Abstract
Postnatal brain development is studded with sensitive periods during which experience dependent plasticity is enhanced. This enables rapid learning from environmental inputs and reorganization of cortical circuits that matches behavior with environmental contingencies. Significant headway has been achieved in characterizing and understanding sensitive period biology in primary sensory cortices, but relatively little is known about sensitive period biology in associative neocortex. One possible mediator is the onset of puberty, which marks the transition to adolescence, when animals shift their behavior toward gaining independence and exploring their social world. Puberty onset correlates with reduced behavioral plasticity in some domains and enhanced plasticity in others, and therefore may drive the transition from juvenile to adolescent brain function. Pubertal onset is also occurring earlier in developed nations, particularly in unserved populations, and earlier puberty is associated with vulnerability for substance use, depression and anxiety. In the present article we review the evidence that supports a causal role for puberty in developmental changes in the function and neurobiology of the associative neocortex. We also propose a model for how pubertal hormones may regulate sensitive period plasticity in associative neocortex. We conclude that the evidence suggests puberty onset may play a causal role in some aspects of associative neocortical development, but that further research that manipulates puberty and measures gonadal hormones is required. We argue that further work of this kind is urgently needed to determine how earlier puberty may negatively impact human health and learning potential.
Keywords: sensitive period, frontal cortex, puberty, steroid hormones, executive function, inhibition
1. Introduction
An organism’s experience interacts with its genetics to significantly impact brain development. For parents, educators, and mental health professionals, it is critical to understand how and when experience maximally impacts childhood brain development in order to improve outcomes from educational and therapeutic interventions by timing them to coincide with periods during which they will have the greatest efficacy.
One particularly important period of development is childhood, which is unusually long in humans. Some hypothesize that this extended period of development evolved to allow children to learn complex foraging skills and adapt to complex social and cultural practices prior to reaching sexual maturity (Bock and Sellen, 2002; Kaplan et al., 2000; Konner, 2010). In modern times, childhood is when most humans learn to speak, read and write, perform arithmetic, and perform numerous other cognitive skills. These high-level skills are thought to rely on the associative neocortex, which, during childhood, may be exceptionally plastic in order to enhance learning capacity and experience dependent sculpting of neural circuits. As childhood ends, we lose the more radical forms of neocortical plasticity and learn in a more conservative manner. We also become more independent while learning new behaviors and skills that aid in the transition to adult life and parental behavior. Here we discuss the evidence that the onset of adolescence may represent a shift in sensitive period for experience dependent plasticity in the neocortex and different forms of associative learning. Given that puberty onset marks the transition from childhood to adolescence, it is a strong candidate in the search for mechanisms regulating developmental transitions in neocortical brain plasticity.
While puberty is a conspicuous biological marker in this transition, it is unclear if gonadal steroids impact neocortical brain plasticity. Correlative evidence ranging from studies of language acquisition to recovery from stroke suggests neocortical neural plasticity and the capacity for some dramatic forms of learning and recovery decrease after the age of puberty onset. However, there is little evidence, either positive or negative, to causally link puberty onset with loss of plasticity. In the present review, we have chosen to focus on studies that examine the associative regions of the neocortex and relate brain or behavioral changes to pubertal status, gonadal steroids, and/or mechanisms that regulate sensitive periods in sensory neocortex. We first review studies of learning, memory, and executive function before covering some of the biological underpinnings that may regulate these effects. Based on the anatomical changes reviewed, we propose a biological model for sensitive period regulation in the associative neocortex inspired by successful models developed for sensory cortices. Overall, we uncover an intriguing array of changes in the neurobiology and function of associative neocortex with inflection points near the ages of pubertal milestones. However, we conclude that more experimental work is needed to fully determine how puberty affects postnatal brain development. Finally, we discuss the urgency of understanding the role of puberty in brain development because the age at puberty onset is advancing in developed nations and early puberty is associated with increased risk for psychopathology and negative behavioral outcomes.
2. Puberty as a life history transition
Life history theory posits that an animal has finite time and resources and thus must selectively allocate these resources toward various evolutionary endpoints, including survival, growth, bodily maintenance, reproduction, and raising offspring (Ellis et al., 2009; Roff, 2002; Stearns, 1992). Evolution has selected for certain species specific life-history patterns that are genetically encoded and hard-wired in the brain, however, many are impacted by experience such that different postnatal experiences may result in alternate life-history strategies. The importance assigned to each behavioral or physiological endpoint varies by age and stage of development (Hochberg and Belsky, 2013). Thus, a successful organism’s life history represents a balance between survival at any given stage of development and lifetime inclusive fitness (Konner, 2010). For example, during childhood and adolescence relatively more energy may be allocated toward somatic growth, while in adulthood growth is restricted so that more energy can be allocated toward reproductive ends (Ellis et al., 2009).
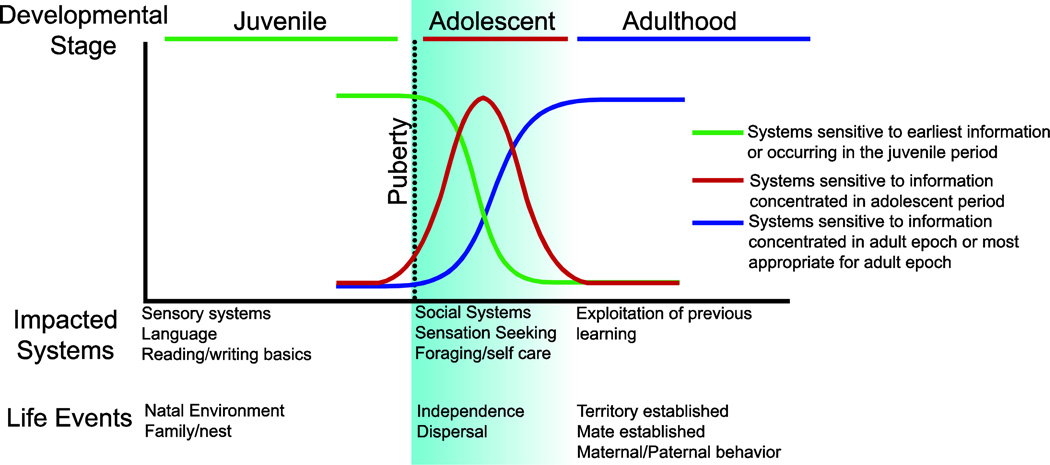
Brain development may also reflect the demands of specific life stages (Fig. 1). At different stages of development, animals must emphasize gaining certain knowledge or developing certain cognitive or physical skills. These skills and knowledge structures will be retained, refined, and combined over time for exploitation at later ages (Spear, 2000). Thus, because their basic needs (e.g. nutrition and protection) are provided by their parents, juveniles may allocate their time and energy toward exploring and learning about their natal environment. Numerous lines of evidence suggest that the brain is highly plastic during this period, presumably to first establish basic sensory processing and then build on this base by attaining further information that is most relevant for their life-stage; for example, in humans, natal language is a basic skill acquired in the early environment. Once the basics of the natal environment are established, animals transition toward independence, exploring more broadly to gather new types of information (particularly social and foraging information) that will be important to navigate dispersal, mate selection, and territory establishment (Lynn and Brown, 2009; Spear, 2000). Thus, development is likely studded with a series of sensitive periods in which experience-dependent plasticity in specific circuits, including those in associative regions of neocortex, is timed to coincide with access to important information.
Figure 1.
Heuristic model of three distinct periods of development: juvenile, adolescent and adult. In this model the juvenile period (Green line) is when an animal is developing the foundations of sensory and cognitive/associational architectures in the brain. To facilitate this process they display enhanced experience dependent plasticity, which serves to organize neural circuits to be best adapted to their natal environment. In humans, this period is also when the basic educational foundations are established, like the ability to read and write and produce or understand language. At the start of puberty (dotted line), which marks the onset of adolescence (Red line), independence is exerted, social networks expand, and sensation seeking and the salience of social feedback increases. This period is important for humans and animals to explore their social world and learn how to survive independently. Finally, adolescents transition into adults (blue line) and exploit the knowledge they gained during childhood and adolescence, to successfully survive, mate, maintain territory, and raise young. These characteristic life stages are conserved across mammalian species.
In models of adaptive developmental plasticity, natural selection sculpts sensitivity to cues that predict the future environment and matches behavioral phenotypes to bodily states (Nettle and Bateson, 2015). One of the great challenges for modern neuroscience is to isolate the specific neural circuits and mechanisms that regulate the multiple cognitive and emotional sensitive periods in human development.
The start of puberty, which marks the end of childhood and the onset of adolescence, is a critical life history transition (Ellison et al., 2012). In mammals, independence from parental care and dispersal typically occur during the peripubertal period, but the timing of independence and dispersal varies by species, season, sex, and even individual factors. As such, it is possible that the onset of puberty and/or independence may herald a shift in sensitive periods for experience dependent plasticity. During this period, plasticity attuned to information from parents or the natal environment may close, while plasticity related to social opportunities and sampling the wider environment may peak (Crone and Dahl, 2012). When adult stability and reproductive status are reached, we may expect brain plasticity to shift to a new mode to fulfill these different needs (Fig. 1). Upon reaching adulthood, the brain may shift again to exploit the skills and knowledge attained across both developmental epochs to successfully survive, compete for mates, reproduce, and raise offspring.
In Figure 1 we present a simplified heuristic model of the duration and timing of different hypothetical sensitive periods matched to life-history stages. Puberty onset is marked as a potential trigger point that causes juvenile plasticity (green line) to decline and enhances plasticity in circuits more appropriate for adolescence (red line). While animals establish mating patterns and are able to care for themselves, adolescent behavioral patterns decline and adult behavioral patterns (blue line) with differential sensitivity and tuning emerge. The causal role of puberty onset as the trigger closing the juvenile period and opening the adolescent period is unknown. Other factors like age, adrenarche, or experience (including feedback from social experience and independent decision making itself), could play a role in this transition. Before we review the evidence, we quickly review the ages of pubertal milestones in humans and experimental rodent models.
3. Comparing pubertal milestones in humans and rodent models
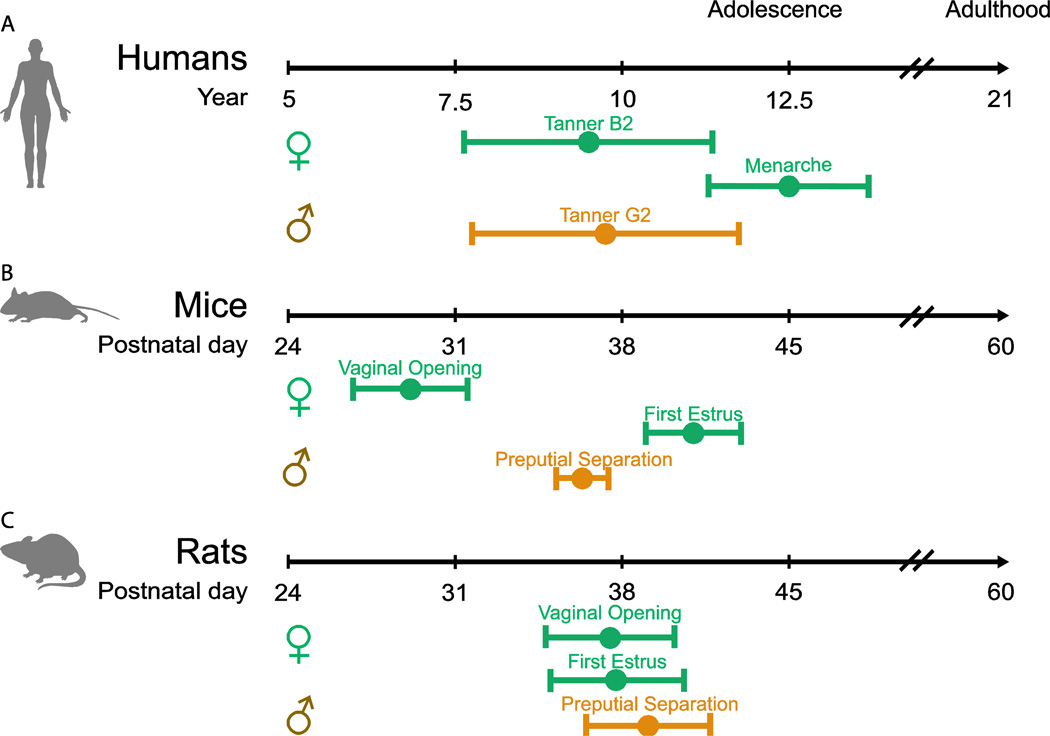
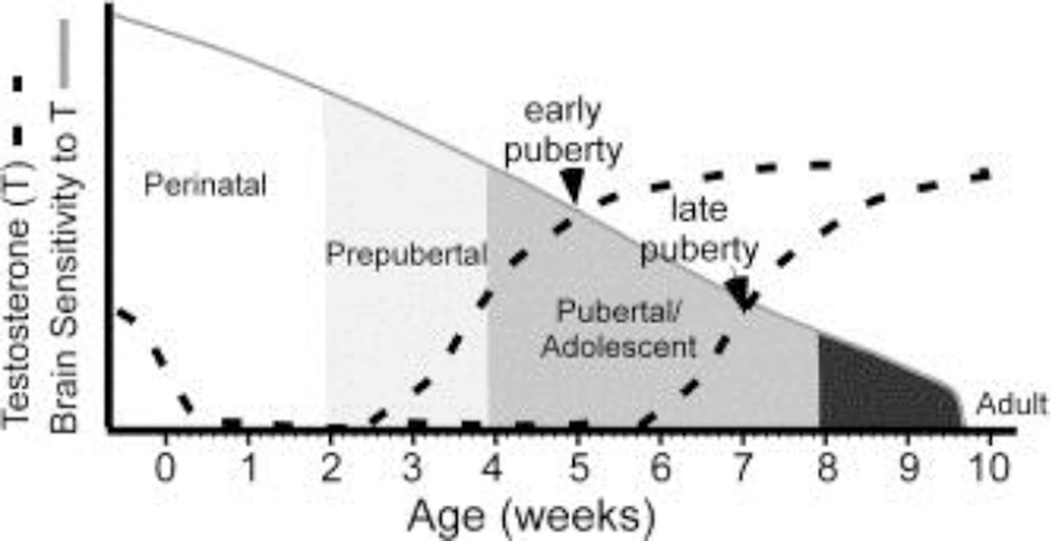
In order to understand if we can meaningfully compare puberty with brain maturation across animals and humans, we must know if pubertal milestones are similar between species and at what ages they are attained. Fortunately, we find comparable milestones that enable us to align development in humans with that of rats and mice (Fig. 2).
Figure 2.
Humans, mice, and rats experience analogous pubertal milestones. A) In girls (green line) the first externally observable pubertal milestone is onset of breast development caused by the pubertal rise in estradiol (Tanner B2; occurring at age 9.5 ± 1.85 SD) which is followed by first menses (menarche; average age 12.5 ±1.2 SD). In boys puberty onset can be observed by attainment of testicular volume greater than 3 ml (Tanner G2; 9.75 ± 2.0 SD). Data for girls reproduced from (Herman-Giddens et al., 1997) and data from boys reproduced from (Herman-Giddens et al., 2012). B) In mice the female milestones are similar to humans. In female mice the first externally visible milestone is vaginal opening in which estradiol causes dissolution of a film of cells covering the vagina (Vaginal opening; 29.125 ± 2.4 SD), which is followed by first estrus, the mouse equivalent of menarche (P41 ± 2 SD) (Piekarski and Wilbrecht, unpublished data). Male rodents exhibit the first external signs of puberty by the separation of the prepuce from the glans penis (Preputial separation; P36.5 ± 1.1; (Deboer and Li, 2011). C) Finally, both female and male rats display the same pubertal milestones as mice: Females (Vaginal opening; 37.5 ± 2.7; First Estrus; 37.8 ± 2.7; (Clark and Price, 1981) and males (Preputial Separation; P36.5 ± 1.1; (Korenbrot et al., 1977). NOTE: Exact age at pubertal milestones varies by strain and between experiments. Specific ages are offered to compare progression through puberty as it relates to pubertal milestones in each species and to provide a reference for the data reviewed below.
Puberty onset in animals, including humans, is initiated by an increase in pulsatile release of gonadotropin releasing hormone (GnRH) from the hypothalamus, which signals the pituitary to release lutenizing hormone and follicle stimulating hormone that mature the gonads and increase production and release of gonadal steroids. The mechanism that determines the age at puberty onset is unknown, but it appears to involve complex changes to regulatory circuits upstream of GnRH neurons, including kisspeptin neurons that stimulate GnRH release (Mayer et al., 2010; Seminara et al., 2003), RFRP-3 neurons that inhibit release (Poling and Kauffman, 2015), and significant roles played by glial regulation of growth factors and GnRH stimulatory molecules (Lomniczi et al., 2013). Once initiated, puberty likely exerts its broad effects on brain and body through the rise in gonadal steroids. In both sexes, circulating gonadal steroids (predominately estradiol and progesterone in girls and testosterone in boys, although both sexes experience rises in all three hormones at puberty) rise across puberty until reaching adult concentrations (Boswell, 2014; Rilling et al., 1996). In girls, the rise in circulating estradiol first manifests externally through the start of breast development (Divall and Radovick, 2008; Marshall and Tanner, 1969), which is followed 1–3 years later by first menses (menarche) (Anderson and Must, 2005; Hansen et al., 1975) and eventual ovulation and regular menstrual cyclicity within another 1–3 years (Boswell, 2014; Divall and Radovick, 2008; Legro et al., 2000). In boys, milestones are more difficult to observe; the first external indicator of puberty onset occurs with testosterone-induced increase in testicular volume along with a change in the color and texture of the scrotal skin (Marshall and Tanner, 1970; Sørensen et al., 2010). After this, a gradual increase in sexual maturity occurs. Although not tied to the same orderly sequence of binary milestones as in girls, the gradual progression of male pubertal development can still be assessed in a semi-continuous manner, e.g. by Tanner staging (Fig. 2).
The sequence of pubertal milestones is similar in humans and rodents, suggesting that rodents may serve as a valid model for human pubertal development (Fig. 2). In female rodents, the first indicator of the pubertal rise in estradiol is vaginal opening, during which increased circulating estradiol induces apoptosis in cells that form a thin sheath over the vaginal opening (Ito et al., 2014; Rodriguez et al., 1997). This is followed some days later by first estrus and a few days later by onset of regular cyclicity (Nelson et al., 1990). These rodent milestones are regulated by the rise in estradiol, just as the milestones in humans. In males, increased testosterone at puberty induces separation of the prepuce from the glans penis (preputial separation; (Korenbrot et al., 1977) accompanied by increasing testicular volumes and eventual attainment of fertility.
Once gonadal steroids rise in the bloodstream, they can access and act on any cells expressing the cognate receptor. Steroid receptors are distributed widely across the cortex in a number of mammalian species (Almey et al., 2014; Blurton-Jones and Tuszynski, 2002; Finley and Kritzer, 1999; Kritzer, 2004; Kritzer, 2006; López and Wagner, 2009; Zsarnovszky and Belcher, 2001) and can affect cell physiology over the long term by acting as transcription factors to regulate gene expression (Heldring et al., 2007) or rapidly through non-genomic second messenger cascades (Micevych and Dominguez, 2009). The mechanisms by which steroids influence their cortical substrates are numerous, complex, and outside the scope of the present review, but regardless of their mechanism, steroid receptor expression in the cortex provides a clear mechanism by which pubertal steroids may directly influence cortical plasticity at puberty. The ontogeny and distribution of gonadal steroid receptors in the cortex will be discussed in more detail in section 7.
4. Puberty is advancing
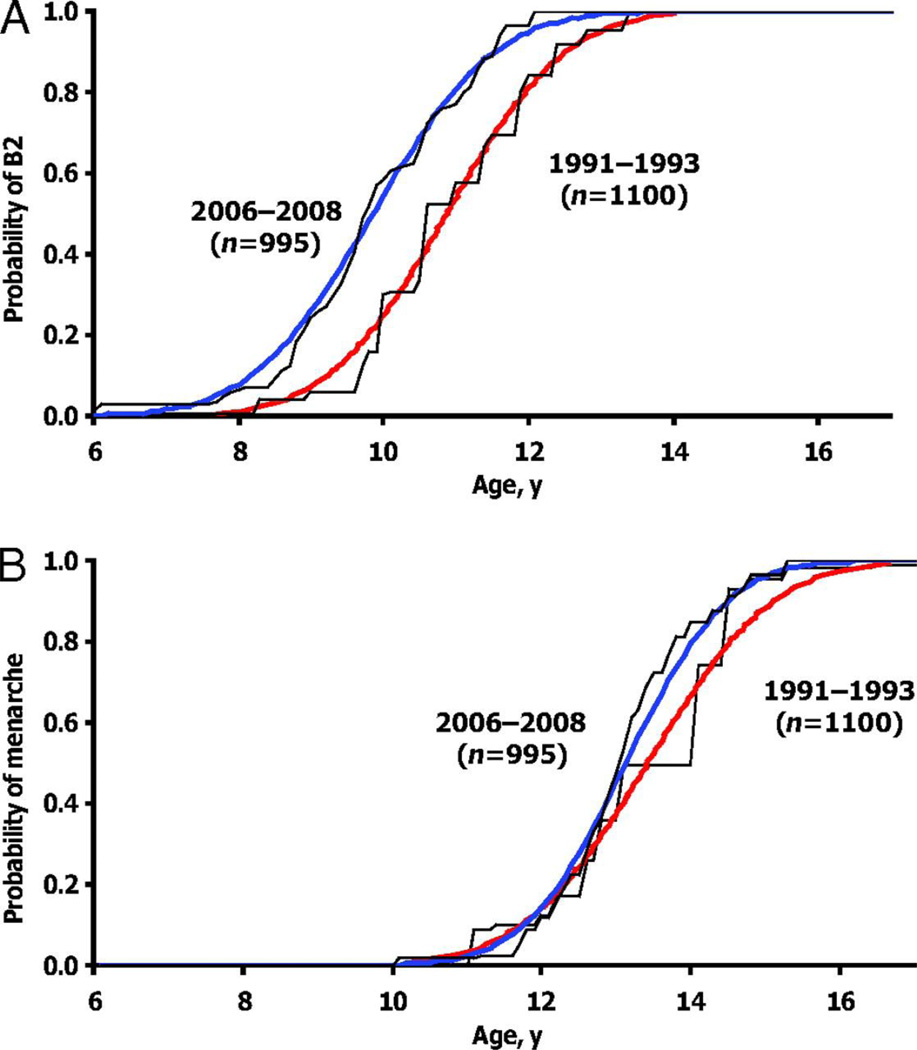
Over the last decades, the average age at puberty onset in girls has advanced by multiple years (Aksglaede et al., 2009; Biro et al., 2013; Herman-Giddens, 2006; Rosenfield et al., 2009). The data for boys is less clear but suggest a similar trend of smaller magnitude (Herman-Giddens et al., 2012; Sørensen et al., 2010). In North American boys and girls, earlier maturation is more common in black, Hispanic and low socioeconomic status children (Biro et al., 2010; Biro et al., 2013; Herman-Giddens, 2006; Herman-Giddens et al., 2012). In one study, the mean age of breast budding in black or Hispanic girls occurred at 8.8 and 9.3 years old (respectively) compared to 9.7 in white or Asian girls (Biro et al., 2013). Earlier puberty may be partly caused by increases in food intake and body mass index, but racial differences may also be independent of differences in body mass index (Rosenfield et al., 2009).
It is unknown what is causing this trend of advancing puberty but numerous factors may accelerate puberty in humans and animals including a stressful early environment or absence of a father in the household (Belsky, 2012), early exposure to endocrine disruptors (Meeker, 2012), or childhood obesity (Ahmed et al., 2009). In rodents, injecting leptin (a hormone released by adipose tissue) in juvenile mice advances puberty onset (Chehab et al., 1997), providing a mechanism by which obesity may induce puberty. From a life history theory standpoint, earlier sexual maturation may reflect an adaptive developmental response and distinct life history strategy that maximizes reproductive fitness in a harsh environment (Belsky, 2012).
Regardless of the underlying etiology of advancing puberty, it is clear that puberty onset is associated with enhanced risk for psychopathology and behavior problems, including anxiety and depression, (Deardorff et al., 2007; Deardorff et al., 2013; Ge et al., 1996; Ge et al., 2001; Graber et al., 2004; Graber et al., 2006; Hayward et al., 1997; Silberg et al., 1999), and substance abuse (Graber et al., 1997; Stice et al., 2001). Unfortunately, the relative roles that biology and social factors play in the development of puberty-related psychopathology is difficult to determine from studies in humans (Graber, 2013).
Even if early puberty onset weren’t associated with psychopathology, there would be reason for concern. If puberty onset closes sensitive periods in associative neocortex, then advanced puberty onset may truncate the juvenile period of early learning and enhanced plasticity. Further, if this biological limit comes earlier in already disadvantaged groups, then it may enhance disparities in learning and future potential for achievement. For example, it is important to know if reading interventions (or other forms of training) should be timed before puberty onset to ensure that they are effective. We might also consider that other forms of experience or interventions (for example, related to enhancing social development) are best timed to occur after puberty onset to achieve maximal impact.
5. How does the function of associative neocortex change during peripubertal development?
The associative cortices are thought to develop later than sensory cortices (Huttenlocher and Dabholkar, 1997), but it is not well understood if the associative cortices have sensitive periods for plasticity of function equivalent to those found in sensory regions. In this section, we will review evidence that humans and animals experience sensitive periods for skills and behaviors that are likely regulated by associative neocortex. Further, we will review the evidence that puberty affects this plasticity. The following is not an exhaustive review of these many functions but rather a brief overview of evidence suggesting that puberty may be important for regulating these sensitive periods.
5.1 Language learning
In the 1960’s, Eric Lenneberg outlined a theory that a sensitive period for language acquisition was closed by the onset of puberty. This argument was based on evidence that children recovered language function after resection of either the cerebral cortex of the right or left hemisphere, but adults undergoing the same procedure experienced aphasia after left but not right hemisphere resection (Lenneberg, 1967). He viewed these data as evidence that puberty crystallized language capacity and prevented interhemispheric transfer of language abilities after left hemisphere damage (Lenneberg, 1967).
A number of studies subsequently measured the age at which acquiring a new language or recovering from trauma to language centers of the brain became impaired. These studies broadly support Lenneberg’s hypothesis that peripubertal development is associated with significant decline in language plasticity. Ability to learn a second language begins to decline around the age of normal puberty onset (~9–11 years old), which continues throughout adolescence before leveling off in adulthood (DeKeyser, 2000; Dekeyser et al., 2010; Johnson and Newport, 1989; Newport, 1990); however, some have argued that a second language acquisition, contrary to primary language acquisition, is not subject to sensitive period learning (Hakuta et al., 2003).
There is biological evidence that the location of language centers in the neocortex is highly plastic, but this plasticity is decreased in groups measured at postpubertal ages. In a case study, researchers scanned a child’s brain before and after he underwent a left hemispherectomy at 9 years old to control intractable epilepsy. Scans at 7.5 and 10 years old revealed that language function, measured through BOLD signal pattern, had transferred from the left to the right hemisphere, suggesting interhemispheric transference was still possible at the age of 9 (Hertz-Pannier et al., 2002). Another study demonstrated that infants and children exposed to a second language showed overlapping BOLD signal patterns when processing both languages in adulthood, but adults who became fluent in a second language after the first decade of life (mean age 11.2 years old) recruited different neural networks to process their second language (Kim et al., 1997). These data suggest that by the second decade of life, language network plasticity had either declined or entered a different state.
Studies of language-specific learning problems (in which other aspects of intellectual function remain intact) have also identified age 10 as a cutoff point for behavioral plasticity; studies show that complex sensory processing is delayed in subjects diagnosed with dyslexia and other language-specific impairments, but improvements can be observed until age 10. After age 10, improvement stalls and subjects never ‘catch up’ with typically developing individuals (Wright and Zecker, 2004).
Together, these studies suggest that sometime around 10 years of age, which is around the onset of puberty in boys and girls, we lose juvenile capacity for plasticity in cortical language centers. It is important to recognize that none of these papers measured pubertal milestones or gonadal steroids. Thus, any inferences about puberty’s role are based on average age at puberty onset (Fig. 2), which varies substantially between individuals and sexes.
5.2 Birdsong learning
Birdsong learning can be considered an animal model of at least the motor aspects of language learning. Song motor plasticity is reduced after sexual maturation in species that learn one song for life (zebra finches), but in species that learn each season (canaries), plasticity is regulated by seasonal changes in gonadal steroids (Doupe and Kuhl, 1999). In zebra finches, pre-pubertal exposure to testosterone impairs song learning by inducing early song crystallization, consistent with the idea that hormones are sufficient to close a sensitive period of song flexibility (Korsia and Bottjer, 1991). In a parallel finding, gonadectomy combined with androgen receptor blockade during adolescence disrupts the development of crystallized adult song, suggesting that hormones are necessary for normal song crystallization (Bottjer and Hewer, 1992); however, gonadectomy and androgen receptor blockade in adulthood have no effect on already crystallized song (Bottjer and Hewer, 1992), although androgen receptor blockade enhances and testosterone reduces the capacity for adult song flexibility after nerve injury (Williams et al., 2003). Taken together, data from these songbirds are consistent with the idea that pubertal hormones close sensitive periods for flexible song learning in birds.
5.3 Stroke and recovery
Researchers using rodent models have systematically varied the age at which they induce lesions in the frontal cortex while measuring executive function outcomes in adulthood. Age clearly affects prospects for recovery of executive function after focal injury in rat models; rats recover poorly from lesions made in the first week of life, but lesions made during the second week of life—roughly analogous to the first year of life in humans (Kolb and Cioe, 2000)—often results in full recovery of executive function in adulthood (Kolb, 1987; Kolb and Gibb, 1990; Kolb et al., 1998; Nonneman and Corwin, 1981; Prins and Hovda, 1998). The data are more sparse during the late juvenile to peripubertal period (~P35-P40), but prepubertal rats recover better from lesions than postpubertal rats (Nemati and Kolb, 2010; Nemati and Kolb, 2012). Still, recovery potential from late juvenile lesions is lower than P7–14 rats (Kolb and Nonneman, 1978; Nemati and Kolb, 2012; Prins and Hovda, 1998). These data suggest that recovery capacity peaks during the second week of life before gradually declining across the juvenile period. Recovery potential then becomes adult-like around the onset of puberty. These same series of studies suggest that the heightened recovery from lesions may be conferred from heightened cellular plasticity and regrowth of dendritic arbors and spines (Dallison and Kolb, 2003; Kolb et al., 1996) and that part of the recovery may occur from partial restitution of the damaged brain area rather than solely from transference of function (Dallison and Kolb, 2003).
5.4 Reading, mathematics, and academic performance
Basic reading and mathematics skills are thought to depend on associative neocortex and are typically established in the prepubertal period from age 4–9 (by grade 3) (Blank, 2006; Hernandez, 2011). In the late 90’s, The LA Times and Baltimore Sun even supported “Reading by 9” campaigns that chose age 9 as a critical cutoff. It is unclear if age 9 (grade 3) is a cutoff for biological reasons or if there are also important curricular changes at this time, shifting pupils from “learning to read” to “reading to learn”(Wanzek et al., 2010). However, studies of reading interventions for readers behind grade level have found diminishing effects of intervention after age 9 (or grade 3) (Wanzek and Vaughn, 2007; Wanzek and Vaughn, 2013). Prepubertal reading and math skills are also thought to predict later outcomes (Hernandez, 2011; Jordan et al., 2009; Jordan and Levine, 2009). Interestingly, earlier reading interventions are not always better. Interventions for children with special needs showed increases in effect size with increasing age, suggesting a potentially later sensitive period (up to age 12, in a cutoff of a meta-analysis; Kroesbergen and Van Luit, 2003). No study of which we are aware has measured the effects of teaching or interventions before and after puberty to establish if puberty onset regulates sensitivity to teaching or intervention.
Studies have looked at pubertal status and school performance, with an eye toward identifying advantaged and/or vulnerable populations. In 1964, Douglas and Ross at the London School of Economics asked, “is the physical spurt at puberty paralleled by a speeding up of mental development? Do those who come into puberty early do better at school, and if so do they retain their advantage in the secondary schools?” Using data from 5000 subjects from a 1946 cohort of the British National Survey on Health and Development, they found that early maturers of both sexes showed superior performance at age 8, 11 and 15, and this appeared to be independent of social class, which in the post world war II years was a potential proxy for nutritional differences (Douglas and Ross, 1964). More recent studies of academic performance show that earlier maturation is still associated with positive effect for boys in terms of academic performance, but earlier maturation is now associated with worse academic performance in girls (Dubas et al., 1991), while early maturers of both sexes reported lower career training aspirations. Dubas et al. (1991) also find that relative pubertal timing (compared to grade level peers) rather than pubertal status (independent of grade level) drives these effects suggesting that extrinsic social factors play a role in the effects of pubertal maturation on academic performance more than intrinsic biological factors.
5.5 Autobiographical memory
Associative neocortex is thought to be the site of memory storage, and a wealth of evidence suggests autobiographical memory storage changes with age. A series of investigations of autobiographical memories have found that people tend to generate more lasting autobiographical memories from late childhood and teenage period of life than later in life (Janssen et al., 2011; Koppel and Berntsen, 2015)—a phenomenon called the “reminiscence bump.” The reminiscence bump was found to peak between ages 6–10 in a recent study that used neutral word cues to elicit memories and had large enough samples to use small age bins (Janssen et al., 2011). Studies that used olfactory cues also place this reminiscence bump at age 6–10 (Chu and Downes, 2000; Willander and Larsson, 2008). Interestingly, studies that use important life events to cue memories find a bump that occurs in later teenage years and into the third decade of life (age 15–28; Koppel and Berntsen, 2015). These data suggest that across development, memories are differentially encoded or encoded in a way that they can be differentially later retrieved (Rubin, 2015). Olfactory-related autobiographical memories are more readily retrieved from the time prior to puberty (Chu and Downes, 2000; Rubin, 2015; Willander and Larsson, 2008), and more general autobiographical memories are retrieved from around the peripubertal period than at later times in life (Janssen et al., 2011). We can speculate that these forms of autobiographical memory may rely more on biological processes related to greater juvenile plasticity (Fig. 1, green curve). In contrast, autobiographical memories related to important life events and life decisions peak after puberty and coincide instead with the time of important stages of early independent decision making (Koppel and Berntsen, 2015; Rubin, 2015). These forms of autobiographical memory may be related to changes in behavior and/or biological processes (Fig. 1, red inverted U curve) associated with independent exploration and the formation of new social bonds during adolescence and early adulthood.
5.6 Executive function
The frontal association cortex is the seat of executive function and continues its development into the third decade of life in humans (Gogtay et al., 2004). Executive function can be divided into sub-domains that include spatial cognition, reasoning, response inhibition, delay discounting, and flexible updating. Together, these processes support cognition and adaptive goal-directed behavior.
Changes to associative neocortex during the transition to adolescence may contribute to the altered executive function observed between juveniles and adolescents. However, subdomains of executive function develop along different trajectories, resulting in a complex pattern of maturation. Here we address how each subdomain changes during the peripubertal period and evaluate the evidence that pubertal hormones affect this development.
5.6.1 Spatial cognition
Spatial cognition includes skills such as mental rotation or perception of embedded figures thought to depend on higher neocortical association areas. Many studies find sex differences in spatial cognition that emerge after puberty. Schulz et al. (2009a) review a number of studies that show higher or lower levels of androgens due to idiopathic hypogonadotropic hypogonadism in men or congenital adrenal hyperplasia in women alters spatial cognition after puberty. Schulz et al. (2009a) suggest developmental changes in spatial cognition may be driven by organizational effects of steroid hormones. Petersen (1983) reviews a number of studies from the 70s-80s that find a decrement in spatial cognition at puberty onset or in earlier maturing children. However, in follow up Petersen (1983) finds null effects of pubertal status and grade (6–8) in a sample of high performing children.
5.6.2 Reasoning and cognitive strategy
Learning rules that guide appropriate behavior in a particular context is critical for successful development and survival. In novel situations, rules must be learned either through explicit instruction or implicitly through trial and error. A recent behavioral study showed that children (age 6–12) and adolescents (age 13–17) excel at learning rules through experience, whereas adults were biased to learn more from explicit instruction (Decker et al., 2015). Ten to eleven year olds are more likely than older adolescents (age 13–14) to use a problem solving strategy that more heavily weights personally experienced recent outcomes (Kokis et al., 2002). Additionally, performance in a feedback learning task increased from age 8–12 before reaching adult levels, but was not explained by pubertal stage, estradiol, or testosterone levels (Peters et al., 2014).
Strategic exploration, which is the purposeful choice to test the outcome of novel experiences during feedback learning, aids in actively determining a particular environment’s rules. Measures of exploration and novelty seeking in both humans and rodents increase during adolescence (Spear, 2004; Steinberg et al., 2008). Within a focused sample of girls ranging from 11–13 years, no association was found between age or pubertal development and propensity for strategic exploration (Kayser et al., 2015). Similarly in rodents, there was no relationship between testosterone, estradiol, progesterone, or physical signs of sexual maturation with novelty seeking after controlling for age (Vetter-O’Hagen and Spear, 2012).
5.6.3 Response inhibition
An important aspect of cognitive control is the ability to inhibit prepotent or inappropriate responses. Numerous studies have shown that response inhibition increases from childhood through young adulthood (Bunge et al., 2002; Luna et al., 2004; Marsh et al., 2006; Rubia et al., 2006; Velanova et al., 2008; Williams et al., 1999). One study showed a transient adolescent (age 13–17) decrease in response inhibition relative to children and adults (Somerville et al., 2010), however there are considerable individual differences in performance across studies that may reflect age independent factors.
There are few longitudinal studies that have specifically spanned the peripubertal age range. An accelerated longitudinal design study of antisaccade performance in humans showed that accuracy improved most steeply between ages 10–14, but the authors did not track the pubertal status of participants (Paulsen et al., 2015). Another longitudinal antisaccade study was performed in macaque male monkeys which were tested during the pubertal transition and again as young adults, using morphometric, radiographic, and hormonal measurements to determine pubertal onset (Zhou et al., 2016). Neural recordings in the prefrontal cortex during behavior suggest that adult improvements in performance may be due to increased preparation for an alternate course of action and not due to suppression of the visual stimulus. Increased neural capacity to represent rules of the task and multiple actions may drive age related increases in task performance. A recent study by Tyborowska et al. (2016) was able to further parse the role of testosterone levels in 14 year-old adolescents’ ability to avoid impulsive actions in an emotional approach-avoidance task. Higher testosterone levels were associated with greater control over emotional actions and increased recruitment of the anterior prefrontal cortex.
5.6.4 Delay discounting
Living independently requires long term planning and anticipating future events. One laboratory test for these abilities involves asking subjects to choose between a small immediate reward and a larger delayed reward. Decreased subjective value of a reward as the delay increases is called delay discounting. Delay discounting generally decreases between young childhood and adulthood, meaning children tend to choose a smaller immediate reward over a larger delayed reward (Christakou et al., 2011; de Water et al., 2014; Green et al., 1994; Olson et al., 2007; Scheres et al., 2006; Steinberg et al., 2009). However, one study found that 13–17 year olds were more willing to wait for the delayed reward compared to children (aged 6–12) and young adults (aged 18–19; Scheres et al., 2014). Pubertal stage and hormone concentrations were not significantly associated with delay discounting in either boys or girls, but prepubertal children were not included in these studies, so the effect of puberty onset could not be determined (Bromberg et al., 2015; de Water et al., 2014).
5.6.5 Flexible updating
A growing body of studies suggests that flexibility in goal-directed behavior increases during adolescence. The ability to switch between sets of simple stimuli emerges by age 5 (Espy, 1997). More complex forms of switching such as shifting between response sets based on abstract categories become adult-like as early as age 7 (Luciana and Nelson, 2002), with other studies suggesting a plateau at age 10 (Chelune and Baer, 1986; Chevalier et al., 2013; De Luca et al., 2003) or 11–12 (Huizinga et al., 2006; Huizinga and van der Molen, 2007; Somsen, 2007), gradual improvement across the teenage years (Crone et al., 2006; Crone et al., 2008), or an inverted U in the teenage years (van der Schaaf et al., 2011). Variability in these studies may be related to aspects of study design, including the real-world motivational salience of rewards offered (Nelson et al., 2005; Vrtička et al., 2014) and the relative amounts of punishment and reward in the task (Cohen et al., 2010; Davidson et al., 2006; van den Bos et al., 2012; van der Schaaf et al., 2011; van Duijvenvoorde et al., 2008). To our knowledge, studies have not directly addressed the relationship between the onset of puberty and behavioral flexibility in humans.
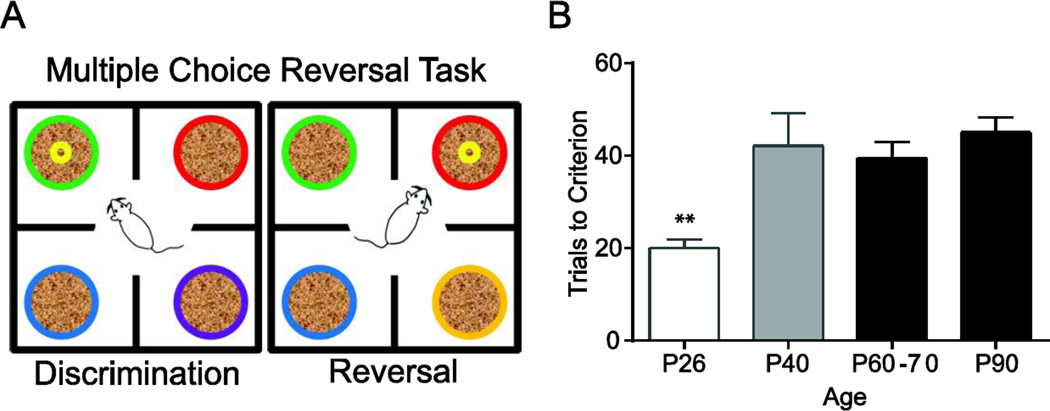
Experimental studies in animal models are well suited to ask how pubertal timing impacts behavioral flexibility because the age of pubertal onset and progression through pubertal milestones can be easily tracked. A challenge for these studies is to find a behavioral task that can be easily trained and tested before the onset of puberty. A digging-based foraging task is a naturalistic method to test behavioral flexibility and is rapidly learned by mice and rats (Birrell and Brown, 2000; Garner et al., 2006; Kim and Ragozzino, 2005). In the version of the task used in our lab, food rewards are buried in one of 4 bowls of scented wood shavings and rodents rapidly learn to forage based on the odor cues (Fig. 4). We observe that pre-pubescent juvenile mice (P26) learn the odor associations more rapidly than adults and that they more quickly update their choice behavior when the reward unexpectedly shifts to a different odor in a reversal phase of the task (Johnson and Wilbrecht, 2011). Behavioral flexibility declines with age and is adult-like after P40, just ~10 days after the onset of puberty in mice (Fig. 4). A similar task in postpubertal rats (but with 2 rather than 4 choices in the task) instead found that older adolescents were less cognitively flexible in reversal and set-shift phases of training than adults, but juveniles were not studied (Newman and McGaughy, 2011). Future studies are needed to test flexibility with greater age resolution and manipulation of puberty to directly understand puberty’s role in this cognitive function.
Figure 4.
Age-dependent changes in behavioral flexibility in a multiple choice reversal task. A) A schematic of the task used in our lab (Johnson and Wilbrecht, 2011). Each color represents an odor. Mice are released from a central cylinder to start a trial. In the discrimination phase mice must learn to dig in the correct odor to receive a food reward. After the mouse completes 8 out of 10 correct trials the reversal phase begins in which a previously unpaired scent predicts the food reward. B) Prepubertal (P26) mice make significantly fewer reversal errors (F3,96=12.00; p<0.01) than mid-adolescent mice (P40) or adult mice (P60–90).
A clear role for pubertal hormones altering executive function has not emerged from the studies reviewed in this section. However, many of these studies did not assess any pubertal measures. Due to the variability in age at pubertal onset, future studies will need to directly assay puberty while including prepubertal subjects. It is also possible that changes in executive function may instead relate to changing experience and accumulation of information, independent of biological effects of puberty. Variation in executive function phenotypes displayed in adolescence and adulthood may also reflect adaptive processes related to cues or resources in the early environment (Nettle and Bateson, 2015). Animal models and experimental manipulations of both experience and puberty will be necessary to untangle the relationships between these variables.
5.7 Effects of stress
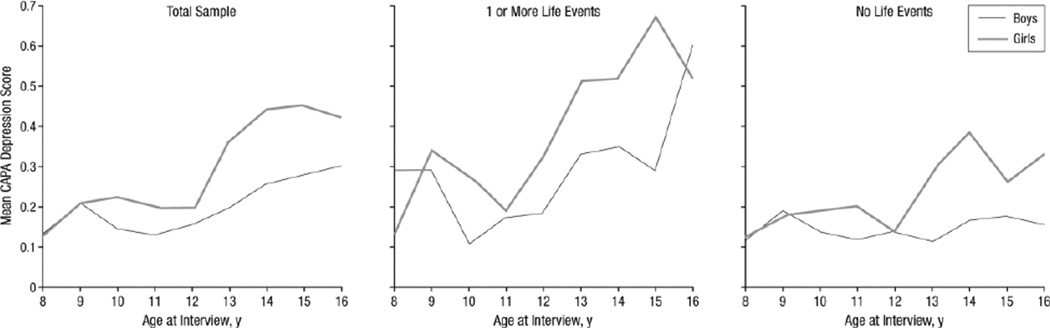
Periods of heightened plasticity can be moments of opportunity for growth, learning, and adaptation, or periods of vulnerability, particularly to stressful experiences. Stressors occurring during sensitive periods may have profound effects on development of brain, cognition, and behavior by altering the organization of neural circuits, which in turn may lead to increased risk for psychopathology and negative health outcomes (Gilbert et al., 2009; Heim and Nemeroff, 2001; Sánchez et al., 2001). Further, early life stress can induce early vaginal opening in female rats (Grassi-Oliveira et al., 2016) and may interact with puberty to exacerbate the rise in psychopathology during early puberty (Fig. 5; Silberg et al., 1999).
Figure 5.
Reprinted from Silberg et al., 1999 without modifications. Cohorts of 1420 boys and 1700 girls were separated by sex and whether they had experienced a stressful-life even within the past year. They found that one or more stressful life-events was associated with a significant increase in depression score starting at age 11 and that stressful life events impacted girls who had started puberty more than prepubertal girls.
Studies assessing the effects of stress during the first weeks of life in rodents indicate that maternal separation or other disruptions to the early environment change neuronal morphology of the frontal cortex (Brenhouse et al., 2013; Bock et al., 2005; Chocyk et al., 2013; Monroy et al., 2010; Muhammad and Kolb, 2011; Yang et al., 2015). These structural changes are accompanied by changes to cognition supported by frontal circuits (Lovic and Fleming, 2004; Thomas et al., 2015; Yang et al., 2015). Interestingly, early life adversity may accelerate brain development (Callaghan et al., 2014; Hostinar and Gunnar, 2013), puberty onset (Belsky et al., 1991; Ellis et al., 1999; Grassi-Oliveira et al., 2016), maturation of fear extinction/recovery behavior (Callaghan and Richardson, 2011), attachment and avoidance learning (Moriceau et al., 2009), reversal learning (Thomas et al., 2015), and maturation of frontal-amygdala connectivity (Gee et al., 2013). This accelerated brain development following early life stress may be an adaptive mechanism that coordinates the development of neural circuits to meet the demands of an adverse environment (Callaghan et al., 2014; Hostinar and Gunnar, 2013).
Heightened responsivity to stress may continue until the period between weaning and mid-puberty in rodents. Some reviews suggest that adolescence, more than early life or adulthood, is a sensitive period for stress-induced programming of the brain (Mccormick et al., 2010), which may contribute to vulnerabilities for developing psychopathologies (Blaustein and Ismail, 2013; Dahl and Gunnar, 2009; Romeo and Mcewen, 2006). Multiple studies indicate that post-weaning social isolation (Powell et al., 2012) leads to reduced dendritic arborization and spine density in frontal cortex (Pascual et al., 2006; Silva-Gómez et al., 2003), changes in cortical dopamine (Novick et al., 2011; Watt et al., 2009; Wright et al., 2008), abnormal activity of pyramidal cells after VTA stimulation (Peters and O’donnell, 2005), and decreased parvalbumin immunoreactivity (Schiavone et al., 2009), which may directly affect plasticity (see section 8). Social isolation from P20–40 in mice disrupts medial PFC function and myelination, but later isolation from P40–60 does not (Makinodan et al., 2012). This may result in stunted social learning from lack of play behavior, which itself peaks between P28-P35 in rats (Meaney and Stewart, 1979; Thor and Holloway, 1984; Vanderschuren et al., 1997).
There is growing evidence that early life stress alters development and perhaps accelerates the development of the brain and body. The onset of puberty may change individuals’ needs and expectations as well as sensitivity to different forms of stress, resulting in different responses to stress between juveniles and adolescents. Future studies that manipulate or assay puberty directly will be needed to resolve the causal role of puberty.
5.8 Effects of drugs with abuse potential
In contrast to puberty’s likely role in closing a critical period for learning or stress responsivity, puberty may open a period of heightened sensitivity to drugs of abuse (Fig. 1). The peripubertal period is a time of exploration outside the home/nest in both humans and rodents, during which animals learn crucial information about the rewards (e.g. food, social contact, drugs) available in their environment. Given that reward circuitry is also developing (Benes et al., 1996; Sinclair et al., 2014; Wahlstrom et al., 2010), it is not surprising that animal models of drug abuse reveal unique patterns of drug-related behaviors (Schramm-Sapyta et al., 2009) and heightened sensitivity to the long-term effects of drug exposure during this period (reviewed below). Pubertal hormones influence a variety of drug-related behaviors in animal models (reviewed below and in Kuhn et al., 2010), suggesting that changes in the timing of puberty may influence peripubertal sensitivity to drugs of abuse.
5.8.1 Effects of pubertal hormones on drug-related behaviors
Pubertal hormones affect or correlate with drug-related behaviors in a variety of animal models. For example, p30–50 female rats show higher levels of cocaine self-administration than p30-p50 male rats, with higher levels of estradiol correlating with higher levels of cocaine self-administration in females (Lynch, 2008). In addition, prepubertal (p25) gonadectomy produces opposite effects on cocaine-induced locomotion in adult (p65) male and female rats, suggesting that pubertal hormones play a role in regulating sex-specific responses to cocaine (Parylak et al., 2008). In the case of nicotine, sex differences in self-administration emerge at the time of puberty (p30–45), with females self-administering more than males and high estradiol to progesterone ratios correlating with higher rates of self-administration in females (Lynch, 2009). For ethanol, pre-pubertal (p23) or adult (p70) gonadectomy does not affect intake in adult female rats, though pre-pubertal gonadectomy increases ethanol preference in adult females (Vetter-O’hagen and Spear, 2011). In contrast, gonadectomy at either timepoint increases both ethanol preference and intake in male rats (Vetter-O’hagen and Spear, 2011), and testosterone replacement in adulthood (p77–93) lowers ethanol preference to control levels (Vetter-O’Hagen et al., 2011). In a separate study involving both sucrose and ethanol consumption, pre-pubertal (p20) gonadectomy increased ethanol consumption in adult male rats but decreased consumption in adult females (Sherrill et al., 2011). Thus, although specific patterns of pubertal drug preference and intake differ between drugs, pubertal hormones regulate drug-related behaviors for a variety of drugs of abuse.
Given the strong relationship between pubertal hormones and drug-related behaviors in animal models, changes in the timing of puberty may influence propensity for drug abuse in humans. For example, estradiol’s activation of drug preference/intake may occur earlier in girls who experience early puberty. Early drug use is consistently associated with higher risk of developing substance use disorders (Chou and Pickering, 1992; Clark et al., 1998; DeWit et al., 2000; Grant and Dawson, 1998; Hawkins et al., 1997; Lynskey et al., 2003; O’Brien and Anthony, 2005; Prescott and Kendler, 1999; SAMHSA, 2013), suggesting that early puberty may further increase risk of drug abuse in already vulnerable populations due to their average earlier puberty onset (Downing and Bellis, 2009).
5.8.2 Peripubertal sensitivity to drug exposure
While pubertal hormones affect propensity for drug self-administration, the peripubertal period also marks a time of unique vulnerability to the long-term effects of drug exposure in a variety of animal models. For example, peripubertal sensitivity to the long-term effects of nicotine, alcohol, and THC on a variety of adult behaviors is discussed extensively in other reviews (Counotte et al., 2011; Goriounova and Mansvelder, 2012; Lisdahl et al., 2013; Malone et al., 2010; Rubino and Parolaro, 2008; Rubino and Parolaro, 2014; Schneider, 2008; Spear, 2015; Witt, 2010). While many studies point to ages surrounding puberty as a vulnerable time for the long-term effects of drug exposure, the extent to which this vulnerability is mediated by pubertal hormones remains largely unknown.
Given that the dopamine system changes markedly during the peripubertal period (Kuhn et al., 2010; Sinclair et al., 2014; Wahlstrom et al., 2010), animals may be primed to organize their reward circuitry in a way that is adapted to the specific environmental contingencies they experience during this period of exploration. Drug-related experiences during this period may therefore impact developmental trajectories in a way that is distinct from the same experiences in childhood or adulthood. To the extent that pubertal hormones regulate the development of reward circuitry (Kuhn et al., 2010; Sinclair et al., 2014), changing the timing of puberty may affect the timing of this sensitive period for the long-term effects of drug exposure.
5.8.3 Peripubertal development of the dopamine system: a unique period of reward exploration and vulnerability to drug abuse
Given the well-established role of dopaminergic function in drug- and other reward-related behaviors, peripubertal changes in drug sensitivity are likely related to peripubertal changes in dopaminergic function (Kuhn et al., 2010). The dopamine system that innervates the frontal association cortex changes in complex ways around puberty, with distinct components showing distinct developmental trajectories (Benes et al., 1996; Kuhn et al., 2010; McCutcheon and Marinelli, 2009; Sinclair et al., 2014; Wahlstrom et al., 2010). For example, dopamine neurons in the ventral tegmental area alter their firing rates in an inverted U-shaped curve that peaks shortly after p40 in male rats (McCutcheon and Marinelli, 2009; McCutcheon et al., 2012). Dopamine receptor expression also peaks around the age of puberty, though only for specific receptor subtypes in specific neuronal populations (reviewed in (Wahlstrom et al., 2010)). For example, a greater number of accumbens-projecting PFC neurons express D1 receptors at p44 compared to p27 and p105 in male rats—a pattern that may directly relate to drug-seeking behavior in adolescent (p44) animals (Brenhouse et al., 2008).
Dopaminergic modulation of both interneurons and pyramidal cells in frontal cortex changes abruptly around the age of puberty. For example, parvalbumin positive (PV+) fast spiking and non-fast spiking interneurons in frontal cortex dramatically increase in excitation in response to the D2 agonist quinpirole between p36 and p50, while non fast spiking interneurons increase in their response to a D1 receptor agonist (Tseng and O’donnell, 2007). In PFC pyramidal neurons, co-application of NMDA and D1 receptor agonists induces plateau depolarizations resembling in vivo up-states in slices from post-pubertal (p45-p65) but not pre-pubertal (p29-p38) rats (Tseng and O’Donnell, 2005). Dopaminergic modulation of cortical projections to striatum also changes across adolescence, with D2 receptor activation exerting opposite effects on striatal responses to stimulation of cortical inputs in early adolescent (p23–38) compared to adult (p50–63) rats (Benoit-Marand and O’Donnell, 2008). Although it is still unknown whether pubertal hormones are necessary and/or sufficient for these changes in neural responses to dopamine receptor activation, these peripubertal alterations in dopamine modulation may have important implications for adolescent patterns of drug-seeking behavior.
While the role of pubertal hormones in many age-related changes in cortical dopamine function has not been tested, several rodent studies point to effects of pubertal estradiol and testosterone on dopaminergic function (reviewed in Becker, 2009; Kuhn et al., 2010; Sinclair et al., 2014). For example, castration or testosterone injections during mid to late adolescence (p45-p60) alter various markers of dopaminergic function in male rats (Purves-Tyson et al., 2014), though gonadal hormones are not necessary for male-specific peaks in striatal dopamine receptor expression (Andersen et al., 2002). While the stimulatory effects of estradiol on dopaminergic function are well-established for adult female rodents (Kuhn et al., 2010), evidence in pubertal females comes largely from the effects of prepubertal ovariectomy on responses to dopaminergic drugs such as cocaine (reviewed above; Parylak et al., 2008). Thus, although scant, literature on the effects of pubertal hormones on the development of the dopamine system suggests that some aspects of dopaminergic function respond to pubertal increases in circulating gonadal hormones.
We know little about the ontogenetic profiles of steroid receptor expression in the ventral tegmental area, but in adult rodents this area expresses steroid receptors in specific spatial and concentration gradients, including progesterone receptor (Frye et al., 2013; Willing and Wagner, 2016), androgen receptor (Kritzer, 1997; Kritzer and Creutz, 2008) and both estrogen receptor alpha and beta ((Creutz and Kritzer, 2002; Kritzer, 1997; Vanderhorst et al., 2005). Many of these cells also express tyrosine hydroxylase, suggesting they produce dopamine. Further, androgen receptor expressing cells in the frontal cortex project to the VTA (Aubele and Kritzer, 2012), providing the potential for puberty to initiate changes in bidirectional regulation of dopaminergic function. If these receptors are expressed during puberty, the rise in gonadal steroids likely significantly alters dopamine function throughout the brain.
Whether mediated by pubertal hormones or by other factors associated with the transition to independence, adolescent development of the dopamine system may facilitate exploration of rewards available in the environment during this time of newfound autonomy. Peripubertal peaks in dopamine neuron firing and dopamine receptor expression suggest a period of enhanced motivation, reinforcement/reward sensitivity, and peripubertal changes in neuronal responses to dopamine receptor activation may be crucial for developing the cognitive strategies appropriate for late adolescent and adult life stages. Determining the extent to which pubertal hormones mediate these changes in dopaminergic function is critical for understanding the impact of early puberty on behaviors associated with reward, exploration, and drug abuse during adolescence.
6 How does the neurobiology of the associative neocortex change during puberty?
Language, reading, mathematics, autobiographical memory, and executive function are presumably regulated, at least in part, by the plasticity of associative cortex. By examining how the various neural systems in neocortex change during development and at the onset of puberty, we hope to develop insight into the mechanisms regulating shifts in behavior and associative neocortex function, while gaining insight into the potential negative health outcomes associated with disruptions to normal pubertal development.
6.1 Gray Matter
In humans, there are significant changes in gray matter volume in associative cortices around the time of puberty (Giedd et al., 2006; Peper et al., 2009b; Herting et al., 2015). In general, brain-wide gray matter increases during early childhood and peaks around the onset of puberty (in frontal cortex of boys and girls peak age is 11–12) before declining gradually to adult levels (Gogtay and Thompson, 2010). In frontal and parietal cortex, this peak occurs around 10–11 years of age in girls, while the peak in boys is shifted about one year later (Giedd et al., 1999), which may relate to the tendency for girls to start puberty earlier than boys (Lenroot et al., 2007). When controlling for age, both pubertal hormones and stage of puberty correlate with changes in gray matter across adolescent development in disparate regions of cortex (De Bellis et al., 2001), including in the frontal cortex (Bramen et al., 2011; Gogtay et al., 2004; Herting et al., 2014; Herting et al., 2015; Koolschijn et al., 2014; Neufang et al., 2009; Peper et al., 2009a; Raznahan et al., 2010). For example, in a longitudinal study in which adolescent girls and boys were scanned once during early puberty and a second time during late puberty, there were effects of rate of change in pubertal status and sex in various subregions of the temporal, frontal, and occipital cortices (Herting et al., 2015). Further, changes in salivary estradiol and testosterone across this time interacted with sex to predict cortical thickness changes in some of these regions (Herting et al., 2015). Another previous study also showed that puberty onset was associated with a significant decrease in frontal and parietal gray matter that was not accounted for by age (Peper et al., 2009b). Thus multiple studies strongly suggest that the rise in pubertal steroids is tied to remodeling of gray matter volume across the associative neocortex.
6.2 White Matter
Measures of adolescent reductions in gray matter volume may be reduced as white matter encroaches and changes the ratio between gray and white matter. White matter volume reflects the myelination of long-range bundles of axons, which dramatically increases the speed of neuronal transmission. Postnatal development in humans is associated with a persistent rise in white matter volume across the brain into adulthood in many white matter tracts (Barnea-Goraly, 2005; Lebel et al., 2008). In addition to age, pubertal status and hormone titers significantly interact with sex to affect white matter volume in multiple tracts in the human brain, including those connecting higher association cortices (Asato et al., 2010; Chavarria et al., 2014; De Bellis et al., 2001; Herting et al., 2012; Peper et al., 2008; Perrin et al., 2008; Perrin et al., 2009).
In rodents, frontal white matter is also affected by age and hormones in both sexes (Markham et al., 2007). Frontal white matter increases in volume in a linear fashion in male rats but jumps in females from P25–35 and then again from p45–60, suggesting specific regulation during the peripubertal period (Willing and Juraska, 2015). In rats, gonadectomy results in no change in white matter volume in male rats but does increase white matter volume in female rats in the frontal cortex (Koss et al., 2015). Further, exposure to ovarian steroids during adolescence reduces the size of the corpus callosum in female rats (Bimonte et al., 2000).
Changes in gray matter and white matter may also reflect changes in the number and composition of cell types and the function of neurons and glia. In animal models and human postmortem tissue, each can be measured directly to better understand which gray matter components are remodeled during puberty.
6.3 Synapses
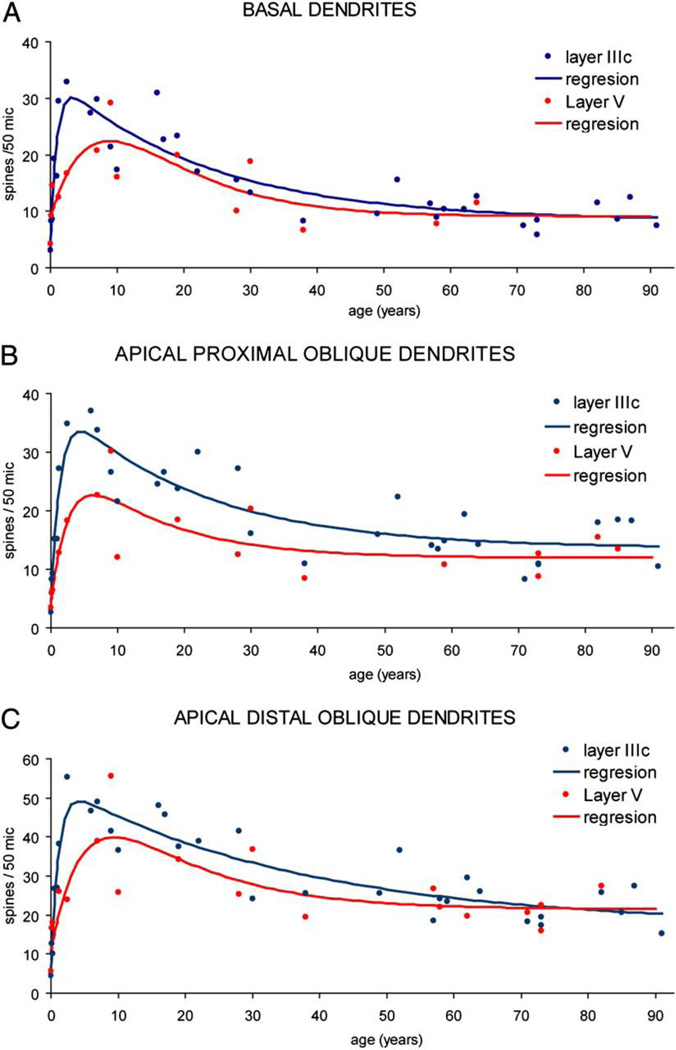
A portion of gray matter volume is made of small dendritic protrusions called dendritic spines. These are the sites of a vast majority of excitatory synapses onto pyramidal cells in the neocortex (Holtmaat and Svoboda, 2009). In humans, there is a rapid increase in synapse formation just after birth, which reaches a plateau in late childhood before declining during adolescence (Huttenlocher, 1979; Petanjek et al., 2011). In the prefrontal cortex, reduction in global synapse density occurs later than in sensory neocortex and declines at its steepest rate around the time of pubertal milestones (Huttenlocher and Dabholkar, 1997). Synaptophysin and PSD-95, markers of excitatory synapses, reach their peak in human cortex ~age 8 (group consisted of 6–10 year olds, mean age 8.3) before declining to adult levels by 18 years of age (Glantz et al., 2007). This general pattern of development has been replicated in greater temporal and spatial resolution in macaques, mice, and rats. Again, the age range in which pubertal milestones are reached is a time of decline in prefrontal cortex synapse density (Anderson et al., 1995; Bourgeois et al., 1994; Drzewiecki et al., 2016; Gourley et al., 2012; Koss et al., 2014). These correlational data suggest that synapse pruning and pubertal milestones may be co-regulated, but some experimental data suggest otherwise. Castration in a very small sample of male monkeys did not clearly impact the general pattern of synapse density decline in the prefrontal principal sulcus (Anderson et al., 1995); however, there were too few samples to draw strong conclusions.
In addition to pruning synapses, neuronal connectivity in the developing neocortex also undergoes a process of stabilization (Holtmaat et al., 2005). In mice, individual dendritic spines can be followed longitudinally using 2-photon in vivo imaging. By repeatedly imaging the same dendritic branch over time, the gains and losses of individual spines can be tracked over many days (Holtmaat et al., 2009). Across the mouse neocortex, rates of spine gain and spine loss (independent of spine density) decrease from peripubertal age (P30) to P60 (Chen et al., 2014; Johnson et al., 2016a; Zuo et al., 2005). Recent evidence suggests regional specificity in this effect. Pattwell et al. (2016) found that dendritic spine formation decreased on pyramidal cells between P30 and P45 in male mice in the medial prefrontal cortex but not the adjacent frontal association cortex. This means juvenile development is characterized by exuberant daily turnover of synaptic structures (Holtmaat et al., 2005; Johnson et al., 2016a; Zuo et al., 2005) that declines during puberty and into adolescence. Daily spine gain and loss in the PFC, sensory, and motor cortices are associated with potential for greater rewiring to support learning and memory (Comeau et al., 2010; Holtmaat et al., 2006; Johnson et al., 2016b; Kasai et al., 2010; Muñoz-Cuevas et al., 2013; Xu et al., 2009; Yang et al., 2010). Lower turnover that comes with age means that neurons come to sample information from fewer potential partners (Stepanyants and Chklovskii, 2005) and exhibit fewer long thin spines that have greater potential to undergo significant Hebbian strengthening (Bourne and Harris, 2007; Kasai et al., 2010). We speculate that greater levels of turnover in the juvenile neocortex allow it to more readily learn or adapt its connectivity to accommodate changing inputs and experience. To our knowledge, there is no published evidence to date that examine the impact of pubertal status or gonadal steroids on in vivo spine dynamics.
6.4 Cell bodies and dendritic arbors
Cell bodies and dendritic arbors also contribute to the overall gray matter volume and therefore may contribute to the age and puberty dependent decline in gray matter. In female rats, the number of neurons and glia in the frontal cortex increases early in postnatal development and then declines to adult levels within a ten day period surrounding puberty onset (Willing and Juraska, 2015). This decline can be eliminated by prepubertal ovariectomy, suggesting that pubertal gonadal steroids induce apoptosis of frontal cortex neurons in females (Koss et al., 2015). There is no age related decline in frontal cortex neuron numbers in males, nor does removing the testes before puberty result in any changes to neuron number (Koss et al., 2015; Willing and Juraska, 2015). This effect also holds true for the number of glial cell bodies in the frontal cortex (Koss et al., 2015). Manipulating ovarian hormone availability has no effect on cell number in the frontal cortex in 12 month old rats, suggesting that the frontal cortex is more sensitive to gonadal steroids earlier in life (Chisholm et al., 2012).
Time lapse imaging studies of dendritic tips in frontal cortex show that dendritic branches of pyramidal neurons are largely stable by P25 in male mice (Johnson et al., 2016a). Dendritic arborization of pyramidal neurons in frontal cortex examined by Golgi declines between P35 and P90 in female rats but not male rats (Koss et al., 2014). A similar pattern is observed in certain subtypes of dendrite in the human prefrontal cortex, which decline in arborization starting between ~10–15 years old, though it is unknown if there is a sex difference (Petanjek et al., 2008).
6.5 Inhibitory and Excitatory neurotransmission
There are also significant shifts in the molecular and electrophysiological underpinnings of excitatory and inhibitory neurotransmission in association cortex during peripubertal development. These changes could significantly alter information processing and plasticity in the frontal cortex.
6.5.1. mRNA and protein expression
Data from tissue punches in the frontal cortex suggest that adolescence is associated with shifts in markers of both excitatory and inhibitory transmission.
In rats, AMPA/Kainate receptor binding sites (Insel et al., 1990) and GluR2 protein (Murphy et al., 2012) begin to decline between ~P24-P30 and P60. NMDA receptor expression also changes during peripubertal development. In humans, mRNA and protein expression of the obligatory subunit of the NMDA receptor, NR1, peaks within the frontal cortex in the 11–15 year old age group before declining until age 21–25 (Catts et al., 2013; Henson et al., 2008).
GABA receptors in frontal cortex also demonstrate peripubertal changes that may influence cortical processing and plasticity. In humans, mRNA for GABA receptor subunits (Duncan et al., 2010; Fillman et al., 2010) and markers of inhibitory cell bodies (Fung et al., 2010) change substantially across postnatal development, with some specific subunits and markers showing either positive or negative inflections around 10 years of age.
6.5.2 Pyramidal neurons
Cell type-specific assays offer a more in-depth understanding of the peripubertal development of frontal cortex. While many measures of excitatory transmission in cortical pyramidal cells do not change across adolescence, some measures of inhibition show marked peripubertal changes (reviewed below).
Functional measures of excitatory AMPA and NMDA mediated currents in frontal cortex pyramidal neurons do not change between the juvenile and adolescent periods in macaques or mice (Gonzalez-Burgos et al., 2008; Vandenberg et al., 2015), in contrast to the reduction in dendritic spines observed at these ages (Johnson et al., 2016a; Zuo et al., 2005). Further, NMDA-mediated current does not change across adolescence in layer 5 pyramidal neurons in rat frontal cortex (Wang et al., 2008). It is possible that AMPA-mediated currents change in non-pyramidal cell types or that changes are occurring on distal dendrites that are not detectable in somatic recordings, which could explain differences in conclusions reached between protein measures and excitatory current measures.
In contrast, inhibition onto pyramidal cells increases between juvenile and adolescent/adult groups in both mice and macaques (Delevich, 2014; Gonzalez-Burgos et al., 2015; Vandenberg et al., 2015). In mice, this increase is dependent on signaling through the TrkB receptor (Vandenberg et al., 2015), which is activated by brain derived neurotrophic factor (BDNF), which itself is known to induce maturation of PV+ interneurons (Huang et al., 1999; Rutherford et al., 1997). BDNF signaling in the cortex is modulated by puberty and directly affected by gonadal steroid availability (Hill et al., 2012), suggesting a possible causal mechanistic link between inhibition and puberty.
In another study of macaques, the GABA alpha 2 subunit declined during postnatal development while the GABA alpha 1 subunit increased in the frontal cortex, resulting in a faster decay of inhibitory currents in pyramidal cells after puberty (Hashimoto et al., 2009). Further, the expression of GABA receptor subunits on layer 3 and 5 pyramidal cells in macaque dlPFC changes substantially across postnatal development, with different subunits reaching maximal levels at ages ranging from perinatal to adult (Datta et al., 2015). These data suggest that peripubertal development is more strongly associated with maturation of inhibition, which implicates changes to inhibition as one mechanism underlying peripubertal changes to cortical plasticity and function.
6.5.3 Interneurons
As reviewed above, inhibition onto pyramidal cells changes during adolescence. It appears that PV+ interneurons may be most affected by puberty onset and thus may underlie the changes in inhibition onto pyramidal cells.
In rats, excitatory postsynaptic currents change across puberty in fast spiking PV+ interneurons (Wang and Gao, 2009). This change is mediated by an increase in AMPA currents onto PV+ interneurons, which, combined with decreased NMDA mediated currents, results in a decreased NMDA/AMPA ratio (Wang and Gao, 2009), suggesting that these neurons are more easily recruited by excitatory input but may be less plastic. Importantly, regular and low threshold spiking interneurons show little modulation of NMDA or AMPA currents with age (Wang and Gao, 2009), suggesting that there is cell type specific regulation of NMDA and AMPA currents during adolescence in frontal cortex. Finally, there is also an increase in calcium-permeable AMPA receptors in adolescent PV+ interneurons in frontal cortex, which is associated with altered short term synaptic plasticity (Wang and Gao, 2010).
In macaque prefrontal cortex, the number of PV+ synapses at the soma increases linearly from early juvenile to adult development (Erickson and Lewis, 2002) while those that cluster on the axon initial segment decrease dramatically ( as visualized by parvalbumin immuno-staining) near macaque puberty onset (Anderson et al., 1995). In mice, the intrinsic electrophysiological properties of PV expressing interneurons in the frontal cortex change significantly during prepubertal development (Yang et al., 2012) and into mid adolescence (P45–49; (Cho et al., 2015)).
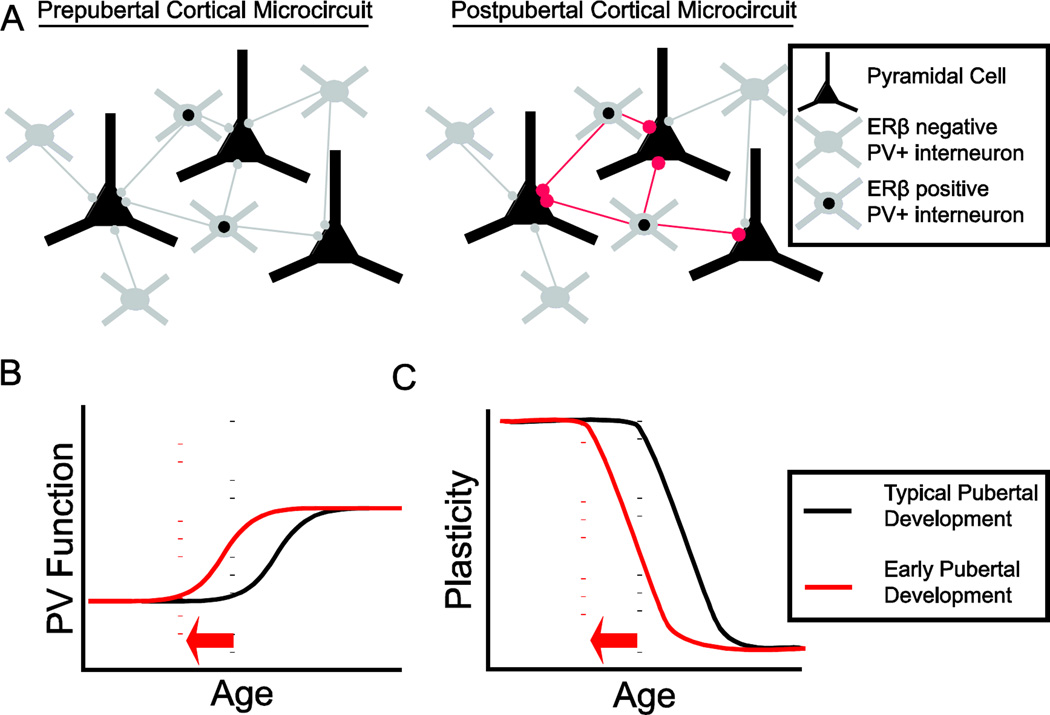
Together, these data highlight the continuing change in cortical circuit function across postnatal development. The maintenance of excitation coupled with the rise in inhibition demonstrated in frontal cortex likely results in altered excitatory to inhibitory balance within frontal networks, which has been associated with regulation of sensitive period plasticity. This shift in inhibition is likely mediated by PV+ interneurons, which are also thought to regulate sensitive period plasticity in sensory cortex (Davis et al., 2015; de Villers-Sidani et al., 2008; Hensch, 2005; Le Magueresse and Monyer, 2013; Werker and Hensch, 2015), and therefore are attractive candidates for pubertal regulation of association cortex plasticity.
7. Steroid Receptor Expression in the Cortex
In order to understand the role of pubertal hormones in association cortex maturation, it is critical to know which steroid hormone receptors are expressed in what cell types during development.
Androgen receptors (AR) are expressed in the cerebral cortex throughout postnatal development, including in frontal regions (McAbee and DonCarlos, 1998; Nunez et al., 2003). There is relatively little known about the peripubertal development of AR expression in cerebral cortex, but one study suggests that AR is expressed in similar density in juvenile (P21), adolescent (P42), and adult (P63) rat cortex (Monbon et al., 1974). In adults AR is expressed across the cerebral cortex, including associative cortex, in both sexes in rodents (Clancy et al., 1992; Dart et al., 2013; DonCarlos et al., 2006; Feng et al., 2010; Kritzer, 2004; Simerly et al., 1990; Young and Chang, 1998), and humans (Bezdickova et al., 2007; Puy et al., 1995; Sarrieau et al., 1990).
In rodents, the two genes that express distinct estrogen receptors, ER alpha and ER beta, are expressed in neocortex, including the frontal cortex (Shughrue et al., 1990). Their postnatal expression patterns suggest interesting and potentially important roles in ontogeny. In neonatal rodents, ERalpha is expressed relatively abundantly in neocortex while ERbeta is mostly absent (Perez et al., 2003; Zsarnovszky and Belcher, 2001). Prior to weaning (~P18), ERalpha expression declines precipitously and shortly after ERbeta rises (Prewitt and Wilson, 2007; Wilson et al., 2011; Zsarnovszky and Belcher, 2001), resulting in higher ERbeta expression in neocortex in adults (Kritzer, 2002; Shughrue et al., 1997), which is localized primarily to inhibitory interneurons (Blurton-Jones and Tuszynski, 2002; Kritzer, 2002). In humans, antibody labeling suggests widespread expression of ERalpha in frontal (Montague et al., 2008) and temporal (Gonzalez et al., 2007) neocortex but one mRNA labeling study found low and diffuse expression of ERalpha mRNA in temporal cortex and restricted expression in deep layers of entorhinal cortex (Osterlund et al., 2000). These studies mirror a study in mice that found that while ERalpha mRNA transcript levels decrease through life, protein expression remains stable (Dietrich et al., 2015). Further studies will be required to understand the extent and ontogeny of estrogen receptor expression in humans and animal models.
The ontogeny of PR expression in the neocortex is less clear. It is unambiguously expressed in the rat frontal cortex at birth and rises in both males and females until it peaks during the second week of life, after which it significantly declines and is maintained at lower levels into adulthood (Hagihara et al., 1992; Kato and Onouchi, 1981; Kato and Onouchi, 1983; Kato et al., 1984; Lopez and Wagner, 2009; Shughrue et al., 1991; Shughrue et al., 1992). Females express more PR than males (Kato et al., 1984; Lopez and Wagner, 2009; Shughrue et al., 1991; Wagner et al., 2001), and it is likely only expressed in neurons (Lopez and Wagner, 2009; Hagihara et al., 1992).
It is difficult to infer the precise pattern of PR expression in post-weaning to adult aged animals due to the literature’s poor age resolution and a diversity of techniques. However, a general theme in the literature is that PR binding decreases before weaning and is maintained at mid to low levels into adulthood (Kato and Onouchi, 1981; Kato and Onouchi, 1983; Kato et al., 1984; Parsons et al., 1982); this general pattern emerges in mRNA experiments as well (Hagihara et al., 1992; Intlekofer and Petersen, 2011). However, in one study of mRNA from homogenized tissue, differential developmental expression patterns of the two PR isoforms (A and B) was seen. They found that expression of the B isoform peaked at P8 and then declined to basal values before weaning, while the A isoform rose, resulting in total PR expression in the cortex remaining into adulthood (Kato et al., 1993).
Canonically, steroids influence cells by activating their cognate receptors that act as transcription factors to alter gene expression. However, it is important to note that steroids can also signal via non-canonical, non-genomic means, both through the familiar nuclear receptors and through novel receptor signaling. Canonical and non-canonical AR, ER, and PR, are expressed on neuronal membranes and in glial cells(Almey et al., 2014; Brinton et al., 2008; Foradori et al., 2008; Mermelstein and Micevych, 2008; Petersen et al., 2013). AR and both ERs are expressed sparsely in axons and dendrites in the frontal cortex, including on both pre and postsynaptic sites (DonCarlos et al., 2003; Wang et al., 2010) where they may directly alter synaptic signaling.
Unfortunately, data describing developmental changes in the various steroid receptors in cortex during peripubertal development is relatively sparse, particularly in association cortex. Further, we do not fully understand the role these receptors play in cortical physiology. However, the literature is sufficient to reasonably conclude the following: 1. Steroid receptors are expressed widely across the cortex, including association cortex. 2. Cortical expression of these receptors is developmentally regulated, suggesting changing function with development. 3. A complement of steroid receptors are present in association cortex (including frontal areas) during peripubertal/adolescent development. This suggests that the rise in gonadal steroids at puberty can locally influence development of association cortices.
8 Proposed Model
Given the diversity of steroid signaling mechanisms present in association cortex during development, the potential for the rise in gonadal steroids at puberty to alter associative cortex circuit function is strong. However, as we’ve discussed, very few studies have attempted to causally relate the pubertal rise in gonadal steroids to cortical sensitive period plasticity. We hypothesize that pubertal steroids may influence the timing and/or duration of the juvenile sensitive period in cortical circuits, primarily through their modulation of PV+ inhibitory interneurons. The following model is speculative and simplified, but provides a framework to guide future studies. Our three main lines of evidence (discussed in greater detail below) are: 1) estrogen receptors specifically localize to PV+ interneurons 2) PV+ interneurons regulate sensitive period plasticity in sensory cortices and 3) inhibitory neurotransmission in frontal association cortex increases during puberty.
Estrogen receptors in frontal cortex are specifically localized to PV+ interneurons: ERbeta, the predominant nuclear estrogen receptor expressed in cortex during puberty and adulthood (Kritzer, 2002; Pérez et al., 2003; Shughrue et al., 1998), is expressed almost exclusively in PV+ interneurons. Across various areas of cortex, including the frontal, parietal, and insular cortices, about 96% of cells that express ERbeta are PV+ interneurons, and this represents between 15–27% of total PV+ interneurons (Blurton-Jones and Tuszynski, 2002; Kritzer, 2002). It is important to note that both males and females show this pattern of estrogen receptor expression (Kritzer, 2002); Testosterone may be converted to estradiol via the aromatase enzyme, which is present in the frontal cortex (Akther et al., 2015; Mitra et al., 2015) or the testosterone metabolite, dihydrotestosterone, may be converted to 3beta-diol, which itself can activate ERbeta (Handa et al., 2008). Thus, the rise in either androgens or estrogens at puberty in both sexes may activate frontal cortex ERbeta where it may act as a transcription factor (Heldring et al., 2007) to alter gene expression that may then regulate the physiological properties of PV+ interneurons and change network inhibition.
PV+ interneurons are implicated in sensitive period plasticity: Increasing inhibition from PV+ interneurons is implicated in regulating sensitive period plasticity in primary sensory cortices (Davis et al., 2015; Heimel et al., 2011; Hensch, 2005; Le Magueresse and Monyer, 2013; Werker and Hensch, 2015). In rodents, the age and timing of sensitive periods varies substantially throughout the cortex; in auditory and somatosensory cortex the sensitive period closes prior to the third week of life (de Villers-Sidani et al., 2007; Wen and Barth, 2011) while the sensitive period for ocular dominance plasticity in rodents closes after puberty onset (Gordon and Stryker, 1996). This suggests that the age and timing of sensitive period plasticity varies substantially throughout the cortex, and while mechanisms underlying these sensitive periods are similar, their triggers, and thus timing, vary substantially. In mice, puberty may overlap with the decline in ocular dominance plasticity, but this is not true for many other species, including ferrets (Issa et al., 1999) monkeys (LeVay et al., 1980), and humans (Sengpiel, 2014). Further, early exposure to testosterone in kittens does not close ocular dominance plasticity (Daw et al., 1987). Thus, although frontal and visual cortices may share common sensitive period plasticity mechanisms involving PV+ interneurons, we only propose a role for pubertal hormones in regulating sensitive periods in associative neocortex.
Inhibition increases peripubertally in the frontal cortex: In the frontal association cortex, measures of inhibitory neurotransmission onto pyramidal neurons increases from P21–25 to P40–50 (Vandenberg et al., 2015), and inhibitory current amplitudes increase around puberty in rhesus macaque dlPFC (Gonzalez-Burgos et al., 2015). Given that PV+ axons preferentially target cell bodies (Hu et al., 2014), these increases in inhibition observed in recordings from pyramidal cell bodies may be mediated by GABA release originating from PV+ interneurons. Further, these cells are recruited differently after puberty. AMPA currents increase substantially during early puberty in PV+ interneurons (Wang and Gao, 2009), while the NMDA/AMPA ratio decreases, suggesting that these neurons may be more readily recruited, but less plastic, after puberty onset. In these same cells, activation of D2 receptors enhances excitability, but only after puberty (Tseng and O’donnell, 2007). Collectively, these changes could increase inhibition in association cortex and mediate transient enhancement and developmental reduction of plasticity (Dorrn et al., 2010; Froemke et al., 2007; Gandhi et al., 2008; Hensch, 2005; Southwell et al., 2010) in a manner similar to sensory cortex (Levelt and Hubener, 2012) . Changes in inhibitory neurotransmission of PV+ cells can potentially be explained by genomic alterations within PV+ interneurons themselves, which may feasibly be linked to activation of ERbeta receptors on PV+ interneurons.
Our model is primarily motivated by the fact that gonadal steroids appear at the right place and time to affect cortical sensitive period plasticity and that these cells exhibit the clearest cell specific changes around puberty onset. While steroid receptor expression is sparse in cortex compared to subcortical regions, PV+ interneuron axons branch extensively (Karube, 2004) and provide dense inhibition to nearby pyramidal neurons in cortical circuits (Packer and Yuste, 2011). This means that steroidal modulation of even a small portion of these neurons could exert large effects over the function of neocortex (Fig. 7). In all, this model has yet to be tested, but based on reports of steroid receptor expression we can surmise that PV+ interneurons are very likely to be affected by the pubertal rise in circulating steroids, which could affect pubertal changes to cortical function.
Figure 7.
Model for the mechanism by which pubertal hormones may increase inhibition in the frontal cortex and close the sensitive period. This model is adapted from work conducted in primary sensory cortices implicating PV expressing interneurons in regulation of sensitive period plasticity. A) Before puberty circulating gonadal steroids are low. At puberty onset, circulating gonadal steroids rise, and estradiol (either produced in the ovaries or locally synthesized from circulating testosterone) binds to estrogen receptor beta (ERβ), which is expressed in a subset of PV+ interneurons. Estrogen receptors beta then acts as a transcription factor to increase these cells’ inhibitory output (red axons and terminals). Because of their dense connectivity, increased inhibition from a minority of PV+ interneurons could affect all local pyramidal cells. B) In this model, early puberty results in earlier increases in PV function and C) premature closure of juvenile plasticity.
Further, it is important to note that pubertal changes in frontal cortex physiology likely rely on the complex interaction of the activation of all steroid receptors present in the cortex at puberty, including ER, AR, and PR. It is also important to appreciate that steroid derivatives can act as neuromodulators; progesterone and androgen metabolites, all of which can be synthesized in the brain, are known to regulate such diverse systems as GABA and NMDA neurotransmission, myelination, and neurite growth (Compagnone and Mellon, 2000), many of which are independent of steroid receptor binding.
The role of pubertal hormones on frontal cortex development resists a simple explanation. However, the specific localization of ERbeta on a cell type that is in flux during puberty and regulates circuit plasticity is a strong candidate system by which puberty may affect some of the behavioral and phenomenological effects on plasticity reviewed above.
9 Does early pubertal maturation negatively affect adolescent cognitive and neural development?
Many neural systems shift their developmental trajectories around the time of puberty onset, but only a handful of studies have sought to causally relate the rise in pubertal steroids with these changes to development. Thus, it is impossible to determine how much of a causal role puberty plays in sensitive period plasticity and the development of associative cortex. This lack of knowledge is troubling because age at puberty onset is advancing (reviewed above), and there is reason to believe that early puberty onset may negatively impact brain development and adult outcomes.
Furthermore, the age at puberty onset may titrate the impact of gonadal steroids on brain development. Sisk and colleagues have demonstrated that the juvenile to adolescent transition is a sensitive period for steroid-induced organization of hypothalamic and limbic circuitry that regulates basic motivated behaviors, including sex and aggression (Schulz-Wilson et al., 2002; Schulz et al., 2004; Schulz et al., 2009a). They have proposed that during postnatal development, the brain gradually becomes less sensitive to the organizing effects of circulating steroids, such that earlier puberty may have greater effects on neural circuits than on-time or late puberty (Fig. 8). For example, they found that male hamsters implanted with testosterone earlier than normal puberty expressed stronger sexual behaviors than hamsters exposed to testosterone at the appropriate time (Schulz et al., 2009b). Gonadectomized hamsters implanted with testosterone later than normal puberty also displayed reduced adult sexual behavior. Together, these data suggest that the timing of puberty onset may result in long lasting effects on hormone sensitive neural circuits (Schulz et al., 2009a).
Figure 8.
Reprinted from Schulz et al., 2009 without modification. In this schematic, put forward by Schulz et al., 2009, the brain’s sensitivity to the organizing effects of gonadal steroids declines continuously across postnatal development and into adulthood. In this case, altering the age at the pubertal rise in gonadal steroids would alter the extent of organization of neural circuits at puberty. These data are supported by behavioral studies that show sexually dimorphic male behaviors are more strongly expressed in males treated with androgens earlier than typical puberty compared to those treated with androgens later. It is unknown if the same declining sensitivity to hormones manifests in the neocortex, but if it does, early puberty may exaggerate organization of cortical regions sensitive to steroids. This provides a biological mechanism by which early puberty could impact sensitivity to the development of psychopathology or cognitive dysfunction at puberty.
It is unknown if the associative neocortex demonstrates a similar age-related decline in sensitivity to gonadal steroids, but early puberty is associated with increased risk for depression, anxiety, negative self-body image, drug use, and symptoms of eating disorders, conduct disorder, and schizophrenia (Burt, 2006; Copeland et al., 2010; Ge et al., 2003; Graber et al., 2004; Kaiser and Gruzelier, 1996; Kaiser and Gruzelier, 1999; Obeidallah et al., 2009; Tschann et al., 1994; Whittle et al., 2012; Zehr et al., 2007), suggesting that early exposure to gonadal steroids may affect cortical development differently than on-time exposure. Earlier maturation itself may also be an adaptive developmental response to cues or stress experienced in the early environment (Hostinar and Gunnar, 2013; Thomas et al., 2015)
Language, reading, mathematics, autobiographical memory, and executive function are complex processes resulting from the interactions of a dizzying array of neural systems in association cortex and connected brain regions. If only a portion of their constituent inputs are sensitive to hormones, then advancing puberty may change the timing and magnitude of development while some other non-hormone sensitive neural systems lag behind. Without knowing which systems are hormone sensitive and how the various systems interact, we will be unable to predict how altered pubertal timing affects associative circuit function. Further, if hormones induce larger effects at earlier ages, then hormone sensitive systems may have exaggerated responses compared to non-hormone responsive systems, possibly altering the balance of function in neural circuits for life. Differences of this kind may explain changes in functional phenotype and vulnerability to disease.
10 Conclusion
It is clear from our survey of the literature that 1) there are dramatic changes in the function of the associative neocortex at ages overlapping with pubertal milestones, and 2) these changes are accompanied by neurobiological changes in the associative neocortex itself. Some studies link these processes and changes to pubertal steroids, while others do not find relationships. The large majority of studies, however, do not manipulate puberty or assess pubertal markers. Our survey of the literature suggests PV+ interneurons are a promising candidate mediator of pubertal effects on cortical development based on their expression of steroid receptors, significant changes during peripubertal development, and their established role in regulating sensitive period plasticity in primary sensory cortices.
The onset of puberty in both humans and animal models is extremely variable, so cross-sectional studies grouping subjects by age lack the resolution to identify links with pubertal development. In order to clarify if and how gonadal steroids affect peripubertal cortical maturation, it will be necessary to use animal models to experimentally manipulate exposure to hormones during development. In humans, studies using gonadal steroid assays and tanner stage determination will be critical for linking the myriad peripubertal changes to brain and behavior with puberty onset. Further, increased emphasis on conducting longitudinal studies with collection of somatic, neural, and hormonal measures will allow researchers to much more strongly delineate puberty-dependent from puberty-independent maturation and avoid errors associated with inferring longitudinal developmental profiles from cross-sectional data (Kraemer et al., 2000). With greater investment in both manipulation and measurement of gonadal steroids, we will better understand the unique role that puberty onset plays in adolescent neocortical development. This question is particularly urgent given clear evidence that the age of puberty onset is advancing in developed nations, and the possible connections between earlier puberty, decreased plasticity, and psychopathology.
Figure 3.
Reprinted from Aksglaede et al. (2009) without modifications. Here, onset of breast development (Tanner breast stage 2) and menarche were collected by pediatricians. Mean age at onset of breast development advanced by 1 year (mean age 10.88 vs 9.86) while mean age at menarche advanced by approximately four months (mean ages of 13.42 vs 13.13). These differences remained significant even after adjustment for BMI.
Figure 6.
Reprinted from Petanjek et al., 2011 without modification. These data were collected from human post-mortem samples of dorsolateral prefrontal cortex in both males and females. Dendritic spines on both the basal (Fig 6A) and the proximal (Fig 6B) and distal (Fig 6C) apical dendrites rise steadily throughout the first decade of life in post-mortem samples of human brain before gradually declining in density in the frontal cortex. This decline began at peripubertal age ranges in these mixed sex samples.
Highlights.
Juvenile and adolescent is demarcated by puberty, a major life-history event.
Puberty may close a sensitive period for enhanced plasticity in associative cortex.
PV+ interneurons may mediate effect of pubertal hormones on cortical plasticity.
Acknowledgments
We thank Silvia Bunge, Jessica Church-Lang, Ron Dahl, Julianna Deardorff, Robert Froemke, Sunil Gandhi, Alison Gopnik, Takao Hensch, Richard Kramer, Lance Kriegsfeld, Jen Pfeifer, Judy Stamps, Ahna Suleiman, Nim Tottenham, and Irving Zucker for discussion. This work was supported by NIH (R01MH087542 and R01DA029150) and the Center on the Developing Adolescent at UC Berkeley.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed ML, Ong KK, Dunger DB. Childhood obesity and the timing of puberty. Trends Endocrinol Metab. 2009;20:237–242. doi: 10.1016/j.tem.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Aksglaede L, Sørensen K, Petersen JH, Skakkebaek NE, Juul A. Recent decline in age at breast development: the Copenhagen Puberty Study. Pediatrics. 2009;123:e932–e939. doi: 10.1542/peds.2008-2491. [DOI] [PubMed] [Google Scholar]
- Akther S, Huang Z, Liang M, Zhong J, Fakhrul AA, Yuhi T, Lopatina O, Salmina AB, Yokoyama S, Higashida C, Tsuji T, Matsuo M, Higashida H. Paternal Retrieval Behavior Regulated by Brain Estrogen Synthetase (Aromatase) in Mouse Sires that Engage in Communicative Interactions with Pairmates. Front Neurosci. 2015;9:450. doi: 10.3389/fnins.2015.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almey A, Cannell E, Bertram K, Filardo E, Milner TA, Brake WG. Medial Prefrontal Cortical Estradiol Rapidly Alters Memory System Bias in Female Rats: Ultrastructural Analysis Reveals Membrane-Associated Estrogen Receptors as Potential Mediators. Endocrinology. 2014;155:4422–4432. doi: 10.1210/en.2014-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Thompson AP, Krenzel E, Teicher MH. Pubertal changes in gonadal hormones do not underlie adolescent dopamine receptor overproduction. Psychoneuroendocrinology. 2002;27:683–691. doi: 10.1016/s0306-4530(01)00069-5. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Classey JD, Condé F, Lund JS, Lewis DA. Synchronous development of pyramidal neuron dendritic spines and parvalbumin-immunoreactive chandelier neuron axon terminals in layer III of monkey prefrontal cortex. Neuroscience. 1995;67:7–22. doi: 10.1016/0306-4522(95)00051-j. [DOI] [PubMed] [Google Scholar]
- Anderson SE, Must A. Interpreting the continued decline in the average age at menarche: results from two nationally representative surveys of U.S. girls studied 10 years apart. The Journal of pediatrics. 2005;147:753–760. doi: 10.1016/j.jpeds.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Asato MR, Terwilliger R, Woo J, Luna B. White Matter Development in Adolescence: A DTI Study. Cerebral Cortex. 2010;20:2122–2131. doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubele T, Kritzer MF. Androgen influence on prefrontal dopamine systems in adult male rats: localization of cognate intracellular receptors in medial prefrontal projections to the ventral tegmental area and effects of gonadectomy and hormone replacement on glutamate-stimulated extracellular dopamine level. Cereb Cortex. 2012;22:1799–1812. doi: 10.1093/cercor/bhr258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Goraly N. White Matter Development During Childhood and Adolescence: A Cross-sectional Diffusion Tensor Imaging Study. Cerebral Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Becker JB. Sexual differentiation of motivation: a novel mechanism? Horm Behav. 2009;55:646–654. doi: 10.1016/j.yhbeh.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Steinberg L, Draper P. Childhood experience, interpersonal development, and reproductive strategy: and evolutionary theory of socialization. Child Dev. 1991;62:647–670. doi: 10.1111/j.1467-8624.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- Belsky J. The Development of Human Reproductive Strategies. Directions in Psychological Science. 2012;21:310–316. [Google Scholar]
- Benes FM, Vincent SL, Molloy R, Khan Y. Increased interaction of dopamine-immunoreactive varicosities with GABA neurons of rat medial prefrontal cortex occurs during the postweanling period. Synapse. 1996;23:237–245. doi: 10.1002/(SICI)1098-2396(199608)23:4<237::AID-SYN1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Benoit-Marand M, O’Donnell P. D2 dopamine modulation of corticoaccumbens synaptic responses changes during adolescence. Eur J Neurosci. 2008;27:1364–1372. doi: 10.1111/j.1460-9568.2008.06107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezdickova M, Molikova R, Bebarova L, Kolar Z. Distribution of nuclear receptors for steroid hormones in the human brain: a preliminary study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2007;151:69–71. doi: 10.5507/bp.2007.012. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Mack CM, Stavnezer AJ, Denenberg VH. Ovarian hormones can organize the rat corpus callosum in adulthood. Brain research Developmental brain research. 2000;121:169–177. doi: 10.1016/s0165-3806(00)00043-2. [DOI] [PubMed] [Google Scholar]
- Biro F, Galvez M, Greenspan L, Succop… P. Pubertal assessment method and baseline characteristics in a mixed longitudinal study of girls. Pediatrics. 2010 doi: 10.1542/peds.2009-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro FM, Greenspan LC, Galvez MP, Pinney SM, Teitelbaum S, Windham GC, Deardorff J, Herrick RL, Succop PA, Hiatt RA, Kushi LH, Wolff MS. Onset of Breast Development in a Longitudinal Cohort. PEDIATrics. 2013;132:1019–1027. doi: 10.1542/peds.2012-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial Frontal Cortex Mediates Perceptual Attentional Set Shifting in the Rat. J. Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank M. Six Essential Skills that will turn your Child into a READER. Hoboke, NJ: Wiley; 2006. The Reading Remedy. Vol. [Google Scholar]
- Blaustein JD, Ismail N. Enduring influence of pubertal stressors on behavioral response to hormones in female mice. Hormones and behavior. 2013;64:390–398. doi: 10.1016/j.yhbeh.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blurton-Jones M, Tuszynski MH. Estrogen receptor-beta colocalizes extensively with parvalbumin-labeled inhibitory neurons in the cortex, amygdala, basal forebrain, and hippocampal formation of intact and ovariectomized adult rats. J Comp Neurol. 2002;452:276–287. doi: 10.1002/cne.10393. [DOI] [PubMed] [Google Scholar]
- Bock J, Sellen DW. Childhood and the evolution of the human life course : An introduction. Human nature (Hawthorne, NY) 2002;13:153–159. doi: 10.1007/s12110-002-1006-5. [DOI] [PubMed] [Google Scholar]
- Bock J, Gruss M, Becker S, Braun K. Experience-induced changes of dendritic spine densities in the prefrontal and sensory cortex: correlation with developmental time windows. Cerebral cortex (New York, NY : 1991) 2005;15:802–808. doi: 10.1093/cercor/bhh181. [DOI] [PubMed] [Google Scholar]
- Boswell HB. Normal Pubertal Physiology in Females. 2014:7–30. [Google Scholar]
- Bottjer SW, Hewer SJ. Castration and antisteroid treatment impair vocal learning in male zebra finches. J Neurobiol. 1992;23:337–353. doi: 10.1002/neu.480230402. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cerebral cortex (New York, NY : 1991) 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? Current Opinion in Neurobiology. 2007;17:381–386. doi: 10.1016/j.conb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Bramen JE, Hranilovich JA, Dahl RE, Forbes EE, Chen J, Toga AW, Dinov ID, Worthman CM, Sowell ER. Puberty Influences Medial Temporal Lobe and Cortical Gray Matter Maturation Differently in Boys Than Girls Matched for Sexual Maturity. Cerebral Cortex. 2011;21:636–646. doi: 10.1093/cercor/bhq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. J Neurosci. 2008;28:2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Lukkes JL, Andersen SL. Early life adversity alters the developmental profiles of addiction-related prefrontal cortex circuitry. Brain Sci. 2013;3:143–158. doi: 10.3390/brainsci3010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J. Progesterone receptors: form and function in brain. Front Neuroendocrinol. 2008;29:313–339. doi: 10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg U, Wiehler A, Peters J. Episodic Future Thinking Is Related to Impulsive Decision Making in Healthy Adolescents. Child Development. 2015;86:1458–1468. doi: 10.1111/cdev.12390. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JDE. Immature Frontal Lobe Contributions to Cognitive Control in Children: Evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, McGue M, DeMarte JA, Krueger RF, Iacono WG. Timing of menarche and the origins of conduct disorder. Arch. Gen. Psychiatry. 2006;63:890–896. doi: 10.1001/archpsyc.63.8.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BL, Richardson R. Maternal separation results in early emergence of adult-like fear and extinction learning in infant rats. Behavioral Neuroscience. 2011;125:20–28. doi: 10.1037/a0022008. [DOI] [PubMed] [Google Scholar]
- Callaghan BL, Sullivan RM, Howell B, Tottenham N. The international society for developmental psychobiology Sackler symposium: early adversity and the maturation of emotion circuits--a cross-species analysis. Developmental Psychobiology. 2014;56:1635–1650. doi: 10.1002/dev.21260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catts VS, Fung SJ, Long LE, Joshi D, Vercammen A, Allen KM, Fillman SG, Rothmond DA, Sinclair D, Tiwari Y, Tsai S-Y, Weickert TW, Shannon Weickert C. Rethinking schizophrenia in the context of normal neurodevelopment. Frontiers in Cellular Neuroscience. 2013;7:1–27. doi: 10.3389/fncel.2013.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarria MC, Sanchez FJ, Chou YY, Thompson PM, Luders E. Puberty in the corpus callosum. Neuroscience. 2014;265:1–8. doi: 10.1016/j.neuroscience.2014.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehab FF, Mounzih K, Lu R, Lim ME. Early onset of reproductive function in normal female mice treated with leptin. Science. 1997;275:88–90. doi: 10.1126/science.275.5296.88. [DOI] [PubMed] [Google Scholar]
- Chelune GJ, Baer RA. Developmental norms for the wisconsin card sorting test. Journal of Clinical and Experimental Neuropsychology. 1986;8:219–228. doi: 10.1080/01688638608401314. [DOI] [PubMed] [Google Scholar]
- Chen C-C, Lu J, Zuo Y. Spatiotemporal dynamics of dendritic spines in the living brain. Frontiers in neuroanatomy. 2014;8:28. doi: 10.3389/fnana.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier N, Huber KL, Wiebe SA, Espy KA. Qualitative change in executive control during childhood and adulthood. Cognition. 2013;128:1–12. doi: 10.1016/j.cognition.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm NC, Packard AR, Koss WA, Juraska JM. The effects of long-term treatment with estradiol and medroxyprogesterone acetate on tyrosine hydroxylase fibers and neuron number in the medial prefrontal cortex of aged female rats. Endocrinology. 2012;153:4874–4882. doi: 10.1210/en.2012-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KKA, Hoch R, Lee AT, Patel T, Rubenstein JLR, Sohal VS. Gamma rhythms link prefrontal interneuron dysfunction with cognitive inflexibility in Dlx5/6(+/−) mice. Neuron. 2015;85:1332–1343. doi: 10.1016/j.neuron.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chocyk A, Bobula B, Dudys D, Przyborowska A, Majcher-Maslanka I, Hess G, Wedzony K. Early-life stress affects the structural and functional plasticity of the medial prefrontal cortex in adolescent rats. Eur J Neurosci. 2013;38:2089–2107. doi: 10.1111/ejn.12208. [DOI] [PubMed] [Google Scholar]
- Chou SP, Pickering RP. Early onset of drinking as a risk factor for lifetime alcohol-related problems. Br J Addict. 1992;87:1199–1204. doi: 10.1111/j.1360-0443.1992.tb02008.x. [DOI] [PubMed] [Google Scholar]
- Christakou A, Brammer M, Rubia K. Maturation of limbic corticostriatal activation and connectivity associated with developmental changes in temporal discounting. NeuroImage. 2011;54:1344–1354. doi: 10.1016/j.neuroimage.2010.08.067. [DOI] [PubMed] [Google Scholar]
- Chu S, Downes J. Long live Proust: The odour-cued autobiographical memory bump. Cognition. 2000 doi: 10.1016/s0010-0277(00)00065-2. [DOI] [PubMed] [Google Scholar]
- Clancy AN, Bonsall RW, Michael RP. Immunohistochemical labeling of androgen receptors in the brain of rat and monkey. Life Sci. 1992;50:409–417. doi: 10.1016/0024-3205(92)90375-y. [DOI] [PubMed] [Google Scholar]
- Clark BR, Price EO. Sexual maturation and fecundity of wild and domestic Norway rats (Rattus norvegicus) Journal of reproduction and fertility. 1981;63:215–220. doi: 10.1530/jrf.0.0630215. [DOI] [PubMed] [Google Scholar]
- Clark DB, Kirisci L, Tarter RE. Adolescent versus adult onset and the development of substance use disorders in males. Drug Alcohol Depend. 1998;49:115–121. doi: 10.1016/s0376-8716(97)00154-3. [DOI] [PubMed] [Google Scholar]
- Cohen JR, Asarnow RF, Sabb FW, Bilder RM, Bookheimer SY, Knowlton BJ, Poldrack RA. A unique adolescent response to reward prediction errors. Nat Neurosci. 2010;13:669–671. doi: 10.1038/nn.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comeau WL, McDonald RJ, Kolb BE. Learning-induced alterations in prefrontal cortical dendritic morphology. Behav Brain Res. 2010;214:91–101. doi: 10.1016/j.bbr.2010.04.033. [DOI] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- Copeland W, Shanahan L, Miller S, Costello EJ, Angold A, Maughan B. Outcomes of early pubertal timing in young women: a prospective population-based study. The American journal of psychiatry. 2010;167:1218–1225. doi: 10.1176/appi.ajp.2010.09081190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counotte DS, Smit AB, Pattij T, Spijker S. Development of the motivational system during adolescence, and its sensitivity to disruption by nicotine. Dev Cogn Neurosci. 2011;1:430–443. doi: 10.1016/j.dcn.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutz LM, Kritzer MF. Estrogen receptor-beta immunoreactivity in the midbrain of adult rats: regional, subregional, and cellular localization in the A10, A9, and A8 dopamine cell groups. J Comp Neurol. 2002;446:288–300. doi: 10.1002/cne.10207. [DOI] [PubMed] [Google Scholar]
- Crone EA, Donohue SE, Honomichl R, Wendelken C, Bunge SA. Brain Regions Mediating Flexible Rule Use during Development. J. Neurosci. 2006;26:11239–11247. doi: 10.1523/JNEUROSCI.2165-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Zanolie K, Van Leijenhorst L, Westenberg MP, Rombouts SARB. Neural mechanisms supporting flexible performance adjustment during development. Cognitive, Affective, & Behavioral Neuroscience. 2008;8:165–177. doi: 10.3758/cabn.8.2.165. [DOI] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat Rev Neurosci. 2012;13:636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Gunnar MR. Heightened stress responsiveness and emotional reactivity during pubertal maturation: implications for psychopathology. Dev Psychopathol. 2009;21:1–6. doi: 10.1017/S0954579409000017. [DOI] [PubMed] [Google Scholar]
- Dallison A, Kolb B. Recovery from infant medial frontal cortical lesions in rats is reversed by cortical lesions in adulthood. Behavioural brain research. 2003;146:57–63. doi: 10.1016/j.bbr.2003.09.026. [DOI] [PubMed] [Google Scholar]
- Dart DA, Waxman J, Aboagye EO, Bevan CL. Visualising androgen receptor activity in male and female mice. PLoS One. 2013;8:e71694. doi: 10.1371/journal.pone.0071694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta D, Arion D, Lewis DA. Developmental Expression Patterns of GABAA Receptor Subunits in Layer 3 and 5 Pyramidal Cells of Monkey Prefrontal Cortex. Cereb Cortex. 2015;25:2295–2305. doi: 10.1093/cercor/bhu040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson MC, Amso D, Anderson LC, Diamond A. Development of cognitive control and executive functions from 4 to 13 years: Evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44:2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MF, Figueroa Velez DX, Guevarra RP, Yang MC, Habeeb M, Carathedathu MC, Gandhi SP. Inhibitory Neuron Transplantation into Adult Visual Cortex Creates a New Critical Period that Rescues Impaired Vision. Neuron. 2015;86:1055–1066. doi: 10.1016/j.neuron.2015.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw NW, Baysinger KJ, Parkinson D. Increased levels of testosterone have little effect on visual cortex plasticity in the kitten. Journal of Neurobiology. 1987;18:141–154. doi: 10.1002/neu.480180203. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM. Sex differences in brain maturation during childhood and adolescence. Cerebral cortex (New York, NY : 1991) 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- De Luca C, Wood S, Anderson V, Buchanan J, Proffitt T, Mahony K, Pantelis C. Normative data from the Cantab. I: Development of executive function over the lifespan. Journal of Clinical and Experimental Neuropsychology. 2003;25:242–254. doi: 10.1076/jcen.25.2.242.13639. [DOI] [PubMed] [Google Scholar]
- de Villers-Sidani E, Chang EF, Bao S, Merzenich MM. Critical period window for spectral tuning defined in the primary auditory cortex (A1) in the rat. J Neurosci. 2007;27:180–189. doi: 10.1523/JNEUROSCI.3227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villers-Sidani E, Simpson KL, Lu YF, Lin RC, Merzenich MM. Manipulating critical period closure across different sectors of the primary auditory cortex. Nat Neurosci. 2008;11:957–965. doi: 10.1038/nn.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Water E, Cillessen AH, Scheres A. Distinct age-related differences in temporal discounting and risk taking in adolescents and young adults. Child Dev. 2014;85:1881–1897. doi: 10.1111/cdev.12245. [DOI] [PubMed] [Google Scholar]
- Deardorff J, Hayward C, Wilson KA, Bryson S, Hammer LD, Agras S. Puberty and gender interact to predict social anxiety symptoms in early adolescence. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2007;41:102–104. doi: 10.1016/j.jadohealth.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deardorff J, Cham H, Gonzales NA, White RM, Tein JY, Wong JJ, Roosa MW. Pubertal timing and Mexican-origin girls’ internalizing and externalizing symptoms: the influence of harsh parenting. Dev Psychol. 2013;49:1790–1804. doi: 10.1037/a0031016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deboer MD, Li Y. Puberty Is Delayed in Male Mice With Dextran Sodium Sulfate Colitis Out of Proportion to Changes in Food Intake, Body Weight, and Serum Levels of Leptin. Pediatric Research. 2011;69:34–39. doi: 10.1203/PDR.0b013e3181ffee6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker J, Lourenco F, Doll B, Hartley C. Experiential reward learning outweighs instruction prior to adulthood. Cognitive, Affective, & Behavioral Neuroscience. 2015;15:310–320. doi: 10.3758/s13415-014-0332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKeyser R. The Robustness of Critical Period Effects in Second Language Acquisition. SSLA. 2000;22:499–533. [Google Scholar]
- Dekeyser R, Alfi-Shabtay I, Ravid D. Cross-linguistic evidence for the nature of age effects in second language acquisition. Applied Psycholinguistics. 2010;31:413–438. [Google Scholar]
- Delevich KM. PhD Thesis. Cold Spring Harbor Laboratory; 2014. Synaptic effects of disrupted-in-schizophrenia 1 loss-of-function in the medial prefrontal cortex and thalamofrontal feedforward inhibitory circuit. [Google Scholar]
- DeWit DJ, Hance J, Offord DR, Ogborne A. The influence of early and frequent use of marijuana on the risk of desistance and of progression to marijuana-related harm. Prev Med. 2000;31:455–464. doi: 10.1006/pmed.2000.0738. [DOI] [PubMed] [Google Scholar]
- Dietrich AK, Humphreys GI, Nardulli AM. Expression of estrogen receptor alpha in the mouse cerebral cortex. Mol Cell Endocrinol. 2015;406:19–26. doi: 10.1016/j.mce.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divall SA, Radovick S. Pubertal Development and Menarche. Annals of the New York Academy of Sciences. 2008;1135:19–28. doi: 10.1196/annals.1429.026. [DOI] [PubMed] [Google Scholar]
- DonCarlos LL, Garcia-Ovejero D, Sarkey S, Garcia-Segura LM, Azcoitia I. Androgen receptor immunoreactivity in forebrain axons and dendrites in the rat. Endocrinology. 2003;144:3632–3638. doi: 10.1210/en.2002-0105. [DOI] [PubMed] [Google Scholar]
- DonCarlos LL, Sarkey S, Lorenz B, Azcoitia I, Garcia-Ovejero D, Huppenbauer C, Garcia-Segura LM. Novel cellular phenotypes and subcellular sites for androgen action in the forebrain. Neuroscience. 2006;138:801–807. doi: 10.1016/j.neuroscience.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Dorrn AL, Yuan K, Barker AJ, Schreiner CE, Froemke RC. Developmental sensory experience balances cortical excitation and inhibition. Nature. 2010;465:932–936. doi: 10.1038/nature09119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas JWB, Ross JM. Age of Puberty Related to Education Ability, Attainment and School Leaving Age. Journal of Child Psychology and Psychiatry. 1964;5:185–195. doi: 10.1111/j.1469-7610.1964.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. Annual review of neuroscience. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- Downing J, Bellis MA. Early pubertal onset and its relationship with sexual risk taking, substance use and anti-social behaviour: a preliminary cross-sectional study. BMC Public Health. 2009;9:446. doi: 10.1186/1471-2458-9-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzewiecki CM, Willing J, Juraska JM. Synaptic number changes in the medial prefrontal cortex across adolescence in male and female rats: A role for pubertal onset. Synapse. 2016;70:361–368. doi: 10.1002/syn.21909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubas JS, Graber JA, Petersen AC. The Effects of Pubertal Development on Achievement during Adolescence American Journal of Education. 1991;99:444–460. [Google Scholar]
- Duncan CE, Webster MJ, Rothmond DA, Bahn S, Elashoff M, Weickert CS. Prefrontal GABAA receptor α-subunit expression in normal postnatal human development and schizophrenia. Journal of psychiatric research. 2010;44:673–681. doi: 10.1016/j.jpsychires.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, McFadyen-Ketchum S, Dodge KA, Pettit GS, Bates JE. Quality of early family relationships and individual differences in the timing of pubertal maturation in girls: a longitudinal test of an evolutionary model. J Pers Soc Psychol. 1999;77:387–401. doi: 10.1037//0022-3514.77.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Figueredo AJ, Brumbach BH, Schlomer GL. Fundamental Dimensions of Environmental Risk : The Impact of Harsh versus Unpredictable Environments on the Evolution and Development of Life History Strategies. Hum Nat. 2009;20:204–268. doi: 10.1007/s12110-009-9063-7. [DOI] [PubMed] [Google Scholar]
- Ellison PT, Reiches MW, Shattuck-Faegre H, Breakey A, Konecna M, Urlacher S, Wobber V. Puberty as a life history transition. Annals of human biology. 2012;39:352–360. doi: 10.3109/03014460.2012.693199. [DOI] [PubMed] [Google Scholar]
- Erickson SL, Lewis DA. Postnatal development of parvalbumin- and GABA transporter-immunoreactive axon terminals in monkey prefrontal cortex. The Journal of comparative neurology. 2002;448:186–202. doi: 10.1002/cne.10249. [DOI] [PubMed] [Google Scholar]
- Espy KA. The shape school: Assessing executive function in preschool children. Developmental Neuropsychology. 1997;13:495–499. [Google Scholar]
- Feng Y, Weijdegard B, Wang T, Egecioglu E, Fernandez-Rodriguez J, Huhtaniemi I, Stener-Victorin E, Billig H, Shao R. Spatiotemporal expression of androgen receptors in the female rat brain during the oestrous cycle and the impact of exogenous androgen administration: a comparison with gonadally intact males. Mol Cell Endocrinol. 2010;321:161–174. doi: 10.1016/j.mce.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Fillman SG, Duncan CE, Webster MJ, Elashoff M, Weickert CS. Developmental co-regulation of the β and γ GABAA receptor subunits with distinct α subunits in the human dorsolateral prefrontal cortex. International Journal of Developmental Neuroscience. 2010;28:513–519. doi: 10.1016/j.ijdevneu.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Finley SK, Kritzer MF. Immunoreactivity for intracellular androgen receptors in identified subpopulations of neurons, astrocytes and oligodendrocytes in primate prefrontal cortex. Journal of Neurobiology. 1999;40:446–457. [PubMed] [Google Scholar]
- Foradori CD, Weiser MJ, Handa RJ. Non-genomic actions of androgens. Front Neuroendocrinol. 2008;29:169–181. doi: 10.1016/j.yfrne.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Merzenich MM, Schreiner CE. A synaptic memory trace for cortical receptive field plasticity. Nature. 2007;450:425–429. doi: 10.1038/nature06289. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA, Kohtz AS, Zhu Y. Membrane progestin receptors in the midbrain ventral tegmental area are required for progesterone-facilitated lordosis of rats. Horm Behav. 2013;64:539–545. doi: 10.1016/j.yhbeh.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, Weickert CS. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. The American journal of psychiatry. 2010;167:1479–1488. doi: 10.1176/appi.ajp.2010.09060784. [DOI] [PubMed] [Google Scholar]
- Gandhi SP, Yanagawa Y, Stryker MP. Delayed plasticity of inhibitory neurons in developing visual cortex. Proc Natl Acad Sci U S A. 2008;105:16797–16802. doi: 10.1073/pnas.0806159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner JP, Thogerson CM, Würbel H, Murray JD, Mench JA. Animal neuropsychology: Validation of the Intra-Dimensional Extra-Dimensional set shifting task for mice. Behavioural Brain Research. 2006;173:53–61. doi: 10.1016/j.bbr.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Ge X, Conger RD, Elder GH. Coming of age too early: pubertal influences on girls’ vulnerability to psychological distress. Child development. 1996;67:3386–3400. [PubMed] [Google Scholar]
- Ge X, Conger RD, Elder GH. Pubertal transition, stressful life events, and the emergence of gender differences in adolescent depressive symptoms. Developmental Psychology. 2001;37:404–417. doi: 10.1037//0012-1649.37.3.404. [DOI] [PubMed] [Google Scholar]
- Ge X, Kim IJ, Brody GH, Conger RD, Simons RL, Gibbons FX, Cutrona CE. It’s about timing and change: pubertal transition effects on symptoms of major depression among African American youths. Dev Psychol. 2003;39:430–439. doi: 10.1037/0012-1649.39.3.430. [DOI] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, Hare TA, Bookheimer SY, Tottenham N. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Clasen LS, Lenroot R, Greenstein D, Wallace GL, Ordaz S, Molloy EA, Blumenthal JD, Tossell JW, Stayer C, Samango-Sprouse CA, Shen D, Davatzikos C, Merke D, Chrousos GP. Puberty-related influences on brain development. Mol Cell Endocrinol. 2006;254–255:154–162. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Gilbert R, Widom CS, Browne K, Fergusson D, Webb E, Janson S. Burden and consequences of child maltreatment in high-income countries. Lancet (London, England) 2009;373:68–81. doi: 10.1016/S0140-6736(08)61706-7. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Gilmore JH, Hamer RM, Lieberman JA, Jarskog LF. Synaptophysin and postsynaptic density protein 95 in the human prefrontal cortex from mid-gestation into early adulthood. NEUROSCIence. 2007;149:582–591. doi: 10.1016/j.neuroscience.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Thompson PM. Mapping gray matter development: Implications for typical development and vulnerability to psychopathology. Brain and Cognition. 2010;72:6–15. doi: 10.1016/j.bandc.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Kroener S, Zaitsev AV, Povysheva NV, Krimer LS, Barrionuevo G, Lewis DA. Functional Maturation of Excitatory Synapses in Layer 3 Pyramidal Neurons during Postnatal Development of the Primate Prefrontal Cortex. Cerebral Cortex. 2008;18:626–637. doi: 10.1093/cercor/bhm095. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Miyamae T, Pafundo DE, Yoshino H, Rotaru DC, Hoftman G, Datta D, Zhang Y, Hammond M, Sampson AR, Fish KN, Ermentrout GB, Lewis DA. Functional Maturation of GABA Synapses During Postnatal Development of the Monkey Dorsolateral Prefrontal Cortex. Cereb Cortex. 2015;25:4076–4093. doi: 10.1093/cercor/bhu122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M, Cabrera-Socorro A, Perez-Garcia CG, Fraser JD, Lopez FJ, Alonso R, Meyer G. Distribution patterns of estrogen receptor alpha and beta in the human cortex and hippocampus during development and adulthood. J Comp Neurol. 2007;503:790–802. doi: 10.1002/cne.21419. [DOI] [PubMed] [Google Scholar]
- Gordon JA, Stryker MP. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J Neurosci. 1996;16:3274–3286. doi: 10.1523/JNEUROSCI.16-10-03274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goriounova NA, Mansvelder HD. Nicotine exposure during adolescence alters the rules for prefrontal cortical synaptic plasticity during adulthood. Front Synaptic Neurosci. 2012;4:3. doi: 10.3389/fnsyn.2012.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Olevska A, Warren MS, Taylor JR, Koleske AJ. Arg Kinase Regulates Prefrontal Dendritic Spine Refinement and Cocaine-Induced Plasticity. Journal of Neuroscience. 2012;32:2314–2323. doi: 10.1523/JNEUROSCI.2730-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber J, Seeley J, Brooks-Gunn… J. Is pubertal timing associated with psychopathology in young adulthood? Journal of the American …. 2004 doi: 10.1097/01.chi.0000120022.14101.11. [DOI] [PubMed] [Google Scholar]
- Graber JA, Lewinsohn PM, Seeley JR, Brooks-Gunn J. Is psychopathology associated with the timing of pubertal development? Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:1768–1776. doi: 10.1097/00004583-199712000-00026. [DOI] [PubMed] [Google Scholar]
- Graber JA, Lewinsohn PM, Seeley JR, Brooks-Gunn J. Is Psychopathology Associated With the Timing of Pubertal Development? 2006;36:1768–1776. doi: 10.1097/00004583-199712000-00026. [DOI] [PubMed] [Google Scholar]
- Graber JA. Pubertal timing and the development of psychopathology in adolescence and beyond. Hormones and behavior. 2013;64:262–269. doi: 10.1016/j.yhbeh.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age of onset of drug use and its association with DSM-IV drug abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1998;10:163–173. doi: 10.1016/s0899-3289(99)80131-x. [DOI] [PubMed] [Google Scholar]
- Grassi-Oliveira R, Honeycutt JA, Holland FH, Ganguly P, Brenhouse HC. Cognitive impairment effects of early life stress in adolescents can be predicted with early biomarkers: Impacts of sex, experience, and cytokines. Psychoneuroendocrinology. 2016;71:19–30. doi: 10.1016/j.psyneuen.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Fry AF, Myerson J. Discounting of Delayed Rewards: A Life-Span Comparison. Psychological Science. 1994;5:33–36. [Google Scholar]
- Hagihara K, Hirata S, Osada T, Hirai M, Kato J. Distribution of cells containing progesterone receptor mRNA in the female rat di- and telencephalon: an in situ hybridization study. Brain Res Mol Brain Res. 1992;14:239–249. doi: 10.1016/0169-328x(92)90179-f. [DOI] [PubMed] [Google Scholar]
- Hakuta K, Bialystok E, Wiley E. Critical evidence: a test of the critical-period hypothesis for second-language acquisition. Psychological science. 2003;14:31–38. doi: 10.1111/1467-9280.01415. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L. An alternate pathway for androgen regulation of brain function: activation of estrogen receptor beta by the metabolite of dihydrotestosterone, 5alpha-androstane-3beta,17beta-diol. Horm Behav. 2008;53:741–752. doi: 10.1016/j.yhbeh.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JW, Hoffman HJ, Ross GT. Monthly gonadotropin cycles in premenarcheal girls. Science (New York, NY) 1975;190:161–163. doi: 10.1126/science.1166307. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Nguyen QL, Rotaru D, Keenan T, Arion D, Beneyto M, Gonzalez-Burgos G, Lewis DA. Protracted Developmental Trajectories of GABAA Receptor α1 and α2 Subunit Expression in Primate Prefrontal Cortex. Biological psychiatry. 2009;65:1015–1023. doi: 10.1016/j.biopsych.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins JD, Graham JW, Maguin E, Abbott R, Hill KG, Catalano RF. Exploring the effects of age of alcohol use initiation and psychosocial risk factors on subsequent alcohol misuse. J Stud Alcohol. 1997;58:280–290. doi: 10.15288/jsa.1997.58.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward C, Killen JD, Wilson DM, Hammer LD, Litt IF, Kraemer HC, Haydel F, Varady A, Taylor CB. Psychiatric risk associated with early puberty in adolescent girls. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:255–262. [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biological Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Heimel JA, Van Versendaal D, Levelt CN. The Role of GABAergic Inhibition in Ocular Dominance Plasticity. Neural Plasticity. 2011;2011:1–11. doi: 10.1155/2011/391763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nature reviews Neuroscience. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Henson MA, Roberts AC, Salimi K, Vadlamudi S, Hamer RM, Gilmore JH, Jarskog LF, Philpot BD. Developmental Regulation of the NMDA Receptor Subunits, NR3A and NR1, in Human Prefrontal Cortex. Cerebral Cortex. 2008;18:2560–2573. doi: 10.1093/cercor/bhn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman-Giddens ME, Slora EJ, Wasserman RC, Bourdony CJ, Bhapkar MV, Koch GG, Hasemeier CM. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics. 1997;99:505–512. doi: 10.1542/peds.99.4.505. [DOI] [PubMed] [Google Scholar]
- Herman-Giddens ME. Recent data on pubertal milestones in United States children: the secular trend toward earlier development. International journal of andrology. 2006;29:241–246. doi: 10.1111/j.1365-2605.2005.00575.x. [DOI] [PubMed] [Google Scholar]
- Herman-Giddens ME, Steffes J, Harris D, Slora E, Hussey M, Dowshen SA, Wasserman R, Serwint JR, Smitherman L, Reiter EO. Secondary sexual characteristics in boys: data from the Pediatric Research in Office Settings Network. Pediatrics. 2012;130:e1058–e1068. doi: 10.1542/peds.2011-3291. [DOI] [PubMed] [Google Scholar]
- Hernandez DJ. Double Jeopardy: How third-grade reading skills and poverty influence high school graduation. The Annie E. Casey Foundation; 2011. Vol., ed.^eds. [Google Scholar]
- Herting MM, Maxwell EC, Irvine C, Nagel BJ. The Impact of Sex, Puberty, and Hormones on White Matter Microstructure in Adolescents. Cerebral Cortex. 2012;22:1979–1992. doi: 10.1093/cercor/bhr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Gautam P, Spielberg JM, Kan E, Dahl RE, Sowell ER. The role of testosterone and estradiol in brain volume changes across adolescence: A longitudinal structural MRI study. Human Brain Mapping. 2014;35:5633–5645. doi: 10.1002/hbm.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Gautam P, Spielberg JM, Dahl RE, Sowell ER. A Longitudinal Study: Changes in Cortical Thickness and Surface Area during Pubertal Maturation. PLOS ONE. 2015;10:e0119774. doi: 10.1371/journal.pone.0119774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Pannier L, Chiron C, Jambaqué I, Renaux-Kieffer V, Van de Moortele P-F, Delalande O, Fohlen M, Brunelle F, Le Bihan D. Late plasticity for language in a child’s non-dominant hemisphere: a pre- and post-surgery fMRI study. Brain : a journal of neurology. 2002;125:361–372. doi: 10.1093/brain/awf020. [DOI] [PubMed] [Google Scholar]
- Hill RA, Wu YWC, Kwek P, Buuse MVD. Modulatory Effects of Sex Steroid Hormones on Brain-Derived Neurotrophic Factor-Tyrosine Kinase B Expression during Adolescent Development in C57Bl/6 Mice. Journal of neuroendocrinology. 2012;24:774–788. doi: 10.1111/j.1365-2826.2012.02277.x. [DOI] [PubMed] [Google Scholar]
- Hochberg Ze, Belsky J. Evo-devo of human adolescence: beyond disease models of early puberty. BMC medicine. 2013;11:113. doi: 10.1186/1741-7015-11-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Wilbrecht L, Knott GW, Welker E, Svoboda K. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature. 2006;441:979–983. doi: 10.1038/nature04783. [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Bonhoeffer T, Chow DK, Chuckowree J, De Paola V, Hofer SB, Hübener M, Keck T, Knott G, Lee W-CA, Mostany R, Mrsic-Flogel TD, Nedivi E, Portera-Cailliau C, Svoboda K, Trachtenberg JT, Wilbrecht L. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat Protoc. 2009;4:1128–1144. doi: 10.1038/nprot.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Holtmaat AJGD, Trachtenberg JT, Wilbrecht L, Shepherd GM, Zhang X, Knott GW, Svoboda K. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005;45:279–291. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Gunnar MR. The Developmental Effects of Early Life Stress: An Overview of Current Theoretical Frameworks. Current Directions in Psychological Science. 2013;22:400–406. doi: 10.1177/0963721413488889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Gan J, Jonas P. Fast-spiking, parvalbumin+ GABAergic interneurons: From cellular design to microcircuit function. Science (New York, NY) 2014;345:1255263–1255263. doi: 10.1126/science.1255263. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Huizinga M, Dolan CV, van der Molen MW. Age-related change in executive function: Developmental trends and a latent variable analysis. Neuropsychologia. 2006;44:2017–2036. doi: 10.1016/j.neuropsychologia.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Huizinga M, van der Molen MW. Age-Group Differences in Set-Switching and Set-Maintenance on the Wisconsin Card Sorting Task. Developmental Neuropsychology. 2007;31:193–215. doi: 10.1080/87565640701190817. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. The Journal of comparative neurology. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Insel TR, Miller LP, Gelhard RE. The ontogeny of excitatory amino acid receptors in rat forebrain--I. N-methyl-D-aspartate and quisqualate receptors. NEUROSCIence. 1990;35:31–43. doi: 10.1016/0306-4522(90)90117-m. [DOI] [PubMed] [Google Scholar]
- Intlekofer KA, Petersen SL. Distribution of mRNAs encoding classical progestin receptor, progesterone membrane components 1 and 2, serpine mRNA binding protein 1, and progestin and ADIPOQ receptor family members 7 and 8 in rat forebrain. Neuroscience. 2011;172:55–65. doi: 10.1016/j.neuroscience.2010.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa NP, Trachtenberg JT, Chapman B, Zahs KR, Stryker MP. The critical period for ocular dominance plasticity in the Ferret’s visual cortex. J Neurosci. 1999;19:6965–6978. doi: 10.1523/JNEUROSCI.19-16-06965.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Bai T, Tanaka T, Yoshida K, Ueyama T, Miyajima M, Negishi T, Kawasaki T, Takamatsu H, Kikutani H, Kumanogoh A, Yukawa K. Estrogen-dependent proteolytic cleavage of semaphorin 4D and plexin-B1 enhances semaphorin 4D–induced apoptosis during postnatal vaginal remodeling in pubescent mice. PLOS ONE. 2014;9:e97909. doi: 10.1371/journal.pone.0097909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen S, Rubin D, Jacques P. The temporal distribution of autobiographical memory: changes in reliving and vividness over the life span do not explain the reminiscence bump. Memory & cognition. 2011 doi: 10.3758/s13421-010-0003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C, Wilbrecht L. Juvenile mice show greater flexibility in multiple choice reversal learning than adults. Dev Cogn Neurosci. 2011;1:540–551. doi: 10.1016/j.dcn.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C, Loucks A, Peckler H, Thomas AW, Janak P, Wilbrecht L. Long-range orbitofrontal and amygdala axons show divergent patterns of maturation in the frontal cortex across adolescence. Developmental cognitive neuroscience. 2016a doi: 10.1016/j.dcn.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C, Peckler H, Tai LH, L W. Rule learning enhances structural plasticity of long range axons in frontal cortex. Nature Communications. 2016b doi: 10.1038/ncomms10785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JS, Newport EL. Critical period effects in second language learning: the influence of maturational state on the acquisition of English as a second language. Cognitive psychology. 1989;21:60–99. doi: 10.1016/0010-0285(89)90003-0. [DOI] [PubMed] [Google Scholar]
- Jordan NC, Kaplan D, Ramineni C, Locuniak MN. Early math matters: kindergarten number competence and later mathematics outcomes. Dev Psychol. 2009;45:850–867. doi: 10.1037/a0014939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan NC, Levine SC. Socioeconomic variation, number competence, and mathematics learning difficulties in young children. Dev Disabil Res Rev. 2009;15:60–68. doi: 10.1002/ddrr.46. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Gruzelier JH. Timing of puberty and EEG coherence during photic stimulation. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 1996;21:135–149. doi: 10.1016/0167-8760(95)00048-8. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Gruzelier JH. Timing of puberty and syndromes of schizotypy: a replication. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 1999;34:237–247. doi: 10.1016/s0167-8760(99)00081-1. [DOI] [PubMed] [Google Scholar]
- Kaplan H, Hill K, Lancaster J, Hurtado A. A Theory of Human Life History Evolution: Diet, Intelligence, and Longevity. Evolutionary Anthropology. 2000:156–185. [Google Scholar]
- Karube F. Axon Branching and Synaptic Bouton Phenotypes in GABAergic Nonpyramidal Cell Subtypes. Journal of Neuroscience. 2004;24:2853–2865. doi: 10.1523/JNEUROSCI.4814-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H, Fukuda M, Watanabe S, Hayashi-Takagi A, Noguchi J. Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci. 2010;33:121–129. doi: 10.1016/j.tins.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Kato J, Onouchi T. Progesterone receptors in the cerebral cortex of neonatal female rats. Dev Neurosci. 1981;4:427–432. doi: 10.1159/000112811. [DOI] [PubMed] [Google Scholar]
- Kato J, Onouchi T. Progestin receptors in female rat brain and hypophysis in the development from fetal to postnatal stages. Endocrinology. 1983;113:29–36. doi: 10.1210/endo-113-1-29. [DOI] [PubMed] [Google Scholar]
- Kato J, Onouchi T, Okinaga S, Takamatsu M. The ontogeny of cytosol and nuclear progestin receptors in male rat brain and its male-female differences. J Steroid Biochem. 1984;20:147–152. doi: 10.1016/0022-4731(84)90201-2. [DOI] [PubMed] [Google Scholar]
- Kato J, Hirata S, Nozawa A, Mouri N. The ontogeny of gene expression of progestin receptors in the female rat brain. J Steroid Biochem Mol Biol. 1993;47:173–182. doi: 10.1016/0960-0760(93)90072-5. [DOI] [PubMed] [Google Scholar]
- Kayser AS, Op de Macks Z, Dahl RE, Frank MJ. A Neural Correlate of Strategic Exploration at the Onset of Adolescence. Journal of Cognitive Neuroscience. 2015;28:199–209. doi: 10.1162/jocn_a_00896. [DOI] [PubMed] [Google Scholar]
- Kim J, Ragozzino ME. The involvement of the orbitofrontal cortex in learning under changing task contingencies. Neurobiology of Learning and Memory. 2005;83:125–133. doi: 10.1016/j.nlm.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Relkin NR, Lee KM, Hirsch J. Distinct cortical areas associated with native and second languages. Nature. 1997;388:171–174. doi: 10.1038/40623. [DOI] [PubMed] [Google Scholar]
- Kokis JV, Macpherson R, Toplak ME, West RF, Stanovich KE. Heuristic and analytic processing: Age trends and associations with cognitive ability and cognitive styles. Journal of Experimental Child Psychology. 2002;83:26–52. doi: 10.1016/s0022-0965(02)00121-2. [DOI] [PubMed] [Google Scholar]
- Kolb B, Nonneman AJ. Sparing of function in rats with early prefrontal cortex lesions. Brain Research. 1978;151:135–148. doi: 10.1016/0006-8993(78)90956-3. [DOI] [PubMed] [Google Scholar]
- Kolb B. Recovery from early cortical damage in rats. I. Differential behavioral and anatomical effects of frontal lesions at different ages of neural maturation. Behavioural brain research. 1987;25:205–220. doi: 10.1016/0166-4328(87)90069-6. [DOI] [PubMed] [Google Scholar]
- Kolb B, Gibb R. Anatomical correlates of behavioural change after neonatal prefrontal lesions in rats. Prog Brain Res. 1990;85:241–255. doi: 10.1016/s0079-6123(08)62683-7. discussion 255-6. [DOI] [PubMed] [Google Scholar]
- Kolb B, Petrie B, Cioe J. Recovery from early cortical damage in rats, VII. Comparison of the behavioural and anatomical effects of medial prefrontal lesions at different ages of neural maturation. Behavioural brain research. 1996;79:1–14. doi: 10.1016/0166-4328(95)00254-5. [DOI] [PubMed] [Google Scholar]
- Kolb B, Gibb R, Gorny G, Whishaw IQ. Possible regeneration of rat medial frontal cortex following neonatal frontal lesions. Behavioural brain research. 1998;91:127–141. doi: 10.1016/s0166-4328(97)00112-5. [DOI] [PubMed] [Google Scholar]
- Kolb B, Cioe J. Recovery from early cortical damage in rats, VIII. Earlier may be worse: behavioural dysfunction and abnormal cerebral morphogenesis following perinatal frontal cortical lesions in the rat. Neuropharmacology. 2000;39:756–764. doi: 10.1016/s0028-3908(99)00260-9. [DOI] [PubMed] [Google Scholar]
- Konner M. The evolution of childhood : relationships, emotion, mind. Cambridge, Mass: Belknap Press of Harvard University Press; 2010. Vol. [Google Scholar]
- Koolschijn PCMP, Peper JS, Crone EA. The Influence of Sex Steroids on Structural Brain Maturation in Adolescence. PloS one. 2014;9:e83929. doi: 10.1371/journal.pone.0083929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel J, Berntsen D. The peaks of life: The differential temporal locations of the reminiscence bump across disparate cueing methods. Journal of Applied Research in Memory and Cognition. 2015;4:66–80. [Google Scholar]
- Korenbrot CC, Huhtaniemi IT, Weiner RI. Preputial separation as an external sign of pubertal development in the male rat. Biology of reproduction. 1977;17:298–303. doi: 10.1095/biolreprod17.2.298. [DOI] [PubMed] [Google Scholar]
- Korsia S, Bottjer SW. Chronic testosterone treatment impairs vocal learning in male zebra finches during a restricted period of development. J Neurosci. 1991;11:2362–2371. doi: 10.1523/JNEUROSCI.11-08-02362.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss WA, Belden CE, Hristov AD, Juraska JM. Dendritic remodeling in the adolescent medial prefrontal cortex and the basolateral amygdala of male and female rats. Synapse. 2014;68:61–72. doi: 10.1002/syn.21716. [DOI] [PubMed] [Google Scholar]
- Koss WA, Lloyd MM, Sadowski RN, Wise LM, Juraska JM. Gonadectomy before puberty increases the number of neurons and glia in the medial prefrontal cortex of female, but not male, rats. Developmental Psychobiology. 2015;57:305–312. doi: 10.1002/dev.21290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Yesavage JA, Taylor JL, Kupfer D. How can we learn about developmental processes from cross-sectional studies, or can we? Am J Psychiatry. 2000;157:163–171. doi: 10.1176/appi.ajp.157.2.163. [DOI] [PubMed] [Google Scholar]
- Kritzer M. The Distribution of Immunoreactivity for Intracellular Androgen Receptors in the Cerebral Cortex of Hormonally Intact Adult Male and Female Rats: Localization in Pyramidal Neurons Making Corticocortical Connections. Cerebral Cortex. 2004;14:268–280. doi: 10.1093/cercor/bhg127. [DOI] [PubMed] [Google Scholar]
- Kritzer MF. Selective colocalization of immunoreactivity for intracellular gonadal hormone receptors and tyrosine hydroxylase in the ventral tegmental area, substantia nigra, and retrorubral fields in the rat. J Comp Neurol. 1997;379:247–260. doi: 10.1002/(sici)1096-9861(19970310)379:2<247::aid-cne6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Kritzer MF. Regional, laminar, and cellular distribution of immunoreactivity for ER alpha and ER beta in the cerebral cortex of hormonally intact, adult male and female rats. Cereb Cortex. 2002;12:116–128. doi: 10.1093/cercor/12.2.116. [DOI] [PubMed] [Google Scholar]
- Kritzer MF. Regional, Laminar and Cellular Distribution of Immunoreactivity for ER in the Cerebral Cortex of Hormonally Intact, Postnatally Developing Male and Female Rats. Cerebral Cortex. 2006;16:1181–1192. doi: 10.1093/cercor/bhj059. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Creutz LM. Region and sex differences in constituent dopamine neurons and immunoreactivity for intracellular estrogen and androgen receptors in mesocortical projections in rats. J Neurosci. 2008;28:9525–9535. doi: 10.1523/JNEUROSCI.2637-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroesbergen EH, Van Luit JEH. Mathematics Interventions for Children with Special Educational Needs. Remedial and Special Education. 2003;24:97–114. [Google Scholar]
- Kuhn C, Johnson M, Thomae A, Luo B, Simon SA, Zhou G, Walker QD. The emergence of gonadal hormone influences on dopaminergic function during puberty. Horm Behav. 2010;58:122–137. doi: 10.1016/j.yhbeh.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Magueresse C, Monyer H. GABAergic interneurons shape the functional maturation of the cortex. Neuron. 2013;77:388–405. doi: 10.1016/j.neuron.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. NeuroImage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Legro RS, Lin HM, Demers LM, Lloyd T. Rapid maturation of the reproductive axis during perimenarche independent of body composition. The Journal of clinical endocrinology and metabolism. 2000;85:1021–1025. doi: 10.1210/jcem.85.3.6423. [DOI] [PubMed] [Google Scholar]
- Lenneberg EH. Biological foundations of language. New York: Wiley; 1967. Vol. [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVay S, Wiesel TN, Hubel DH. The development of ocular dominance columns in normal and visually deprived monkeys. J Comp Neurol. 1980;191:1–51. doi: 10.1002/cne.901910102. [DOI] [PubMed] [Google Scholar]
- Levelt CN, Hubener M. Critical-period plasticity in the visual cortex. Annu Rev Neurosci. 2012;35:309–330. doi: 10.1146/annurev-neuro-061010-113813. [DOI] [PubMed] [Google Scholar]
- Lisdahl KM, Gilbart ER, Wright NE, Shollenbarger S. Dare to delay? The impacts of adolescent alcohol and marijuana use onset on cognition, brain structure, and function. Front Psychiatry. 2013;4:53. doi: 10.3389/fpsyt.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomniczi A, Wright H, Castellano JM, Sonmez K, Ojeda SR. A system biology approach to identify regulatory pathways underlying the neuroendocrine control of female puberty in rats and nonhuman primates. Hormones and behavior. 2013;64:175–186. doi: 10.1016/j.yhbeh.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez V, Wagner CK. Progestin receptor is transiently expressed perinatally in neurons of the rat isocortex. J Comp Neurol. 2009;512:124–139. doi: 10.1002/cne.21883. [DOI] [PubMed] [Google Scholar]
- López V, Wagner CK. Progestin receptor is transiently expressed perinatally in neurons of the rat isocortex. The Journal of comparative neurology. 2009;512:124–139. doi: 10.1002/cne.21883. [DOI] [PubMed] [Google Scholar]
- Lovic V, Fleming AS. Artificially-reared female rats show reduced prepulse inhibition and deficits in the attentional set shifting task--reversal of effects with maternal-like licking stimulation. Behavioural brain research. 2004;148:209–219. doi: 10.1016/s0166-4328(03)00206-7. [DOI] [PubMed] [Google Scholar]
- Luciana M, Nelson CA. Assessment of neuropsychological function through use of the Cambridge Neuropsychological Testing Automated Battery: performance in 4- to 12-year-old children. Dev Neuropsychol. 2002;22:595–624. doi: 10.1207/S15326942DN2203_3. [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of Cognitive Processes From Late Childhood to Adulthood. Child Development. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology (Berl) 2008;197:237–246. doi: 10.1007/s00213-007-1028-0. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Sex and ovarian hormones influence vulnerability and motivation for nicotine during adolescence in rats. Pharmacol Biochem Behav. 2009;94:43–50. doi: 10.1016/j.pbb.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn DA, Brown GR. The ontogeny of exploratory behavior in male and female adolescent rats (Rattus norvegicus) Dev Psychobiol. 2009;51:513–520. doi: 10.1002/dev.20386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynskey MT, Heath AC, Bucholz KK, Slutske WS, Madden PA, Nelson EC, Statham DJ, Martin NG. Escalation of drug use in early-onset cannabis users vs co-twin controls. Jama. 2003;289:427–433. doi: 10.1001/jama.289.4.427. [DOI] [PubMed] [Google Scholar]
- Makinodan M, Rosen KM, Ito S, Corfas G. A Critical Period for Social Experience-Dependent Oligodendrocyte Maturation and Myelination. Science (New York, NY) 2012;337:1357–1360. doi: 10.1126/science.1220845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone DT, Hill MN, Rubino T. Adolescent cannabis use and psychosis: epidemiology and neurodevelopmental models. Br J Pharmacol. 2010;160:511–522. doi: 10.1111/j.1476-5381.2010.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Morris JR, Juraska JM. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. NEUROSCIence. 2007;144:961–968. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Marsh R, Zhu H, Schultz RT, Quackenbush G, Royal J, Skudlarski P, Peterson BS. A developmental fMRI study of self-regulatory control. Human Brain Mapping. 2006;27:848–863. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Archives of disease in childhood. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Archives of disease in childhood. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer C, Acosta-Martinez M, Dubois SL, Wolfe A, Radovick S, Boehm U, Levine JE. Timing and completion of puberty in female mice depend on estrogen receptor -signaling in kisspeptin neurons. Proceedings of the National Academy of Sciences. 2010;107:22693–22698. doi: 10.1073/pnas.1012406108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAbee MD, DonCarlos LL. Ontogeny of region-specific sex differences in androgen receptor messenger ribonucleic acid expression in the rat forebrain. Endocrinology. 1998;139:1738–1745. doi: 10.1210/endo.139.4.5940. [DOI] [PubMed] [Google Scholar]
- Mccormick CM, Mathews IZ, Thomas C, Waters P. Investigations of HPA function and the enduring consequences of stressors in adolescence in animal models. Brain and cognition. 2010;72:73–85. doi: 10.1016/j.bandc.2009.06.003. [DOI] [PubMed] [Google Scholar]
- McCutcheon JE, Marinelli M. Age matters. Eur J Neurosci. 2009;29:997–1014. doi: 10.1111/j.1460-9568.2009.06648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Conrad KL, Carr SB, Ford KA, McGehee DS, Marinelli M. Dopamine neurons in the ventral tegmental area fire faster in adolescent rats than in adults. J Neurophysiol. 2012;108:1620–1630. doi: 10.1152/jn.00077.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney M, Stewart J. Environmental factors influencing the affiliative behavior of male and female rats (Rattus norvegicus) Anim Learn Behav. 1979;7:397–405. [Google Scholar]
- Meeker JD. Exposure to environmental endocrine disruptors and child development. Arch Pediatr Adolesc Med. 2012;166:952–958. [PubMed] [Google Scholar]
- Mermelstein PG, Micevych PE. Nervous system physiology regulated by membrane estrogen receptors. Rev Neurosci. 2008;19:413–424. doi: 10.1515/revneuro.2008.19.6.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych P, Dominguez R. Membrane estradiol signaling in the brain. Front Neuroendocrinol. 2009;30:315–327. doi: 10.1016/j.yfrne.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S, Ghosh N, Sinha P, Chakrabarti N, Bhattacharyya A. Alteration in Nuclear Factor-KappaB Pathway and Functionality of Estrogen via Receptors Promote Neuroinflammation in Frontal Cortex after 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine Treatment. Sci Rep. 2015;5:13949. doi: 10.1038/srep13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monbon M, Loras B, Reboud JP, Bertrand J. Binding and metabolism of testosterone in the rat brain during sexual maturation. I. Macromolecular binding of androgens. J Steroid Biochem. 1974;5:417–423. doi: 10.1016/0022-4731(74)90038-7. [DOI] [PubMed] [Google Scholar]
- Monroy E, Hernández-Torres E, Flores G. Maternal separation disrupts dendritic morphology of neurons in prefrontal cortex, hippocampus, and nucleus accumbens in male rat offspring. Journal of chemical neuroanatomy. 2010;40:93–101. doi: 10.1016/j.jchemneu.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Montague D, Weickert CS, Tomaskovic-Crook E, Rothmond DA, Kleinman JE, Rubinow DR. Oestrogen receptor alpha localisation in the prefrontal cortex of three mammalian species. J Neuroendocrinol. 2008;20:893–903. doi: 10.1111/j.1365-2826.2008.01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Shionoya K, Jakubs K, Sullivan RM. Early-life stress disrupts attachment learning: the role of amygdala corticosterone, locus ceruleus corticotropin releasing hormone, and olfactory bulb norepinephrine. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:15745–15755. doi: 10.1523/JNEUROSCI.4106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad A, Kolb B. Maternal separation altered behavior and neuronal spine density without influencing amphetamine sensitization. Behavioural brain research. 2011;223:7–16. doi: 10.1016/j.bbr.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Muñoz-Cuevas FJ, Athilingam J, Piscopo D, Wilbrecht L. Cocaine-induced structural plasticity in frontal cortex correlates with conditioned place preference. Nat Neurosci. 2013;16:1367–1369. doi: 10.1038/nn.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KM, Tcharnaia L, Beshara SP, Jones DG. Cortical development of AMPA receptor trafficking proteins. Frontiers in Molecular Neuroscience. 2012;5:1–12. doi: 10.3389/fnmol.2012.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol Med. 2005;35:163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Karelus K, Felicio LS, Johnson TE. Genetic influences on the timing of puberty in mice. Biology of reproduction. 1990;42:649–655. doi: 10.1095/biolreprod42.4.649. [DOI] [PubMed] [Google Scholar]
- Nemati F, Kolb B. Motor cortex injury has different behavioral and anatomical effects in early and late adolescence. Behavioral Neuroscience. 2010;124:612–622. doi: 10.1037/a0020911. [DOI] [PubMed] [Google Scholar]
- Nemati F, Kolb B. Recovery from medial prefrontal cortex injury during adolescence: Implications for age-dependent plasticity. Behavioural brain research. 2012;229:168–175. doi: 10.1016/j.bbr.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Nettle D, Bateson M. Adaptive developmental plasticity: what is it, how can we recognize it and when can it evolve? Proceedings Biological sciences / The Royal Society. 2015;282:20151005. doi: 10.1098/rspb.2015.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufang S, Specht K, Hausmann M, Gunturkun O, Herpertz-Dahlmann B, Fink GR, Konrad K. Sex Differences and the Impact of Steroid Hormones on the Developing Human Brain. Cerebral Cortex. 2009;19:464–473. doi: 10.1093/cercor/bhn100. [DOI] [PubMed] [Google Scholar]
- Newman LA, McGaughy J. Adolescent rats show cognitive rigidity in a test of attentional set shifting. Developmental Psychobiology. 2011;53:391–401. doi: 10.1002/dev.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport E. Maturational Constraints on Language Learning. Cognitive Science. 1990;14:11–28. [Google Scholar]
- Nonneman AJ, Corwin JV. Differential effects of prefrontal cortex ablation in neonatal, juvenile, and young adult rats. Journal of comparative and physiological psychology. 1981;95:588–602. doi: 10.1037/h0077800. [DOI] [PubMed] [Google Scholar]
- Novick AM, Forster GL, Tejani-Butt SM, Watt MJ. Adolescent social defeat alters markers of adult dopaminergic function. Brain research bulletin. 2011;86:123–128. doi: 10.1016/j.brainresbull.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez JL, Huppenbauer CB, McAbee MD, Juraska JM, DonCarlos LL. Androgen receptor expression in the developing male and female rat visual and prefrontal cortex. J Neurobiol. 2003;56:293–302. doi: 10.1002/neu.10236. [DOI] [PubMed] [Google Scholar]
- O’Brien MS, Anthony JC. Risk of becoming cocaine dependent: epidemiological estimates for the United States, 2000–2001. Neuropsychopharmacology. 2005;30:1006–1018. doi: 10.1038/sj.npp.1300681. [DOI] [PubMed] [Google Scholar]
- Obeidallah D, Brennan RT, Brooks-Gunn J, Earls F. Links Between Pubertal Timing and Neighborhood Contexts: Implications for Girls’ Violent Behavior. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;43:1460–1468. doi: 10.1097/01.chi.0000142667.52062.1e. [DOI] [PubMed] [Google Scholar]
- Olson EA, Hooper CJ, Collins P, Luciana M. Adolescents’ performance on delay and probability discounting tasks: contributions of age, intelligence, executive functioning, and self-reported externalizing behavior. Pers Individ Dif. 2007;43:1886–1897. doi: 10.1016/j.paid.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund MK, Keller E, Hurd YL. The human forebrain has discrete estrogen receptor alpha messenger RNA expression: high levels in the amygdaloid complex. Neuroscience. 2000;95:333–342. doi: 10.1016/s0306-4522(99)00443-1. [DOI] [PubMed] [Google Scholar]
- Packer AM, Yuste R. Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: a canonical microcircuit for inhibition? The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:13260–13271. doi: 10.1523/JNEUROSCI.3131-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons B, Rainbow TC, MacLusky NJ, McEwen BS. Progestin receptor levels in rat hypothalamic and limbic nuclei. J Neurosci. 1982;2:1446–1452. doi: 10.1523/JNEUROSCI.02-10-01446.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parylak SL, Caster JM, Walker QD, Kuhn CM. Gonadal steroids mediate the opposite changes in cocaine-induced locomotion across adolescence in male and female rats. Pharmacol Biochem Behav. 2008;89:314–323. doi: 10.1016/j.pbb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual R, Zamora-León SP, Valero-Cabré A. Effects of postweaning social isolation and re-socialization on the expression of vasoactive intestinal peptide (VIP) and dendritic development in the medial prefrontal cortex of the rat. Acta neurobiologiae experimentalis. 2006;66:7–14. doi: 10.55782/ane-2006-1582. [DOI] [PubMed] [Google Scholar]
- Pattwell SS, Liston C, Jing D, Ninan I, Yang RR, Witztum J, Murdock MH, Dincheva I, Bath KG, Casey BJ, Deisseroth K, Lee FS. Dynamic changes in neural circuitry during adolescence are associated with persistent attenuation of fear memories. Nat Commun. 2016:7. doi: 10.1038/ncomms11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen DJ, Hallquist MN, Geier CF, Luna B. Effects of incentives, age, and behavior on brain activation during inhibitory control: a longitudinal fMRI study. Dev Cogn Neurosci. 2015;11:105–115. doi: 10.1016/j.dcn.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper JS, Brouwer RM, Schnack HG, van Baal GCM, van Leeuwen M, van den Berg SM, Delemarre-Van de Waal HA, Janke AL, Collins DL, Evans AC, Boomsma DI, Kahn RS, Hulshoff Pol HE. Cerebral white matter in early puberty is associated with luteinizing hormone concentrations. Psychoneuroendocrinology. 2008;33:909–915. doi: 10.1016/j.psyneuen.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Peper JS, Brouwer RM, Schnack HG, van Baal GC, van Leeuwen M, van den Berg SM, Delemarre-Van de Waal HA, Boomsma DI, Kahn RS, Hulshoff Pol HE. Sex steroids and brain structure in pubertal boys and girls. Psychoneuroendocrinology. 2009a;34:332–342. doi: 10.1016/j.psyneuen.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Peper JS, Schnack HG, Brouwer RM, Van Baal GCM, Pjetri E, Székely E, van Leeuwen M, van den Berg SM, Collins DL, Evans AC, Boomsma DI, Kahn RS, Hulshoff Pol HE. Heritability of regional and global brain structure at the onset of puberty: A magnetic resonance imaging study in 9-year-old twin pairs. Human brain mapping. 2009b;30:2184–2196. doi: 10.1002/hbm.20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez SE, Chen EY, Mufson EJ. Distribution of estrogen receptor alpha and beta immunoreactive profiles in the postnatal rat brain. Brain Res Dev Brain Res. 2003;145:117–139. doi: 10.1016/s0165-3806(03)00223-2. [DOI] [PubMed] [Google Scholar]
- Pérez SE, Chen E-Y, Mufson EJ. Distribution of estrogen receptor alpha and beta immunoreactive profiles in the postnatal rat brain. Brain research Developmental brain research. 2003;145:117–139. doi: 10.1016/s0165-3806(03)00223-2. [DOI] [PubMed] [Google Scholar]
- Perrin JS, Herve P-Y, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, Veillette S, Pausova Z, Paus T. Growth of White Matter in the Adolescent Brain: Role of Testosterone and Androgen Receptor. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:9519–9524. doi: 10.1523/JNEUROSCI.1212-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin JS, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, Veillette S, Pausova Z, Paus T. Sex differences in the growth of white matter during adolescence. NeuroImage. 2009;45:1055–1066. doi: 10.1016/j.neuroimage.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judas M, Kostovic I, Uylings HBM. Lifespan Alterations of Basal Dendritic Trees of Pyramidal Neurons in the Human Prefrontal Cortex: A Layer-Specific Pattern. Cerebral Cortex. 2008;18:915–929. doi: 10.1093/cercor/bhm124. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HBM, Rakic P, Kostovic I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S, Braams BR, Raijmakers MEJ, Koolschijn PCMP, Crone EA. The Neural Coding of Feedback Learning across Child and Adolescent Development. Journal of Cognitive Neuroscience. 2014;26:1705–1720. doi: 10.1162/jocn_a_00594. [DOI] [PubMed] [Google Scholar]
- Peters YM, O’donnell P. Social isolation rearing affects prefrontal cortical response to ventral tegmental area stimulation. Biological Psychiatry. 2005;57:1205–1208. doi: 10.1016/j.biopsych.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Petersen A. In: Pubertal change and cognition. In Girls at puberty: Biological and psychosocial perspectives. Brooks-Gunn J, Petersen A, editors. New York: Plenum Press; 1983. pp. 179–198. Vol. [Google Scholar]
- Petersen SL, Intlekofer KA, Moura-Conlon PJ, Brewer DN, Del Pino Sans J, Lopez JA. Novel progesterone receptors: neural localization and possible functions. Front Neurosci. 2013;7:164. doi: 10.3389/fnins.2013.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poling MC, Kauffman AS. Regulation and Function of RFRP-3 (GnIH) Neurons during Postnatal Development. Frontiers in Endocrinology. 2015;6:150. doi: 10.3389/fendo.2015.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell SB, Sejnowski TJ, Behrens MM. Behavioral and neurochemical consequences of cortical oxidative stress on parvalbumin-interneuron maturation in rodent models of schizophrenia. Neuropharmacology. 2012;62:1322–1331. doi: 10.1016/j.neuropharm.2011.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Age at first drink and risk for alcoholism: a noncausal association. Alcohol Clin Exp Res. 1999;23:101–107. [PubMed] [Google Scholar]
- Prewitt AK, Wilson ME. Changes in estrogen receptor-alpha mRNA in the mouse cortex during development. Brain Research. 2007;1134:62–69. doi: 10.1016/j.brainres.2006.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins ML, Hovda DA. Traumatic brain injury in the developing rat: effects of maturation on Morris water maze acquisition. Journal of neurotrauma. 1998;15:799–811. doi: 10.1089/neu.1998.15.799. [DOI] [PubMed] [Google Scholar]
- Purves-Tyson TD, Owens SJ, Double KL, Desai R, Handelsman DJ, Weickert CS. Testosterone induces molecular changes in dopamine signaling pathway molecules in the adolescent male rat nigrostriatal pathway. PLoS One. 2014;9:e91151. doi: 10.1371/journal.pone.0091151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puy L, MacLusky NJ, Becker L, Karsan N, Trachtenberg J, Brown TJ. Immunocytochemical detection of androgen receptor in human temporal cortex characterization and application of polyclonal androgen receptor antibodies in frozen and paraffin-embedded tissues. J Steroid Biochem Mol Biol. 1995;55:197–209. doi: 10.1016/0960-0760(95)00165-v. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Lee Y, Stidd R, Long R, Greenstein D, Clasen L, Addington A, Gogtay N, Rapoport JL, Giedd JN. Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proc Natl Acad Sci U S A. 2010;107:16988–16993. doi: 10.1073/pnas.1006025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Worthman CM, Campbell BC, Stallings JF, Mbizva M. Ratios of plasma and salivary testosterone throughout puberty: production versus bioavailability. Steroids. 1996;61:374–378. doi: 10.1016/0039-128x(96)00043-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez I, Araki K, Khatib K, Martinou JC, Vassalli P. Mouse vaginal opening is an apoptosis-dependent process which can be prevented by the overexpression of Bcl2. Developmental biology. 1997;184:115–121. doi: 10.1006/dbio.1997.8522. [DOI] [PubMed] [Google Scholar]
- Roff DA. Life History Evolution. Sunderland, MA: Sinauer; 2002. Vol. [Google Scholar]
- Romeo RD, Mcewen BS. Stress and the adolescent brain. Annals of the New York Academy of Sciences. 2006;1094:202–214. doi: 10.1196/annals.1376.022. [DOI] [PubMed] [Google Scholar]
- Rosenfield RL, Lipton RB, Drum ML. Thelarche, Pubarche, and Menarche Attainment in Children With Normal and Elevated Body Mass Index. Pediatrics. 2009;123:84–88. doi: 10.1542/peds.2008-0146. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, Brammer M. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Human Brain Mapping. 2006;27:973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DC. One bump, two bumps, three bumps, four? Using retrieval cues to divide one autobiographical memory reminiscence bump into many. Journal of Applied Research in Memory and Cognition. 2015;4:87–89. [Google Scholar]
- Rubino T, Parolaro D. Long lasting consequences of cannabis exposure in adolescence. Mol Cell Endocrinol. 2008;286:S108–S113. doi: 10.1016/j.mce.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Rubino T, Parolaro D. Cannabis abuse in adolescence and the risk of psychosis: a brief review of the preclinical evidence. Prog Neuropsychopharmacol Biol Psychiatry. 2014;52:41–44. doi: 10.1016/j.pnpbp.2013.07.020. [DOI] [PubMed] [Google Scholar]
- Rutherford LC, DeWan A, Lauer HM, Turrigiano GG. Brain-derived neurotrophic factor mediates the activity-dependent regulation of inhibition in neocortical cultures. J Neurosci. 1997;17:4527–4535. doi: 10.1523/JNEUROSCI.17-12-04527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings U.S. Department of Health and Human Services; Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. 2013 [Google Scholar]
- Sánchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Development and Psychopathology. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sarrieau A, Mitchell JB, Lal S, Olivier A, Quirion R, Meaney MJ. Androgen binding sites in human temporal cortex. Neuroendocrinology. 1990;51:713–716. doi: 10.1159/000125415. [DOI] [PubMed] [Google Scholar]
- Scheres A, Dijkstra M, Ainslie E, Balkan J, Reynolds B, Sonuga-Barke E, Castellanos FX. Temporal and probabilistic discounting of rewards in children and adolescents: effects of age and ADHD symptoms. Neuropsychologia. 2006;44:2092–2103. doi: 10.1016/j.neuropsychologia.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Scheres A, Tontsch C, Thoeny AL, Sumiya M. Temporal reward discounting in children, adolescents, and emerging adults during an experiential task. Frontiers in Psychology. 2014;5:711. doi: 10.3389/fpsyg.2014.00711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavone S, Sorce S, Dubois-Dauphin M, Jaquet V, Colaianna M, Zotti M, Cuomo V, Trabace L, Krause K-H. Involvement of NOX2 in the development of behavioral and pathologic alterations in isolated rats. Biological Psychiatry. 2009;66:384–392. doi: 10.1016/j.biopsych.2009.04.033. [DOI] [PubMed] [Google Scholar]
- Schneider M. Puberty as a highly vulnerable developmental period for the consequences of cannabis exposure. Addict Biol. 2008;13:253–263. doi: 10.1111/j.1369-1600.2008.00110.x. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Walker QD, Caster JM, Levin ED, Kuhn CM. Are adolescents more vulnerable to drug addiction than adults? Evidence from animal models. Psychopharmacology (Berl) 2009;206:1–21. doi: 10.1007/s00213-009-1585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz-Wilson KM, Menard TA, Sisk CL. Gonadal steroid hormones during puberty influence the social, submissive, and aggressive behaviors of adult male Syrian hamsters. Horm Behav. 2002;41:477–483. [Google Scholar]
- Schulz KM, Richardson HN, Zehr JL, Osetek AJ, Menard TA, Sisk CL. Gonadal hormones masculinize and defeminize reproductive behaviors during puberty in the male Syrian hamster. Horm Behav. 2004;45:242–249. doi: 10.1016/j.yhbeh.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Hormones and Behavior. 2009a;55:597–604. doi: 10.1016/j.yhbeh.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Zehr JL, Salas-Ramirez KY, Sisk CL. Testosterone programs adult social behavior before and during, but not after, adolescence. Endocrinology. 2009b;150:3690–3698. doi: 10.1210/en.2008-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MBL, Crowley WF, Aparicio SAJR, Colledge WH. The GPR54 gene as a regulator of puberty. The New England journal of medicine. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Sengpiel F. Plasticity of the visual cortex and treatment of amblyopia. Curr Biol. 2014;24:R936–R940. doi: 10.1016/j.cub.2014.05.063. [DOI] [PubMed] [Google Scholar]
- Sherrill LK, Koss WA, Foreman ES, Gulley JM. The effects of pre-pubertal gonadectomy and binge-like ethanol exposure during adolescence on ethanol drinking in adult male and female rats. Behavioural brain research. 2011;216:569–575. doi: 10.1016/j.bbr.2010.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shughrue PJ, Stumpf WE, MacLusky NJ, Zielinski JE, Hochberg RB. Developmental changes in estrogen receptors in mouse cerebral cortex between birth and postweaning: studied by autoradiography with 11 beta-methoxy-16 alpha-[125I]iodoestradiol. Endocrinology. 1990;126:1112–1124. doi: 10.1210/endo-126-2-1112. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Stumpf WE, Elger W, Schulze PE, Sar M. Progestin receptor cells in mouse cerebral cortex during early postnatal development: a comparison with preoptic area and central hypothalamus using autoradiography with [125I]progestin. Brain Res Dev Brain Res. 1991;59:143–155. doi: 10.1016/0165-3806(91)90094-y. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Sar M, Stumpf WE. Progestin target cell distribution in forebrain and midbrain regions of the 8-day postnatal mouse brain. Endocrinology. 1992;130:3650–3659. doi: 10.1210/endo.130.6.1597162. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Scrimo PJ, Merchenthaler I. Comparative distribution of estrogen receptor-alpha (ER-alpha) and beta (ER-beta) mRNA in the rat pituitary, gonad, and reproductive tract. Steroids. 1998;63:498–504. doi: 10.1016/s0039-128x(98)00054-3. [DOI] [PubMed] [Google Scholar]
- Silberg J, Pickles A, Rutter M, Hewitt J, Simonoff E, Maes H, Carbonneau R, Murrelle L, Foley D, Eaves L. The influence of genetic factors and life stress on depression among adolescent girls. Arch Gen Psychiatry. 1999;56:225–232. doi: 10.1001/archpsyc.56.3.225. [DOI] [PubMed] [Google Scholar]
- Silva-Gómez AB, Rojas D, Juárez I, Flores G. Decreased dendritic spine density on prefrontal cortical and hippocampal pyramidal neurons in postweaning social isolation rats. Brain Research. 2003;983:128–136. doi: 10.1016/s0006-8993(03)03042-7. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Sinclair D, Purves-Tyson TD, Allen KM, Weickert CS. Impacts of stress and sex hormones on dopamine neurotransmission in the adolescent brain. Psychopharmacology (Berl) 2014;231:1581–1599. doi: 10.1007/s00213-013-3415-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Hare T, Casey BJ. Frontostriatal Maturation Predicts Cognitive Control Failure to Appetitive Cues in Adolescents. Journal of Cognitive Neuroscience. 2010;23:2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somsen RJM. The development of attention regulation in the Wisconsin Card Sorting Task. Developmental Science. 2007;10:664–680. doi: 10.1111/j.1467-7687.2007.00613.x. [DOI] [PubMed] [Google Scholar]
- Sørensen K, Aksglaede L, Petersen JH, Juul A. Recent Changes in Pubertal Timing in Healthy Danish Boys: Associations with Body Mass Index. The Journal of clinical endocrinology and metabolism. 2010;95:263–270. doi: 10.1210/jc.2009-1478. [DOI] [PubMed] [Google Scholar]
- Southwell DG, Froemke RC, Alvarez-Buylla A, Stryker MP, Gandhi SP. Cortical plasticity induced by inhibitory neuron transplantation. Science. 2010;327:1145–1148. doi: 10.1126/science.1183962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescent Brain Development and Animal Models. Annals of the New York Academy of Sciences. 2004;1021:23–26. doi: 10.1196/annals.1308.002. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescent alcohol exposure: Are there separable vulnerable periods within adolescence? Physiol Behav. 2015;148:122–130. doi: 10.1016/j.physbeh.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns S. The evolution of life histories. Oxford, UK: Oxford University Press; 1992. Vol. [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: Evidence for a dual systems model. Developmental Psychology. 2008;44:1764–1778. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Graham S, O’Brien L, Woolard J, Cauffman E, Banich M. Age Differences in Future Orientation and Delay Discounting. Child Development. 2009;80:28–44. doi: 10.1111/j.1467-8624.2008.01244.x. [DOI] [PubMed] [Google Scholar]
- Stepanyants A, Chklovskii DB. Neurogeometry and potential synaptic connectivity. Trends in neurosciences. 2005;28:387–394. doi: 10.1016/j.tins.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Stice E, Presnell K, Bearman SK. Relation of early menarche to depression, eating disorders, substance abuse, and comorbid psychopathology among adolescent girls. Developmental Psychology. 2001;37:608–619. doi: 10.1037//0012-1649.37.5.608. [DOI] [PubMed] [Google Scholar]
- Thomas AW, Caporale N, Wu C, Wilbrecht L. Early maternal separation impacts cognitive flexibility at the age of first independence in mice. Developmental Cognitive Neuroscience. 2015 doi: 10.1016/j.dcn.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor DH, Holloway WR. Social play in juvenile rats: a decade of methodological and experimental research. Neuroscience and Biobehavioral Reviews. 1984;8:455–464. doi: 10.1016/0149-7634(84)90004-6. [DOI] [PubMed] [Google Scholar]
- Tschann JM, Adler NE, Irwin CE, Millstein SG, Turner RA, Kegeles SM. Initiation of substance use in early adolescence: the roles of pubertal timing and emotional distress. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 1994;13:326–333. doi: 10.1037//0278-6133.13.4.326. [DOI] [PubMed] [Google Scholar]
- Tseng K-Y, O’donnell P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb Cortex. 2007;17:1235–1240. doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. Post-pubertal emergence of prefrontal cortical up states induced by D1-NMDA co-activation. Cereb Cortex. 2005;15:49–57. doi: 10.1093/cercor/bhh107. [DOI] [PubMed] [Google Scholar]
- Tyborowska A, Volman I, Smeekens S, Toni I, Roelofs K. Testosterone during Puberty Shifts Emotional Control from Pulvinar to Anterior Prefrontal Cortex. The Journal of Neuroscience. 2016;36:6156–6164. doi: 10.1523/JNEUROSCI.3874-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos W, Cohen MX, Kahnt T, Crone EA. Striatum-Medial Prefrontal Cortex Connectivity Predicts Developmental Changes in Reinforcement Learning. Cerebral Cortex. 2012;22:1247–1255. doi: 10.1093/cercor/bhr198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Schaaf ME, Warmerdam E, Crone EA, Cools R. Distinct linear and non-linear trajectories of reward and punishment reversal learning during development: Relevance for dopamine’s role in adolescent decision making. Developmental Cognitive Neuroscience. 2011;1:578–590. doi: 10.1016/j.dcn.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijvenvoorde ACK, Zanolie K, Rombouts SARB, Raijmakers MEJ, Crone EA. Evaluating the Negative or Valuing the Positive? Neural Mechanisms Supporting Feedback-Based Learning across Development. The Journal of Neuroscience. 2008;28:9495–9503. doi: 10.1523/JNEUROSCI.1485-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg A, Piekarski DJ, Caporale N, Munoz-Cuevas FJ, Wilbrecht L. Adolescent maturation of inhibitory inputs onto cingulate cortex neurons is cell-type specific and TrkB dependent. Frontiers in Neural Circuits. 2015;9:1–10. doi: 10.3389/fncir.2015.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhorst VG, Gustafsson JA, Ulfhake B. Estrogen receptor-alpha and -beta immunoreactive neurons in the brainstem and spinal cord of male and female mice: relationships to monoaminergic, cholinergic, and spinal projection systems. J Comp Neurol. 2005;488:152–279. doi: 10.1002/cne.20569. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Niesink RJ, Van Ree JM. The neurobiology of social play behavior in rats. Neuroscience and Biobehavioral Reviews. 1997;21:309–326. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. Maturational Changes in Anterior Cingulate and Frontoparietal Recruitment Support the Development of Error Processing and Inhibitory Control. Cerebral Cortex. 2008;18:2505–2522. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen CS, Sanders KW, Spear LP. Evidence for suppressant effects of testosterone on sex-typical ethanol intake in male Sprague-Dawley rats. Behav Brain Res. 2011;224:403–407. doi: 10.1016/j.bbr.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen CS, Spear LP. Hormonal and physical markers of puberty and their relationship to adolescent-typical novelty-directed behavior. Developmental Psychobiology. 2012;54:523–535. doi: 10.1002/dev.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’hagen CS, Spear LP. The Effects of Gonadectomy on Age- and Sex-Typical Patterns of Ethanol Consumption in Sprague-Dawley Rats. Alcoholism: Clinical and Experimental Research. 2011;35:2039–2049. doi: 10.1111/j.1530-0277.2011.01555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrtička P, Sander D, Anderson B, Badoud D, Eliez S, Debbané M. Social feedback processing from early to late adolescence: influence of sex, age, and attachment style. Brain and Behavior. 2014;4:703–720. doi: 10.1002/brb3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner CK, Pfau JL, De Vries GJ, Merchenthaler IJ. Sex differences in progesterone receptor immunoreactivity in neonatal mouse brain depend on estrogen receptor alpha expression. J Neurobiol. 2001;47:176–182. doi: 10.1002/neu.1025. [DOI] [PubMed] [Google Scholar]
- Wahlstrom D, Collins P, White T, Luciana M. Developmental changes in dopamine neurotransmission in adolescence: behavioral implications and issues in assessment. Brain Cogn. 2010;72:146–159. doi: 10.1016/j.bandc.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AC, Hara Y, Janssen WG, Rapp PR, Morrison JH. Synaptic estrogen receptor-alpha levels in prefrontal cortex in female rhesus monkeys and their correlation with cognitive performance. J Neurosci. 2010;30:12770–12776. doi: 10.1523/JNEUROSCI.3192-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H-X, Gao W-J. Cell Type-Specific Development of NMDA Receptors in the Interneurons of Rat Prefrontal Cortex. 2009;34:2028–2040. doi: 10.1038/npp.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H-X, Gao W-J. Development of calcium-permeable AMPA receptors and their correlation with NMDA receptors in fast-spiking interneurons of rat prefrontal cortex. The Journal of Physiology. 2010;588:2823–2838. doi: 10.1113/jphysiol.2010.187591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Stradtman GG, Wang X-J, Gao W-J. A specialized NMDA receptor function in layer 5 recurrent microcircuitry of the adult rat prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:16791–16796. doi: 10.1073/pnas.0804318105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanzek J, Vaughn S. Research-based implications from extensive early reading interventions. School Psychology Review. 2007;36:541–561. [Google Scholar]
- Wanzek J, Wexler J, Vaughn S, Ciullo S. Reading interventions for struggling readers in the upper elementary grades: a synthesis of 20 years of research. Read Writ. 2010;23:889–912. doi: 10.1007/s11145-009-9179-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanzek J, Vaughn S. Research-Based-Implications from extensive early reading interventions. 2013:1–21. [Google Scholar]
- Watt MJ, Burke AR, Renner KJ, Forster GL. Adolescent male rats exposed to social defeat exhibit altered anxiety behavior and limbic monoamines as adults. Behavioral Neuroscience. 2009;123:564–576. doi: 10.1037/a0015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen JA, Barth AL. Input-specific critical periods for experience-dependent plasticity in layer 2/3 pyramidal neurons. J Neurosci. 2011;31:4456–4465. doi: 10.1523/JNEUROSCI.6042-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werker JF, Hensch TK. Critical periods in speech perception: new directions. Annual review of psychology. 2015;66:173–196. doi: 10.1146/annurev-psych-010814-015104. [DOI] [PubMed] [Google Scholar]
- Whittle S, Yücel M, Lorenzetti V, Byrne ML, Simmons JG, Wood SJ, Pantelis C, Allen NB. Pituitary volume mediates the relationship between pubertal timing and depressive symptoms during adolescence. Psychoneuroendocrinology. 2012;37:881–891. doi: 10.1016/j.psyneuen.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Willander J, Larsson M. The mind’s nose and autobiographical odor memory. Chemosensory perception. 2008 [Google Scholar]
- Williams BR, Ponesse JS, Schachar RJ, Logan GD, Tannock R. Development of inhibitory control across the life span. Dev Psychol. 1999;35:205–213. doi: 10.1037//0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]
- Williams H, Connor DM, Hill JW. Testosterone decreases the potential for song plasticity in adult male zebra finches. Horm Behav. 2003;44:402–412. doi: 10.1016/j.yhbeh.2003.06.005. [DOI] [PubMed] [Google Scholar]
- Willing J, Juraska JM. The timing of neuronal loss across adolescence in the medial prefrontal cortex of male and female rats. NEUROSCIence. 2015;301:268–275. doi: 10.1016/j.neuroscience.2015.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing J, Wagner CK. Exposure to the Synthetic Progestin, 17alpha-Hydroxyprogesterone Caproate During Development Impairs Cognitive Flexibility in Adulthood. Endocrinology. 2016;157:77–82. doi: 10.1210/en.2015-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ME, Westberry JM, Trout AL. Estrogen receptor-alpha gene expression in the cortex: Sex differences during development and in adulthood. Hormones and behavior. 2011;59:353–357. doi: 10.1016/j.yhbeh.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt ED. Research on alcohol and adolescent brain development: opportunities and future directions. Alcohol. 2010;44:119–124. doi: 10.1016/j.alcohol.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Wright BA, Zecker SG. Learning problems, delayed development, and puberty. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:9942–9946. doi: 10.1073/pnas.0401825101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright LD, Hébert KE, Perrot-Sinal TS. Periadolescent stress exposure exerts long-term effects on adult stress responding and expression of prefrontal dopamine receptors in male and female rats. Psychoneuroendocrinology. 2008;33:130–142. doi: 10.1016/j.psyneuen.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, Jones T, Zuo Y. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462:915–919. doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Pan F, Gan W-B. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2010;462:920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J-M, Zhang J, Yu Y-Q, Duan S, Li X-M. Postnatal Development of 2 Microcircuits Involving Fast-Spiking Interneurons in the Mouse Prefrontal Cortex. Cerebral Cortex. 2012:1–12. doi: 10.1093/cercor/bhs291. [DOI] [PubMed] [Google Scholar]
- Yang X-D, Liao X-M, Uribe-Mariño A, Liu R, Xie X-M, Jia J, Su Y-A, Li J-T, Schmidt MV, Wang X-D, Si T-M. Stress during a critical postnatal period induces region-specific structural abnormalities and dysfunction of the prefrontal cortex via CRF1. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015;40:1203–1215. doi: 10.1038/npp.2014.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young WJ, Chang C. Ontogeny and autoregulation of androgen receptor mRNA expression in the nervous system. Endocrine. 1998;9:79–88. doi: 10.1385/ENDO:9:1:79. [DOI] [PubMed] [Google Scholar]
- Zehr JL, Culbert KM, Sisk CL, Klump KL. An association of early puberty with disordered eating and anxiety in a population of undergraduate women and men. Hormones and behavior. 2007;52:427–435. doi: 10.1016/j.yhbeh.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Zhu D, King SG, Lees CJ, Bennett AJ, Salinas E, Stanford TR, Constantinidis C. Behavioral response inhibition and maturation of goal representation in prefrontal cortex after puberty. Proc Natl Acad Sci U S A. 2016;113:3353–3358. doi: 10.1073/pnas.1518147113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsarnovszky A, Belcher SM. Identification of a developmental gradient of estrogen receptor expression and cellular localization in the developing and adult female rat primary somatosensory cortex. Brain research Developmental brain research. 2001;129:39–46. doi: 10.1016/s0165-3806(01)00180-8. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Lin A, Chang P, Gan W-B. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron. 2005;46:181–189. doi: 10.1016/j.neuron.2005.04.001. [DOI] [PubMed] [Google Scholar]