Abstract
Background
The role of donor-specific HLA antibodies (DSA) following pediatric liver transplantation (LTx) is not clearly established. We completed a cross-sectional study to characterize DSA in long-term survivors of pediatric LTx and assess the impact of C1q-binding DSA on allograft outcomes.
Methods
Serum samples were collected at routine clinic visits from fifty pediatric LTx recipients classified into three clinical phenotypes: non-tolerant (n=18) with de-novo autoimmune hepatitis (DAIH) and/or late acute cellular rejection (ACR); stable (n=25) on maintenance tacrolimus; operationally tolerant (n=7). Samples were blinded and antibody detection was performed using Luminex single antigen class I and II beads. Patients with positive DSA were tested for C1q-binding DSA.
Results
DSA were detected in 54% (n=27) of patients, with the majority directed at HLA class II antigens (41% DR, 53% DQ). Patients with DSA were younger at time of LTx (p=0.016) and time of study (p=0.024). Mean AST, ALT, total bilirubin, and GGT were higher in DSA positive patients, though did not reach statistical significance. Non-tolerant patients were significantly more likely to have DQ DSA (61%) compared to stable (20%) and tolerant (29%) patients (p=0.021). The non-tolerant phenotype was associated with DSA and C1q-binding DSA, with odds ratios of 13 (p=0.015) and 8.6 (p=0.006), respectively. The presence of DQ DSA was associated with DAIH and late ACR, with odds ratios of 12.5 (p=0.004) and 10.8 (p=0.006), respectively.
Conclusions
Allograft dysfunction is not always evident in patients with DSA, but DQ DSA are strongly associated with DAIH, late ACR, and chronic rejection.
INTRODUCTION
Post-transplant donor-specific anti-human leukocyte antigen (HLA) antibodies (DSA) are associated with deleterious effects following kidney, heart, and lung transplantation (1, 2). The role of DSA following liver transplantation (LTx) is less clear, but increasing evidence suggests that DSA are associated with both acute (3, 4) and chronic (4–7) liver allograft rejection. Despite mounting data showing that post-LTx DSA are associated with liver allograft dysfunction, monitoring of HLA antibodies is not routinely performed. As such, there is limited data in pediatric LTx recipients.
A recent study of pediatric living donor LTx recipients found that DSA were present in 32/67 (48%) of long-term survivors, and patients with DSA were more likely to have biopsy findings of fibrosis and inflammation (8). A second study in pediatric living donor LTx recipients investigated whether HLA antibodies impeded the development of operational tolerance. This study found that HLA antibodies, especially those with higher mean florescence intensity (MFI), were associated with the absence of operational tolerance (9). A third study of 73 liver transplant recipients (both children and adults) also found that operationally tolerant liver transplant recipients lacked DSA. In contrast, DSA were detected in the majority of patients on maintenance immunosuppression as well as those undergoing weaning of immunosuppression (10).
Combined, published data suggests that humoral alloreactivity may contribute to late liver allograft dysfunction. However, it is also clear that not all DSA are immediately injurious. One mechanism through which DSA mediate graft injury is by fixing complement and initiating the complement cascade. The classical complement pathway is activated when the first component of complement C1q binds two subunits of immunoglobulin (Ig). This initiates the complement cascade, ultimately resulting in the membrane attack complex which leads to cell injury and death (11). Subclasses of IgG have varying ability to fix complement. IgG1 and 3 are the strongest complement fixing subclasses, while IgG2 binding is less strong, and IgG4 is considered unable to fix complement. DSA also promote allograft damage via complement independent mechanisms. Crosslinking of HLA class I antigens by antibodies triggers intracellular signaling pathways resulting in endothelial cell and smooth muscle cell proliferation (12). HLA ligation also induces Weibel Palade Body exocytosis, p-Selectin expression, and recruitment of leukocytes to the allograft (13). HLA antibody subclass appears to influence the degree of inflammation via Fc receptor interactions. In particular, IgG1 and IgG3 have a greater capacity to trigger monocyte infiltration into the allograft due to enhanced Fc receptor interactions (14).
Retrospective analysis of adult LTx recipients has shown that DSA predominantly of the IgG3 subtype are associated with increased risk of graft loss, while those with a predominance of IgG1 correlate with normal graft function(6). Data in pediatric cohorts is lacking, largely because previous studies have relied on sensitive solid phase assays such as ELISA (10) or Luminex based single-HLA-antigen-coated beads (SAB) (8, 9) that measure the antigen specific IgG component in patient sera. However, these methods do not differentiate between complement-fixing and non-complement-fixing antibodies. The Luminex-based HLA SAB-C1q assay is a novel platform that detects HLA antibodies in patient sera capable of binding to the first component of the complement cascade, C1q, using an anti-C1q detection antibody (15, 16). Presence of C1q-binding DSA has been shown to more positively predict acute allograft rejection and loss, or late graft loss, in pediatric or adult kidney transplant recipients and antibody mediated rejection in heart transplant recipients (15, 17–21) when compared to DSA that are not C1q-binding. The utility of SAB-C1q in pediatric LTx patients to evaluate complement binding DSA has not yet been studied.
To date, the relationship between DSA and de-novo autoimmune hepatitis (DAIH) also remains undefined. DAIH is a source of late graft dysfunction not explained by other causes. It is typically diagnosed in the setting of elevated transaminases associated with characteristic biopsy findings (a lymphocytic portal infiltrate with plasma cells, periportal hepatitis, and parenchymal bridging fibrosis) and auto-antibodies (anti-nuclear, double stranded DNA, smooth muscle, mitochondrial, liver-kidney microsomal) (22, 23). It has also been associated with rejection and steroid dependence (22).
Our aims were first to characterize DSA in long-term pediatric LTx survivors, and second to assess the impact of C1q-binding DSA on long-term allograft outcomes. We hypothesized that patients with DAIH and/or episodes of late acute or chronic rejection would be more likely to have C1q-binding DSA.
MATERIALS AND METHODS
Patients
This study was approved by the University of California, Los Angeles (UCLA) institutional review board, IRB# 11–002792. Informed consent was obtained from patients and/or their parents at the time of enrollment. Inclusion criteria included all children followed by the UCLA Liver Transplant Program who had received an isolated LTx prior to age 18 years. Exclusion criteria included a primary diagnosis of immune-mediated liver disease (autoimmune hepatitis and/or sclerosing cholangitis). Patients with HLA antibodies where donor HLA typing was not available to determine whether the antibodies were donor-specific were also excluded. A total of 50 LTx recipients were enrolled in this cross-sectional study. Three additional patients declined to consent to the study. A single serum sample was collected from each patient at one routine clinic visit when patients were in their baseline state of health at a mean of 12.3 (range 1.8–25.4) years post-LTx. No protocol biopsies were performed, and clinical phenotypes based on laboratory trends, past biopsies, and immunosuppression at the time of study were defined as:
- Non-tolerant patients (n=18) with a history of late allograft dysfunction ≥1 year post-LTx requiring dual or triple immunosuppression. This group included 3 patients with biopsy-proven chronic rejection. Non-tolerant patients were further classified as:
- Patients with DAIH (n=3), based on the presence of histopathological criteria and autoantibodies,
- Patients without DAIH (n=5) but with a history of biopsy-proven late acute cellular rejection (ACR) ≥1 year post-LTx, or
- Patients with both DAIH and late ACR (n=10).
Stable patients (n=25) with normal liver function on tacrolimus monotherapy and no history of late ACR ≥1 year post-LTx.
Operationally tolerant patients (n=7) with normal liver function off chronic immunosuppression ≥2 years. Immunosuppression had been discontinued due to post-transplant lymphoproliferative disease (PTLD, n=5) or medication non-adherence (n=2). All seven of these patients were spontaneously tolerant and had not undergone any protocol weaning of immunosuppression.
Immunosuppression Management
Before 1994, cyclosporine formulations (Sandimmune or Neoral; Novartis Pharmaceuticals) were used as the primary maintenance immunosuppressant. Since then, tacrolimus (Prograf; Astellas Pharmaceuticals Inc) has been the primary maintenance agent. Most recipients receive dual immunotherapy with steroids in the early post-LTx period. If allograft function remains normal, steroids are weaned starting at 4–6 months post-LTx. Biopsy-proven ACR is typically treated with a seven-day steroid pulse. In select instances, patients have received a third agent consisting of azathioprine (Imuran; Prometheus Laboratories) or mycophenolate mofetil (Cellcept; Roche Pharmaceuticals). DAIH is typically treated with reinitiation of steroids and addition of a third agent (azathioprine or mycophenolate mofetil). Once allograft function normalizes, steroids are slowly weaned. Historically, monitoring of HLA antibodies was not routinely performed and therefore patients received no treatment for DSA.
Detection of HLA Antibodies and DSA
All patient samples were blinded and antibody detection against HLA-A, B, DR, and DQ was performed using Luminex-based SAB kits (LABScreen; One Lambda, Canoga Park, CA) as previously described (24). In brief, 5 µL of HLA class I or II antigen-coated Luminex beads were incubated with 20 µL of DTT treated patient serum for 30 minutes. Unbound excess serum was removed by washing and the microparticles were stained with 100 µL phycoerythrin-conjugated goat antihuman IgG antibody and incubated in the dark on a rotating platform for 30 min. Particle florescence was assessed by Luminex 100 IS (Luminex, Austin, TX). For patients with detectable HLA antibodies, donor-recipient mismatched HLAs were compared, and positive DSA were defined as a normalized value >1000 MFI.
Measurement of Complement-Fixing DSA
Serum samples from patients with positive DSA were tested for C1q-binding DSA using commercially available kits (C1qScreen; One Lambda, Canoga Park, CA) according to the manufacturer’s specifications. In brief, 5 µL of HLA class I or II antigen-coated Luminex beads were incubated with 5 µL of heat inactivated patient sera for 20 minutes at room temperature with shaking. Phycoerythrin-conjugated anti-C1q (5 µl) was then added, and samples incubated an additional 20 minutes. As above, particle florescence was assessed by Luminex 100 IS (Luminex, Austin, TX). Normalized values >1000 MFI were considered positive.
Data Analysis
The relationships between demographic data, laboratory data, clinical phenotype, DSA, and C1q-binding DSA were analyzed. Statistical analyses were performed using chi-square, Fisher’s exact, Kruskal-wallis, and t-tests. Multivariate logistic regression analyses were used to assess the effects of our key variables (DSA and C1q-binding DSA) on non-tolerance. In the logistic models, additional factors were initially included to determine if they would alter the main effects of the key parameters listed above. These factors were selected based on other studies showing their significance and included a combination of pre-transplant (primary diagnosis of cholestatic liver disease, male sex, and race), transplant (recipient age at transplant, time since transplant, retransplant recipient), and post-transplant (history of early rejection, history of PTLD, non-tolerant clinical phenotype, DSA, and C1q-binding DSA) factors. Stepwise regression with backward selection was used to produce the final regression results. Results are expressed as percentages, mean ± SD, or mean (range) when appropriate. Statistical significance was defined as a p-value <0.05. All statistical analyses were performed with Stata version 11 (StataCorp LP, College Station, TX).
RESULTS
Clinical Characteristics
This study included a total of 50 patients who received their most recent LTx prior to 18 years of age. The mean age at the time of most recent LTx was 3.7 ± 4.4 years, while the mean age at the time of the study was 16 ± 4.9 years. Forty-two percent of patients were male. The most common indication for primary LTx was cholestatic liver disease (biliary atresia, n=35; Alagille syndrome, n=1), followed by acute liver failure (n=5), hepatoblastoma (n=3), neonatal hepatitis (n=2), criggler najjar (n=1), neuroblastoma (n=1), cryptogenic liver failure (n=1), and tyrosinemia (n=1).
The clinical characteristics of patients with and without DSA are summarized in Table 1. Patients with DSA were younger at the time of transplant (p=0.016) and time of study (p=0.024). The majority of patients in each group was transplanted for a primary diagnosis of cholestatic liver disease and received allografts from cadaveric donors. There was no difference in sex or race, and the distribution of patients requiring re-transplantation for early graft loss due to vascular complications (hepatic artery and/or portal vein thrombosis) was similar between the two groups. Markers of allograft function including mean AST, ALT, total bilirubin, and GGT were slightly higher in DSA positive patients, though not statistically significant. Mean tacrolimus troughs were similar between the two groups.
Table 1.
Clinical characteristics of patients
| DSA Negative (n=23) |
DSA Positive (n=27) |
p-value | |
|---|---|---|---|
| Age at transplant in years (mean ± SD)a | 5.4 ± 5.3 | 2.4 ± 3 | 0.016 |
| Age at study in years (mean ± SD) | 17.7 ± 5.1 | 14.6 ± 4.3 | 0.024 |
| Time since transplant in years (mean ± SD)a | 12.3 ± 6.4 | 12.2 ± 4.4 | 0.944 |
|
Primary diagnosis of cholestatic liver disease n (%) |
15 (65%) | 21 (78%) | 0.324 |
| Cadaveric donor, n (%) | 21 (91%) | 26 (96%) | 0.588 |
| Retransplant recipient, n (%) | 6 (26%) | 5 (19%) | 0.733 |
| Male, n (%) | 11 (48%) | 10 (37%) | 0.441 |
| Race, n (%) | 0.240 | ||
| Hispanic | 8 (35%) | 17 (63%) | |
| White | 8 (35%) | 6 (22%) | |
| African-American | 5 (22%) | 3 (11%) | |
| Asian | 2 (8%) | 1 (4%) | |
| Laboratory markers | |||
| AST (U/L) | 26 ± 13 | 36 ± 26 | 0.091 |
| ALT (U/L) | 24 ± 16 | 33 ± 29 | 0.169 |
| Total bilirubin (mg/dL) | 0.7 ± 0.4 | 0.8 ± 1.4 | 0.682 |
| GGT (U/L) | 26 ± 23 | 86 ± 157 | 0.078 |
| Tacrolimus trough in ng/mL (mean ± SD) | 5.8 ± 4.1 | 4.9 ± 3.2 | 0.421 |
| Clinical phenotype, n (%) | 0.226 | ||
| Non-tolerant | 6 (26%) | 12 (44%) | |
| Stable | 12 (52%) | 13 (48%) | |
| Tolerant | 5 (22%) | 2 (8%) | |
| History of PTLD, n (%) | 5 (22%) | 2 (8%) | 0.225 |
| Early rejection (<1 year post-transplant), n (%) | 10 (43%) | 14 (52%) | 0.584 |
| Late rejection (≥1 year post-transplant), n (%) | 5 (22%) | 10 (37%) | 0.355 |
| Number of late rejection episodes (mean ± SD) |
0.2 ± 0.4 | 0.7 ± 1.2 | 0.078 |
| Time since most recent rejection episode in years (mean ± SD) |
3.7 ± 6 | 7 ± 4.8 | 0.267 |
| DAIH, n (%) | 3 (13%) | 10 (37%) | 0.104 |
| Time since DAIH diagnosis in years (mean ± SD) |
3.7 ± 4.9 | 6.8 ± 3.9 | 0.277 |
| Chronic rejection, n (%) | 0 | 3 (11%) | 0.240 |
For retransplant recipients, age at transplant and time since transplant are based on the date of the most recent transplant.
Patients were stratified into three cohorts based on clinical phenotype: 1)non-tolerant, with a history of late allograft dysfunction ≥1 year post-LTx requiring dual or triple immunosuppression; 2) stable, with normal liver function on tacrolimus monotherapy and no history of late ACR ≥1 year post-LTx; and 3) operationally tolerant, with normal liver function off chronic immunosuppression ≥2 years. More non-tolerant patients than stable and tolerant patients had DSA, although this difference was not statistically significant (Table 1). The proportion of patients with a history of PTLD, early rejection, and late rejection were also similar across groups. However, there was a trend toward a greater number of late rejection episodes and higher proportion of patients with DAIH in the group with DSA. All three patients with chronic rejection were also noted to have DSA.
DSA and C1q-binding DSA
The prevalence of DSA and C1q-binding DSA in the study population is shown in Table 2. Fifty-four percent of patients (n=27) were determined to have DSA. These patients had a mean of 1.9 DSA each (range 1–4 per patient). Most DSA were directed at HLA class II antigens, with predominant specificity toward DQ (2% HLA-A, 4% HLA-B, 41% HLA-DR, 53% HLA-DQ). There was no statistically significant difference in the percentage of non-tolerant (67%), stable (52%), and tolerant (29%) patients with positive DSA (p=0.226). Non-tolerant patients however were significantly more likely to have DQ DSA, with at least one DQ DSA detected in 61% of non-tolerant patients compared to only 20% of stable and 29% of tolerant patients (p=0.021).
Table 2.
DSA and C1q-binding DSA in non-tolerant, stable, and tolerant patients
| Class I | Class II | |||
|---|---|---|---|---|
| Clinical phenotype | DSA (n) |
C1q-binding DSA (n) |
DSA (n) |
C1q-binding DSA (n) |
| Non-tolerant | 0 | Not tested | 12 | 8 |
| (n=18) | 1 DR | 0 DR | ||
| 6 DQ | 5 DQ | |||
| 5 DR and DQ | 3 DR and DQ | |||
| (15954 ± 8290)a | ||||
| Stable | 2 | 0 | 12 | 4 |
| (n=25) | 0 A | 7 DR | 1 DR | |
| 1 B | 1 DQ | 3 DQ | ||
| 1 A and B | 4 DR and DQ | 0 DR and DQ | ||
| (1617 ± 255)a | (10903 ± 8308)a | |||
| Tolerant | 0 | Not tested | 2 | 1 |
| (n=7) | 0 DR | 0 DR | ||
| 2 DQ | 1 DQ | |||
| 0 DR and DQ | 0 DR and DQ | |||
| (19149 ± 5106)a | ||||
Represents mean ± SD of peak MFI for patients with detectable DSA. Note: A total of 27 patients were found to have DSA, including one stable patient with a combination of both class I and II DSA.
Only two stable patients had detectable class I DSA with a mean peak MFI of 1617 ± 255. In patients with detectable class II DSA, there was no statistically significant difference in peak antibody strength in the non-tolerant group (15954 ± 8290), compared to the stable and tolerant groups (10903 ± 8308 and 19149 ± 5106, respectively; kruskal wallis test, p=0.211). Nor was there a statistically significant difference in mean or cumulative MFI across groups.
Of the 27 patients with detectable DSA, 48% (n=13) were determined to have C1q-binding DSA. These patients had a mean of 1.7 (range 1–4) C1q-binding DSA each. All C1q-binding DSA were directed at HLA class II antigens, predominantly DQ (18% HLA-DR, 82% HLA-DQ). There was a weak trend towards a differential distribution of patients with C1q-binding DSA (p=0.101), with 44% of the non-tolerant group compared to only 16% of the stable and 14% of the tolerant groups.
Predictors associated with DSA and C1q-binding DSA
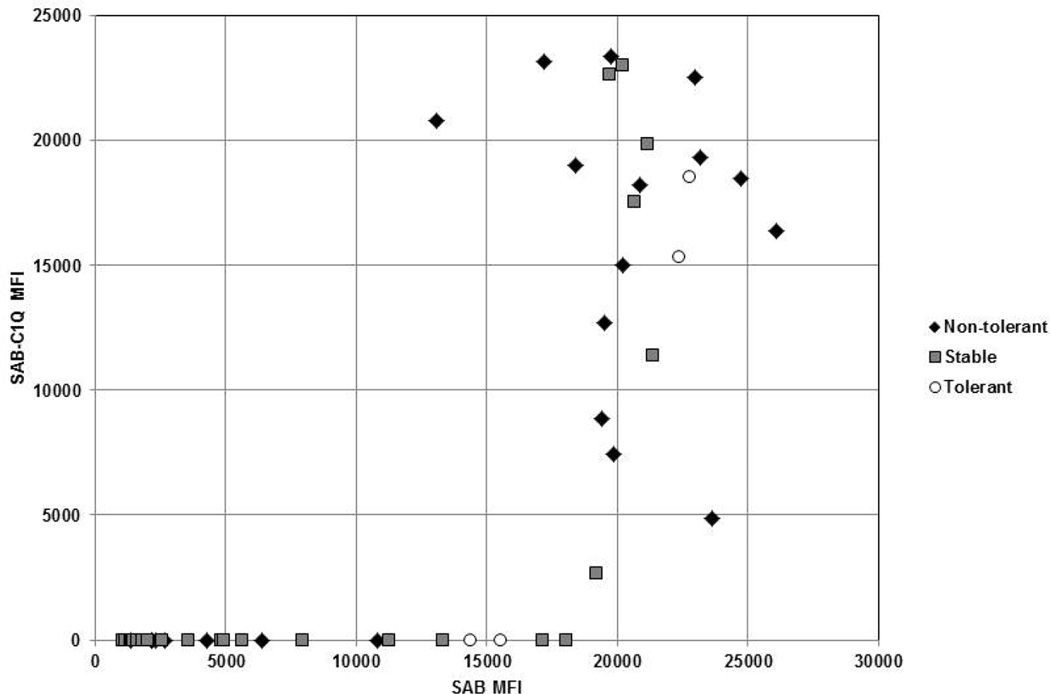
Figure 1 demonstrates the relationship between the measured MFI for DSA and C1q-binding DSA detected by the SAB and SAB-C1q assays, respectively. As shown, the SAB-C1q assay only detected DSA that were strongly positive on the SAB assay, with a threshold MFI of 13000. Univariate analysis showed that the presence of DQ DSA and C1q-binding DSA had the strongest associations with non-tolerance (odds ratios of 5.6 and 4.3, respectively) compared to the presence of any DSA and DSA with >13000 threshold MFI (Table 3).
Figure 1. Scatter plot of SAB vs SAB-C1q MFI.
MFI for DSA and C1q-binding DSA detected by the SAB and SAB-C1q assays, respectively. Most DSA above a threshold of 13000 MFI in the SAB assay also had a positive result in the SAB-C1q assay.
Note: JPEG format uploaded as separate document.
Table 3.
Associations between type of DSA and non-tolerance
| Type of DSA | Odds ratio (95% CI) |
p-value |
|---|---|---|
| Any DSA | 2.3 (0.7–7.5) |
0.175 |
| DSA with MFI>13000 | 2.4 (0.7–8.2) |
0.161 |
| DQ DSA | 5.6 (1.6–19.9) |
0.006 |
| C1q-binding DSA | 4.3 (1.1–16.4) |
0.028 |
The multivariate predictors of DSA and C1q-binding DSA are shown in Table 4. The non-tolerant phenotype was the strongest predictor associated with both DSA and C1q-binding DSA, with an odds ratio of 13 and 8.6, respectively (p=0.015 and 0.006, respectively). Older age at time of transplant was significantly associated with lower risk of developing DSA (OR=0.7, p=0.004), and showed a trend toward association with lower risk of developing C1q-binding DSA (OR=0.8, p=0.066).
Table 4.
Multivariate predictors of DSA and C1q-binding DSA
| Predictors of DSA | Predictors of C1q-binding DSA | |||
|---|---|---|---|---|
| Odds ratio (95% CI) |
p-value | Odds ratio (95% CI) |
p-value | |
| Age at transplant (years) | 0.7 (0.6–0.9) |
0.004 | 0.8 (0.7–1) |
0.066 |
| Non-tolerant phenotype | 13 (1.6–103) |
0.015 | 8.6 (1.8–40) |
0.006 |
Predictors associated with DAIH and late ACR
Multivariate logistic regression was also performed to assess predictors associated specifically with DAIH and late ACR. As shown in Table 5, the presence of DQ DSA was the strongest predictor associated with both clinical phenotypes. Older age at the time of transplant was also associated with a higher risk for DAIH and late ACR.
Table 5.
Multivariate predictors associated with DAIH and late ACR
| Predictors of DAIH | Predictors of late ACR | |||
|---|---|---|---|---|
| Odds ratio (95% CI) |
p-value | Odds ratio (95% CI) |
p-value | |
| Age at transplant (years) | 1.2 (1–1.4) |
0.038 | 1.3 (1.1–1.6) |
0.005 |
| Positive DQ DSA | 12.5 (2.3–69) |
0.004 | 10.8 (2–59.3) |
0.006 |
Chronic Rejection
Three non-tolerant patients also had biopsy-proven chronic rejection. All three of these patients had been diagnosed with late ACR (range of 1–5 episodes per patient), and two of these patients also had DAIH. Class II DSA were seen in all three patients with chronic rejection (see Table 1). Interestingly, each of these patients had strongly positive DQ DSA, with an MFI ranging from 6397 to 20190. Two of these three patients had DQ antibodies that were C1q-binding. One patient also had a DR antibody with an MFI of 1333.
DISCUSSION
This study demonstrates that DSA are common in long-term survivors of pediatric LTx. The most important finding was that non-tolerant patients with a history of DAIH, late ACR, and/or chronic rejection were more likely to have DSA compared to stable and tolerant patients, with DSA seen in two-thirds of non-tolerant patients, but only 52% of stable patients and 29% of tolerant patients. Class II antibodies were seen more often than class I antibodies. In particular, DQ DSA were more often associated with the non-tolerant phenotype, suggesting that they may be more pathogenic. Complement-fixing DSA tend to be DQ antibodies with high MFI (>13000) and also have a strong association with a non-tolerant phenotype. However, the strength and ability to fix complement alone does not appear to be the only discriminating feature between pathogenic and non-pathogenic DSA.
This study contributes to a growing body of literature establishing the importance of DSA in LTx recipients. Over a decade ago, Kasahara et al found that 12/58 (21%) of living donor LTx recipients developed DSA within the first month following LTx, and all 12 of these patients were diagnosed with acute rejection compared to only 8 (17%) of those without DSA (3). This association was again reported more recently by Musat et al who found that 27/43 (63%) of LTx recipients were found to have DSA, and those with DSA and diffuse portal C4d deposition were more likely to have acute rejection and steroid resistant rejection (4).
DSA have also been linked to chronic rejection in adult LTx recipients. O’Leary et al found that 36/39 (92%) of patients with chronic rejection had DSA compared to only 24/39 (61%) of comparator patients (5). Similarly, Musat et al reported an association between DSA and ductopenia due to chronic rejection (4). Biliary complications have also been linked to DSA. In a cross-sectional study of 95 adult LTx recipients, 23 (24%) had HLA antibodies but only four (4%) had DSA (7). There was a trend towards a higher prevalence of DSA in long-term survivors, and three of the patients with DSA had biliary complications.
Importantly, our results add to the limited data describing the clinical significance of DSA in long term survivors of pediatric LTx. Patients with DSA represent 54% of our cohort, which is similar to what has been reported in the literature (8–10). In addition, we found the absence of DSA to be most common in tolerant patients which has also been reported (8–10). We also describe that the presence of DSA, particularly DQ DSA, was associated with DAIH. Interestingly, the Kyoto group found DSA to be associated with biopsy findings of fibrosis and inflammation (8). Though they did not comment on the presence of DAIH, it is quite likely that some of their patients with DSA and abnormal biopsy findings also met criteria for DAIH. A recent study of adult LTx recipients described IgG4+ rich plasma cell infiltrates in patients with DAIH which, when combined with demographic and other histopathologic differences, was suggested to represent an overlap between allo- and auto-immunity in patients with DAIH (25). Our observation that DAIH is associated with detectable DSA has led us to hypothesize that DAIH may be a form of antibody-mediated rejection. Mechanistically, DSA may lead to allograft injury, subsequently resulting in the development of autoantibodies. This is consistent with recent data that supports an interplay between HLA and non-HLA antibodies in the pathogenesis of chronic rejection. In the setting of favorable cytokines such as interleukin-17, inflammation and subsequent tissue remodeling following an alloimmune response may allow for the development of de novo autoimmune responses (26).
Interestingly, the presence of DSA is not always associated with allograft dysfunction, but DSA strength does appear to carry some relevance. In our cohort, there was a non-significant trend towards non-tolerant patients being more likely to have DSA above a higher threshold MFI of 2000. In addition, there was a strong association between complement-fixing DSA and the non-tolerant phenotype, with most complement-fixing DSA being DQ antibodies with high MFI (>13000). Similarly, O’Leary et al found that higher MFI DSA were associated with chronic rejection in adult LTx recipients (5). The importance of antibody strength has also been demonstrated in recipients of pediatric heart transplantation, where DSA with low MFI have been reported to occur frequently (in 50% of patients), but alone are insufficient to predict C4d deposition and antibody mediated rejection (27). In our cohort, the SAB-C1q assay only detected DSA that were strongly positive (MFI > 13000). It therefore remains unclear as to whether this assay better identifies pathogenic DSA compared to the SAB assay. Further studies are needed to assess the clinical significance of the SAB-C1q assay in comparison to other DSA properties such as MFI, titer, and subclass.
A previous study established that DSA positive LTx recipients with normal graft function most often had isolated IgG subclass 1, and patients with IgG subclass 3 DSA had an increased risk of graft loss (6). Patients with chronic rejection were noted to have a combination of IgG subclasses (6). In addition to IgG subclass, DSA target loci are also likely to play an important role in pathogenicity. Our study suggests that class II DSA, and in particular DQ DSA, are most strongly associated with worse allograft outcomes. This finding is consistent with what has been reported in liver (4, 28) as well as other solid organ transplants. For example, in renal transplantation DQ DSA are the most frequent HLA DSA to develop and tend to be associated with antibody mediated rejection, transplant glomerulopathy, and allograft loss (29). In cardiac transplantation de novo persistent DSA, the majority of which have DQ specificities, are an independent predictor of poor survival (30). The reasons that DQ DSA are associated with poor outcomes remain unclear, but may be related to binding affinity and clearance kinetics, properties that were not analyzed in this study.
The main limitation of our study was its cross-sectional design and associated lack of longitudinal data. In particular, we were unable to determine if the DSA in our cohort were pre-formed DSA or de novo DSA because pre-transplant antibody testing was not performed. Likewise, we were unable to determine the incidence of new DSA and the persistence of pre-existing DSA. A recent study in adult LTx recipients found that 13% of patients had DSA at a median time of 51 months following LTx, and that 9% of patients developed de novo DSA on retesting a mean of 36.5 months later (31). Future longitudinal studies are needed to further establish the temporal relationship between the development of DSA and the diagnosis of DAIH, late ACR, and/or chronic rejection. Longitudinal follow-up is also needed to determine whether DSA are an independent predictor for patient and graft loss in pediatric LTx recipients which has been shown in adult LTx recipients (28). An additional limitation of our study was the lack of liver biopsies. Even in the setting of normal liver biochemistry, hepatitis and fibrosis can be present in protocol biopsies (32). It is therefore possible that some of our patients may have been mis-classified in their respective clinical phenotype groups. Likewise, our lack of biopsies limits our ability to determine if the presence of DSA translates to allograft pathology despite an absence of laboratory abnormalities. A third limitation of our study was lack of data on patient adherence and how this may have influenced the presence of DSA. Non-adherence has been found to be an independent risk factor for development of DSA and allograft loss in kidney transplant recipients (33, 34). All DSA including HLA and non-HLA antibodies, have the potential to be pathogenic. However, our study was limited by the restriction of DSA analysis to HLA-A, B, DR, and DQ antigens due to unavailability of complete donor DP and Cw HLA typing. In addition, we were unable to assess the degree of HLA mismatches. And finally, we were unable to define antibody subclass profiles given that the SAB-C1q used in this study to identify complement binding antibodies does not distinguish between IgG antibody subclasses. The ideal study to address these issues would longitudinally correlate the presence and/or development of DSA with histopathology seen on protocol biopsies. Such a study would also include a larger cohort of patients from multiple centers which would allow for improved statistical power, therefore strengthening the findings of our multivariate analyses.
In conclusion, HLA class II DSA are common in long-term survivors of pediatric LTx. While allograft dysfunction is not always evident in patients with DSA, DSA are strongly associated with the non-tolerant phenotype. In particular, DQ DSA are associated with DAIH, late ACR, and chronic rejection. Complement-fixing DSA, which are most often DQ antibodies with high MFI (>13000), also tend to be associated with the non-tolerant phenotype. Further study is needed to longitudinally assess DSA and their associations with liver histopathology in pediatric LTx recipients to determine the long-term effects of DSA and better define the discriminating features between pathogenic and non-pathogenic DSA.
Acknowledgments
This work is supported by grants from the Translational Research Fund from the UCLA Department of Pathology and the National Center for Advancing Translational Sciences UCLA CTSI Grant UL1TR000124. L.J.W is supported by the American Association for the Study of Liver Diseases (AASLD Career Development Award in Liver Transplantation in Memory of the University of Michigan Transplant Team). M.J.H is supported by a Histocompatibility Laboratory Director Trainee Fellowship from the UCLA Immunogenetics Center Fellowship Program. E.F.R. is supported by NIH RO1 AI042819.
Abbreviations
- ACR
acute cellular rejection
- DAIH
de novo autoimmune hepatitis
- DSA
donor-specific HLA antibodies
- Ig
immunoglobulin
- LTx
liver transplantation
- MFI
mean florescence intensity
- PTLD
post-transplant lymphoproliferative disease
- SAB
single-HLA-antigen-coated beads
Footnotes
Disclosure: The authors of this manuscript have no conflicts of interest to disclose other than the funding sources included above.
Author Contributions: L.J.W., M.J.H, S.V.M., and E.F.R. participated in research design, performance of the research, data analysis, and writing of the paper. R.S.V., J.H.V., D.G.F., and R.W.B. participated in performance of the research and writing of the paper.
REFERENCES
- 1.McKenna RM, Takemoto SK, Terasaki PI. Anti-HLA antibodies after solid organ transplantation. Transplantation. 2000;69(3):319–326. doi: 10.1097/00007890-200002150-00001. [DOI] [PubMed] [Google Scholar]
- 2.Girnita AL, Webber SA, Zeevi A. Anti-HLA alloantibodies in pediatric solid organ transplantation. Pediatr Transplant. 2006;10(2):146–153. doi: 10.1111/j.1399-3046.2005.00425.x. [DOI] [PubMed] [Google Scholar]
- 3.Kasahara M, Kiuchi T, Takakura K, Uryuhara K, Egawa H, Asonuma K, et al. Postoperative flow cytometry crossmatch in living donor liver transplantation: clinical significance of humoral immunity in acute rejection. Transplantation. 1999;67(4):568–575. doi: 10.1097/00007890-199902270-00014. [DOI] [PubMed] [Google Scholar]
- 4.Musat AI, Agni RM, Wai PY, Pirsch JD, Lorentzen DF, Powell A, et al. The significance of donor-specific HLA antibodies in rejection and ductopenia development in ABO compatible liver transplantation. Am J Transplant. 2011;11(3):500–510. doi: 10.1111/j.1600-6143.2010.03414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Leary JG, Kaneku H, Susskind BM, Jennings LW, Neri MA, Davis GL, et al. High Mean Fluorescence Intensity Donor-Specific Anti-HLA Antibodies Associated With Chronic Rejection Postliver Transplant. American Journal of Transplantation. 2011;11(9):1868–1876. doi: 10.1111/j.1600-6143.2011.03593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaneku H, O’Leary JG, Taniguchi M, Susskind BM, Terasaki PI, Klintmalm GB. Donor-specific HLA antibodies of IgG3 subclass are associated with chronic rejection and graft loss after liver transplantation. Liver Transplantation. 2012 doi: 10.1002/lt.23451. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 7.Fontana M, Moradpour D, Aubert V, Pantaleo G, Pascual M. Prevalence of anti-HLA antibodies after liver transplantation. Transpl Int. 2010;23(8):858–859. doi: 10.1111/j.1432-2277.2009.01022.x. [DOI] [PubMed] [Google Scholar]
- 8.Miyagawa-Hayashino A, Yoshizawa A, Uchida Y, Egawa H, Yurugi K, Masuda S, et al. Progressive graft fibrosis and donor-specific HLA antibodies in pediatric late liver allografts. Liver Transpl. 2012 doi: 10.1002/lt.23534. [DOI] [PubMed] [Google Scholar]
- 9.Waki K, Sugawara Y, Mizuta K, Taniguchi M, Ozawa M, Hirata M, et al. Predicting operational tolerance in pediatric living-donor liver transplantation by absence of HLA antibodies. Transplantation. 2013;95(1):177–183. doi: 10.1097/TP.0b013e3182782fef. [DOI] [PubMed] [Google Scholar]
- 10.Girnita A, Mazariegos GV, Castellaneta A, Reyes J, Bentlejewski C, Thomson AW, et al. Liver transplant recipients weaned off immunosuppression lack circulating donor-specific antibodies. Hum Immunol. 2010;71(3):274–276. doi: 10.1016/j.humimm.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Stegall MD, Chedid MF, Cornell LD. The role of complement in antibody-mediated rejection in kidney transplantation. Nat Rev Nephrol. 2012;8(11):670–678. doi: 10.1038/nrneph.2012.212. [DOI] [PubMed] [Google Scholar]
- 12.Tsai EW, Reed EF. MHC class I signaling: new functional perspectives for an old molecule. Tissue Antigens. 2014;83(6):375–381. doi: 10.1111/tan.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valenzuela NM, Hong L, Shen XD, Gao F, Young SH, Rozengurt E, et al. Blockade of p-selectin is sufficient to reduce MHC I antibody-elicited monocyte recruitment in vitro and in vivo. Am J Transplant. 2013;13(2):299–311. doi: 10.1111/ajt.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valenzuela NM, Mulder A, Reed EF. HLA class I antibodies trigger increased adherence of monocytes to endothelial cells by eliciting an increase in endothelial P-selectin and, depending on subclass, by engaging FcgammaRs. J Immunol. 2013;190(12):6635–6650. doi: 10.4049/jimmunol.1201434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loupy A, Lefaucheur C, Vernerey D, Prugger C, van Huyen JP, Mooney N, et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med. 2013;369(13):1215–1226. doi: 10.1056/NEJMoa1302506. [DOI] [PubMed] [Google Scholar]
- 16.Otten HG, Verhaar MC, Borst HP, Hene RJ, van Zuilen AD. Pretransplant donor-specific HLA class-I and -II antibodies are associated with an increased risk for kidney graft failure. Am J Transplant. 2012;12(6):1618–1623. doi: 10.1111/j.1600-6143.2011.03985.x. [DOI] [PubMed] [Google Scholar]
- 17.Chen G, Sequeira F, Tyan DB. Novel C1q assay reveals a clinically relevant subset of human leukocyte antigen antibodies independent of immunoglobulin G strength on single antigen beads. Hum Immunol. 2011;72(10):849–858. doi: 10.1016/j.humimm.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Zeevi A, Lunz J, Feingold B, Shullo M, Bermudez C, Teuteberg J, et al. Persistent strong anti-HLA antibody at high titer is complement binding and associated with increased risk of antibody-mediated rejection in heart transplant recipients. J Heart Lung Transplant. 2013;32(1):98–105. doi: 10.1016/j.healun.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutherland SM, Chen G, Sequeira FA, Lou CD, Alexander SR, Tyan DB. Complement-fixing donor-specific antibodies identified by a novel C1q assay are associated with allograft loss. Pediatr Transplant. 2012;16(1):12–17. doi: 10.1111/j.1399-3046.2011.01599.x. [DOI] [PubMed] [Google Scholar]
- 20.Chin C, Chen G, Sequeria F, Berry G, Siehr S, Bernstein D, et al. Clinical usefulness of a novel C1q assay to detect immunoglobulin G antibodies capable of fixing complement in sensitized pediatric heart transplant patients. J Heart Lung Transplant. 2011;30(2):158–163. doi: 10.1016/j.healun.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 21.Yabu JM, Higgins JP, Chen G, Sequeira F, Busque S, Tyan DB. C1q–fixing human leukocyte antigen antibodies are specific for predicting transplant glomerulopathy and late graft failure after kidney transplantation. Transplantation. 2011;91(3):342–347. doi: 10.1097/TP.0b013e318203fd26. [DOI] [PubMed] [Google Scholar]
- 22.Venick RS, McDiarmid SV, Farmer DG, Gornbein J, Martin MG, Vargas JH, et al. Rejection and steroid dependence: unique risk factors in the development of pediatric posttransplant de novo autoimmune hepatitis. Am J Transplant. 2007;7(4):955–963. doi: 10.1111/j.1600-6143.2006.01717.x. [DOI] [PubMed] [Google Scholar]
- 23.Pongpaibul A, Venick RS, McDiarmid SV, Lassman CR. Histopathology of de novo autoimmune hepatitis. Liver Transpl. 2012;18(7):811–818. doi: 10.1002/lt.23422. [DOI] [PubMed] [Google Scholar]
- 24.Blumberg JM, Gritsch HA, Reed EF, Cecka JM, Lipshutz GS, Danovitch GM, et al. Kidney paired donation in the presence of donor-specific antibodies. Kidney Int. 2013;84(5):1009–1016. doi: 10.1038/ki.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castillo-Rama M, Sebagh M, Sasatomi E, Randhawa P, Isse K, Salgarkar AD, et al. “Plasma cell hepatitis” in liver allografts: identification and characterization of an IgG4-rich cohort. Am J Transplant. 2013;13(11):2966–2977. doi: 10.1111/ajt.12413. [DOI] [PubMed] [Google Scholar]
- 26.Angaswamy N, Tiriveedhi V, Sarma NJ, Subramanian V, Klein C, Wellen J, et al. Interplay between immune responses to HLA and non-HLA self-antigens in allograft rejection. Hum Immunol. 2013;74(11):1478–1485. doi: 10.1016/j.humimm.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng DM, Law YM, Kemna MS, Warner P, Nelson K, Boucek RJ. Donor-specific antibodies: can they predict C4d deposition in pediatric heart recipients? Pediatr Transplant. 2013;17(5):429–435. doi: 10.1111/petr.12075. [DOI] [PubMed] [Google Scholar]
- 28.Kaneku H, O’Leary JG, Banuelos N, Jennings LW, Susskind BM, Klintmalm GB, et al. De Novo Donor-Specific HLA Antibodies Decrease Patient and Graft Survival in Liver Transplant Recipients. Am J Transplant. 2013 doi: 10.1002/ajt.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willicombe M, Brookes P, Sergeant R, Santos-Nunez E, Steggar C, Galliford J, et al. De novo DQ donor-specific antibodies are associated with a significant risk of antibody-mediated rejection and transplant glomerulopathy. Transplantation. 2012;94(2):172–177. doi: 10.1097/TP.0b013e3182543950. [DOI] [PubMed] [Google Scholar]
- 30.Smith JD, Banner NR, Hamour IM, Ozawa M, Goh A, Robinson D, et al. De novo donor HLA-specific antibodies after heart transplantation are an independent predictor of poor patient survival. Am J Transplant. 2011;11(2):312–319. doi: 10.1111/j.1600-6143.2010.03383.x. [DOI] [PubMed] [Google Scholar]
- 31.Del Bello A, Congy-Jolivet N, Muscari F, Lavayssiere L, Esposito L, Cardeau-Desangles I, et al. Prevalence, incidence and risk factors for donor-specific anti-HLA antibodies in maintenance liver transplant patients. Am J Transplant. 2014;14(4):867–875. doi: 10.1111/ajt.12651. [DOI] [PubMed] [Google Scholar]
- 32.Evans HM, Kelly DA, McKiernan PJ, Hubscher S. Progressive histological damage in liver allografts following pediatric liver transplantation. Hepatology. 2006;43(5):1109–1117. doi: 10.1002/hep.21152. [DOI] [PubMed] [Google Scholar]
- 33.Wiebe C, Gibson IW, Blydt-Hansen TD, Karpinski M, Ho J, Storsley LJ, et al. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant. 2012;12(5):1157–1167. doi: 10.1111/j.1600-6143.2012.04013.x. [DOI] [PubMed] [Google Scholar]
- 34.Sellares J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12(2):388–399. doi: 10.1111/j.1600-6143.2011.03840.x. [DOI] [PubMed] [Google Scholar]