Abstract
Objective
Antiphospholipid antibody syndrome (APS) is one of the most common acquired thrombophilic disorders resulting in arterial and venous thromboses. APS is a major cause for cerebrovascular accidents or stokes, myocardial infarction, venous thromboembolism and recurrent abortions/pregnancy losses especially in young patients. APS patients have an increased risk of atherosclerotic cardiovascular events. There are only two studies on lipid abnormalities in APS patients. None of them have studied the relationship between individual laboratory tests for APS and lipid profile abnormalities. Here we describe the significance of the relationship between various APS tests and lipid profile abnormalities in a subset of APS patients who presented with arterial thrombosis in a tertiary care hospital.
Material and Methods
The study was conducted at Government Medical College, which is a tertiary care referral hospital. All patients who presented to the medicine department with APS during a two-year period were studied. A patient was considered to be positive for anticardiolipin (aCL) antibody or anti-β2 glycoprotein (anti-β2G) if the titer was more than 15 IU/mL, and a high titer was considered to be more than 40 IU/mL for Immunoglobulin (lg) IgG and IgM isotypes. The fasting lipid profile was measured in all patients, and lipid profile abnormalities were defined with cutoffs of low-density lipoprotein (LDL) levels of >150 mg/dL, triglyceride (TG) levels of >150 mg/dL, and high-density lipoprotein (HDL) levels of <40 mg/dL. The relationship between lipid abnormalities and individual tests for APS, aCL IgG and IgM and anti-β2G IgG and IgM, were determined by statistical analysis.
Results
The study population included 77 APS patients, with 53% of patients between 20 and 40 years. The commonest abnormality in the lipid profile test was elevated TG levels of >150 mg/dL in 51.9% of the patients, followed by low HDL levels (<40 mg/dL) in 38.9% of the patients and high LDL levels (>150 mg/dL) in 40.2% of the patients. There was a statistically significant relationship between anti-β2G IgG levels and HDL and LDL levels, but not TG levels. Only LDL levels had a statistically significant relationship with aCL IgM levels. None of the lipid abnormalities had any statistically significant relationship with aCL IgG levels.
Conclusion
This study highlights the importance of testing lipid profile abnormalities in APS patients and the existence of a statistically significant relationship between antiphospholipid antibody tests and lipid profile abnormalities.
Keywords: APS, dyslipidemia, anti cardiolipin antibodies, anti beta 2 glycoprotein, fasting lipid profile
Introduction
Antiphospholipid antibody syndrome (APS) is one of the most common acquired thrombophilic disorder resulting in major arterial thrombotic events such as stroke, myocardial infarction, venous thrombosis, and recurrent pregnancy loss (1, 2). APS is an important cause of cerebrovascular accidents (stroke) or myocardial infraction in young patients particularly in those with no other significant risk factor for thrombosis. Arterial thrombosis patients most commonly present with transient ischemic attack or stroke (50%) or myocardial infarction (23%) (1, 2). APS is diagnosed if patients are persistently positive for antiphospholipid antibodies (aPLs) and satisfy clinical criteria (1). These antibodies are a heterogeneous group of autoantibodies, and tests commonly detect anticardiolipin (aCL) antibody IgG and IgM, anti-β2 glycoprotein I (anti-β2G) IgG and IgM, and lupus anticoagulant test (LA) (1).
Antiphospholipid antibody syndrome is a largely unrecognized problem in many patients, particularly in the Indian subcontinent. Many cases of APS have not been recognized as patients are not evaluated due to the lack of awareness and also due to economic constraints. Many patients with young stroke, recurrent stroke, young myocardial infarction, venous thromboembolic diseases, and recurrent pregnancy losses may have APS as an etiological factor. Systemic lupus erythematosus (SLE) and APS patients have a high risk of atherosclerotic cardiovascular events as part of the inflammatory response (3). These autoimmune disorders may contribute by themselves to thrombotic manifestations in addition to other risk factors for atherosclerosis such as dyslipidemia, smoking, obesity, and hypertension. There are very few publications on lipid abnormalities in APS patients (4, 5). It has been noted in two studies that lipid abnormalities in APS patients were more than those in the general population (4, 5). The finding noted in one of the studies was that patients with APS had lower high-density lipoprotein (HDL) levels than those without APS. However, the study did not test the relationship between individual laboratory tests for APS and lipid profile abnormalities (5). No study has determined the relationship between individual laboratory tests for APS and lipid profile abnormalities (6–8). We analyzed the relationship between various tests for APS and lipid profile abnormalities in a subset of APS patients who presented with major arterial thrombotic events in a tertiary care hospital.
Material and Methods
The study was conducted at Government Medical College, which is the largest tertiary level referral hospital and teaching institution in the state of Kerala, South India. The study was approved by the Institutional Ethics Committee, and signed informed consent was taken from all included patients. All patients who presented to the Medicine Department of the Government Medical College with major arterial thrombotic events in the form of stroke or myocardial infarction as the clinical manifestation of APS during the two-year period from September 2009 to August 2011 were studied. The aCL and anti-β2G tests of IgG and IgM performed done on the patients 12 weeks apart, and the higher of the two values of each parameter was taken for analysis to determine the relationship with lipid abnormalities. The tests were performed using quantitative enzyme-linked immunosorbant assay kits (EUROIMMUN Medizinische Labordiagnostika AG; Lübeck, Germany). Patients were considered to be positive for aCL antibodies or anti-β2G if the titer was more than 15 IU/mL, and a high titer was considered to be more than 40 IU/mL for IgG and IgM isotypes (1, 2). The lupus anticoagulant test was performed by a dilute Russell Viper Venom Time-based lupus anticoagulant detection system (Tulip Diagnostics (P) Ltd; Goa, India). The fasting lipid profile was measured in all patients using a fully automated biochemistry analyzer (Biolis 24i, Tokyo Boeki Medical Systems Ltd.; Tokyo, Japan). Lipid profile abnormalities were defined with cutoffs of low-density lipoprotein (LDL) levels of >150 mg/dL, triglyceride (TG) levels of >150 mg/dL, and HDL levels of <40 mg/dL. The relationship between lipid abnormalities and individual tests for APS, aCL IgG and IgM and anti-β2G IgG and IgM, was determined by statistical analysis. Data obtained from the study were subjected to statistical scrutiny using Statistical Package for Social Science 16, windows version 16 (SPSS Inc.; Chicago, IL, USA). There were only very few lupus anticoagulant-positive patients (n=11) considered in the study. The lupus anticoagulant test could not be performed on all patients due to technical issues as many of them were on oral anticoagulants.
Results
The study population comprised 77 APS patients, a majority of whom (53%) were in the age group of 20–40 years. In total, 58% of the patients were males and 42% were females. The following were our salient observations.
Lipid profile abnormalities among APS patients
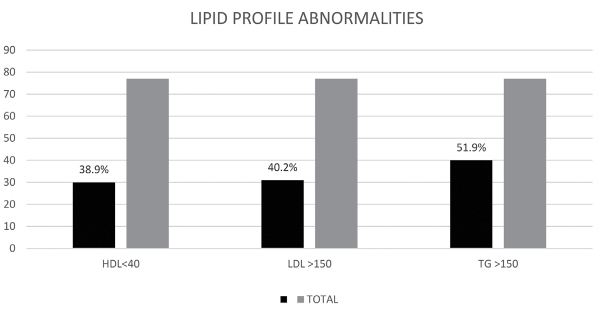
The commonest abnormality noted was elevated TG levels of >150 mg/dL seen in 51.9% of the patients. Low HDL levels (<40mg/dL) were seen in 38.9% of the patients, while 40.2% of the patients had high LDL levels (>150 mg/dL) (Figure 1).
Figure 1.
Abnormalities in lipid profile among APS patients
APS: antiphospholipid antibody syndrome; HDL: high-density lipoprotein level of <40mg/dL; LDL: low-density lipoprotein level of >150mg/dL; TG: triglyceride level of >150mg/dL
Relationship between APS tests and individual lipid profile abnormalities
Relationship between aCL IgG levels and various lipid abnormalities
As 72.1% of the patients with moderate-to-high aCL IgG levels had normal HDL levels, there was no statistically significant relationship between the two parameters (p=0.4865). Similar results were obtained for LDL levels (p=0.199). Regarding TG levels, a majority of patients with low aCL IgG levels had TG levels above 150 mg/dL, showing no significance (p=0.489).
Thus, no lipid abnormality had any statistically significant relationship with aCL IgG levels.
Relationship between aCL IgM levels and lipid abnormalities
On analyzing the relationship between aCL IgM and LDL levels, 67.4% of the patients with low aCL IgM levels had normal LDL levels and that this relationship was statistically significant with p value of 0.0146 (Table 1). As only 35.7% of the patients with moderate-to-high aCL IgM levels had HDL levels below 40 mg/dL, the relationship between the two parameters was not statistically significant, and the p value was 0.984. Moreover, there was no statistically significant relationship between aCL IgM levels and TG levels as 68.4% of the patients with low aCL IgM levels had TG levels above 150 mg/dL (p=0.613).
Table 1.
Relationship between aCL IgM and LDL levels
| LDL mg/dL | aCL IgM levels IU/mL | |||
|---|---|---|---|---|
|
| ||||
| <15 | 15–40 | >40 | TOTAL | |
| <150 | 29 | 2 | 12 | 43 |
| >150 | 16 | 8 | 4 | 28 |
| TOTAL | 45 | 10 | 16 | 71 |
aCL: anticardiolipin antibody; LDL: low-density lipoprotein
Thus, only LDL level had statistically significant relationship with aCL IgM levels.
Relationship between anti-β2G IgG levels and lipid abnormalities
Unlike aCL IgG, there was a statistically significant relationship between anti-β2G IgG and HDL (Table 2). This was because 65.4% of the patients with moderate-to-high anti-β2G IgG levels had low HDL levels (p=0.034). A similar significant relationship between anti-β2G IgG levels and LDL was seen (p=0.011) (Table 3, Figure 2). However, there was no significant relationship between anti-β2G IgG levels and TG levels (p=0.833).
Table 2.
Relationship between anti-β2G IgG and HDL levels
| HDL mg/dL | Anti-β2G IgG levels IU/mL | |||
|---|---|---|---|---|
|
| ||||
| <15 | 15–40 | >40 | TOTAL | |
| <40 | 9 | 7 | 10 | 26 |
| >40 | 15 | 20 | 2 | 37 |
| TOTAL | 24 | 27 | 12 | 63 |
Anti-β2G: anti-β2 glycoprotein; HDL: high-density lipoprotein
Table 3.
Relationship between anti-β2G IgG and LDL levels
| LDL mg/dL | Anti-β2G IgG levels IU/mL | |||
|---|---|---|---|---|
|
| ||||
| <15 | 15–40 | >40 | TOTAL | |
| <150 | 13 | 14 | 12 | 39 |
| >150 | 11 | 13 | 0 | 24 |
| TOTAL | 24 | 27 | 12 | 63 |
Anti-β2G: anti-β2 glycoprotein; HDL: high-density lipoprotein
Figure 2.

Relationship between anti-β2G IgG and LDL levels
anti-β2G: anti-β2 glycoprotein; LDL: low-density lipoprotein
Thus, there was a statistically significant relationship between anti-β2G IgG levels and HDL and LDL levels, but not TG levels.
Relationship between anti-β2G IgM levels and lipid abnormalities
There was no statistically significant relationship between anti-β2G IgM levels and HDL (p=0.225), LDL (p=0.563), and TG (p=0.083) levels.
Relationship between the lupus anticoagulant test and lipid abnormalities
There was no statistically significant relationship between lupus anticoagulant test and HDL (p=0.857), LDL (p=0.316), and TG (p=0.882) levels.
Discussion
As serum lipoproteins also contain phospholipids, they may also act as a target for antiphospholipid antibodies. Cross-reactions between antibodies to oxidized LDLs and cardiolipins have been described in SLE from as early as 1993 (9). In our study, we found that aCL IgM levels had a statistically significant relationship with LDL levels. Although isolated IgM aCL levels have no direct correlation with thrombosis, their significance in atherosclerotic diseases has not been studied till date. Our study also did not study this aspect. If any cross-reactivity occurs with LDLs or oxidized LDLs, there may be a possible role for isolated aCL IgM antibodies in atherosclerotic diseases. As LDL contain phospholipids, LDL or oxidized LDL may cross react with anti cardiolipin/anti phospholipid antibodies, thus contributing to atherosclerotic diseases.
β2G is also called apolipoprotein H (10). This terminology highlights the special relationship of this glycoprotein with lipoproteins. In our study, we found a statistically significant relationship between anti-β2G IgG levels and HDL and LDL levels, but not TG levels.
The mechanisms by which anti-β2G antibodies cause thrombosis have been well described (11). They enhance the thrombotic activity of aCL antibodies and the lupus anticoagulant (12) and have also been suggested to induce procoagulant proteins (13).
We propose that anti-β2G has a role in atherothrombotic complications by promoting formation of antibodies against LDL or HDL. Also the cross reactions between LDL or HDL and anti-β2G antibody may result in atherothrombotic complications
Conclusion
There are very few published articles to compare the lipid abnormalities of APS patients with the results obtained in the present study (4, 5). Both mentioned studies have not compared individual lipid abnormalities with separate laboratory parameters of the APS test. The finding noted in one of the studies was that patients with APS had lower HDL levels than those without APS. It was noted in the two studies that lipid abnormalities in APS patients was more than those in the general population.
In our study, we found that aCL IgM levels have a statistically significant relationship with LDL levels and also that anti-β2G IgG levels had a statistically signification relationship with both HDL and LDL levels. The remainder of the APS tests did not show any significant relationship with lipid profile abnormalities.
This study highlights the relationship between various antiphospholipids and lipoproteins. This suggests a pathogenic role of these antibodies in atherosclerotic diseases in addition to its well-established role in thrombotic disorders.
Formation of antibodies against lipoproteins or direct binding of antibodies to lipoproteins may result in activation of lipoproteins causing thrombotic and atherosclerotic complications in APS or the direct binding of antibodies to lipoproteins leading to their activation may be a connecting link between thrombotic and atherosclerotic complications in APS.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Calicut Medical College.
Informed Consent: Written informed consent was obtained from who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - S.S., B.J.P.; Design - S.S., B.J.P.; Supervision - S.S., B.J.P.; Resource - S.S., B.J.P., E.J.T.; Materials - S.S., B.J.P., R.M.; Data Collection and/or Processing - S.S., B.J.P., R.M.; Analysis and/or Interpretation - S.S., B.J.P., E.J.T.; Literature Search - S.S., B.J.P., E.J.T.; Writing - S.S., B.J.P., E.J.T.; Critical Reviews - S.S., B.J.P., E.J.T.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Saigal R, Kansal A, Mittal M, Singh Y, Ram H. Antiphospholipid antibody syndrome. J Assoc Physicians India. 2010;58:176–84. [PubMed] [Google Scholar]
- 2.Cojocaru IM, Cojocaru M, Muşuroi C, Botezat M. Study of anti-cardiolipin and anti-beta2-glycoprotein I antibodies in patients with ischemic stroke. Rom J Intern Med. 2003;41:189–204. [PubMed] [Google Scholar]
- 3.Jiménez S, García-Criado MA, Tàssies D, Reverter JC, Cervera R, Gilabert MR, et al. Preclinical vascular disease in systemic lupus erythematosus and primary antiphospholipid syndrome. Rheumatology (Oxford) 2005;44:756–61. doi: 10.1093/rheumatology/keh581. https://doi.org/10.1093/rheumatology/keh581. [DOI] [PubMed] [Google Scholar]
- 4.Medina G, Gutiérrez-Moreno AL, Vera-Lastra O, Saavedra MA, Jara LJ. Prevalence of metabolic syndrome in primary antiphospholipid syndrome patients. Autoimmun Rev. 2011;10:214–7. doi: 10.1016/j.autrev.2010.10.004. https://doi.org/10.1016/j.autrev.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Rodrigues C, Bonfá E, Caleiro M, Vendramini M, Bueno C, Lopes JB, et al. Association of arterial events with the coexistence of metabolic syndrome and primary antiphospholipid syndrome. Arthritis Care Res (Hoboken) 2012;64:1576–83. doi: 10.1002/acr.21701. https://doi.org/10.1002/acr.21701. [DOI] [PubMed] [Google Scholar]
- 6.Jara LJ, Medina G, Vera-Lastra O, Shoenfeld Y. Atherosclerosis and antiphospholipid syndrome. Clin Rev Allergy Immunol. 2003;25:79–88. doi: 10.1385/CRIAI:25:1:79. https://doi.org/10.1385/CRIAI:25:1:79. [DOI] [PubMed] [Google Scholar]
- 7.Spronk PE, Overbosch EH, Schut NH. Severe atherosclerotic changes, including aortic occlusion, associated with hyperhomocysteinaemia and antiphospholipid antibodies. Ann Rheum Dis. 2001;60:699–701. doi: 10.1136/ard.60.7.699. https://doi.org/10.1136/ard.60.7.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ames PR, Margarita A, Sokoll KB, Weston M, Brancaccio V. Premature atherosclerosis in primary antiphospholipid syndrome: preliminary data. Ann Rheum Dis. 2005;64:315–7. doi: 10.1136/ard.2004.023952. https://doi.org/10.1136/ard.2004.023952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaarala O, Alfthan G, Jauhiainen M, Leirisalo-Repo M, Aho K, Palosuo T. Cross reaction between antibodies to oxidised low-density lipoprotein and to cardiolipin in systemic lupus erythematosus. Lancet. 1993;341:923–5. doi: 10.1016/0140-6736(93)91213-6. https://doi.org/10.1016/0140-6736(93)91213-6. [DOI] [PubMed] [Google Scholar]
- 10.Pruitt K, Tatusova T, Maglott DR. NCBI Reference Sequence (RefSeq): a curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res. 2005;33 doi: 10.1093/nar/gki025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackworth-Young C. Antiphospholipid syndrome: multiple mechanisms. Clin Exp Immunol. 2004;136:393–401. doi: 10.1111/j.1365-2249.2004.02497.x. https://doi.org/10.1111/j.1365-2249.2004.02497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Laat HB, Derksen RH, Urbanus RT, Roest M, de Groot PG. Beta2-glycoprotein I-dependent lupus anticoagulant highly correlates with thrombosis in the antiphospholipid syndrome. Blood. 2004;104:3598–602. doi: 10.1182/blood-2004-03-1107. https://doi.org/10.1182/blood-2004-03-1107. [DOI] [PubMed] [Google Scholar]
- 13.Espinosa G, Cervera R, Font J, Reverter JC, Shoenfeld Y. Mechanisms of thrombosis in the antiphospholipid syndrome. Immunología. 2003;22:53–6. [Google Scholar]