). The correlation of diary pain with CPM efficiency was minimal at −.01 (p = .97).
Figure 2 illustrates abdominal pain in concert with thermal sensitivity at 47°C with the emphasis on the IBS-CM versus IBS-D contrast. Within the IBS group, there is a positive correlation of .38 between visceral WLSP pain and visceral thermal pain (p = .009). As shown, the majority of IBS-D participants fall in the lower left indicating both decreased visceral and thermal pain sensitivity while the majority of the IBS-CM participants fall in the upper right indicating both increased visceral and thermal pain sensitivity. In our three groups, 52% of IBS-CM, 17% of IBS-D, and 16% HCs had both increased visceral sensitivity (> 20) and increased thermal sensitivity (> 5).
Figure 2.
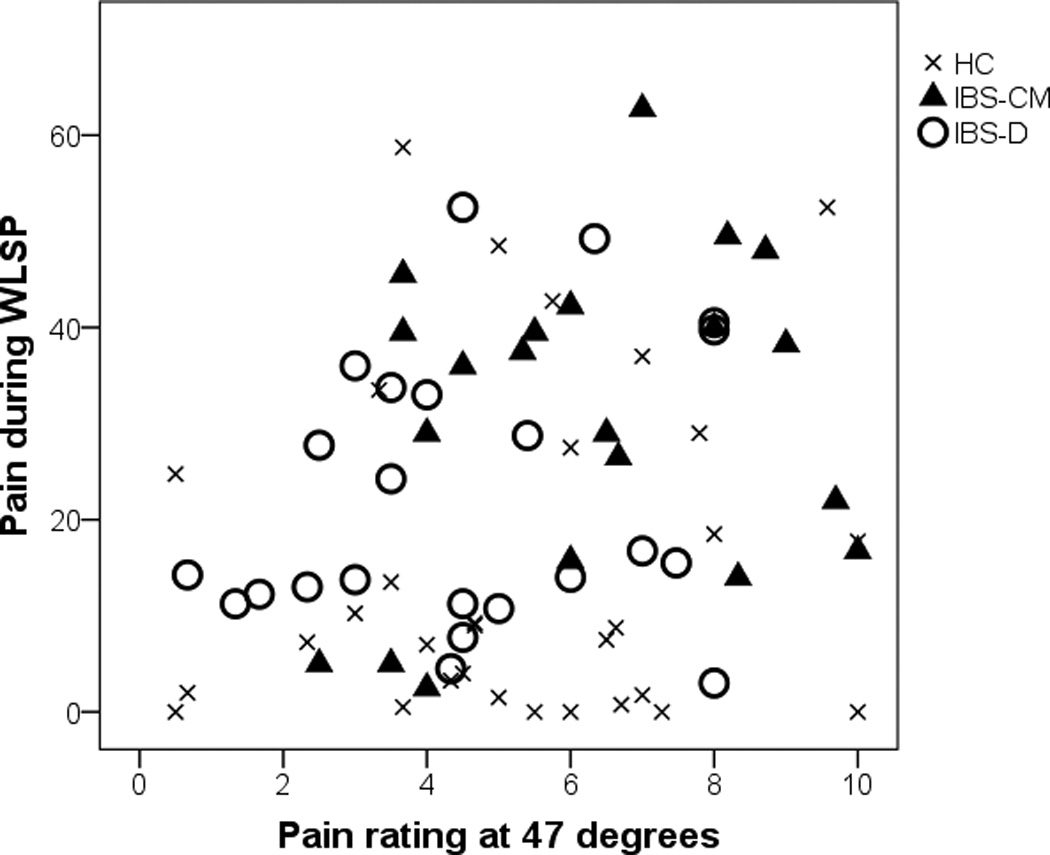
Average pain rating during the Water Load Symptom Provocation (WLSP) procedure versus pain rating from thermal pain at 47° C by group. HC = Healthy Control. IBS-CM = Constipation- and Mixed-irritable bowel syndrome (IBS) group. IBS-D = Diarrhea-predominant IBS group.
Comparison of HRV by groups and subgroups
Table 3 shows the means of HRV indices for HC and IBS groups. There were no significant differences between HC and IBS groups for any of the HRV indices. Subgroup analyses showed HF to be lower and LF/HF higher in IBS-CM than in HC though these differences did not reach statistical significance (p = .182), but did for LF/HF (p = .046), respectively.
Table 3.
Comparison of HRV in women with IBS and HCs
| HC vs. IBS | IBS Subgroups | |||||||
|---|---|---|---|---|---|---|---|---|
| HC n = 36 |
IBS n = 54 |
pa | IBS-CM n = 25 |
IBS-D n = 29 |
pb | pc | pd | |
| HF (ms2) | 1183 (1243) | 871 (795) | NS | 677 (555) | 1039 (933) | .182 | NS | .140 |
| LF (ms2) | 1453 (992) | 1429 (1047) | NS | 1295 (843) | 1544 (1198) | NS | NS | NS |
| TP (ms2) | 5118 (4487) | 4069 (2622) | NS | 3638 (1887) | 4440 (3106) | NS | NS | NS |
| LF/HF (ratio) | 2.6 (2.6) | 2.9 (2.1) | .095 | 3.0 (1.9) | 2.8 (2.3) | .046 | NS | NS |
| Heart Rate (beats/min.) | 62.3 (8.6) | 64.1 (8.9) | NS | 65.5 (8.3) | 63.0 (9.4) | .133 | NS | .184 |
Note. HRV = heart rate variability. HC = healthy controls. IBS = irritable bowel syndrome. IBS-CM = Constipation- and Mixed-predominant irritable bowel syndrome (IBS) group. IBS-D = Diarrhea-predominant IBS group. HF = high frequency power. LF = low frequency power. TP = Total power (very low power + LF + HF). Comparisons were made with an analysis of covariance using the rank transformation, controlling for age and BMI.
pa= 2 group comparison of IBS vs. HC; pb = IBS-CM vs. HC; pc = IBS-D vs. HC; pd = IBS-CM vs. IBS-D. NS = non-significant (p > .20).
Relationships of symptoms and HRV
The relationship of HRV with daily symptoms was not tested in the HC group because they had no or rare GI symptoms except at menses. In the IBS group, LF and TP were significantly (positively) associated with abdominal pain/discomfort and there was a trend for an association between LF and abdominal pain/ discomfort after eating (Table 4). LF was also related to diarrhea in the total IBS group.
Table 4.
Relationships of heart rate variability to diary GI symptoms, thermal sensitivity, conditioned pain modulation and upper GI visceral sensitivity symptoms in women with irritable bowel syndrome and healthy controls
| HF (ms2) | LF (ms2) | TP (ms2) | LF/HF (ratio) | HR (bpm) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HC | IBS | HC | IBS | HC | IBS | HC | IBS | HC | IBS | |
| Percent of days with moderate to very severe symptoms | ||||||||||
| Abdominal pain/discomfort | NA | .22 | NA | .39* | NA | .31* | NA | .00 | NA | −.10 |
| Abdominal pain/discomfort after eating |
NA | .13 | NA | .31* | NA | .24 | NA | .17 | NA | −.12 |
| Constipation | NA | .17 | NA | .08 | NA | .09 | NA | −.07 | NA | −.06 |
| Diarrhea | NA | .15 | NA | .30† | NA | .24 | NA | .04 | NA | −.01 |
| Bloating | NA | .03 | NA | .15 | NA | .16 | NA | .07 | NA | .07 |
| Thermal pain sensitivity | ||||||||||
| 45 °C | .03 | .07 | −.19 | .07 | −.36† | −.07 | −.35† | −.07 | .04 | −.09 |
| 46 °C | .15 | −.02 | −.09 | −.14 | −.28 | −.22 | −.36† | −.12 | .08 | .01 |
| 47 °C | .10 | −.04 | −.14 | −.20 | −.35† | −.29† | −.31† | −.13 | .10 | .10 |
| 48 °C | .13 | .18 | −.09 | −.29† | −.29 | −.38* | −.33† | −.06 | .17 | .20 |
| CPM Efficiency Score | −.09 | .24 | −.17 | .08 | −.11 | .11 | .11 | −.13 | .15 | −.08 |
| Visceral Pain Sensitivity | ||||||||||
| Pain | .22 | .16 | .09 | .03 | .11 | −.02 | −.21 | −.12 | .02 | .07 |
| Bloating | .37† | −.06 | .20 | .10 | .18 | −.12 | −.29 | .10 | −.03 | .18 |
| Fullness | .17 | −.09 | −.04 | −.07 | .07 | −.22 | −.26 | .13 | .06 | .16 |
| Nausea | .25 | −.23 | .03 | −.08 | .16 | −.28† | −.32† | .16 | .02 | .11 |
Note. IBS = irritable bowel syndrome. HC = healthy controls, CPM = conditioned pain modulation. GI = gastrointestinal. HF = high frequency power. LF = low frequency power. TP = total power. HR = Heart rate. Bpm = beats/min. NA = Not applicable because too few HC women had a non-zero symptom score. Rank-based partial correlations are shown, controlled for age and body mass index (BMI). Number of participants: HC = 31; IBS = 44
p <. 05,
p ≥.05 and p < .10.
Relationships of symptoms and pain sensitivity measures with HRV
Consistent negative relationships of LF/HF to both visceral WLSP test symptoms and thermal sensitivity were found in the HC group (Table 4). In the IBS group, pain sensitivity at 48°C was negatively associated with TP. CPM efficiency scores did not show a relationship with HRV indices in the IBS, HC, or IBS subgroups.
Discussion
Based on our prior work,(18, 19, 21, 26, 27) we hypothesized that participants with IBS would have increased thermal and visceral pain sensitivity, reduced CPM efficiency, and lower vagal HRV indices during the night as compared to HCs. We found that the IBS group reported increased severity of abdominal pain, bloating, fullness and nausea measured during the WLSP test compared to HCs. However, we found no difference in thermal pain sensitivity between IBS and HCs. Additionally, both visceral and thermal pain sensitivity were higher in the IBS-CM than the IBS-D group. There were no statistical significant differences between IBS versus HCs in HRV indices, however, there was a trend for IBS-CM women to have lower night-time vagal measures (HF), higher LF/HF ratio, and reduced CPM efficiency. Although we hypothesized that there would be a negative correlation between daily abdominal pain and CPM and HF, we found trends in the opposite direction.
Previous studies have demonstrated that thermal sensitivity is increased in at least a subset of patients with IBS(6, 28) and that this sensitivity is site specific (i.e., only the foot). For example, Zhou studying 78 IBS-D patients found that the IBS group had decreased thermal pain tolerance relative to controls when the foot was studied, but did not find the same results when the hand was tested. Our overall lack of difference in the thermal stimulation of the arm between HC and IBS groups is consistent with these findings. What is novel about our findings is that there are bowel pattern subgroup differences in thermal sensitivity in the arm. This divergence between the two bowel subgroups in thermal sensitivity is not likely to be due to psychological distress levels since there were no bowel pattern subgroup differences in daily measures of feeling stressed, anxiety, and depression.
In this study we did find increased upper GI visceral sensitivity with the WLSP test in the IBS group. Visceral hypersensitivity to liquid ingestion can be the result of alterations in peripheral afferents, spinal cord transmission, exaggerated gastrocolonic reflex, altered central processing of afferent input and/or decreased descending inhibitory input. Other researchers have found reduced CPM efficiency in IBS-D patients compared to HCs.(29–31) However, we did not find this reduction. Instead we found that the CPM efficiency tended to be reduced in the IBS-CM group when compared to IBS-D and HCs. The observations of increased thermal sensitivity, heightened upper GI visceral sensitivity, and a trend toward reduced CPM in the IBS-CM group may suggest an overall hyperalgesia in women with constipation and/or mixed bowel pattern. However, whether this visceral hyperalgesia is specific to the upper GI tract is unknown. Based on the literature it is uncertain whether our observations would have been similar if a rectal distention model had been used. Wilder-Smith and Robert-Yap found greater rectal sensitivity (barostat) in patients with IBS-D as compared to IBS-C (31). Several investigators have suggested that the gastrocolonic reflex in response to liquid non nutrient intake may be greater in patients with predominantly IBS-constipation due to increases in serum motilin levels (32). This could explain the increase abdominal pain reported by the IBS-CM group relative to the IBS-D group in response to WLST as opposed to an overall heightened visceral sensitivity.
With regard to night-time HRV we did not find the hypothesized relationship of reduced HRV (low HF) and increased GI symptoms. However, we did find that the diary report of abdominal pain was positively associated with LF and TP. LF represents a mix of both parasympathetic and sympathetic influences, and TP represents overall autonomic function(32) and, as such, it does suggest physiological hyperarousal during sleep may contribute to pain-related symptoms.
The observed trend of lower HF and higher LF/HF ratio in those with IBS-CM relative to IBS-D is consistent with both the recent meta-analysis by Liu and our prior study. In the meta-analyses, Liu et al found 11 studies that overall showed that IBS patients had lower HF power, and higher LF:HF compared to controls. However, the authors pointed out that IBS versus control differences were more consistently observed when short day time assessments were performed. Protocols that include HRV assessment both night-time and during pain testing may better clarify the relationship of ANS to visceral and thermal pain perception along with symptoms. For example, Iovino(33) in an early study demonstrated that when ANS activity was experimentally altered (i.e., increased SNS activity) sensitivity to rectal stimulation was increased.
The subtle night-time differences in HRV indices found in our study are not unique to patients with IBS but are also noted in patients with fibromyalgia, interstitial cystitis, and chronic fatigue syndrome(34). The sympathetic nervous system is the main component of the stress response system. Along these lines, the presence of these syndromes could be viewed as a failed attempt of our main complex adaptive system (the ANS) to adjust to a hostile environment.3
Whether the trends toward reduced HF noted in the IBS-CM is the cause of the constipation or vice versa remains unknown. One plausible explanation put forth by Liu et al. is that chronic stress leads to vagal suppression and subsequent reduction of motility and increased transit time. Strategies such as exercise and controlled respiration that enhance vagal activity may improve constipation but would require sustained patient involvement.(35, 36). Such research remains to be done.
Limitations
There are several important limitations to our study. First, the sample size is relatively small for the number of comparisons that were made. Thus, the results should be interpreted with caution. The sample was recruited from the community and not specifically from tertiary care centers. As such, the findings may not be generalizable to the patients with the most severe symptoms seen in tertiary care settings. The sample for this study only included women who were still menstruating and no efforts were made to control for oral contraceptive or other hormone contraception. Men were not included in this study and hence it is unknown whether the results found here would also be found in men.
Conclusions
In this study of pain sensitivity in women with IBS, we found that thermal pain sensitivity did not differ between those with IBS and HCs. Although within the IBS group, the IBS-CM subgroup had significantly higher pain sensitivity than those in the IBS-D subgroup. In the visceral test, WLSP, the IBS group experienced significantly more symptom distress than HCs and the IBS-CM subgroup had more distress than the HCs. There were no differences in HRV indicators between IBS and HCs or within in the subgroups. However, daily abdominal pain was positively correlated with LF and TP in the IBS group. Further research with large samples and standardized methodology may help to elucidate the pain mechanism active in this heterogeneous group.
Key Messages.
Irritable bowel syndrome (IBS) is associated with visceral and somatic pain hypersensitivity
The aim of this study was to determine if women with IBS and the stool pattern subgroups differed from controls on measures of thermal, visceral, and conditioned pain modulation (CPM), and night-time heart rate variability (HRV).
A heat stimulus on the forearm was used to test thermal pain sensitivity and CPM while a water load symptom provocation was used to test visceral pain sensitivity and a Holter monitor was used to assess HRV during the night.
Visceral sensitivity was significantly higher in the IBS group compared to controls. The constipation-mixed group reported greater thermal sensitivity and a trend towards reduced CPM compared to the diarrhea subgroup.
Acknowledgments
Supported by a grant from the NINR, NIH (NR04101).
Footnotes
Author Contributions:
Monica E. Jarretta, PhD.- Planned and conducted the study, assisted in data analyses, and lead author of paper
Claire J. Hana, MSN. - Assisted in the data analyses and data interpretation with manuscript preparation.
Kevin C. Cainb, PhD. – Assisted with design of the study and was the lead data analyst
Robert L. Burra, PhD.–Planned and conducted the heart rate variability analyses
Robert J. Shulmanc, MD. – Planned the study and assisted with manuscript preparation and interpretation of data.
Pamela G. Barneya, MN.– Planned and conducted the study
Bruce D. Naliboffd, PhD. – Involved in the design of the study and the interpretation of study findings
Jasmine Ziae, MD.– Involved in the clinical interpretation of the data and the data analyses.
Margaret M. Heitkempera,*, PhD. - Wrote the grant that funded the study, assisted with data interpretation and writing of the manuscript.
References
- 1.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712.e714–721.e714. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Drossman D. The role of psychosocial factors in gastrointestinal illness. Scand J Gastroenterol Suppl. 1996;221:1–4. doi: 10.3109/00365529609095542. [DOI] [PubMed] [Google Scholar]
- 3.Drossman D, Richter J, Talley N, Corazziari E, Thompson W, Whitehead W. Functional Gastrointestinal Disorders. Boston: Little, Brown and Company; 1994. [Google Scholar]
- 4.Tanaka Y, Kanazawa M, Fukudo S, Drossman DA. Biopsychosocial model of irritable bowel syndrome. J Neurogastroenterol Motil. 2011;17:131–139. doi: 10.5056/jnm.2011.17.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Q, Wang EM, Yan XJ, Chen SL. Autonomic functioning in irritable bowel syndrome measured by heart rate variability: A meta-analysis. J Dig Dis. 2013;14:638–646. doi: 10.1111/1751-2980.12092. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Q, Fillingim RB, Riley JL, Malarkey WB, Verne GN. Central and peripheral hypersensitivity in the irritable bowel syndrome. Pain. 2010;148:454–461. doi: 10.1016/j.pain.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarrett ME, Shulman RJ, Cain KC, et al. Conditioned Pain Modulation in Women With Irritable Bowel Syndrome. Biol Res Nurs. 2014;16:368–377. doi: 10.1177/1099800413520486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King CD, Wong F, Currie T, Mauderli AP, Fillingim RB, Riley JL. Deficiency in endogenous modulation of prolonged heat pain in patients with irritable bowel syndrome and temporomandibular disorder. PAIN®. 2009;143:172–178. doi: 10.1016/j.pain.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heymen S, Maixner W, Whitehead WE, Klatzkin RR, Mechlin B, Light KC. Central processing of noxious somatic stimuli in patients with irritable bowel syndrome compared with healthy controls. Clin J Pain. 2010;26:104–109. doi: 10.1097/AJP.0b013e3181bff800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King CD, Goodin B, Kindler LL, et al. Reduction of conditioned pain modulation in humans by naltrexone: an exploratory study of the effects of pain catastrophizing. J Behav Med. 2013;36:315–327. doi: 10.1007/s10865-012-9424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang R, Cui L, Zhou W, et al. Reliability study of thermal quantitative sensory testing in healthy Chinese. Somatosens Mot Res. 2014;31:198–203. doi: 10.3109/08990220.2014.914485. [DOI] [PubMed] [Google Scholar]
- 12.Mulak A. Testing of visceral sensitivity. J Physiol Pharmacol. 2003;55:55–72. [PubMed] [Google Scholar]
- 13.Van Oudenhove L, Vandenberghe J, Vos R, Holvoet L, Tack J. Factors associated with co-morbid irritable bowel syndrome and chronic fatigue-like symptoms in functional dyspepsia. Neurogastroenterol Motil. 2011;23:524-e202. doi: 10.1111/j.1365-2982.2010.01667.x. [DOI] [PubMed] [Google Scholar]
- 14.Trimble KC, Farouk R, Pryde A, Douglas S, Heading RC. Heightened visceral sensation in functional gastrointestinal disease is not site-specific. Evidence for a generalized disorder of gut sensitivity. Dig Dis Sci. 1995;40:1607–1613. doi: 10.1007/BF02212678. [DOI] [PubMed] [Google Scholar]
- 15.Bruehl S, Chung OY. Interactions between the cardiovascular and pain regulatory systems: an updated review of mechanisms and possible alterations in chronic pain. Neurosci Biobehav Rev. 2004;28:395–414. doi: 10.1016/j.neubiorev.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Chalaye P, Goffaux P, Lafrenaye S, Marchand S. Respiratory effects on experimental heat pain and cardiac activity. Pain Med. 2009;10:1334–1340. doi: 10.1111/j.1526-4637.2009.00681.x. [DOI] [PubMed] [Google Scholar]
- 17.De Kooning M, Daenen L, Roussel N, et al. Endogenous pain inhibition is unrelated to autonomic responses in acute whiplash-associated disorders. J Rehabil Res Dev. 2015;52:431–440. doi: 10.1682/JRRD.2014.06.0154. [DOI] [PubMed] [Google Scholar]
- 18.Burr RL, Heitkemper M, Jarrett M, Cain KC. Comparison of Autonomic Nervous System Indices Based on Abdominal Pain Reports in Women with Irritable Bowel Syndrome. Biol Res Nurs. 2000;2:97–106. doi: 10.1177/109980040000200203. [DOI] [PubMed] [Google Scholar]
- 19.Heitkemper MM, Jarrett M, Cain KC, et al. Autonomic nervous system function in women with irritable bowel syndrome. Dig Dis Sci. 2001;46:1276–1284. doi: 10.1023/a:1010671514618. [DOI] [PubMed] [Google Scholar]
- 20.Cain KC, Jarrett ME, Burr RL, Hertig VL, Heitkemper MM. Heart rate variability is related to pain severity and predominant bowel pattern in women with irritable bowel syndrome. Neurogastroenterol Motil. 2007;19:110–118. doi: 10.1111/j.1365-2982.2006.00877.x. [DOI] [PubMed] [Google Scholar]
- 21.Jarrett M, Burr R, Cain K, Rothermel J, Landis C, Heitkemper M. Autonomic Nervous System Function During Sleep Among Women with Irritable Bowel Syndrome. Dig Dis Sci. 2008;53:694–703. doi: 10.1007/s10620-007-9943-9. [DOI] [PubMed] [Google Scholar]
- 22.Drossman D, Corazziari E, Delvaux M, et al. Rome III: The functional gastrointestinal disorders. 3rd. McLean, VA: Degnon Associates Inc.; 2006. [Google Scholar]
- 23.Chey W, Paré P, Viegas A, Ligozio G, Shetzline M. Tegaserod for female patients suffering from IBS with mixed bowel habits or constipation: a randomized controlled trial. Am J Gastroenterol. 2008;103:1217–1225. doi: 10.1111/j.1572-0241.2008.01808.x. [DOI] [PubMed] [Google Scholar]
- 24.Berntson GG, Thomas Bigger J, Eckberg DL, et al. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 25.Convoer WJ, Iman RL. Analysis of covariance using the rank transformation. Biometrics. 1982;38:715–724. [PubMed] [Google Scholar]
- 26.Heitkemper M, Burr R, Jarrett M, Hertig V, Lustyk M, Bond E. Evidence for Autonomic Nervous System Imbalance in Women with Irritable Bowel Syndrome. Dig Dis Sci. 1998;43:2093–2098. doi: 10.1023/a:1018871617483. [DOI] [PubMed] [Google Scholar]
- 27.Heitkemper M, Cain KC, Shulman R, Burr R, Poppe A, Jarrett M. Subtypes of irritable bowel syndrome based on abdominal pain/discomfort severity and bowel pattern. Dig Dis Sci. 2011;56:2050–2058. doi: 10.1007/s10620-011-1567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verne GN, Price DD, Callam CS, Zhang B, Peck J, Zhou Q. Viscerosomatic facilitation in a subset of IBS patients, an effect mediated by N-methyl-D-aspartate receptors. J Pain. 2012;13:901–909. doi: 10.1016/j.jpain.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong RK, Van Oudenhove L, Li X, Cao Y, Ho KY, Wilder-Smith CH. Visceral pain perception in patients with irritable bowel syndrome and healthy volunteers is affected by the MRI scanner environment. European Gastroenterol J. 2015 doi: 10.1177/2050640615580888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanazawa M, Hongo M, Fukudo S. Visceral hypersensitivity in irritable bowel syndrome. J Gastroenterol Hepatol. 2011;26:119–121. doi: 10.1111/j.1440-1746.2011.06640.x. [DOI] [PubMed] [Google Scholar]
- 31.Wilder-Smith CH, Robert-Yap J. Abnormal endogenous pain modulation and somatic and visceral hypersensitivity in female patients with irritable bowel syndrome. World J Gastroenterol. 2007;13:3699–3704. doi: 10.3748/wjg.v13.i27.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simren M, Abrahamsson H, Bjornsson ES. An exaggerated sensory component of the gastrocolonic response in patients with irritable bowel syndrome. Gut. 2001;48:20–27. doi: 10.1136/gut.48.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iovino P, Azpiroz F, Domingo E, Malagelada J-R. The sympathetic nervous system modulates perception and reflex responses to gut distention in humans. Gastroenterology. 1995;108:680–686. doi: 10.1016/0016-5085(95)90439-5. [DOI] [PubMed] [Google Scholar]
- 34.Martínez-Martínez L-A, Mora T, Vargas A, Fuentes-Iniestra M, Martínez-Lavín M. Sympathetic Nervous System Dysfunction in Fibromyalgia, Chronic Fatigue Syndrome, Irritable Bowel Syndrome, and Interstitial Cystitis: A Review of Case-Control Studies. J Clin Rheumatol. 2014;20:146–150. doi: 10.1097/RHU.0000000000000089. [DOI] [PubMed] [Google Scholar]
- 35.Barbosa R, Netto Júnior J, Cassemiro BM, et al. Impact of functional training on cardiac autonomic modulation, cardiopulmonary parameters and quality of life in healthy women. Clin Physiol Funct Imaging. 2015 doi: 10.1111/cpf.12235. [DOI] [PubMed] [Google Scholar]
- 36.Daley A, Grimmett C, Roberts L, et al. The effects of exercise upon symptoms and quality of life in patients diagnosed with irritable bowel syndrome: a randomised controlled trial. Int J Sports Med. 2008;29:778–782. doi: 10.1055/s-2008-1038600. [DOI] [PubMed] [Google Scholar]


