Summary
The dendritic arbor is subject to continual activity-dependent remodeling, requiring a balance between directed cargo trafficking and dynamic restructuring of the underlying microtubule tracks. How cytoskeletal components are able to dynamically regulate these processes to maintain this balance remains largely unknown. By combining single molecule assays and live imaging in rat hippocampal neurons, we have identified the kinesin-4 KIF21B as a molecular regulator of activity-dependent trafficking and microtubule dynamicity in dendrites. We find that KIF21B contributes to the retrograde trafficking of BDNF/TrkB complexes, and also regulates microtubule dynamics through a separable, non-motor microtubule-binding domain. Neuronal activity enhances the motility of KIF21B at the expense of its role in cytoskeletal remodeling, the first example of a kinesin whose function is directly tuned to neuronal activity state. These studies suggest a model in which KIF21B navigates the complex cytoskeletal environment of dendrites by compartmentalizing functions in an activity-dependent manner.
eTOC Blurb
Ghiretti et al. demonstrate that the dendritic kinesin KIF21B independently regulates transport and microtubule dynamicity through discrete microtubule binding sites. Uniquely, regulation of dynamicity is independent of motor activity, and neuronal activity differentially regulates the dual functions of this kinesin.
Introduction
Neurons are structurally and functionally polarized cells, receiving information via dendrites and sending information through axons. To maintain proper function, protein cargoes must be accurately sorted to and trafficked within specific subcellular compartments: presynaptic components must reach axon terminals, and postsynaptic components must be sorted into dendrites (Kapitein and Hoogenraad, 2011). Simultaneously, cargoes from distal axonal and dendritic regions undergo sustained transport back to the soma (Cohen et al., 2011; Maday et al., 2012). This long-range bidirectional trafficking is primarily mediated by molecular motors that transit along the polarized microtubule cytoskeleton. Kinesin motors generally travel from the minus to plus-ends of microtubules, while dynein travels from plus to minus-ends (Hirokawa et al., 2010). Axonal microtubules are uniformly arranged in a “plus-end-outward” fashion (Baas et al., 1988), which has made the study of axonal trafficking and characterization of axon-specific motors relatively straightforward. In contrast, the microtubule population in mammalian neuronal dendrites is, at least proximally, of mixed polarity (Baas et al., 1988).
A further layer of complexity lies in the fact that the dendritic arbor is subject to constant remodeling, with new branches extended or retracted in response to cues such as changes in neuronal activity (Wong and Ghosh, 2002). This structural plasticity is supported and maintained by the underlying microtubule cytoskeleton, which is itself continually remodeled through cycles of stochastic growth and catastrophe known as dynamic instability (Mitchison and Kirschner, 1984). In addition to invading newly-formed dendritic branches (Szebenyi et al., 2005), dynamic microtubules repeatedly sample dendritic spines and are essential for activity-dependent spine maturation (Hu et al., 2008). Neuronal activity affects dendritic microtubule function in multiple ways, including enhanced transport of specific cargoes into dendrites (Hoerndli et al., 2015; Ichinose et al., 2015; Neupert et al., 2015) and stabilization of microtubule dynamics (Kapitein et al., 2011). However, the mechanism by which the dendritic cytoskeleton balances processive long-range transport with dynamic remodeling of the underlying microtubule network remains unknown.
In both axons and dendrites, brain-derived neurotrophic factor (BDNF), in complex with its receptor TrkB, is a key long-range cargo critical for neuronal survival and function. Upon BDNF binding, TrkB dimers are endocytosed to form signaling endosomes that are retrogradely transported to the nucleus to affect gene transcription (Zweifel et al., 2005). BDNF/TrkB signaling is well-studied in axons, where dynein is the sole retrograde motor (Zhou et al., 2012). While dynein contributes to the transport of signaling endosomes in dendrites (Liot et al., 2013), the possible role of kinesin motors in this motility has not been explored. Importantly, BDNF signaling and neuronal activity are inextricably linked: application of soluble BDNF phenocopies many of the downstream affects of neuronal depolarization (Huang and Reichardt, 2001), and BDNF expression is upregulated in response to activity (Tao et al., 1998). Furthermore, depolarization results in increased surface expression of TrkB and prolonged activation of the BDNF/TrkB signaling complex (Guo et al., 2014), but how activity affects retrograde BDNF/TrkB trafficking in dendrites, and particularly, how processive motility persists in the face of activity-induced dendrite dynamics, is unclear.
Intriguingly, the neuronal kinesin-4s KIF21A and KIF21B exhibit distinct subcellular localizations despite ~65% amino acid homology. KIF21A is restricted to the axon, and KIF21B localizes to both axons and dendrites (Jenkins et al., 2012; Marszalek et al., 1999), making it one of only a few kinesins to exhibit significant dendritic localization (Huang and Banker, 2012; Setou et al., 2002). The recent characterization of the KIF21B knockout mouse highlights the importance of this kinesin, as KIF21B −/− neurons exhibit decreased dendritic branching and spine density, coupled with a reduction in surface expression of synaptic receptors (Muhia et al., 2016). These cellular deficits are reflected functionally in learning and memory deficits. While KIF21B has been reported to act as both a canonical kinesin motor and a regulator of microtubule dynamics (Labonte et al., 2013; Muhia et al., 2016), a mechanistic understanding of how KIF21B regulates these processes to affect neuronal function remains unknown.
Through a combination of single molecule assays and live imaging in rat hippocampal neurons, we have identified KIF21B as a key molecular regulator of activity-dependent changes in dendritic trafficking and microtubule dynamics. KIF21B is biased towards retrograde motility in dendrites and contributes to the retrograde trafficking of TrkB-positive signaling endosomes activated by BDNF. Intriguingly, while motor activity is determined by the canonical kinesin motor domain, KIF21B-dependent regulation of microtubule dynamics is an entirely separable function regulated through the ATP-independent activity of the C-terminal domain of the protein. Crucially, neuronal activity enhances motor activity of KIF21B at the expense of microtubule dynamic regulation, the first example of a neuronal kinesin that is directly regulated by the activity state of the neuron. Overall, our studies show that KIF21B is uniquely tuned to navigate the complex cytoskeletal environment of dendrites and is regulated by changes in neuronal activity.
Results
KIF21B displays processive motility with a retrograde bias in dendrites of hippocampal neurons
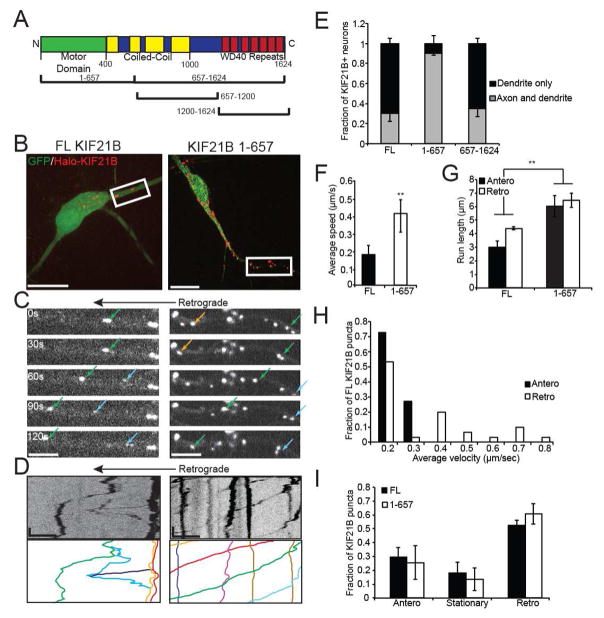
To directly visualize KIF21B in neurons, we transfected cultured rat hippocampal neurons with full-length (FL) HaloTag-KIF21B or one of two KIF21B truncation constructs for comparison. These include KIF21B 1-657, which contains the canonical N-terminal kinesin motor domain (van der Vaart et al., 2013); and KIF21B 657-1624, containing the cargo-binding WD40 repeats at the C-terminus of the protein (Figure 1A). At 10 days in vitro (DIV), we assessed the subcellular localization and motility of these constructs in neurons by confocal microscopy (Movie S1). Both KIF21B FL and 1-657 were punctate and motile throughout the axon, soma, and dendritic arbor of neurons (Figure 1B–D), consistent with the reported localization of endogenous KIF21B to both the axonal and dendritic compartments (Marszalek et al., 1999). However, when comparing axonal and dendritic KIF21B signal from the same neuron, FL KIF21B displayed a higher preference for the dendritic compartment (Figure 1E), with expression restricted to the dendrites in nearly 70% of neurons examined. KIF21B 657-1624 was also widely distributed with a dendritic preference, but puncta were smaller and nonmotile (Figure S1A–C). Overall, these data demonstrate that KIF21B is predominantly dendritic, and this localization requires the C-terminus.
Figure 1. KIF21B is motile and exhibits a retrograde bias in hippocampal dendrites.
A: Schematic of KIF21B, with 1-657 and 657-1624 and additional constructs indicated. B: Representative images of 10 DIV hippocampal neurons co-expressing GFP and TMR-labeled HaloTag-KIF21B, full-length (FL; left) or 1-657 (right). Scale bar = 25 μm. C: Time-lapse images of dendritic segments indicated by white boxes in B, showing FL (left) and 1-657 (right) motility (colored arrows). Scale bar = 10 μm. D: Kymographs of KIF21B motility shown in C. Line colors correspond to arrows in C. Scale bar = 10 μm, 30 sec. E: Fraction of HaloTag-KIF21B observed in both dendrites and axons or dendrites only. n = 10 neurons/condition. F: Average speed and, G: average run length of motile KIF21B puncta. ** = p < 0.01 from FL, by ANOVA with Tukey posthoc test. H: Frequency distribution of average velocity of net anterograde or retrograde FL KIF21B puncta. I: Fraction of anterograde, stationary, or retrograde KIF21B puncta. Anterograde/retrograde = >5 μm in corresponding direction during the imaging period. For F–I: n = 18–20 neurons per condition. Error bars = S.E.M. See also Figure S1.
We focused on proximal dendritic regions within 50–60 μm of the soma, identified morphologically by expression of a soluble EGFP fill, to observe how KIF21B navigates in a mixed microtubule array. While both KIF21B FL and 1-657 exhibited robust motility in this region, KIF21B 1-657 was significantly faster and more processive compared to the full-length kinesin, in regard to both average speed and run length (200 nm/sec and 3.5 μm versus 400 nm/sec and 6 μm, respectively; Figure 1F,G). This suggests that the N-terminal KIF21B 1-657 construct is constitutively active, while the C terminus acts as a tether or autoinhibitory domain to limit motility of the full-length kinesin. Both FL and 1-657 KIF21B displayed sustained motility over the imaging period (Figure S1D,E,G).
While FL and 1-657 KIF21B underwent runs in both the anterograde (towards the dendritic terminal) and retrograde (towards the soma) directions, there was a bias for retrograde motility. While the average speed of full-length KIF21B was relatively slow (200 nm/sec; Figure 1F), distributions of anterograde and retrograde velocities revealed a tail of faster-moving KIF21B puncta in the retrograde direction (Figure 1H); a similar directional bias was not observed for KIF21B 1-657 (Figure S1F). Moreover, 85% of both FL and 1-657 KIF21B puncta were motile (defined as net displacement of greater than 5 μm over the imaging period), and 60% of those moved in the retrograde direction (Figure 1I). This observation is surprising given that the mixed microtubule array of proximal dendrites is biased towards plus-end-outward microtubules (Yau et al., 2016), yet KIF21B has an unexpected preference for the smaller plus-end-inward microtubule fraction. Thus, KIF21B is able to navigate the mixed microtubule network of the proximal dendrite to favor retrograde motility.
KIF21B is highly processive in vitro and binds to microtubules at both its N- and C-termini
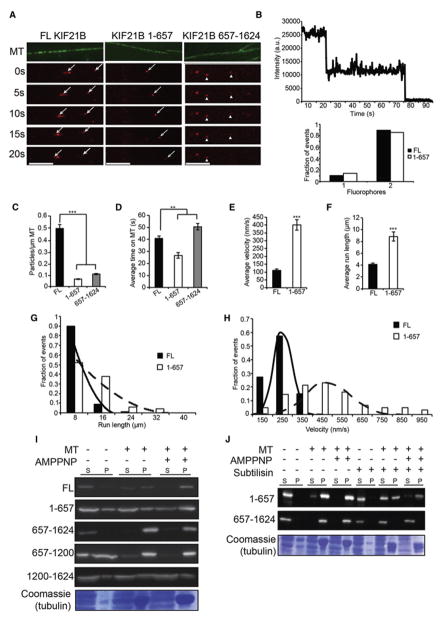
The apparent ability of the C terminus of KIF21B to slow the activity of the N-terminal motor domain suggested to us that there may be a secondary microtubule binding site, a unique property shared by only a few kinesins (Mayr et al., 2011; Su et al., 2011). To investigate this possibility, we turned to a minimal in vitro system for single molecule motility assays using Halo-tagged KIF21B constructs expressed in HeLa cell lysates (Blasius et al., 2007). HeLa cell lysates expressing either HaloTag-FL, −1-657, or -657-1624 KIF21B were flowed into chambers containing Alexa 488-labeled Taxol-stabilized microtubules (MT), allowing visualization of tetramethylrhodamine (TMR) ligand-labeled HaloTag-KIF21B with total internal reflection fluorescence (TIRF) microscopy (Figure 2A; Movie S2). While FL and KIF21B 657-1624 were expressed at comparable levels in these lysates, KIF21B 1-657 was more highly expressed (Figure S2A). Photobleaching analysis of the TMR ligand bound to kinesin molecules yielded two decay steps for both FL and 1-657 KIF21B, consistent with both constructs acting as dimers (Figure 2B).
Figure 2. Characterization of single molecule motility and microtubule binding of KIF21B in vitro.
A: Time-lapse images of FL, 1-657, and 657-1624 HaloTag-KIF21B (red) binding and motility along microtubules (MT; green). Motile particles indicated by arrows, nonmotile by arrowheads. Scale bar = 5 μm. B: Photobleaching of HaloTag-KIF21B particles; sample trace from a 1-657 particle (top) and distribution of fluorophores for both FL and 1-657 (bottom). n = 30 particles/condition. C: Average number of particles per μm MT, D: average dwell time on MT, E: average velocity, F: and average run length for HaloTag-KIF21B particles. ** = p < 0.01, *** = p < 0.001 from FL, by ANOVA with Tukey posthoc test. G: Frequency distribution for run length of motile HaloTag-KIF21B particles with exponential decay fit. H: Frequency distribution for velocity of motile HaloTag-KIF21B particles with Gaussian fit. Both run length and velocity distributions significantly different between FL and 1-657 (KS test; p < 0.001). For C–H: n = 416 (FL), 125 (1-657), 76 (657-1624) particles; N = 90 MTs/condition. I: Western blot (anti-HaloTag, 1:1000) of supernatant (S) and pellet (P) fractions from MT pelleting assay; 5 μM MTs, 10 mM AMPPNP. J: Western blot (anti-HaloTag, 1:1000) of supernatant (S) and pellet (P) fractions from MT pelleting assay; 5 μM MTs, 10 mM AMPPNP, MTs in right lanes digested with 200 μg/mL subtilisin before pelleting. Error bars = S.E.M. See also Figure S2 and S3.
FL KIF21B decorated microtubules at a density of 0.5 particles/μm MT (Figure 2A,C), and moved slowly but processively with 4 μm runs at 100 nm/sec on average (Figure 2E–H) towards the microtubule plus-end (Figure S2B). Despite the high density of binding, motility was not negatively impacted by crowding on the microtubule, as further dilution of the HeLa cell lysate did not significantly enhance motility parameters (Figure S2C–F). The robust binding and motility of full-length KIF21B was unexpected, given that full-length conventional kinesin-1 motors exhibit low binding and motility in this assay due to autoinhibition (Verhey and Hammond, 2009). These results suggest that KIF21B may not be autoinhibited in the same way as observed for kinesin-1. Truncated KIF21B 1-657 also bound to microtubules, but at a significantly lower density than the full-length motor despite its higher level of expression in cell lysates (0.067 particles/μm MT; Figure 2A,C; Figure S2A). Despite this lower binding frequency, the observed runs were highly processive, averaging over 8 μm at approximately 400 nm/sec (Figure 2E–H). Thus, full-length KIF21B has a higher affinity for microtubules, but truncated KIF21B 1-657 is faster and more processive.
Surprisingly, the C-terminal construct, KIF21B 657-1624, bound to microtubules at a similar density to KIF21B 1-657, despite lacking a canonical kinesin motor domain (Figure 2A,C). KIF21B 657-1624 remained bound to microtubules for significantly longer than either full-length KIF21B or KIF21B 1-657, suggesting a higher binding affinity under assay conditions of 10 mM Mg-ATP (Figure 2D). A straightforward explanation for the behavior of FL KIF21B is that it binds microtubules robustly, due to its dual binding sites, but moves slowly, due to anchoring or tethering by its nonmotile C terminus. Overall, these results characterize KIF21B as a processive kinesin motor, with microtubule interactions mediated by both N- and C-terminal binding sites.
Microtubule binding of the KIF21B C terminus is nucleotide-independent and mediated by interaction with the acidic tail of tubulin
A key feature of previously identified kinesin-8 and kinesin-13 C-terminal microtubule binding sites is that they are nucleotide-independent, not reliant on ATP/ADP cycling to bind and detach from microtubules (Mayr et al., 2011; Seeger and Rice, 2010; Su et al., 2011). Since the C terminus of KIF21B also lacks motor function, we tested its nucleotide dependence by performing microtubule pelleting assays with Taxol-stabilized microtubules and lysates from HeLa cells expressing KIF21B constructs. For both FL and 1-657 KIF21B, microtubule binding was enhanced in the presence of 5 mM AMPPNP, a non-hydrolyzable ATP analog (Figure 2I). This behavior is consistent with the properties of canonical kinesin motor domains, which bind to microtubules with high affinity in the presence of ATP or AMPPNP (Schief and Howard, 2001). In contrast, robust microtubule binding of KIF21B 657-1624 occurred even in the absence of AMPPNP (Figure 2I). This nucleotide-independent interaction with microtubules was also apparent with purified recombinant KIF21B 657-1624 (Figure S3A,B), verifying a direct interaction between microtubules and the C terminus of KIF21B.
KIF21B has seven WD40 repeats at its C terminus, which may facilitate cargo binding and could also mediate nucleotide-independent microtubule binding. Thus, we designed two additional KIF21B constructs (Figure 1A), 657–1200 (lacking the WD40 repeats) and 1200–1624 (containing the WD40 repeats), and tested them in the microtubule pelleting assay. Surprisingly, the 657–1200 construct lacking the WD40 repeats retained robust, nucleotide-independent microtubule binding, which was abolished in the 1200–1624 construct (Figure 2I). Thus, KIF21B interacts with microtubules through a previously unidentified domain in its C terminus.
We identified a number of basic residues (Figure S3D; green) in a region of the KIF21B C terminus that is highly conserved amongst vertebrates (Figure S3D; grey). This led us to wonder if KIF21B may interact with the acidic tails of tubulin, similar to the binding mechanism of CLIP170 (Mishima et al., 2007). To test this hypothesis, we performed microtubule pelleting assays using Taxol-stabilized microtubules treated with subtilisin, which cleaves off ~20 acidic amino acid residues located at the C-terminal tail of both alpha and beta tubulin (Lobert and Correia, 1992). While the binding properties of KIF21B 1-657 were unaffected by subtilisin digestion, KIF21B 657-1624 binding was completely abolished (Figure 2J; compare left six and right six lanes). We confirmed successful digestion with subtilisin by Coomassie and Western blotting, demonstrating that the microtubules remained intact despite the absence of their C terminal tails (Figure S3C). Thus, our results indicate that KIF21B is tethered directly to microtubules through a basic domain in its C terminus.
KIF21B motor activity is required for efficient retrograde transport of functional BDNF/TrkB signaling complexes
Only recently have cargoes of KIF21B-dependent transport begun to be identified in neurons (Labonte et al., 2013; Muhia et al., 2016). To identify potential KIF21B dendritic cargoes, we screened a number of candidates for transport defects in 10 DIV cultured hippocampal neurons in which KIF21B was depleted via RNAi. We co-transfected fluorescently-tagged cargoes with either a scrambled siRNA or a pool of KIF21B-targeted siRNAs, which decreased KIF21B expression by ~70% (Figure S4A). While there were no significant effects of KIF21B RNAi on the dendritic trafficking of Rab7-GFP (late endosomes), LAMP1-mCherry (late endosomes/lysosomes), or dsRed2-Mito (mitochondria), we observed a significant effect on the directionality of transport of TrkB-RFP-positive signaling endosomes (Table S1).
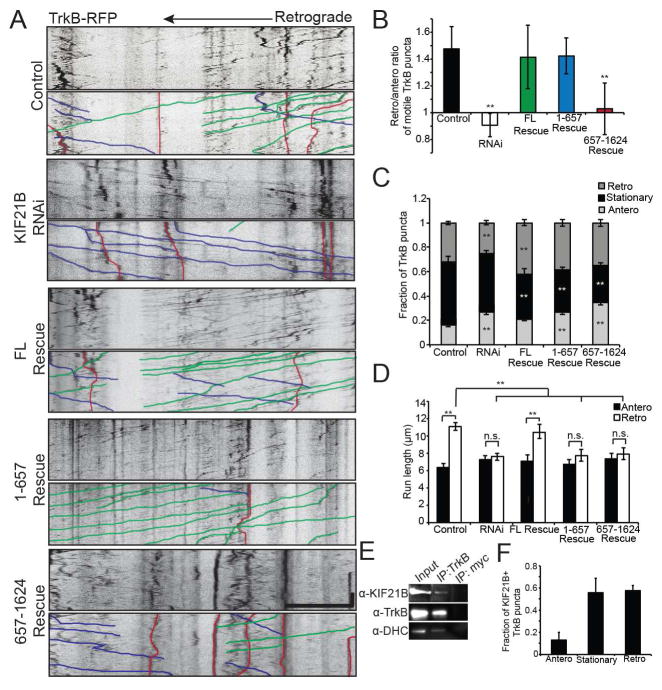
We confirmed this initial result by repeating our analysis of TrkB-RFP using a single siRNA to decrease expression of KIF21B (Figure S4A). Under control conditions, motile TrkB-RFP puncta displayed a retrograde bias (60% retrograde/40% anterograde; Figure 3A–C). However, this bias was lost in KIF21B RNAi neurons, with motile TrkB-RFP puncta equally likely to travel in the anterograde or retrograde direction (Figure 3A–C; Movie S3). This increase in anterograde TrkB-RFP puncta came primarily at the expense of otherwise retrograde puncta (Figure 3C). These data suggest that KIF21B, which displays a retrograde bias itself (Figure 1), contributes to the retrograde directionality of TrkB+ signaling endosome trafficking in proximal dendrites. This decrease in retrograde TrkB-RFP trafficking was also reflected as a significant rightward shift in the distribution of instantaneous velocities (indicating more anterograde motility), as well as a significant decrease in retrograde run length (Figure 3D; Figure S4B). KIF21B RNAi had no effect on the density or average speed of TrkB-RFP puncta (Figure S4C,D), suggesting that KIF21B serves primarily to maintain the retrograde directionality of TrkB+ signaling endosomes, rather than regulating frequency or speed.
Figure 3. KIF21 is required for proper retrograde trafficking of TrkB+ signaling endosomes in dendrites.
A: Kymographs of TrkB-RFP motility in 10 DIV Control, KIF21B RNAi, Full-Length (FL) Rescue, 1-657 Rescue, and 657-1624 Rescue hippocampal neuron dendrites, with sample events traced below. Scale bar = 10 μm, 30 sec. B: Ratio of retrograde to anterograde TrkB-RFP puncta. ** = p < 0.01 from Control, by ANOVA with Tukey posthoc test. C: Fraction of anterograde, stationary, and retrograde TrkB-RFP puncta. ** = p < 0.01 from appropriate Control, by ANOVA with Tukey posthoc test. D: Average run length of motile TrkB-RFP puncta. ** = p < 0.01, by ANOVA with Tukey posthoc test. n.s. = not significant. For A–D: n = 15–22 neurons/condition. E: Western blot of co-immunoprecipitation samples from 8 DIV hippocampal neuronal lysates (anti-TrkB,1:200; anti-KIF21B, 1:100; anti-DHC, 1:250). 10% input loaded. F: Average fraction of anterograde, stationary, or retrograde TrkB-RFP puncta colocalized with HaloTag-KIF21B. n = 10 neurons/condition. Error bars = S.E.M. See also Figure S4.
To confirm that KIF21B and TrkB interact in neurons, we performed co-immunoprecipitation experiments using lysates from 8 DIV cultured hippocampal neurons. A TrkB-specific antibody, but not an irrelevant IgG antibody, pulled down both KIF21B and cytoplasmic dynein, which has been previously implicated in TrkB motility in dendrites (Figure 3E) (Liot et al., 2013). In addition, ~50% of both stationary and retrograde TrkB-RFP puncta colocalize and move together with HaloTag-KIF21B puncta in dendrites. In contrast, only ~12% of anterograde TrkB-RFP puncta colocalized with KIF21B (Figure 3F; Figure S4E), further suggesting a functional interaction between TrkB and KIF21B in retrograde motility.
To verify a role for KIF21B in the dendritic trafficking of TrkB+ signaling endosomes, we performed rescue experiments, co-transfecting the single siRNA along with RNAi-resistant KIF21B cDNA constructs (FL, 1-657, and 657-1624). Expression of FL KIF21B fully restored the retrograde bias in TrkB-RFP motility, as well as the retrograde run length and distribution of instantaneous velocities (Figure 3A–D; Figure S4B–D). In fact, there was an increased fraction of retrograde TrkB-RFP puncta in the FL Rescue condition compared to the control condition, suggesting an enhancement in retrograde bias over endogenous levels, likely due to slightly higher levels of KIF21B expression (Figure 3C). Surprisingly, we also saw partial rescue with KIF21B 1-657, which restored the retrograde bias without affecting run length or instantaneous velocity (Figure 3A–D; Figure S4B–D). Although KIF21B 1-657 lacks a cargo-binding domain, it may be capable of direct cargo binding through its coiled-coils, and is also capable of dimerization (Figure 2B), and so could affect transport by dimerizing with endogenous KIF21B. Consistently, mCherry-tagged FL KIF21B co-immunoprecipitated with Halo-tagged KIF21B 1-657 when overexpressed in HeLa cells (Figure S4G), suggesting KIF21B 1-657 either dimerizes with endogenous KIF21B or that they are bound to the same cargo. In contrast, KIF21B 657-1624 failed to rescue any of the RNAi phenotypes (Figure 3A–D; Figure S4B–D). Thus, the motor domain of KIF21B is required for the retrograde bias in TrkB trafficking in dendrites.
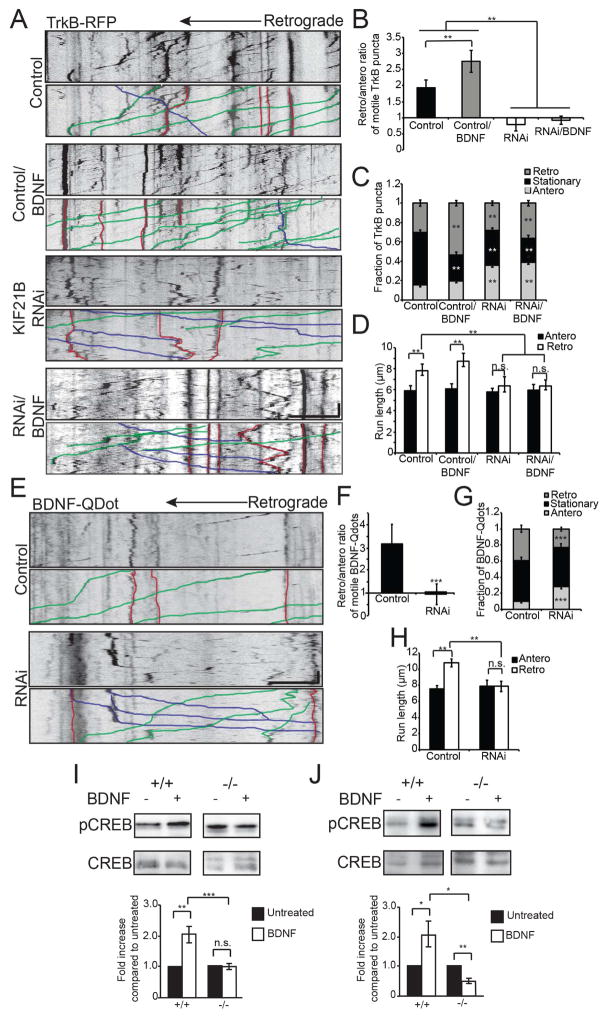
The majority of TrkB-RFP puncta that exhibit dendritic motility are thought to be signaling endosomes, carrying endocytosed, BDNF-bound TrkB from the periphery back to the cell body to influence gene transcription (Liot et al., 2013). Therefore, we wondered whether the TrkB puncta that interact with KIF21B might represent functionally relevant signaling compartments. To test this hypothesis, we bath-applied soluble BDNF (100 nM) to 10 DIV cultured hippocampal neurons co-transfected with TrkB-RFP and either a scrambled siRNA or a KIF21B siRNA. BDNF treatment significantly upregulated the number and retrograde bias of TrkB-RFP puncta (30% in untreated versus 60% in BDNF treated), largely at the expense of stationary puncta (50% in untreated versus 20% in BDNF treated; Figure 4A–D; Figure S5A–C). However, this BDNF-dependent increase in retrograde motility was completely blocked in KIF21B RNAi neurons (Figure 4A–D; Figure S5A–C).
Figure 4. KIF21B regulates trafficking of functional BDNF-bound TrkB.
A: Kymographs of TrkB-RFP motility in 10 DIV Control and KIF21B RNAi hippocampal neuron dendrites, with or without 100 nM BDNF, with sample events traced below. Scale bar = 10 μm, 30 sec. B: Ratio of retrograde to anterograde TrkB-RFP puncta. ** = p < 0.01, by ANOVA with Tukey posthoc test. C: Fraction of anterograde, stationary, and retrograde TrkB-RFP puncta. ** = p < 0.01 from appropriate Control, by ANOVA with Tukey posthoc test. D: Average run length of motile TrkB-RFP puncta. ** = p < 0.01 from Control, by ANOVA with Tukey posthoc test. n.s. = not significant. For A–D: n = 25–28 neurons/condition. E: Kymographs of BDNF-quantum dot (Qdot) motility in 10 DIV Control and KIF21B RNAi hippocampal neuron dendrites, with sample events traced below. Scale bar = 10 μm, 30 sec. F: Ratio of retrograde to anterograde BDNF-Qdot puncta. *** = p < 0.001 from Control, by ANOVA with Tukey posthoc test. G: Fraction of anterograde, stationary, and retrograde BDNF-Qdots. *** = p < 0.001 from appropriate Control, by ANOVA with Tukey posthoc test. H: Average run length of motile BDNF-Qdots. ** = p < 0.01 from Control, by ANOVA with Tukey posthoc test. n.s. = not significant. For E–H: n = 28–30 neurons/condition. I: Western blot (top; anti-pCREB and anti-CREB, 1:1000) and quantification (below) of lysates from 22 DIV wild-type (+/+) or KIF21B knockout (−/−) hippocampal neurons, with or without 100 ng/mL BDNF. J: Western blot (top) and quantification (below) of lysates from wild-type (+/+) or KIF21B knockout (−/−) acute hippocampal slices, with or without 100 ng/mL BDNF. * = p < 0.05, ** = p < 0.01, *** = p < 0.001 by student’s T-test. n.s. = not significant. Error bars = S.E.M. See also Figure S5.
To more directly test the functional role of KIF21B-dependent transport of BDNF/TrkB complexes, we used quantum dots (Qdots) to assess the motility of BDNF newly endocytosed by 10 DIV hippocampal neurons, using soluble EGFP as a cell fill to identify dendritic processes. After 2 hours of incubation with BDNF-Qdots, we observed robust retrograde motility within dendrites (Figure 4E–H). Importantly, these BDNF-Qdots colocalized with both TrkB-RFP (Figure S5D, left) and HaloTag-KIF21B (Figure S5E). Consistent with our observations of TrkB-RFP, colocalization with KIF21B was restricted to primarily stationary and retrograde BDNF-Qdots (Figure S5F). Similar to the observed effects on TrkB-RFP, KIF21B RNAi increased the anterograde fraction of motile BDNF-Qdots at the expense of the retrograde fraction (Figure 4E–G), leading to a rightward shift in the distribution of instantaneous velocities and a decrease in retrograde run length without altering the average speed of motile puncta (Figure 4H; Figure S5F-H). Thus, KIF21B is required for the efficient retrograde transport of functional BDNF-bound TrkB receptors in dendrites.
Finally, we examined the consequences of decreased KIF21B-dependent retrograde BDNF/TrkB transport on neuronal function. We generated primary hippocampal cultures and acute hippocampal slices from wild-type (+/+) and KIF21B knockout (−/−) mice (Muhia et al., 2016) and examined the levels of BDNF-induced S133 phosphorylation of the nuclear transcription factor CREB as a readout for efficient transport of BDNF/TrkB signaling complexes to the nucleus (Finkbeiner et al., 1997; Pizzorusso et al., 2000). We observed robust induction of CREB S133 phosphorylation following BDNF treatment of wild-type cultures and slices, but strikingly, these effects were abolished in KIF21B knockouts (Figure 4I,J). While these data do not distinguish between the potential contributions of axonal and dendritic BDNF signaling, they demonstrate that in the absence of KIF21B, the BDNF/TrkB signal fails to reach the nucleus as efficiently.
KIF21B regulates microtubule dynamics via its C-terminal microtubule binding site
With the motor activity of KIF21B mapped to its N terminus, we wondered if microtubule tethering through its C-terminal microtubule binding site might also have a functional role. Other members of the kinesin-4 family, including KIF4A and KIF21A, have been implicated in the regulation of microtubule dynamics (He et al., 2014; van der Vaart et al., 2013), so we investigated a possible role for KIF21B in the regulation of the microtubule cytoskeleton in dendrites using EB3 comet dynamics. EB3 is a microtubule plus-end binding protein (+TIP) that tracks the growing ends of microtubules; the characteristic “comets” visible by live imaging provide a readout of microtubule polymerization in cells (Stepanova et al., 2003). We co-transfected hippocampal neurons with EB3-GFP and either a scrambled siRNA or an siRNA specific for KIF21B, and assessed EB3 comet appearance at 10 DIV by confocal microscopy (Figure 5A). In agreement with previous reports (Yau et al., 2016), we observed ~70% anterograde/30% retrograde EB3-GFP comets- which correspond to plus-end-outward and plus-end-inward microtubules, respectively- consistent with the mixed microtubule polarity of proximal dendrites (Figure S7A). Moreover, KIF21B depletion had no effect on comet directionality, suggesting it does not play a role in the establishment or maintenance of the overall architecture of the microtubule cytoskeleton (Figure S7A).
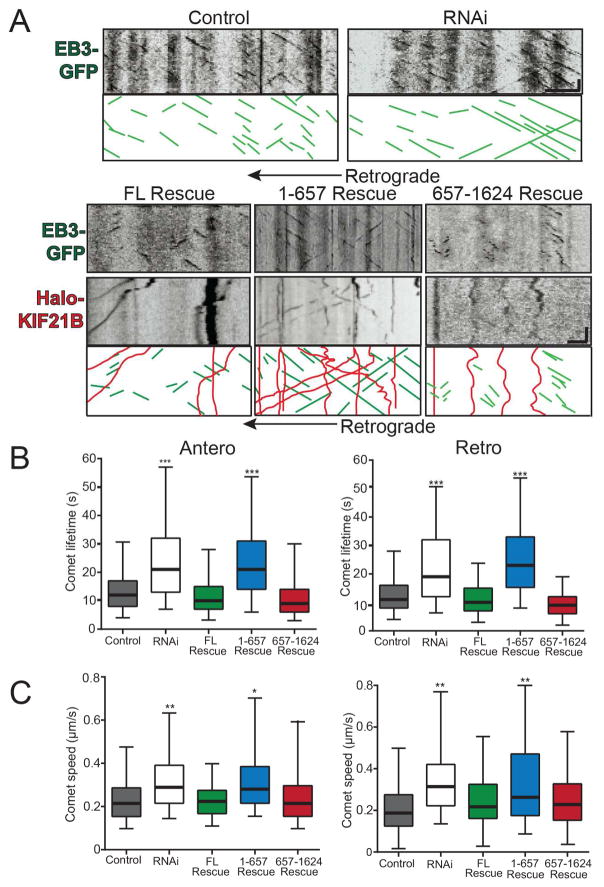
Figure 5. KIF21B regulates microtubule dynamics through its C terminus in hippocampal neuron dendrites.
A: Kymographs of EB3-GFP comets (green) and HaloTag-KIF21B (red) motility for 10 DIV Control, KIF21B RNAi, FL Rescue, 1-657 Rescue, and 657-1624 Rescue hippocampal neurons. Scale bar = 1 μm, 30 sec. B: Average lifetime, C: and average speed of anterograde (left) and retrograde (right) EB3-GFP comets. * = p < 0.05, ** = p < 0.01, *** = p < 0.001 from Control, by ANOVA with Tukey posthoc test. n = 500–754 comets/condition; N = 20–23 neurons/condition. Error bars = 95% confidence intervals.
Depletion of KIF21B via RNAi significantly increased the speed and lifetime of both anterograde and retrograde EB3 comets (Figure 5A–C; Movie S4), demonstrating a role for KIF21B in the regulation of microtubule dynamics; these data are consistent with KIF21B-dependent inhibition of microtubule polymerization, promotion of catastrophe, or both. To verify this KIF21B depletion phenotype, we performed rescue experiments in which we co-transfected the KIF21B siRNA with RNAi-resistant KIF21B constructs (FL, 1-657, or 657-1624) and evaluated EB3 comet dynamics. FL KIF21B was able to rescue the speed and lifetime phenotypes of KIF21B RNAi (Figure 5A–C; Movie S4), confirming that KIF21B does play a role in the regulation of microtubule dynamics in neurons. Interestingly, KIF21B 1-657 was unable to rescue the KIF21B RNAi phenotypes, while KIF21B 657-1624 rescued both the speed and lifetime of EB3 comets to the same extent as FL KIF21B (Figure 5A–C; Movie S4). This suggests that not only does KIF21B regulate microtubule dynamics through its C-terminal microtubule binding site, but that, unexpectedly, the motor domain is completely dispensable for this process, a previously undescribed mechanism of action for kinesins.
KIF21B is a positive regulator of microtubule dynamicity in vitro
We turned to a minimal in vitro system to determine whether KIF21B affects microtubule dynamics by regulating polymerization, catastrophe, or both. We purified recombinant HaloTag-KIF21B FL, 1-657, and 657-1624 (Figure S3A), and combined 50, 100, or 300 nM of each TMR-labeled protein with Alexa 488-labeled tubulin. When flowed into chambers containing Alexa 647-labeled GMPCPP-stabilized microtubule seeds affixed to coverslips, the dynamics of free tubulin polymerization from seeds can be measured via kymograph analysis (Figure 6A).
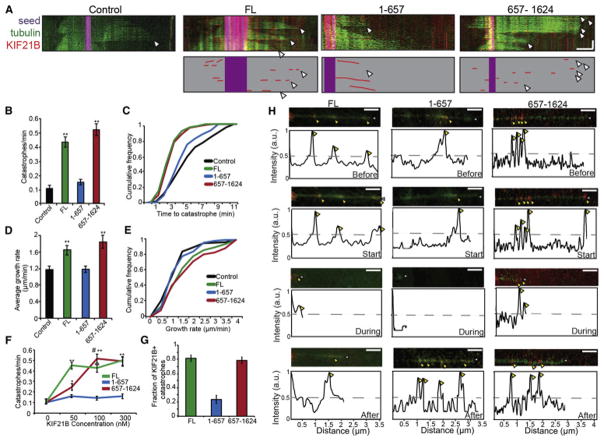
Figure 6. C-terminal KIF21B is a positive regulator of microtubule dynamicity in vitro.
A: Kymographs of microtubule dynamics in vitro. GMPCPP seeds (purple), tubulin (green), and KIF21B (red) above; cartoon representation of seeds and KIF21B below. White arrowheads = catastrophe. Scale bar = 2 μm, 3 min. B: Catastrophes per minute for Control and KIF21B (100 nM) microtubules. ** = p < 0.01 from Control, by ANOVA with Tukey posthoc test. C: Cumulative frequency plot of time to catastrophe, corresponding to B. Distributions for FL and 657-1624 significant from Control, by KS test (p < 0.01). D: Average growth rate for Control and KIF21B (100 nM) microtubules. ** = p < 0.01 from Control, by ANOVA with Tukey posthoc test. E: Cumulative frequency plot of average growth rate, corresponding to D. Distributions for FL and 657-1624 significant from Control, by KS test (p < 0.01). For A–E: n = 28–30 microtubules/condition. F: Catastrophes per minute with varying concentrations of KIF21B protein. * = p < 0.05, ** = p < 0.01 from 0 nM, # = p < 0.01 from 50 nM, by ANOVA with Tukey posthoc test. n = 12–15 microtubules/condition for 50 nM and 300 nM, 28–30 microtubules/condition for 100 nM. G: Fraction of catastrophes positive for KIF21B (100 nM). n = 28–30 microtubules. Error bars = S.E.M. H: Still frames and corresponding line scans of fluorescence intensity of microtubule dynamics 2 frames (20 seconds) prior to (“Before”), at (“Start”), during (“During”), and 10 frames (100 seconds) after (“After”) catastrophe, showing KIF21B localization (red) along microtubule (green). Scale bar = 2 μm. Yellow arrowheads = KIF21B, White arrowheads = plus-end. Line scans = normalized KIF21B fluorescence over the length of the microtubule at each time.
Both the growth rate and catastrophe frequency of microtubules were enhanced in the presence of FL KIF21B (Figure 6A–E; Movie S5); this effect saturated by 50 nM protein (Figure 6F). This suggests that KIF21B is a positive regulator of microtubule dynamicity, promoting both the polymerization and catastrophe of microtubules. While the increase in catastrophe frequency induced by FL KIF21B in vitro correlates nicely with the increased EB3-GFP comet lifetime with KIF21B RNAi in neurons (Figure 5), we were surprised by the seemingly contradictory effects on the speed of microtubule growth in neurons and in vitro, which could be due to compensatory effects of other microtubule regulators in cells. In addition, due to the density of EB3-GFP comets in dendrites, it is unclear whether a given set of comets represents the same microtubule repeatedly growing and shrinking, or multiple microtubules growing in close proximity. Thus, individual microtubule growth rates in vitro are not always easily correlated with comet speed in cells.
Surprisingly, we did not observe tip-tracking behavior of KIF21B with growing microtubules in vitro, unlike what has been reported for other regulators of microtubule dynamic instability (Lazarus et al., 2013). Instead, single molecules of FL KIF21B exhibited short runs along the length of dynamic microtubules. Interestingly, we also observed frequent localization of FL KIF21B to the plus-tips of microtubules at catastrophe, or along the collapsing edge of depolymerizing microtubules during these events (~80% of catastrophes; Figure 6A,G,H). This suggests motile KIF21B associates with the microtubule plus-end at times of catastrophe or rapid shortening.
In agreement with our EB3 comet observations in neurons, KIF21B 1-657 had no affect on microtubule dynamicity in vitro (Figure 6A–E), even at concentrations of up to 300 nM (Figure 6F). In contrast, the C-terminal non-motor KIF21B 657-1624 phenocopied FL KIF21B (Figure 6A–E; Movie S5), reaching saturation with 100 nM protein (Figure 6F). KIF21B 1-657 localization was most apparent as motile events along the length of dynamic microtubules, only localizing to the plus-tips of microtubules either at or during catastrophe about 20% of the time (Figure 6A,G,H). In contrast, C-terminal KIF21B 657-1624 frequently localized to plus-tips during catastrophe, and displayed the same preference for the collapsing edge as FL KIF21B (Figure 6A,G,H). Thus, the C-terminal microtubule binding site of KIF21B is both necessary and sufficient for KIF21B-dependent enhancement of microtubule dynamicity. Overall, our results demonstrate a motor function for KIF21B that is dependent on canonical N-terminal microtubule binding, and a separable, independent function for KIF21B in the regulation of microtubule dynamics through a second C-terminal microtubule binding site.
Neuronal activity shifts the balance of KIF21B function from the regulation of microtubule dynamicity to cargo trafficking
The dendritic arbor remains highly dynamic even beyond development, and is subject to constant remodeling under the influence of intracellular and external cues, such as changes in neuronal network activity (Wong and Ghosh, 2002). As KIF21B mediates two distinct processes in dendrites, trafficking and microtubule dynamicity, we wondered how a particular KIF21B molecule is biased towards one function or the other at any given time. An intriguing hypothesis is that neuronal activity acts as an instructive signal, biasing KIF21B towards participation in either trafficking or microtubule regulation. To test this possibility, we first transfected 10 DIV hippocampal neurons with Halo-tagged FL or 1-657 KIF21B and then treated these neurons with 50 mM potassium chloride (KCl) to look for depolarization-dependent changes in KIF21B motility or localization.
The motility of FL KIF21B was significantly enhanced by neuronal depolarization. FL KIF21B puncta in KCl-treated neurons exhibited longer run lengths and higher speeds compared to untreated neurons, comparable to the baseline motility of KIF21B 1-657 (Figure 7A,B; Figure S6A–D). In contrast, there was no effect of depolarization on the instantaneous velocity or fraction of motile vesicles, and the retrograde bias seen under basal conditions was retained (Figure S6C–D). The motility of KIF21B 1-657 was unaffected by depolarization (Figure 7A,B; Figure S6A–D).
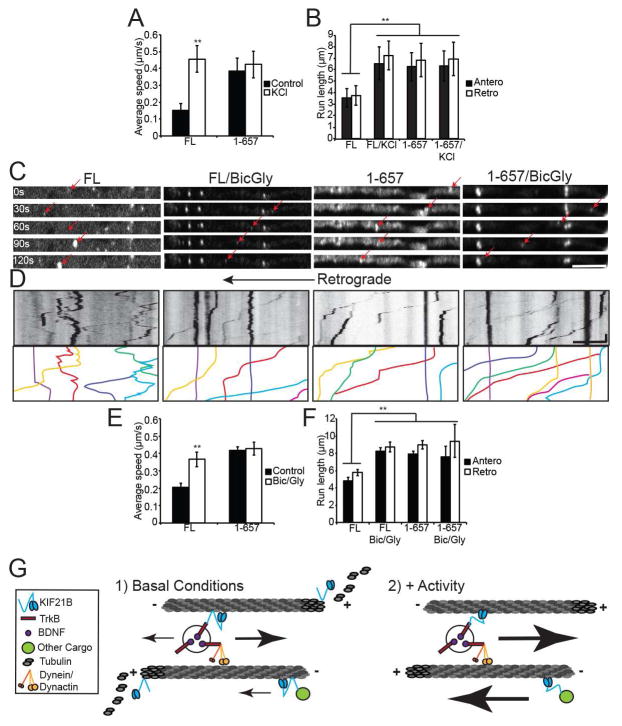
Figure 7. Neuronal activity enhances KIF21B motility.
A: Average speed, B: and average run length of motile HaloTag-KIF21B (FL and 1-657) puncta in 10 DIV hippocampal neuron dendrites, with and without 50 mM KCl. ** = p < 0.01 from appropriate FL condition, by ANOVA with Tukey posthoc test. n = 20–23 neurons/condition. C: Time-lapse images of dendritic segments from 10 DIV hippocampal neurons expressing HaloTag-KIF21B (FL or 1-657), showing motility with and without 50 μM bicuculline/200 μM glycine (red arrows). Scale bar = 10 μm. D: Kymographs of KIF21B motility shown in C. Line colors correspond to arrows in C. Scale bar = 10 μm, 30 sec. E: Average speed, F: and average run length of motile HaloTag-KIF21B puncta. ** = p < 0.01 from appropriate FL condition, by ANOVA with Tukey posthoc test. n = 20–23 neurons/condition. Error bars = S.E.M. G: Model for KIF21B-dependent regulation of dendritic trafficking and microtubule dynamics. See also Figure S6.
To confirm this activity-dependent enhancement of KIF21B velocity and run length using a more physiological stimulus, we performed parallel experiments in 10 DIV hippocampal neurons treated with 50 μM bicuculline/200 μM glycine (bic/gly), which induces synchronized action potential firing in cultured neurons (Hardingham et al., 1997), as verified by calcium imaging (Movie S6). Strikingly, we observed the same significant enhancement of FL KIF21B motility as seen with KCl treatment. FL KIF21B puncta in bic/gly neurons exhibited longer run lengths and higher speeds compared to untreated neurons, while the instantaneous velocity and fraction of motile vesicles, as well as the motility of KIF21B 1-657, were not affected (Figure 7C–F; Figure S6E,F). Parsing the average speed of KIF21B puncta into distributions of anterograde and retrograde-directed motility revealed that while bic/gly enhanced the motility of KIF21B in both directions, retrograde puncta were more strongly affected (Figure S6G,H; note the tail of higher-velocity FL KIF21B puncta with Bic/Gly treatment in Figure S6G). Overall, these data suggest that increased neuronal activity either upregulates the motile behavior of the N terminus or downregulates the tethering behavior of the C terminus of KIF21B, allowing full-length KIF21B to move constitutively (Figure 7G).
To test this model, we looked at activity-dependent changes in KIF21B function by comparing the effects of KIF21B RNAi or KIF21B overexpression on EB3-GFP comet dynamics and TrkB-RFP motility in bic/gly neurons. Neuronal activity enhanced the fraction of anterograde EB3-GFP comets, and therefore plus-end-outward microtubules, regardless of KIF21B manipulation (from 70% anterograde/30% retrograde to 80%/20%; Figure S7B). While the source of these additional anterograde comets is unclear, they may indicate new microtubules that will serve as cytoskeletal support for newly-generated dendritic branches induced by activity.
In control neurons, bic/gly also affected comet dynamics, with enhanced activity leading to a significant increase in the lifetime and speed of both anterograde and retrograde EB3-GFP comets (Figure 8A–C), consistent with the previously reported role of activity in regulating microtubule dynamics (Kapitein et al., 2011). Interestingly, treatment of KIF21B RNAi neurons with bic/gly was not additive, resulting in the same increases in comet lifetime and speed as either bic/gly or KIF21B RNAi alone (Figure 8A–C). This suggests that one mechanism through which neuronal activity mediates microtubule dynamics is the downregulation of KIF21B C-terminal function. Consistently, overexpression of KIF21B was not able to counter the effects of bic/gly on EB3-GFP comet lifetime and speed (Figure 8A–C), suggesting the overexpressed KIF21B is biased towards a function other than the regulation of microtubule dynamics.
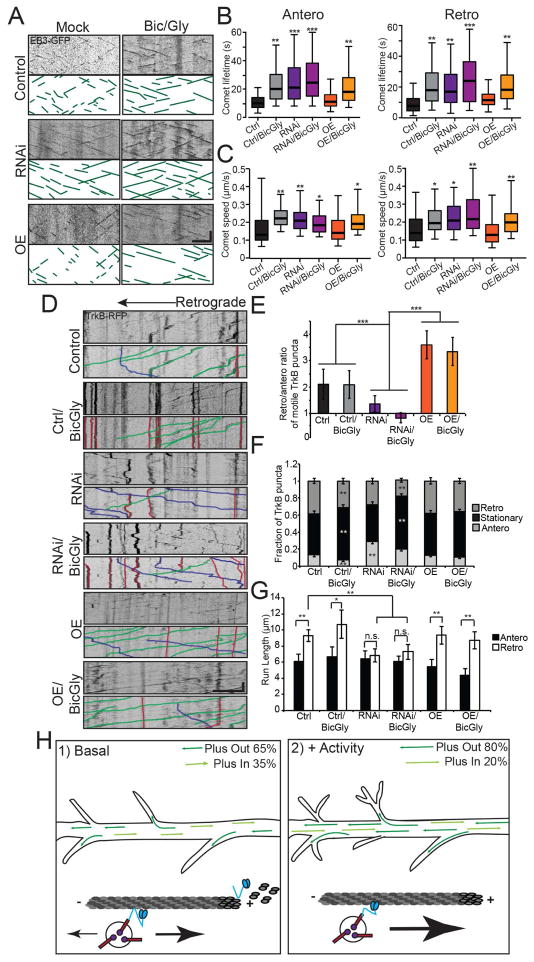
Figure 8. Neuronal activity promotes the function of KIF21B in TrkB trafficking at the expense of microtubule dynamicity regulation.
A: Kymographs of EB3-GFP comets for Control, KIF21B RNAi, and KIF21B-overexpressing (OE) 10 DIV hippocampal neurons, with and without 50 μM bicuculline/200 μM glycine. Scale bar = 1 μm, 30 sec. B: Average lifetime, C: and average speed of anterograde (left) and retrograde (right) EB3-GFP comets. * = p < 0.05, ** = p < 0.01, *** = p < 0.001 from Control, by ANOVA with Tukey posthoc test. n > 500 comets/condition; N = 27–32 neurons/condition. D: Kymographs of TrkB-RFP motility for conditions described in A, with sample events traced below. Scale bar = 10 μm, 30 sec. E: Ratio of retrograde to anterograde TrkB-RFP puncta. *** = p < 0.001 from Control, by ANOVA with Tukey posthoc test. F: Fraction of anterograde, stationary, and retrograde TrkB-RFP. ** = p < 0.01 from appropriate Control, by ANOVA with Tukey posthoc test. G: Average run length of motile TrkB-RFP puncta. * = p < 0.05, ** = p < 0.01, by ANOVA with Tukey posthoc test. n.s. = not significant. n = 27–32 neurons/condition. In B–C, error bars = 95% confidence interval; in E–G, error bars = S.E.M. H: KIF21B plays a dual role in dendrites (left): regulation of cargo trafficking, including the retrograde transport of BDNF/TrkB, and regulation of microtubule dynamicity. KIF21B moves along the microtubule, attached by both N-terminal (which determines motor activity) and C-terminal binding sites. At the plus-end, microtubule dynamics are mediated through the C-terminal binding site. With increased neuronal activity (right): KIF21B-dependent regulation of microtubule dynamicity is downregulated but motility is preserved. See also Figure S7.
Interestingly, enhancing the activity of control neurons led to a significant increase in the stationary fraction of TrkB-RFP puncta, although the retrograde bias of the smaller motile fraction was maintained (Figure 8D–G). This correlated with a significant increase in TrkB-RFP puncta density along dendrites, suggesting that while transport of signaling endosomes out of dendrites into the soma may be affected, the average speed of motile TrkB-RFP puncta was not altered (Figure S7C–E). Treatment of KIF21B RNAi neurons with bic/gly phenocopied treatment of control neurons, with a significant increase in the stationary fraction of TrkB-RFP puncta and the overall puncta density in dendrites (Figure 8D–G; Figure S7C–E). However, the KIF21B RNAi-mediated increase in anterograde and decrease in retrograde motility was maintained in the smaller motile fraction (Figure 8D–G; Figure S7C). This suggests that KIF21B-mediated motility may persist despite an overall decrease in motility following enhanced activity.
Consistently, overexpression of KIF21B in untreated neurons led to a significant increase in the fraction of retrograde TrkB-RFP puncta at the expense of stationary puncta; anterograde motility was largely unaffected (Figure 8D–G), consistent with a rate-limiting role for KIF21B in retrograde-directed transport of BDNF/TrkB signaling complexes. Overexpression of KIF21B also increased the average speed of motile TrkB-RFP puncta under basal conditions (Figure S7C–E). Strikingly, the bic/gly-induced increase in stationary puncta was largely abrogated by KIF21B overexpression, as a significant retrograde fraction of TrkB-RFP puncta was maintained and overall TrkB-RFP puncta density was not increased (Figure 8D–G; Figure S7C–E). Overall, these results indicate that retrograde KIF21B-mediated motility is maintained even in the face of conditions that otherwise decrease the overall transport of a particular cargo. Taken together, these data suggest that neuronal activity suppresses the microtubule dynamic regulation function of KIF21B while preserving its role in regulating the transport of BDNF/TrkB signaling complexes, changing the overall balance of trafficking versus microtubule remodeling and allowing KIF21B-dependent motility to proceed in the face of changing dendritic environments (Figure 8H).
Discussion
In this study, we provide a comprehensive mechanistic characterization of the neuronal kinesin KIF21B, mapping its functions to distinct microtubule binding sites. Despite the mixed polarity of proximal dendritic microtubules, KIF21B exhibits a retrograde bias in motility, and contributes to the retrograde directionality of BDNF-bound TrkB receptors in signaling endosomes. KIF21B also enhances microtubule dynamic instability both in vitro and in neurons. Crucially, the motor domain of KIF21B is dispensable for its role in regulating microtubule dynamics, which instead requires a C-terminal, nucleotide-independent basic region that interacts with the acidic tails of tubulin. KIF21B function is tightly regulated by neuronal activity, as depolarization or action potential firing decreased KIF21B-dependent regulation of microtubule dynamics while preserving its role in regulating BDNF/TrkB transport. Overall, our studies of KIF21B provide key insights into how efficient transport is maintained in the face of the complex and dynamic dendritic environment of neurons.
Signaling endosomes containing neurotrophic signals such as the BDNF/TrkB complex originate distally, but their functional role is to affect gene transcription in the nucleus (Zweifel et al., 2005). Thus, the processive retrograde transport of these organelles is crucial for their function. While our data point to a role for KIF21B in the regulation of this retrograde transport, it is not clear that this role is as a canonical motor. The discrepancy observed between the slow motility of most KIF21B puncta (Figure 1 and 2) and the relatively fast motility of TrkB/BDNF (Figure 3 and 4) suggests that KIF21B is likely not engaged during periods of sustained endosome motility. Instead, KIF21B may facilitate specific aspects of transport, such as run initiation or continuation following a pause, as has been shown for other slow motors in vitro (McIntosh et al., 2015).
There is an emerging appreciation for the hypothesis that the net transport of a given cargo is determined by multiple types of motor proteins that differentially regulate distinct motility properties (Arpag et al., 2014; Prevo et al., 2015). Similarly, our data suggest that KIF21B specifically confers directionality, rather than speed, on the motility of BDNF/TrkB complexes. This is borne out by the fact that KIF21B is a slow motor, both in neurons (Figure 1) and in vitro (Figure 2), and that the major effect of KIF21B RNAi is not related to the speed of motile BDNF/TrkB signaling complexes, but rather directionality (Figure 3). The minus-end directed motor cytoplasmic dynein has also been implicated in the transport of BDNF/TrkB complexes, affecting both the anterograde and retrograde velocities (Liot et al., 2013). Therefore, an attractive hypothesis is that dynein, which displays equivalent motility in both the anterograde and retrograde directions in dendrites (Kapitein et al., 2010), is not sufficient to maintain processive unidirectional motility toward the soma in the mixed microtubule array of the proximal dendrite. KIF21B may assist dynein in maintaining the sustained retrograde-directed motility necessary for neurotrophic signaling endosomes to reach the soma.
Alternatively, the observed effects of KIF21B on this transport may be a result of changes in microtubule dynamics via a signaling pathway that remains to be identified. Only axonally-localized KIF2A and KIF18A have been previously implicated in microtubule dynamicity in post-mitotic neurons (Homma et al., 2003; Kevenaar et al., 2016). Thus, KIF21B is the first kinesin shown to promote microtubule dynamic instability in dendrites. The ability of KIF21B to affect both microtubule growth rate and catastrophe runs counter to the established “GTP cap” model of dynamic instability, which assumes growth rate and catastrophe frequency trend in opposite directions (Bowne-Anderson et al., 2015). KIF21B joins a number of regulators of microtubule dynamicity that do not fit this model, including the yeast kinesin-4 Xklp1 (Bringmann et al., 2004), suggesting this may be a unifying feature of the kinesin-4 family and providing further evidence that microtubule dynamics are more complex than the current model would suggest (Bowne-Anderson et al., 2015).
Other members of the kinesin-4 family, including KIF21A and KIF7 (He et al., 2014; van der Vaart et al., 2013) have also recently emerged as regulators of microtubule dynamics. However, in contrast to the effects of KIF21B promoting microtubule dynamicity, these other kinesin-4s limit growth and/or catastrophe (Bringmann et al., 2004; He et al., 2014; van der Vaart et al., 2013). Given that axonal microtubules are generally less dynamic than microtubules in dendrites (Conde and Caceres, 2009; Witte et al., 2008), the regulatory needs of dendritic microtubules are likely to be higher. Indeed, overexpression of KIF21B did not have a significant effect on EB3-GFP comet length (Figure 5), suggesting that dendritic microtubules dynamics may be under particularly tight control under endogenous conditions. Only upon a loss of function manipulation, such as RNAi or a change in neuronal activity, is it possible to dramatically alter the microtubule architecture of dendrites. This could explain why dendritic KIF21B promotes microtubule dynamicity, while other kinesin-4s, which localize to less dynamic structures (KIF21A: axons, Kif7: cilia) do not.
The functional mapping of KIF21B-dependent regulation of microtubule dynamicity to the tethering activity of its C-terminus is a unique mechanism of action for a kinesin family member, and is consistent with a recent report on the KIF21B knockout mouse, which showed that the motor domain of KIF21B was not sufficient to rescue certain loss-of-function phenotypes (Muhia et al., 2016). While both kinesin-8s and kinesin-13s also bind microtubules outside of their canonical motor domains, these kinesins still require ATP hydrolysis to affect microtubule dynamics. The secondary microtubule binding site of kinesin-13 is nucleotide-independent, but this kinesin still utilizes the hydrolysis of ATP to destabilize microtubules (Zhang et al., 2013), while the kinesin-8 Kip3 can either stabilize or destabilize microtubules through discrete binding sites in the motor domain (Fukuda et al., 2014; Su et al., 2011). In contrast, the C-terminal dependent tethering of KIF21B to microtubules is completely sufficient for regulation of dynamic instability, independent of motor function or ATP hydrolysis.
Our KIF21B overexpression studies show that the number of motors available for transport is a limiting factor in the trafficking of BDNF/TrkB complexes, and that neuronal activity increases this pool by downregulating the microtubule remodeling function of KIF21B (Figure 8D–G). Why would a dendritic kinesin such as KIF21B need to respond functionally to neuronal activity in this way? The long-range transport of cargoes from dendrite to soma and back is crucial for many aspects of neuronal function, including synaptic plasticity. Changes that occur locally at synapses must be communicated to the nucleus to affect gene transcription, including the activation of CREB-dependent pathways (Deisseroth et al., 1996). In turn, the delivery of postsynaptic receptors to synaptic sites in dendrites relies on long-range transport from elsewhere in the somatodendritic compartment, also involving the microtubule cytoskeleton (Kennedy and Ehlers, 2006). Thus, it may be that dendritic kinesins enhance transport in response to neuronal activity in order to ensure these signals reach the appropriate target (Hoerndli et al., 2015; Ichinose et al., 2015; Neupert et al., 2015). These results fit nicely with the synaptic defects and learning and memory deficits of KIF21B knockout mice (Muhia et al., 2016), reflected at least in part as a loss of CREB activation (Figure 4). In the absence of KIF21B, these neurons may be unable to properly balance transport of synaptic proteins and regulation of the dendritic cytoskeleton in the context of altered neuronal activity
Alternatively, the enhanced motility and decreased microtubule regulation by KIF21B in response to activity could be a mechanism to limit excessive microtubule remodeling, which impedes directed transport during periods of depolarization. The microtubule cytoskeleton of dendrites in rodent hippocampal neurons is of mixed polarity in proximal regions, with a bias towards plus-end-outward microtubules (Baas et al., 1988; Yau et al., 2016). Indeed, we consistently observed a ratio of 70% anterograde and 30% retrograde EB3-GFP comets in our experiments (Figure 5). We also observed an activity-dependent increase in this anterograde EB3-GFP comet fraction, suggesting an increase in plus-end-outward microtubules to support newly-formed dendritic branches (Figure S7B). Thus, it is surprising that a plus-end directed motor like KIF21B would show a bias for retrograde motility (Figure 1), suggesting it preferentially utilizes the smaller population of plus-end-inward microtubules. In the context of enhanced activity, KIF21B-driven motility along these tracks may be supported through enhanced cargo binding, decreased binding of the tethered KIF21B C-terminus, or differential microtubule post-translational modification or stability. The characterization of additional KIF21B cargoes (Muhia et al., 2016) may help to distinguish these hypotheses.
The differential regulation of axonal and dendritic transport remains a key area of research in the trafficking field. This question is clearly illustrated by the incongruity between KIF21A and KIF21B, which are highly homologous but differentially compartmentalized to axons and dendrites. Our study illuminates a number of key differences between these kinesins. While our data suggest that KIF21B is not autoinhibited, the autoinhibition of KIF21A has been previously described (Cheng et al., 2014). In addition, while both KIF21A and KIF21B regulate microtubule dynamics, they differ in their mechanism of action. KIF21A inhibits microtubule growth while KIF21B promotes dynamicity, and the motor domain of KIF21A is sufficient for this function while that of KIF21B is completely dispensable (van der Vaart et al., 2013). Thus, a complete characterization of the differential regulation of the full repertoire of axonal and dendritic kinesins is essential to our understanding of neuronal function.
Experimental Procedures
Neuronal culture and treatments
E18 rat hippocampal neurons were obtained from the Neuron Culture Service Center at the University of Pennsylvania and plated at a density of 750,000 neurons per 35 mm glass-bottom dish (MatTek). Neurons were maintained in Neurobasal (Gibco) supplemented with 2mM GlutaMax, 100 units/mL penicillin/100 ug/mL streptomycin, and 2% B27 (ThermoFisher) at 37°C incubator. See Supplemental Experimental Procedures for details of in a 5% CO2 mouse neuronal culture, acute hippocampal slices, and all experimental treatments.
Neuronal transfection and live imaging
Neurons were transfected using Lipofectamine 2000 (Invitrogen). Neurons were prepared for live imaging 24–36 hours later by changing media to low fluorescence HibernateE (BrainBits) supplemented with 2% B27 and 2 mM GlutaMax. For experiments with HaloTag constructs, neurons were labeled with tetramethylrhodamine or Oregon Green HaloTag ligands (Promega). See Supplemental Experimental Procedures for details on plasmid and siRNA reagents. Neurons were imaged in an environmental chamber at 37°C on a Perkin Elmer UltraView Vox spinning disk confocal on a Nikon Eclipse Ti inverted microscope with the Perfect Focus system using an apochromat 100x 1.49 NA oil-immersion objective and a Hamamatsu EMCCD C9100-50 camera driven by Volocity (Perkin Elmer). Kymographs were generated using the MultipleKymograph plugin for Fiji (NIH) and analyzed using custom MATLAB software. See Supplemental Experimental Procedures for details of dendrite selection and motility parameters.
Single molecule assay and analysis
Motility assays were performed as described (Ayloo et al., 2014) and detailed in Supplemental Experimental Procedures. Movies were acquired at room temperature using a Nikon TIRF system (Perkin Elmer) on a Ti inverted microscope with the Perfect Focus system, using an apochromat 100x 1.49 NA oil-immersion objective and a Hamamatsu ImagEM C9100-13 camera driven by Volocity software (Perkin Elmer). Particle tracking was performed using Fiji TrackMate (NIH).
Protein purification, dynamic microtubule assay, and analysis
Recombinant HaloTag-KIF21B (FL, 1-657, and 657-1624) fused to a FLAG tag were purified from Sf9 insect cells using baculovirus following standard methods. Dynamic microtubule assays were performed as described (Lazarus et al., 2013) and detailed in Supplemental Experimental Procedures. Kymographs of microtubule dynamics were prepared using the MultipleKymograph plugin in Fiji (NIH) and manually analyzed.
MT pelleting assay and subtilisin digestion
HeLa cells were transfected, lysed (as described in Supplemental Experimental Procedures), and subjected to centrifugation. Lysates were incubated with 5 mM Taxol-stabilized microtubules with or without 10 mM AMPPNP at 37°C for 20 min, then centrifuged at 18k rpm at 25°C for 20 minutes. In a subset of experiments, 5 mM Taxol-stabilized microtubules were pretreated with 200 ug/mL subtilisin (Sigma) for 1 hr at 37°C.
Supplementary Material
Highlights.
KIF21B dually regulates trafficking and microtubule dynamics in dendrites.
KIF21B is involved in proper retrograde trafficking of BDNF-bound TrkB receptors.
Regulation of microtubule dynamicity by KIF21B is independent of motor activity.
Activity downregulates KIF21B microtubule remodeling activity but not motor function.
Acknowledgments
The authors acknowledge Jeffery Nirschl, Pedro Guedes-Dias, and Margie Maronski (University of Pennsylvania Neuron Culture Service Center) for technical assistance. We thank Sandra Maday, Swathi Ayloo, Mara Olenick, Pallavi Gopal, and Jaime Fox for insight and discussion. This work was funded by NIH Grant F32MH108187 to A.E.G., NIH Grant R01GM048661 to E.L.F.H., and DFG Grant KN556/11-1 to M.K.
Footnotes
Author Contributions: A.E.G., E.T., M.K., and E.L.F.H. designed experiments; A.E.G. and E.T. performed and analyzed experiments; M.K.T., T.L., and E.M.O. contributed new reagents; A.E.G. and E.L.F.H wrote the paper.
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arpag G, Shastry S, Hancock WO, Tuzel E. Transport by populations of fast and slow kinesins uncovers novel family-dependent motor characteristics important for in vivo function. Biophysical journal. 2014;107:1896–1904. doi: 10.1016/j.bpj.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayloo S, Lazarus JE, Dodda A, Tokito M, Ostap EM, Holzbaur EL. Dynactin functions as both a dynamic tether and brake during dynein-driven motility. Nature communications. 2014;5:4807. doi: 10.1038/ncomms5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Deitch JS, Black MM, Banker GA. Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:8335–8339. doi: 10.1073/pnas.85.21.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasius TL, Cai D, Jih GT, Toret CP, Verhey KJ. Two binding partners cooperate to activate the molecular motor Kinesin-1. The Journal of cell biology. 2007;176:11–17. doi: 10.1083/jcb.200605099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowne-Anderson H, Hibbel A, Howard J. Regulation of Microtubule Growth and Catastrophe: Unifying Theory and Experiment. Trends in cell biology. 2015 doi: 10.1016/j.tcb.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringmann H, Skiniotis G, Spilker A, Kandels-Lewis S, Vernos I, Surrey T. A kinesin-like motor inhibits microtubule dynamic instability. Science. 2004;303:1519–1522. doi: 10.1126/science.1094838. [DOI] [PubMed] [Google Scholar]
- Cheng L, Desai J, Miranda CJ, Duncan JS, Qiu W, Nugent AA, Kolpak AL, Wu CC, Drokhlyansky E, Delisle MM, et al. Human CFEOM1 mutations attenuate KIF21A autoinhibition and cause oculomotor axon stalling. Neuron. 2014;82:334–349. doi: 10.1016/j.neuron.2014.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS, Bas Orth C, Kim HJ, Jeon NL, Jaffrey SR. Neurotrophin-mediated dendrite-to-nucleus signaling revealed by microfluidic compartmentalization of dendrites. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11246–11251. doi: 10.1073/pnas.1012401108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde C, Caceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nature reviews Neuroscience. 2009;10:319–332. doi: 10.1038/nrn2631. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Bito H, Tsien RW. Signaling from synapse to nucleus: postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron. 1996;16:89–101. doi: 10.1016/s0896-6273(00)80026-4. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S, Tavazoie SF, Maloratsky A, Jacobs KM, Harris KM, Greenberg ME. CREB: a major mediator of neuronal neurotrophin responses. Neuron. 1997;19:1031–1047. doi: 10.1016/s0896-6273(00)80395-5. [DOI] [PubMed] [Google Scholar]
- Fukuda Y, Luchniak A, Murphy ER, Gupta ML., Jr Spatial control of microtubule length and lifetime by opposing stabilizing and destabilizing functions of Kinesin-8. Current biology : CB. 2014;24:1826–1835. doi: 10.1016/j.cub.2014.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Ji Y, Wang S, Sun Y, Lu B. Neuronal activity alters BDNF-TrkB signaling kinetics and downstream functions. Journal of cell science. 2014;127:2249–2260. doi: 10.1242/jcs.139964. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Chawla S, Johnson CM, Bading H. Distinct functions of nuclear and cytoplasmic calcium in the control of gene expression. Nature. 1997;385:260–265. doi: 10.1038/385260a0. [DOI] [PubMed] [Google Scholar]
- He M, Subramanian R, Bangs F, Omelchenko T, Liem KF, Jr, Kapoor TM, Anderson KV. The kinesin-4 protein Kif7 regulates mammalian Hedgehog signalling by organizing the cilium tip compartment. Nature cell biology. 2014;16:663–672. doi: 10.1038/ncb2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Niwa S, Tanaka Y. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron. 2010;68:610–638. doi: 10.1016/j.neuron.2010.09.039. [DOI] [PubMed] [Google Scholar]
- Hoerndli FJ, Wang R, Mellem JE, Kallarackal A, Brockie PJ, Thacker C, Madsen DM, Maricq AV. Neuronal Activity and CaMKII Regulate Kinesin-Mediated Transport of Synaptic AMPARs. Neuron. 2015;86:457–474. doi: 10.1016/j.neuron.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma N, Takei Y, Tanaka Y, Nakata T, Terada S, Kikkawa M, Noda Y, Hirokawa N. Kinesin superfamily protein 2A (KIF2A) functions in suppression of collateral branch extension. Cell. 2003;114:229–239. doi: 10.1016/s0092-8674(03)00522-1. [DOI] [PubMed] [Google Scholar]
- Hu X, Viesselmann C, Nam S, Merriam E, Dent EW. Activity-dependent dynamic microtubule invasion of dendritic spines. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:13094–13105. doi: 10.1523/JNEUROSCI.3074-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CF, Banker G. The translocation selectivity of the kinesins that mediate neuronal organelle transport. Traffic. 2012;13:549–564. doi: 10.1111/j.1600-0854.2011.01325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annual review of neuroscience. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose S, Ogawa T, Hirokawa N. Mechanism of Activity-Dependent Cargo Loading via the Phosphorylation of KIF3A by PKA and CaMKIIa. Neuron. 2015;87:1022–1035. doi: 10.1016/j.neuron.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Jenkins B, Decker H, Bentley M, Luisi J, Banker G. A novel split kinesin assay identifies motor proteins that interact with distinct vesicle populations. The Journal of cell biology. 2012;198:749–761. doi: 10.1083/jcb.201205070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitein LC, Hoogenraad CC. Which way to go? Cytoskeletal organization and polarized transport in neurons. Molecular and cellular neurosciences. 2011;46:9–20. doi: 10.1016/j.mcn.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Kapitein LC, Schlager MA, Kuijpers M, Wulf PS, van Spronsen M, MacKintosh FC, Hoogenraad CC. Mixed microtubules steer dynein-driven cargo transport into dendrites. Current biology : CB. 2010;20:290–299. doi: 10.1016/j.cub.2009.12.052. [DOI] [PubMed] [Google Scholar]
- Kapitein LC, Yau KW, Gouveia SM, van der Zwan WA, Wulf PS, Keijzer N, Demmers J, Jaworski J, Akhmanova A, Hoogenraad CC. NMDA receptor activation suppresses microtubule growth and spine entry. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:8194–8209. doi: 10.1523/JNEUROSCI.6215-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MJ, Ehlers MD. Organelles and trafficking machinery for postsynaptic plasticity. Annual review of neuroscience. 2006;29:325–362. doi: 10.1146/annurev.neuro.29.051605.112808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevenaar JT, Bianchi S, van Spronsen M, Olieric N, Lipka J, Frias CP, Mikhaylova M, Harterink M, Keijzer N, Wulf PS, et al. Kinesin-Binding Protein Controls Microtubule Dynamics and Cargo Trafficking by Regulating Kinesin Motor Activity. Current biology : CB. 2016 doi: 10.1016/j.cub.2016.01.048. [DOI] [PubMed] [Google Scholar]
- Labonte D, Thies E, Pechmann Y, Groffen AJ, Verhage M, Smit AB, van Kesteren RE, Kneussel M. TRIM3 regulates the motility of the kinesin motor protein KIF21B. PloS one. 2013;8:e75603. doi: 10.1371/journal.pone.0075603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus JE, Moughamian AJ, Tokito MK, Holzbaur EL. Dynactin subunit p150(Glued) is a neuron-specific anti-catastrophe factor. PLoS biology. 2013;11:e1001611. doi: 10.1371/journal.pbio.1001611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liot G, Zala D, Pla P, Mottet G, Piel M, Saudou F. Mutant Huntingtin alters retrograde transport of TrkB receptors in striatal dendrites. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:6298–6309. doi: 10.1523/JNEUROSCI.2033-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobert S, Correia JJ. Subtilisin cleavage of tubulin heterodimers and polymers. Archives of biochemistry and biophysics. 1992;296:152–160. doi: 10.1016/0003-9861(92)90557-d. [DOI] [PubMed] [Google Scholar]
- Maday S, Wallace KE, Holzbaur EL. Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. The Journal of cell biology. 2012;196:407–417. doi: 10.1083/jcb.201106120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalek JR, Weiner JA, Farlow SJ, Chun J, Goldstein LS. Novel dendritic kinesin sorting identified by different process targeting of two related kinesins: KIF21A and KIF21B. The Journal of cell biology. 1999;145:469–479. doi: 10.1083/jcb.145.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr MI, Storch M, Howard J, Mayer TU. A non-motor microtubule binding site is essential for the high processivity and mitotic function of kinesin-8 Kif18A. PloS one. 2011;6:e27471. doi: 10.1371/journal.pone.0027471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima M, Maesaki R, Kasa M, Watanabe T, Fukata M, Kaibuchi K, Hakoshima T. Structural basis for tubulin recognition by cytoplasmic linker protein 170 and its autoinhibition. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10346–10351. doi: 10.1073/pnas.0703876104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T, Kirschner M. Dynamic instability of microtubule growth. Nature. 1984;312:237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- Muhia M, Thies E, Labonte D, Ghiretti AE, Gromova KV, Xompero F, Lappe-Siefke C, Hermans-Borgmeyer I, Kuhl D, Schweizer M, et al. The Kinesin KIF21B Regulates Microtubule Dynamics and Is Essential for Neuronal Morphology, Synapse Function, and Learning and Memory. Cell reports. 2016;15:968–977. doi: 10.1016/j.celrep.2016.03.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neupert C, Schneider R, Klatt O, Reissner C, Repetto D, Biermann B, Niesmann K, Missler M, Heine M. Regulated Dynamic Trafficking of Neurexins Inside and Outside of Synaptic Terminals. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:13629–13647. doi: 10.1523/JNEUROSCI.4041-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzorusso T, Ratto GM, Putignano E, Maffei L. Brain-derived neurotrophic factor causes cAMP response element-binding protein phosphorylation in absence of calcium increases in slices and cultured neurons from rat visual cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:2809–2816. doi: 10.1523/JNEUROSCI.20-08-02809.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevo B, Mangeol P, Oswald F, Scholey JM, Peterman EJ. Functional differentiation of cooperating kinesin-2 motors orchestrates cargo import and transport in C. elegans cilia. Nature cell biology. 2015;17:1536–1545. doi: 10.1038/ncb3263. [DOI] [PubMed] [Google Scholar]
- Schief WR, Howard J. Conformational changes during kinesin motility. Current opinion in cell biology. 2001;13:19–28. doi: 10.1016/s0955-0674(00)00169-1. [DOI] [PubMed] [Google Scholar]
- Seeger MA, Rice SE. Microtubule-associated protein-like binding of the kinesin-1 tail to microtubules. The Journal of biological chemistry. 2010;285:8155–8162. doi: 10.1074/jbc.M109.068247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setou M, Seog DH, Tanaka Y, Kanai Y, Takei Y, Kawagishi M, Hirokawa N. Glutamate-receptor-interacting protein GRIP1 directly steers kinesin to dendrites. Nature. 2002;417:83–87. doi: 10.1038/nature743. [DOI] [PubMed] [Google Scholar]
- Stepanova T, Slemmer J, Hoogenraad CC, Lansbergen G, Dortland B, De Zeeuw CI, Grosveld F, van Cappellen G, Akhmanova A, Galjart N. Visualization of microtubule growth in cultured neurons via the use of EB3-GFP (end-binding protein 3-green fluorescent protein) The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:2655–2664. doi: 10.1523/JNEUROSCI.23-07-02655.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Qiu W, Gupta ML, Jr, Pereira-Leal JB, Reck-Peterson SL, Pellman D. Mechanisms underlying the dual-mode regulation of microtubule dynamics by Kip3/kinesin-8. Molecular cell. 2011;43:751–763. doi: 10.1016/j.molcel.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szebenyi G, Bollati F, Bisbal M, Sheridan S, Faas L, Wray R, Haferkamp S, Nguyen S, Caceres A, Brady ST. Activity-driven dendritic remodeling requires microtubule-associated protein 1A. Current biology : CB. 2005;15:1820–1826. doi: 10.1016/j.cub.2005.08.069. [DOI] [PubMed] [Google Scholar]
- Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- van der Vaart B, van Riel WE, Doodhi H, Kevenaar JT, Katrukha EA, Gumy L, Bouchet BP, Grigoriev I, Spangler SA, Yu KL, et al. CFEOM1-associated kinesin KIF21A is a cortical microtubule growth inhibitor. Developmental cell. 2013;27:145–160. doi: 10.1016/j.devcel.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Verhey KJ, Hammond JW. Traffic control: regulation of kinesin motors. Nature reviews Molecular cell biology. 2009;10:765–777. doi: 10.1038/nrm2782. [DOI] [PubMed] [Google Scholar]
- Witte H, Neukirchen D, Bradke F. Microtubule stabilization specifies initial neuronal polarization. The Journal of cell biology. 2008;180:619–632. doi: 10.1083/jcb.200707042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RO, Ghosh A. Activity-dependent regulation of dendritic growth and patterning. Nature reviews Neuroscience. 2002;3:803–812. doi: 10.1038/nrn941. [DOI] [PubMed] [Google Scholar]
- Yau KW, Schatzle P, Tortosa E, Pages S, Holtmaat A, Kapitein LC, Hoogenraad CC. Dendrites In Vitro and In Vivo Contain Microtubules of Opposite Polarity and Axon Formation Correlates with Uniform Plus-End-Out Microtubule Orientation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2016;36:1071–1085. doi: 10.1523/JNEUROSCI.2430-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Asenjo AB, Greenbaum M, Xie L, Sharp DJ, Sosa H. A second tubulin binding site on the kinesin-13 motor head domain is important during mitosis. PloS one. 2013;8:e73075. doi: 10.1371/journal.pone.0073075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Cai Q, Xie Y, Sheng ZH. Snapin recruits dynein to BDNF-TrkB signaling endosomes for retrograde axonal transport and is essential for dendrite growth of cortical neurons. Cell reports. 2012;2:42–51. doi: 10.1016/j.celrep.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel LS, Kuruvilla R, Ginty DD. Functions and mechanisms of retrograde neurotrophin signalling. Nature reviews Neuroscience. 2005;6:615–625. doi: 10.1038/nrn1727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.