Keywords: Axonal bouton, Dendritic spine, Development, Two-photon microscopy, Structural plasticity, Pruning
Abstract
The adolescent transition from juvenile to adult is marked by anatomical and functional remodeling of brain networks. Currently, the cellular and synaptic level changes underlying the adolescent transition are only coarsely understood. Here, we use two-photon imaging to make time-lapse observations of long-range axons that innervate the frontal cortex in the living brain. We labeled cells in the orbitofrontal cortex (OFC) and basolateral amygdala (BLA) and imaged their axonal afferents to the dorsomedial prefrontal cortex (dmPFC). We also imaged the apical dendrites of dmPFC pyramidal neurons. Images were taken daily in separate cohorts of juvenile (P24–P28) and young adult mice (P64–P68), ages where we have previously discovered differences in dmPFC dependent decision-making. Dendritic spines were pruned across this peri-adolescent period, while BLA and OFC afferents followed alternate developmental trajectories. OFC boutons showed no decrease in density, but did show a decrease in daily bouton gain and loss with age. BLA axons showed an increase in both bouton density and daily bouton gain at the later age, suggesting a delayed window of enhanced plasticity. Our findings reveal projection specific maturation of synaptic structures within a single frontal region and suggest that stabilization is a more general characteristic of maturation than pruning.
1. Introduction
A process of neural circuit reorganization begins in late childhood and spans the adolescent transition into young adulthood. Maturation of the frontal cortex is thought to parallel behavioral changes in cognition and decision-making that occur in adolescence (Paus et al., 2008, Somerville and Casey, 2010, Johnson and Wilbrecht, 2011). Cerebral cortex gray matter volume peaks in late childhood and then declines across adolescence (Gogtay et al., 2004), while subcortical structures follow heterogeneous patterns of maturation, with the amygdala increasing in volume and the nucleus accumbens decreasing in volume over adolescence (Ostby et al., 2009, Mills et al., 2014). Human imaging studies also show that functional and structural connectivity matures across peri-adolescence (Fair et al., 2008, Power et al., 2010, Lebel and Beaulieu, 2011), with a general trend of increased connectivity among distant regions with age. Dendritic spines, the sites of most excitatory synapses in the brain, show pruning (defined as a decrease in density) in the frontal cortex across adolescence (Huttenlocher, 1979, Huttenlocher and Dabholkar, 1997, Zuo et al., 2005, Petanjek et al., 2011).
MRI technology cannot resolve the precise cellular rewiring underlying the developmental changes in functional and structural measures observed with human imaging techniques. Human imaging studies lack the resolution of individual cells or synapses and cannot identify cell types. Two-photon in vivo microscopy allows chronic imaging of neuronal structures in vivo with submicron resolution in animal models (Denk and Svoboda, 1997, De Paola et al., 2006, Holtmaat et al., 2009, Holtmaat and Svoboda, 2009, Chen et al., 2014). This technique allows us to track the specific wiring of identified circuits across development and with experience.
Critical periods are developmental windows in which circuits are more plastic and may be sculpted by the environment or experience (Hensch, 2005). A circuit or brain region might be considered mature when greater stability is achieved after a period of experience-dependent sculpting during an earlier critical period (Takesian and Hensch, 2013). The best-studied model of the developmental transition from plasticity to stability is the adaptation of primary visual cortex to monocular deprivation (Espinosa and Stryker, 2012). It remains an open question whether there is a critical period in associative cortices, particularly in frontal cortex circuits. There is some evidence that the frontal cortex may remain plastic to some degree throughout the lifespan in response to environmental conditions such as enrichment (Kolb et al., 2003), stress (McEwen and Morrison, 2013), or learning (Lai et al., 2012, Munoz-Cuevas et al., 2013, Johnson et al., 2016). To further the understanding of typical and pathological frontal cortex development, it is important to map the timecourse of plasticity in subcircuits connecting the frontal cortex. By identifying circuits that are reorganizing at different developmental stages, we may better understand when subcircuits are more vulnerable to adverse experiences or when developmental changes may unmask pre-existing pathology (Paus et al., 2008).
In the current study, we use two-photon microscopy to perform longitudinal imaging of limbic circuits proposed to be important for decision-making (Bechara et al., 1999, Kim and Ragozzino, 2005, Johnson and Wilbrecht, 2011, Sul et al., 2011, Gremel and Costa, 2013, Izquierdo et al., 2013, Luk and Wallis, 2013, Johnson et al., 2016). These subcircuits include long-range axonal projections to dorsomedial frontal cortex (dmPFC) from the basolateral amygdala (BLA) and the orbitofronal cortex (OFC). We also study the apical dendrites of local dmPFC layer 5 pyramidal cells. The dmPFC is an accessible frontal subregion including secondary motor cortex (M2) and frontal association area (FrA) (Franklin and Paxinos, 2008) and is implicated in associative learning (Sul et al., 2011, Lai et al., 2012). We observe these different subcircuits in the dmPFC during two developmental time windows, the late juvenile and early adult period. The juvenile period we selected (postnatal days 24–28) is when rodents transition to independence after weaning and can be considered analogous to late childhood in humans. The young adult period we selected (P64–68) is a time when we have previously shown that dmPFC dependent decision-making strategies differ from the juvenile period (Johnson and Wilbrecht, 2011). Here, we show that intermingled circuits within the dmPFC show unique patterns of change in density, plasticity, and stability before and after the adolescent transition.
2. Materials and methods
2.1. Animals
Mice were bred in-house and weaned at postnatal day P21. C57Bl/6 mice were used for axonal viral labeling experiments and Thy1-YFP-H mice (strain 00378 H line; http://jaxmice.jax.org/strain/003782.html) for imaging of dendritic spines. Only male mice were included and they were group housed 2–5 to a cage with nesting material and plastic huts on a 12/12 reverse light cycle (lights off at 10 a.m.). Mouse weights were monitored closely following all surgical and imaging procedures to ensure animal health and that young mice gained weight at a developmentally appropriate rate. All animal procedures were approved by the Ernest Gallo Clinic and Research Center and UC Berkeley Animal Care and Use Committees.
2.2. Viral injections
Viral labeling was used to identify ipsilateral projections from the BLA and contralateral projections from the OFC to the dmPFC (Fig. 1, Fig. 2). Stereotaxic injections were made under isoflurane anesthesia at P10 for the juvenile group and at P51 for the adult group. Sparse labeling of cells was achieved using a Nanoject II injector (Drummond Scientific Company, Broomall, PA) to deliver 50 nl of AAV2/1-CAG-eGFP (Addgene plasmid 28014; UNC Vector Core) to either the right BLA (P10: −4.3 AP, 2.9 ML, 4.25 V; P51: −1.3 AP, 3.35 ML, 4.25 V) or the left OFC (P10: 1.0 AP, −1.6 ML, 1.9 V; P51: 2.3 AP, −1.7 ML, 2.5 V). The coordinates for P10 injection are given relative to the midline suture and the front of the brain, identified by the blood sinus visible under the skull. The coordinates for P51 injection are given relative to bregma. After surgery, mice were given access to 0.5 mg/ml acetaminophen solution (Perrigo, Allegan, MI) and 0.7 mg/ml oral sulfamethoxazole with 0.1 mg/ml trimethoprim antibiotic solution (Hi-Tech Pharmacal, Amityville, NY) in drinking water. The virus was allowed to express for two weeks prior to the start of imaging procedures for both age groups of mice.
Fig. 1.
Two-photon microscopy of local dendritic arbors and long-range afferents in the dmPFC of juvenile and adult mice. (a) Schematic of the different circuits imaged in superficial layers of dmPFC through a cranial window. (b, c), Long-range axonal projections to dmPFC were labeled by viral injections in the BLA (b) or the OFC (c). (d) We imaged the apical dendrites of layer 5 pyramidal cells in Thy1-YFP-H mice. (e) BLA axons ramify in layer 2/3 and deep layers of the dmPFC ipsilateral to the injection. (f) OFC axons ramify in layers 1 and 2 of the dmPFC contralateral to the injection. In images (d–f) the medial wall of the frontal cortex is on the left side of image. (g–i) Representative maximum intensity projections of image stacks of daily imaged dmPFC spines (g), BLA axons (h), and OFC axons (i) in juvenile and adult mice. Closed arrowheads indicate gain and open arrowheads indicate loss. Image analysis was performed on three-dimensional image stacks. Scale bars: (d–f) 100 μm, (g–i) 5 μm.
Fig. 2.
Extent of viral labeling and imaging locations. (a, b), Atlas images overlaid with tracings of the bounds of viral labeling at the injection site in the BLA (a) or OFC (b). Histology of injection sites was performed approximately 3 weeks after injection following the completion of in vivo imaging experiment. (c, d), In vivo imaging regions of interest (ROIs) for BLA (c) and OFC (d) axons. Coordinates are given lateral to the medial wall and rostral from bregma. Sampling in the dmPFC focused on the superficial layers of secondary motor cortex (M2) and extended rostrally into frontal association cortex (FrA) (Franklin and Paxinos, 2008).
2.3. Cranial window implantation
In a separate procedure 13 days after viral injection, a ∼3 mm craniotomy was made bilaterally over the dmPFC and rostral to bregma as previously described (Holtmaat et al., 2009). The dura was left intact during this procedure. Our previous data suggest that this procedure does not disrupt spine density when compared with window naïve controls (Holtmaat et al., 2009, Munoz-Cuevas et al., 2013). The craniotomy was overlaid with a thin layer of agarose solution (1% in cortex buffer) and sealed with a glass coverslip. Mice were given subcutaneous injections of an NSAID diluted in sterile saline (5 mg/kg, Rimadyl, Pfizer, New York, NY) following surgery and for the subsequent imaging days. Mice were allowed to recover one day before imaging procedures. This short recovery time was necessary to capture early developmental time points before the regrowth of bone over the craniotomy. While cranial windows remain clear for months in adult mice (Holtmaat et al., 2009), skull regrowth over dmPFC in young mice typically allows 5 days of imaging, and up to two weeks in some animals.
2.4. Two-photon longitudinal imaging
Long-range axonal projections or dendritic spines were imaged daily in juvenile (P24) and adult (P64) mice within a region extending 1 mm from midline and 3 mm rostral from bregma (Fig. 2c and d) using previously described techniques (Holtmaat et al., 2009). Dendrites or axons were imaged in the superficial layers of cortex within 250 μm of the surface (Fig. 1d–i). Neural structures were imaged using an Ultima IV laser scanning microscope (Prairie Technologies) and a water immersion 40× magnification 0.8 NA water immersion objective. A Mai Tai HP laser (Spectra Physics) was tuned to 910 nm for excitation of GFP and 950 nm for excitation of YFP. ∼80 μm segments of axon or ∼40 μm segments of dendrite were imaged with high resolution (12.05 pixels μm−1). Image stacks were obtained using a 1 μm z-step.
There are developmental changes in expression levels of YFP in layer 5 neurons (Feng et al., 2000). However, based on our previous experiments using imaging followed by electron microscopy reconstructions, we are reasonably confident that cells are bright enough such that we can detect the smallest spines at both ages (Holtmaat et al., 2006, Knott et al., 2006). Also, the number of YFP-labeled cells is adult-like by P30 (Porrero et al., 2010) and is restricted to layer 5 in frontal cortices, suggesting we are selecting from a similar subpopulation of layer 5 cells in both juvenile and adult groups.
2.5. Image processing
Images for analysis were median filtered three-dimensional z-stacks. The brightest in focus z-section was selected for measurement of each individual spine or bouton. For image presentation (Fig. 5a and b), images were median filtered and then relevant sections were projected to a two-dimensional image. The featured neurite was cropped from the full size image. Finally, the image was Gaussian filtered and contrasted for presentation.
Fig. 5.
Growth and retraction of neurite arbors is more dynamic in juveniles. (a, b) Examples of tip length change of a dmPFC apical dendrite in a juvenile (a) and a BLA → dmPFC axon in a juvenile (b). Scale bars are 5 μm. (c) Unsigned average daily change in dendrite length showed a difference with age but changes observed were not significantly greater than the measurement noise for juveniles or adults (see Section 2). (d) Unsigned average daily change in length of distal axon tips. Axons in juveniles were more dynamic than in adults. (e) Net average daily change in dendrite length showed no change with age, and changes observed were below measurement noise. (f) Net average daily growth or retraction of axon tips showed BLA axons grew on a daily basis in juveniles and not adults, while OFC axons did not show overall growth or retraction above measurement noise. Graphs show mean ± SEM. Dotted lines in (c–f) show the standard deviation of the measurement noise (σL, see Section 2). * P < 0.05, ** P < 0.01, **** P < 0.0001. # indicates not significantly different than measurement noise (P > 0.05).
2.6. Image analysis
Axonal boutons and dendritic spines were scored according to established criteria (Holtmaat et al., 2009) using custom Matlab software (Mathworks). Briefly, varicosities on axons were scored as bouton gains if the intensity was more than 3 times as bright as the adjacent axon shaft, and as bouton losses if the intensity fell below 1.3 times the shaft brightness. Dendritic spines were scored if they protruded laterally more than 0.4 μm from the dendritic shaft. We measured the total number of structures gained or lost compared to the previous imaging session and divided by the length of the neurite. Values of gain and loss were averaged across two consecutive measurements to improve reliability. Tip length of axons or dendrites was measured relative to a stationary fiducial point. For each tip, measurements of length change were averaged for all days measured. We considered the absolute value of length change as a measure of motility and the net length change as a measure of arbor expansion or retraction. To calculate measurement noise, we took the standard deviation of length measurements between two fiducial points across five days (25 ROIs from 9 mice; absolute change: mean = 1.04 μm, σL = 0.85 μm; net change: mean = 0.14 μm, σL = 1.37 μm). File names of all ROIs were recoded for analysis so that analysis was performed blind to age and axon origin. In total, we recorded 1,907 BLA boutons (from 12 mice), 2,742 OFC boutons (from 17 mice), and 2,428 dmPFC spines (from 15 mice).
2.7. Histology
All mice were transcardially perfused with 4% paraformaldehyde in PB (0.1 M, pH 7.4). Brains were removed and post-fixed in 4% paraformaldehyde overnight, and then rinsed in PB. Coronal sections (100 μm) were cut on a vibratome and mounted on slides to check injection site accuracy (Fig. 2a and b).
2.8. Statistics
Statistical analyses were performed using Matlab (Mathworks) or Graphpad Prism 5 (Graphpad Software). Distributions were tested for normality using the D’Agostino and Pearson omnibus normality test. Values are reported as mean ± SEM. Comparisons were made between age groups of the same circuit using the Mann Whitney U test. We used Kaplan–Meier survival analysis to compare stability of boutons or spines across imaging sessions. The survival curves between two groups were compared using the log-rank test, and by comparing the slope of the curves using the hazard ratio. Changes in tip length were first compared against the measurement noise using a two-way ANOVA followed by Bonferroni post hoc tests. Then the change in tip length for each circuit was compared between juveniles and adults using the Mann Whitney U test.
3. Results
3.1. Density
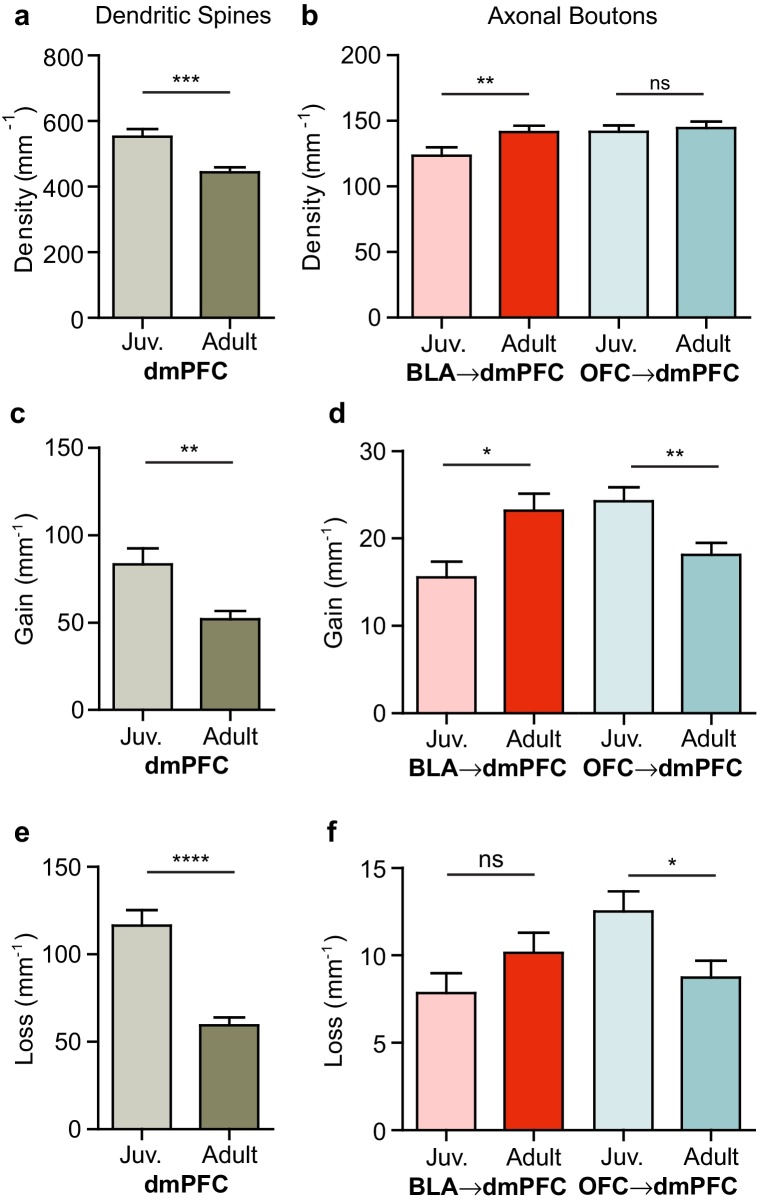
We used viral labeling or the Thy1-YFP-H transgenic line to fluorescently label specific circuits and observe synaptic structures in different circuits in the frontal cortex in the living brain (Fig. 1, Fig. 2). We imaged cohorts of juvenile (P24–P28) and adult (P64–P68) mice with labeling of BLA or OFC axonal projections to dmPFC, or of dendrites of local layer 5 pyramidal cells in dmPFC. We used in vivo two-photon microscopy to obtain high resolution images of pre-synaptic axonal boutons or post-synaptic dendritic spines from the upper layers of dmPFC (Fig. 1g–i). First, we compared the densities of boutons or spines between juveniles and adults for each circuit. In accordance with previous studies (Zuo et al., 2005, Petanjek et al., 2011), we observed a net decrease in spine density in adults compared to juveniles (juvenile: 552 ± 23 spines mm−1, adult: 444 ± 15 spines mm−1; U(77) = 399, P = 0.0005; Fig. 3a). However, densities of axonal boutons followed distinct trajectories. We found that the density of boutons on axons projecting from the BLA to the dmPFC increased in adulthood (juvenile: 124 ± 6 boutons mm−1, adult: 142 ± 5 boutons mm−1, U(124) = 1,392, P = 0.008; Fig. 3b). In contrast, we found no difference in OFC → dmPFC bouton density across ages (juvenile: 142 ± 5 boutons mm−1, adult: 145 ± 5 boutons mm−1, U(154) = 2,817, P = 0.44; Fig. 3b). These observations indicate that not all subcircuits of excitatory synapses in the frontal cortex show net pruning during adolescent development.
Fig. 3.
Circuit-specific developmental windows of plasticity and growth. (a) The density of spines on apical dendrites of local layer 5 pyramidal cells in dmPFC declined with age. (b) The density of BLA boutons increased with age while the density of OFC boutons was stable. (c) Daily spine gain on dmPFC apical dendrites was enhanced in juveniles compared to adults. (d) Daily bouton gain on BLA afferents was enhanced in adults and bouton gain on OFC afferents was enhanced in juveniles. (e) Daily spine loss on dmPFC apical dendrites was enhanced in juveniles. (f) Daily bouton loss on BLA afferents was not significantly different between ages and bouton loss on OFC afferents was increased in juveniles. Data in (a–f) are normalized to the neurite length. Graphs show mean ± SEM. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001.
3.2. Spine and bouton plasticity
Two-photon longitudinal imaging allows us to go beyond traditional static measures, such as density, to probe the dynamics of a circuit over time. To capture these dynamics, we measured the numbers of spines or boutons gained or lost from the previous day. Consistent with the finding of decreasing spine density with age, we found that the rates of both spine gain (juvenile: 83 ± 9, adult: 52 ± 5; U(77) = 467, P = 0.006; Fig. 3c) and loss (juvenile: 117 ± 9, adult: 59 ± 4, U(77) = 257, P < 0.0001; Fig. 3e) were higher in juveniles, with losses outpacing gains. Similarly, OFC → dmPFC axons also had elevated rates of both bouton gain (juvenile: 24 ± 2, adult: 18 ± 1; U(154) = 2,289, P = 0.008; Fig. 3d) and bouton loss in juveniles compared to adults (juvenile: 13 ± 1, adult: 9 ± 1; U(154) = 2,363, P = 0.02; Fig. 3f). Following the opposite pattern, BLA → dmPFC axons increased their rate of bouton gain from the juvenile to the adult period (juvenile: 16 ± 2, adult: 23 ± 2; U(124) = 842.5, P = 0.02; Fig. 3d) with no significant change in the rate of bouton loss (juvenile: 8 ± 1, adult: 10 ± 1; U(124) = 1,039, P = 0.32; Fig. 3f). These data were consistent with the developmental increase in bouton density in BLA → dmPFC axons. In sum, we found that BLA → dmPFC axons showed enhanced plasticity in adulthood, while OFC → dmPFC axons and dmPFC dendrites were more dynamic in juveniles.
3.3. Spine and bouton stability
We next calculated the stable fraction of individual spines or boutons from the first session to subsequent sessions to measure the relative size of the stable versus the dynamic pool (defined as the inverse of the stable fraction) of synapses over time. This measure adds additional information about gain and loss, indicating if a small proportion of structures turnover (large stable fraction) or whether most of the structures are vulnerable to turnover (small stable fraction). The stable fraction of dmPFC spines was significantly lower in juveniles (juvenile: 0.66 ± 0.01, adult: 0.70 ± 0.01 at 4 days; χ2 = 6.91, P = 0.009, hazard ratio = 1.26; Fig. 4a). We found no difference in the stable fraction of BLA → dmPFC boutons comparing juvenile and adult animals (juvenile: 0.85 ± 0.02, adult: 0.83 ± 0.02 at 4 days; χ2 = 0.89, P = 0.34; Fig. 4b). The stable fraction of OFC → dmPFC boutons was significantly lower in juvenile mice (juvenile: 0.78 ± 0.02, adult: 0.86 ± 0.01 at 4 days; χ2 = 21.70, P < 0.0001; Fig. 4c), with bouton destabilization occurring at nearly twice the rate as in adults (hazard ratio = 1.74).
Fig. 4.
The stable fraction of total synaptic structures increased with age, but stabilization of new structures was age independent. (a–c) The stable fraction of total structures present on the first day of imaging that were present on subsequent days in apical dmPFC spines (a), BLA boutons (b), or OFC boutons (c). Open symbols are juveniles and closed symbols are adults. (d–f), There was no effect of age on stabilization of newly gained dmPFC spines (d), BLA boutons (e), or OFC boutons (f). Graphs show mean ± SEM.
The stable fraction of new synaptic structures is also an informative metric because new persistent synapses could alter the circuitry to a greater extent than transient connections. We found that age of the mice did not affect the stabilization rate of new spines or boutons. New dmPFC spines showed no differences in stabilization between ages (juveniles: 206 spines, 0.50 ± 0.05; adults: 95 spines, 0.49 ± 0.06; χ2 = 0.34, P = 0.56; Fig. 4d). We also found no age differences in the stability of new BLA → dmPFC boutons (juveniles: 45 boutons, 0.32 ± 0.08; adults: 116 boutons, 0.25 ± 0.05; χ2 = 1.28, P = 0.26; Fig. 4e) or in new OFC → dmPFC boutons (juveniles: 162 boutons, 0.70 ± 0.04; adults: 129 boutons, 0.65 ± 0.04; χ2 = 1.22, P = 0.27; Fig. 4f). Comparing circuits, we did find that overall new BLA → dmPFC boutons were significantly less stable than new OFC → dmPFC boutons (pooled juvenile and adult, BLA: 0.28 ± 0.04, OFC: 0.68 ± 0.03; χ2 = 42.81, P < 0.0001).
3.4. Tip dynamics
In addition to quantifying the dynamics of synaptic structures, we also studied larger scale changes in the axonal or dendritic arbors. We measured the change in length of the tips of dendrites and axons across days in the same animals (Fig. 5a and b). First, we considered the absolute amount that length changed on average over 24 hours, irrespective of whether it was growth or retraction. Apical dendrite tips were more dynamic in adults compared to juveniles (juveniles: 1.4 ± 0.2 μm, adults: 2.3 ± 0.2 μm, U(65) = 364.0, P = 0.02; Fig. 5c). However these values were small and they were not significantly different than the measurement noise (P > 0.05). We found that axon tips were significantly more motile in juveniles than in adults for both BLA axons (juveniles: 7.3 ± 1.5 μm, adults: 1.0 ± 0.3 μm, U(18) = 11.0, P = 0.004; Fig. 5d) and OFC axons (juveniles: 6.0 ± 1.2 μm, adults: 1.2 ± 0.3 μm, U(42) = 47.0, P < 0.0001; Fig. 5d). By considering the density of boutons or spines at a developmental stage, we calculate that these changes in distal tip length represent additional plasticity of ∼1 bouton (juvenile BLA: 0.9 boutons/day; juvenile OFC: 0.86 boutons/day) or ∼1 spine per day (dmPFC dendrites: 1.0 spines/day). However, we often observed a greater density of structures near the distal tips such that these calculations likely underestimate the synaptic plasticity from tip remodeling.
Next, we calculated the net change in tip length to quantify whether overall the arbors were expanding or shrinking. Apical dmPFC dendrites (juveniles: −0.4 ± 0.2 μm, adults: −0.01 ± 0.24 μm, U(65) = 499.0, P = 0.55; Fig. 5e) and OFC axons (juveniles: 0.1 ± 1.5 μm, adults: −0.3 ± 0.3 μm, U(42) = 175.0, P = 0.50; Fig. 5f) had no net change in length, indicating that growth and retraction events were balanced at both ages. BLA axons showed net growth in juvenile animals compared to adults (juveniles: 5.0 ± 2.1 μm, adults: −0.08 ± 0.3 μm, U(18) = 17.0, P = 0.02; Fig. 5f), suggesting active arbor expansion during the juvenile imaging period (P24–28).
4. Discussion
We labeled specific subcircuits in the limbic-frontal affective system and used chronic two-photon microscopy to follow the density and stability of axons and dendrites in the living brains of juvenile and adult mice. We measured the gain and loss of pre-synaptic boutons and post-synaptic spines, and measured the length of axonal and dendritic tips across time. We replicated the finding from human and rodent studies that dendritic spines show net pruning across this peri-adolescent period (Huttenlocher and Dabholkar, 1997, Zuo et al., 2005, Majewska et al., 2006, Petanjek et al., 2011). Critically, we show that pruning is not the universal developmental pattern for excitatory subcircuits in the frontal cortex. We found that long-range BLA → dmPFC axons increased their bouton density across this time period, while the density of boutons on OFC → dmPFC axons remained stable over the juvenile to adult transition. Furthermore, while both dmPFC spines and OFC boutons showed greater daily gains and losses in juvenile animals, BLA boutons were gained at a greater rate in adult mice, indicating late developmental enhancement of plasticity for the BLA → dmPFC circuit. Taken together, we show that neural processes present within the same cortical territory follow unique patterns of maturation.
A benefit of two-photon imaging techniques over traditional postmortem histology is that we are able to follow the stability of individual synaptic structures over multiple days. Analysis revealed that the stability of previously established structures was typically age-dependent while stabilization of new structures was age-independent. This suggests that different mechanisms regulate early versus long term stability of synaptic structures, and that only the latter is altered during development. We propose that long-term stabilization of synaptic structures may be a more general property of developmental maturation than pruning.
Sampling at only two ages, we cannot rule out non-linear changes that may occur over the pubertal transition, such as an inverted U shape. Imaging studies at additional ages could further characterize the pattern of adolescent maturation of these circuits. We were unable to follow the same neurites from the juvenile period through adulthood because the skull regrows over the medial parts of the cranial window in juvenile mice after ∼5 days. An alternative methodology of skull thinning could be used, though this is generally restricted to a smaller cortical area and few imaging sessions and is therefore not as suitable to capture the daily dynamics of sparse density synaptic structures on long-range axons or rare axonal tips. Xu et al. (2007) have argued that the process of opening a craniotomy and implanting a cranial window may lead to persistent increases in spine turnover, and it is unknown how this may interact with synapse and axon maturation in the developing brain. Further studies, however, have suggested that cranial windows do not alter spine density compared to naïve brains but these studies have focused on adults (Holtmaat et al., 2009, Munoz-Cuevas et al., 2013). New technologies for transcranial imaging may facilitate future developmental studies of dendrites and axons in vivo.
In addition to the gain and loss of synaptic structures, we also measured the change in length of distal tips of axons and dendrites to test whether the neurite arbors were growing or retracting during this developmental time period. We found that in juvenile mice, axons of both BLA and OFC projections to dmPFC were more dynamic than in adult animals. These observations of tip dynamics made from P24 to P28 can be contrasted with findings from barrel cortex showing that axonal tip dynamics decrease to minimal levels by P19 in somatosensory cortex (Portera-Cailliau et al., 2005). This is consistent with the idea that the frontal cortex matures later than more caudal primary sensory regions (Gogtay et al., 2004). In particular, BLA axons had a net increase in length in juveniles, showing that this connection is still actively growing during the peri-adolescent period. These data are consistent with histological studies of BLA innervation of frontal cortex in rats showing increased density of fibers and axo-dendritic contacts with age (Cunningham et al., 2002, Cunningham et al., 2008). Our findings also support human imaging studies showing a developmental increase in amygdala volume (Ostby et al., 2009) and enhanced amygdala functional connectivity with mPFC beginning at the transition from childhood to adolescence (Gabard-Durnam et al., 2014). Our data provide in vivo synaptic level extension and confirmation of these studies and reveal the circuit specificity of this process.
The gain and loss of dendritic spines and axonal boutons can be interpreted as a process of exploration of potential wiring diagrams. ‘Potential synapses’ are sites where pre-synaptic and post-synaptic structures are within close proximity and the outgrowth of spines may bridge the gap and allow synapse formation (Stepanyants et al., 2002). The number of potential synapses is regulated by arbor sizes and overlap, occupancy by existing synapses (density), and the rate of gain and loss of synaptic structures (Stepanyants et al., 2002, Chklovskii et al., 2004, Stepanyants and Chklovskii, 2005). The developmental changes in spine and bouton turnover which we document here likely reflect sampling of potential synapses. The increase in BLA axonal arbor size in the juvenile period along with increasing gains in the adult period is particularly interesting as it suggests increased growth and sampling of potential connectivity between the BLA and its targets in the dmPFC with development. In contrast, developmental declines in gain and loss of OFC boutons and dmPFC spines suggest stabilization of these circuit ‘diagrams’ and less potential for change within these circuits with development.
A model of cortical development derived from years of research in the visual system proposes that inputs to cortex compete for resources to form stable synapses (Espinosa and Stryker, 2012, Trachtenberg, 2015). The adolescent frontal cortex could be a similar battleground for competing inputs, with the consequences of skirmishes resulting in synapse stabilization or elimination. The dmPFC integrates inputs from many brain regions (DeNardo et al., 2015), making it challenging to study this kind of competition. A recent study of BLA and ventral hippocampal (vHC) inputs to mPFC shows that vHC lesions in early life increased adult BLA bouton density and fiber density in mPFC, consistent with the idea that inputs compete (Guirado et al., 2016). Both OFC and BLA long range axons make functional synapses onto layer 5 pyramidal cells in dmPFC (DeNardo et al., 2015, Johnson et al., 2016). OFC and BLA inputs may potentially compete for synaptic partners on the same layer 5 pyramidal cells.
Following this model, alterations in the developmental trajectory of any of these three circuits could result in downstream effects in competing circuits. For example, early life adversity results in adult-like decision-making during adolescence (Thomas et al., 2016) as well as adult-like amygdala-mPFC connectivity in human adolescents (Gee et al., 2013). Future studies may test whether accelerated maturation of BLA → dmPFC connectivity accompanies premature pruning of other frontal circuits, such as OFC inputs or local dmPFC pyramidal cells. We have previously shown that the density and plasticity of OFC → dmPFC axonal boutons relates to individual differences in decision-making strategies in adult mice (Johnson et al., 2016), though additional studies are needed to test if this structure–function relationship extends to adolescent animals and if it may be disrupted by early life stress.
5. Conclusions
Neural circuit maturation in the frontal cortex is a rich dynamic process of gain and loss, likely reflective of experience-related neural activity. Not all excitatory circuits in the frontal cortex undergo net pruning during the adolescent period. BLA inputs to dmPFC show a developmental pattern of growth while OFC inputs and local dmPFC cells show decreased plasticity with age. Stabilization of synaptic turnover may be a more generalizable developmental phenomenon than pruning. We also extend the growing corpus of data obtained using viral and transgenic methods to reveal the importance of cell type and circuit specificity in the study of neural development and plasticity. Further work on these developmental transitions in structural plasticity may also illuminate what makes brains vulnerable to psychopathology and inform efforts to promote positive development. Our subcircuit specific normative data thus provide a foundation on which to build a better model of brain development, function, and pathology.
Acknowledgements
This work was supported by the State of California, the US National Institutes of Health (R01MH087542 and R01DA029150), the National Science Foundation (NSF GRFP), and the P. Royer and K. Clayton Family.
References
- Bechara A., Damasio H., Damasio A.R., Lee G.P. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J. Neurosci. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.-C., Lu J., Zuo Y. Spatiotemporal dynamics of dendritic spines in the living brain. Front. Neuroanat. 2014:8. doi: 10.3389/fnana.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chklovskii D.B., Mel B.W., Svoboda K. Cortical rewiring and information storage. Nature. 2004;431:782–788. doi: 10.1038/nature03012. [DOI] [PubMed] [Google Scholar]
- Cunningham M.G., Bhattacharyya S., Benes F.M. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J. Comp. Neurol. 2002;453:116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Cunningham M.G., Bhattacharyya S., Benes F.M. Increasing interaction of amygdalar afferents with gabaergic interneurons between birth and adulthood. Cereb. Cortex. 2008;18:1529–1535. doi: 10.1093/cercor/bhm183. [DOI] [PubMed] [Google Scholar]
- De Paola V., Holtmaat A., Knott G., Song S., Wilbrecht L., Caroni P., Svoboda K. Cell type-specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron. 2006;49:861–875. doi: 10.1016/j.neuron.2006.02.017. [DOI] [PubMed] [Google Scholar]
- DeNardo L.A., Berns D.S., DeLoach K., Luo L. Connectivity of mouse somatosensory and prefrontal cortex examined with trans-synaptic tracing. Nat. Neurosci. 2015;18:1687–1697. doi: 10.1038/nn.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk W., Svoboda K. Photon upmanship: why multiphoton imaging is more than a gimmick. Neuron. 1997;18:351–357. doi: 10.1016/s0896-6273(00)81237-4. [DOI] [PubMed] [Google Scholar]
- Espinosa J.S., Stryker M.P. Development and plasticity of the primary visual cortex. Neuron. 2012;75:230–249. doi: 10.1016/j.neuron.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Cohen A.L., Dosenbach N.U.F., Church J.A., Miezin F.M., Barch D.M., Raichle M.E., Petersen S.E., Schlaggar B.L. The maturing architecture of the brain's default network. Proc. Natl. Acad. Sci. U. S. A. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G., Mellor R.H., Bernstein M., Keller-Peck C., Nguyen Q.T., Wallace M., Nerbonne J.M., Lichtman J.W., Sanes J.R. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Franklin K.B.J., Paxinos G. Academic Press; 2008. The Mouse Brain in Stereotaxic Coordinates. [Google Scholar]
- Gabard-Durnam L.J., Flannery J., Goff B., Gee D.G., Humphreys K.L., Telzer E., Hare T., Tottenham N. The development of human amygdala functional connectivity at rest from 4 to 23 years: a cross-sectional study. Neuroimage. 2014;95:193–207. doi: 10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Gabard-Durnam L.J., Flannery J., Goff B., Humphreys K.L., Telzer E.H., Hare T.A., Bookheimer S.Y., Tottenham N. Early developmental emergence of human amygdala–prefrontal connectivity after maternal deprivation. Proc. Natl. Acad. Sci. U. S. A. 2013;110:15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Nugent T.F., Herman D.H., Clasen L.S., Toga A.W., Rapoport J.L., Thompson P.M. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel C., Costa R. Premotor cortex is critical for goal-directed actions. Front. Comput. Neurosci. 2013;7 doi: 10.3389/fncom.2013.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirado R., Umemori J., Sipilä P., Castrén E. Evidence for competition for target innervation in the medial prefrontal cortex. Cereb. Cortex. 2016;3:1287–1294. doi: 10.1093/cercor/bhv280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch T.K. Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Holtmaat A., Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat. Rev. Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Holtmaat A., Wilbrecht L., Knott G.W., Welker E., Svoboda K. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature. 2006;441:979–983. doi: 10.1038/nature04783. [DOI] [PubMed] [Google Scholar]
- Holtmaat A., Bonhoeffer T., Chow D.K., Chuckowree J., De Paola V., Hofer S.B., Hubener M., Keck T., Knott G., Lee W.-C.A., Mostany R., Mrsic-Flogel T.D., Nedivi E., Portera-Cailliau C., Svoboda K., Trachtenberg J.T., Wilbrecht L. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat. Protoc. 2009;4:1128–1144. doi: 10.1038/nprot.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher P.R. Synaptic density in human frontal cortex – developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher P.R., Dabholkar A.S. Regional differences in synaptogenesis in human cerebral cortex. J. Comp. Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Izquierdo A., Darling C., Manos N., Pozos H., Kim C., Ostrander S., Cazares V., Stepp H., Rudebeck P.H. Basolateral amygdala lesions facilitate reward choices after negative feedback in rats. J. Neurosci. 2013;33:4105–4109. doi: 10.1523/JNEUROSCI.4942-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C., Wilbrecht L. Juvenile mice show greater flexibility in multiple choice reversal learning than adults. Dev. Cogn. Neurosci. 2011;1:540–551. doi: 10.1016/j.dcn.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C., Peckler H., Tai L.-H., Wilbrecht L. Rule learning enhances structural plasticity of long range axons in frontal cortex. Nat. Commun. 2016;7:10785. doi: 10.1038/ncomms10785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Ragozzino M.E. The involvement of the orbitofrontal cortex in learning under changing task contingencies. Neurobiol. Learn. Mem. 2005;83:125–133. doi: 10.1016/j.nlm.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott G.W., Holtmaat A., Wilbrecht L., Welker E., Svoboda K. Spine growth precedes synapse formation in the adult neocortex in vivo. Nat. Neurosci. 2006;9:1117–1124. doi: 10.1038/nn1747. [DOI] [PubMed] [Google Scholar]
- Kolb B., Gorny G., Soderpalm A.H., Robinson T.E. Environmental complexity has different effects on the structure of neurons in the prefrontal cortex versus the parietal cortex or nucleus accumbens. Synapse. 2003;48:149–153. doi: 10.1002/syn.10196. [DOI] [PubMed] [Google Scholar]
- Lai C.S., Franke T.F., Gan W.B. Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature. 2012;483:87–91. doi: 10.1038/nature10792. [DOI] [PubMed] [Google Scholar]
- Lebel C., Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J. Neurosci. 2011;31:10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk C.-H., Wallis J.D. Choice coding in frontal cortex during stimulus-guided or action-guided decision-making. J. Neurosci. 2013;33:1864–1871. doi: 10.1523/JNEUROSCI.4920-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska A.K., Newton J.R., Sur M. Remodeling of synaptic structure in sensory cortical areas in vivo. J. Neurosci. 2006;26:3021–3029. doi: 10.1523/JNEUROSCI.4454-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Morrison J.H. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79:16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills K.L., Goddings A.L., Clasen L.S., Giedd J.N., Blakemore S.J. The developmental mismatch in structural brain maturation during adolescence. Dev. Neurosci. 2014;36:147–160. doi: 10.1159/000362328. [DOI] [PubMed] [Google Scholar]
- Munoz-Cuevas F.J., Athilingam J., Piscopo D., Wilbrecht L. Cocaine-induced structural plasticity in frontal cortex correlates with conditioned place preference. Nat. Neurosci. 2013;16:1367–1369. doi: 10.1038/nn.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostby Y., Tamnes C.K., Fjell A.M., Westlye L.T., Due-Tonnessen P., Walhovd K.B. Heterogeneity in subcortical brain development: a structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J. Neurosci. 2009;29:11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T., Keshavan M., Giedd J.N. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petanjek Z., Judaš M., Šimić G., Rašin M.R., Uylings H.B.M., Rakic P., Kostović I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc. Natl. Acad. Sci. U. S. A. 2011;108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrero C., Rubio-Garrido P., Avendaño C., Clascá F. Mapping of fluorescent protein-expressing neurons and axon pathways in adult and developing Thy1-eYFP-H transgenic mice. Brain Res. 2010;1345:59–72. doi: 10.1016/j.brainres.2010.05.061. [DOI] [PubMed] [Google Scholar]
- Portera-Cailliau C., Weimer R.M., De Paola V., Caroni P., Svoboda K. Diverse modes of axon elaboration in the developing neocortex. PLoS Biol. 2005;3:e272. doi: 10.1371/journal.pbio.0030272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Fair D.A., Schlaggar B.L., Petersen S.E. The development of human functional brain networks. Neuron. 2010;67:735–748. doi: 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Casey B.J. Developmental neurobiology of cognitive control and motivational systems. Curr. Opin. Neurobiol. 2010;20:236–241. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanyants A., Chklovskii D.B. Neurogeometry and potential synaptic connectivity. Trends Neurosci. 2005;28:387–394. doi: 10.1016/j.tins.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Stepanyants A., Hof P.R., Chklovskii D.B. Geometry and structural plasticity of synaptic connectivity. Neuron. 2002;34:275–288. doi: 10.1016/s0896-6273(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Sul J.H., Jo S., Lee D., Jung M.W. Role of rodent secondary motor cortex in value-based action selection. Nat. Neurosci. 2011;14:1202–1208. doi: 10.1038/nn.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takesian A.E., Hensch T.K. Chapter 1 – balancing plasticity/stability across brain development. Prog. Brain Res. 2013;207:3–34. doi: 10.1016/B978-0-444-63327-9.00001-1. [DOI] [PubMed] [Google Scholar]
- Thomas A.W., Caporale N., Wu C., Wilbrecht L. Early maternal separation impacts cognitive flexibility at the age of first independence in mice. Dev. Cogn. Neurosci. 2016;18:49–56. doi: 10.1016/j.dcn.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtenberg J.T. Competition, inhibition, and critical periods of cortical plasticity. Curr. Opin. Neurobiol. 2015;35:44–48. doi: 10.1016/j.conb.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H.-T., Pan F., Yang G., Gan W.-B. Choice of cranial window type for in vivo imaging affects dendritic spine turnover in the cortex. Nat. Neurosci. 2007;10:549–551. doi: 10.1038/nn1883. [DOI] [PubMed] [Google Scholar]
- Zuo Y., Lin A., Chang P., Gan W.-B. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron. 2005;46:181–189. doi: 10.1016/j.neuron.2005.04.001. [DOI] [PubMed] [Google Scholar]