Abstract
Mutations in the otoferlin (OTOF) gene lead to profound hearing loss in humans. Interestingly, a number of missense otoferlin mutations cause hearing defects but only at higher body temperature, and the reasons for this have been elusive until now. A study published in this issue of The EMBO Journal (Strenzke et al, 2016) adds insight into the underlying mechanisms for this heat‐dependent hearing loss.
Subject Categories: Molecular Biology of Disease, Neuroscience
Imagine lying in bed with a fever, tossing, and turning, trying to find a comfortable position for your aching body. To make matters worse, you are having more trouble hearing than usual and you fear the effect will be lasting. Thankfully, when the fever finally subsides, you find that your hearing returns, so that the relief you yearned for from the aches and pains is even more gratifying.
Hearing is essential for our ability to perceive the outside world, to be warned of danger, and to communicate with fellow humans. Hearing loss is therefore a crucial impairment and even more difficult to cope with if the loss occurs after the acquisition of hearing and language. It means having to adapt to an entirely new situation and, in many ways, a new life. Such is the case for patients with specific mutations of the OTOF gene. These individuals have normal hearing threshold that allows them to hear sounds well enough to avoid dangerous situations, but suffer from compromised speech recognition. They can hear a voice, but often are not able to make out what the person is saying. Moreover, for a subset of these patients, their hearing problems worsen upon exposure to heat, such as a fever.
Otoferlin is a member of the FER‐1 family of transmembrane proteins distinguished by C2 domains that are also found in synaptotagmin, PKC, and PLC isoforms. It binds calcium and membranes and triggers the fusion of neurotransmitter‐filled vesicles with the plasma membrane, presumably in conjunction with a molecular machinery that remains elusive (Roux et al, 2006; Johnson & Chapman, 2010). An article in this issue of The EMBO Journal also reports on the mechanism of membrane insertion of otoferlin and shows that this is mediated via the tail‐anchored protein insertion pathway TRC40 (Vogl et al, 2016). The primary otoferlin defect in hearing‐impaired persons affects the inner ear's hair cells and compromises otoferlin's role in exocytosis of synaptic vesicles at the auditory inner hair cell ribbon synapse (Roux et al, 2006). However, the connection between deafness and excessive heat has remained elusive. Now, Strenzke and colleagues have helped to provide an answer and, furthermore, defined the molecular basis for differences in hearing sensitivity between humans and mice (Fig 1; Strenzke et al, 2016).
Figure 1. The link between otoferlin, heat and hearing loss.
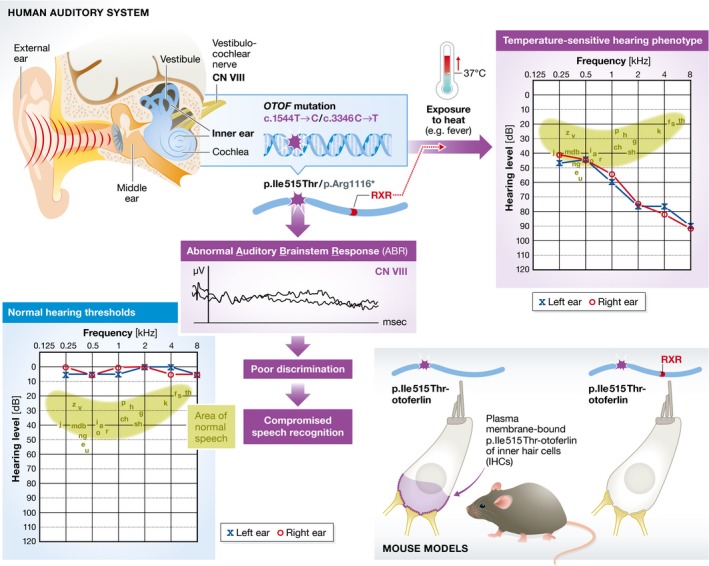
Auditory neuropathy (AN) is a relatively common form of hearing loss, accounting for approximately 10% of childhood deafness. Sensory hair cell activity, as measured by otoacoustic emissions representing mechanical amplification of outer hair cells, and cochlear microphonics, which examines electrical potentials of both inner and outer hair cells, is normal in AN patients. However, auditory nerve activity is compromised, as measured by auditory brainstem response (ABR). The speech discrimination score, which evaluates understanding of speech, is often low. Some patients with OTOF mutations suffer from an exacerbated hearing impairment when they have a fever. Surprisingly, mice with the same mutation retain the same level of hearing upon exposure to heat. Strenzke et al (2016) determined that the culprit of this difference lies in a 20‐amino acid RXR motif. Transfection of otoferlin cDNA with an OTOF mutation and RXR motif in cochlear cultures, introduced by a gene gun, demonstrated that otoferlin disappeared from the inner hair cell plasma membrane. The levels of plasma membrane‐bound otoferlin correlate with functional synaptic function and sound encoding. Note that the mutations c.1544T>C (p.Ile515Thr) and c.3346C>T (p.Arg1116*) are present in the long isoform of otoferlin (NP_001274418).
Otoferlin first appeared in 1999, when it was discovered that mutations of the OTOF gene cause a nonsyndromic form of deafness (Yasunaga et al, 1999). These mutations were found in the pre‐high‐throughput sequencing era, based on a genomewide linkage study using microsatellite markers that led to the identification of a 2‐cM interval on chromosome 2 likely harboring the gene responsible for deafness in affected Lebanese families. A contig of various artificial chromosomes revealed a number of candidate genes, but further analysis was difficult, because the mammalian form of otoferlin itself had not been characterized, other than by finding sequence homologies with the spermatogenesis factor FER‐1 in C. elegans. Eventually, a nonsense mutation was identified in several families. Subsequent genomic analysis led to the discovery of both long and short isoforms of OTOF and, based on a new mutation found in a southwestern Indian family, the long isoform was deemed essential for inner ear function (Yasunaga et al, 2000).
Mutations in OTOF have since been found to cause profound sensorineural hearing loss in patients in many parts of the world, including Spain, Columbia, Argentina, and the Middle East (Adato et al, 2000; Rodríguez‐Ballesteros et al, 2003). Moreover, many patients with OTOF mutations have auditory neuropathy, a diagnosis based on an abnormal auditory brainstem response (ABR) and impaired speech discrimination, but with initially normal otoacoustic emissions, which measures the outer hair cells' response to sound (Varga et al, 2003; Fig 1). First described in 1996 by the scientist (and sometimes jazz musician) Charles (Chuck) Berlin and colleagues as a defect involving the auditory portion of the VIII cranial nerve (Starr et al, 1996), it is now defined as an auditory synaptopathy. But how do temperature sensitivity and hearing loss fit into this picture? Two siblings with auditory neuropathy were reported to have an increase in the severity of their hearing loss when they had a fever (Starr et al, 1998). A subsequent screen of patients with auditory neuropathy several years later led to the identification of OTOF mutations, with one specific mutation, p.Ile515Thr, associated with a temperature‐sensitive hearing phenotype.
The key to discovering the function of otoferlin in humans eventually came from mouse models. Many of the organ systems and physiological mechanisms are remarkably similar among mice and humans, especially the inner ear (Friedman et al, 2007). Moreover, the human and mouse genomes show high sequence conservation. Finally, the ability to perform gene‐targeted mutagenesis and create mouse models to replicate human disease is unsurpassed in comparison with other model organisms.
Strenzke et al (2016) generated a novel Otof mutant mouse with a p.Ile515Thr point mutation, leading to a reduction but not complete loss of otoferlin in the organ of Corti. Auditory brainstem responses indicated a hearing loss and mild impairment of synchronous auditory signaling, with an apparent defect of the inner hair cell synapse, suggested by intact distortion product otoacoustic emissions. This mouse was then crossed with a knockout mimicking patients with a heat‐sensitive hearing loss. As in humans, these mice had an intermediate hearing defect, enabling a more detailed analysis of the function of otoferlin in synaptic sound encoding than in other Otof mutants that are profoundly deaf (Moser & Vogl, 2016).
Sound encoding is a process whereby a temporal sequence of sound frequencies is translated into a specialized format, that is, nerve activation patterns. Inner ear hair cell synapses encode the frequency content and intensity fluctuations of sound and rapidly transmit the information to spiral ganglion neurons with extreme temporal precision. Otoferlin plays a significant role in this synaptic transmission by tethering synaptic vesicles to the plasma membrane, exemplified by its presumed function as a Ca2+ sensor, although in its absence only short tethers are absent (Vogl et al, 2015). The authors were able to further define another critical factor in otoferlin function, namely reformation of synaptic vesicles from endocytosed membrane. Their measurements obtained at normal body temperature highlight the extremely high vesicle turnover rates at these synapses. Inner ear hair cell function and sound encoding appear to be directly related to the amount of plasma membrane otoferlin. Once this amount is reduced, as in OTOF patients, synaptic sound encoding is compromised, leading to a hearing loss.
As the mouse model appears to have a very similar phenotype compared to humans with the same mutations, it may have been a surprise that the heat exposure did not exacerbate the hearing of the mice as it does in humans. The goal was then to elucidate where the difference may come from. Differential isoforms were identified in humans and mice, which include an arginine‐rich 20‐amino acid stretch containing an RXR motif. An elegant transfection experiment revealed that this isoform, when expressed along with the p.Ile515Thr mutation, removed otoferlin from the plasma membrane. This contrasts the earlier description that the relative distribution of otoferlin between plasma membrane and cytoplasm remains unchanged in p.Ile515Thr mice. It seems remarkable that such a small stretch of DNA would dictate the difference in heat sensitivity to hearing loss in humans and mice.
Fortunately, with the exception of this region that seems to cause the discrepancy of the heat‐sensitivity phenomena between human and mouse, the Otof mouse mutant provides a worthy model. It helps explain why patients hear fairly well—have normal sound sensitivity but have difficulties perceiving continuous sounds and speech. Even though the thresholds of individual spiral ganglion neurons—and behavioral thresholds—are normal, ABR amplitudes are reduced because of a reduction of spike rates and irregular action potential firing.
The work presented by Strenzke et al (2016) has clinical implications as well. Patients with auditory neuropathy, particularly those with heat sensitivity, may be misdiagnosed by conventional hearing tests that involve threshold or sensitivity testing. Thus, tests to measure how sound is encoded by the auditory system, such as the ability of neurons to adapt to sound or the ability to detect silent gaps in sound stimuli (“gap detection”), should be added to the diagnostic test repertoire. Moreover, therapies to help OTOF patients will require alternatives to conventional hearing aids, to address their problems with speech and sound processing.
See also: N Strenzke et al (December 2016) and C Vogl et al (December 2016)
References
- Adato A, Raskin L, Petit C, Bonne‐Tamir B (2000) Deafness heterogeneity in a Druze isolate from the Middle East: novel OTOF and PDS mutations, low prevalence of GJB2 35delG mutation and indication for a new DFNB locus. Eur J Hum Genet 8: 437–442 [DOI] [PubMed] [Google Scholar]
- Friedman LM, Dror AA, Avraham KB (2007) Mouse models to study inner ear development and hereditary hearing loss. Int J Dev Biol 51: 609–631 [DOI] [PubMed] [Google Scholar]
- Johnson CP, Chapman ER (2010) Otoferlin is a calcium sensor that directly regulates SNARE‐mediated membrane fusion. J Cell Biol 191: 187–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser T, Vogl C (2016) New insights into cochlear sound encoding. F1000 Res 5: 2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez‐Ballesteros M, del Castillo FJ, Martín Y, Moreno‐Pelayo MA, Morera C, Prieto F, Marco J, Morant A, Gallo‐Terán J, Morales‐Angulo C, Navas C, Trinidad G, Tapia MC, Moreno F, del Castillo I (2003) Auditory neuropathy in patients carrying mutations in the otoferlin gene (OTOF). Hum Mutat 22: 451–456 [DOI] [PubMed] [Google Scholar]
- Roux I, Safieddine S, Nouvian R, Grati M, Simmler MC, Bahloul A, Perfettini I, Le Gall M, Rostaing P, Hamard G, Triller A, Avan P, Moser T, Petit C (2006) Otoferlin, defective in a human deafness form, is essential for exocytosis at the auditory ribbon synapse. Cell 127: 277–289 [DOI] [PubMed] [Google Scholar]
- Starr A, Picton TW, Sininger Y, Hood LJ, Berlin CI (1996) Auditory neuropathy. Brain 119: 741–753 [DOI] [PubMed] [Google Scholar]
- Starr A, Sininger Y, Winter M, Derebery MJ, Oba S, Michalewski HJ (1998) Transient deafness due to temperature‐sensitive auditory neuropathy. Ear Hear 19: 169–179 [DOI] [PubMed] [Google Scholar]
- Strenzke N, Chakrabarti R, Al‐Moyed H, Müller A, Hoch G, Pangrsic T, Yamanbaeva G, Lenz C, Pan K‐T, Auge E, Geiss‐Friedlander R, Urlaub H, Brose N, Wichmann C, Reisinger E (2016) Hair cell synaptic dysfunction, auditory fatigue and thermal sensitivity in otoferlin Ile515Thr mutants. EMBO J 35: 2519–2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga R, Kelley PM, Keats BJ, Starr A, Leal SM, Cohn E, Kimberling WJ (2003) Non‐syndromic recessive auditory neuropathy is the result of mutations in the otoferlin (OTOF) gene. J Med Genet 40: 45–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogl C, Cooper BH, Neef J, Wojcik SM, Reim K, Reisinger E, Brose N, Rhee JS, Moser T, Wichmann C (2015) Unconventional molecular regulation of synaptic vesicle replenishment in cochlear inner hair cells. J Cell Sci 128: 638–644 [DOI] [PubMed] [Google Scholar]
- Vogl C, Panou I, Yamanbaeva G, Wichmann C, Mangosing SJ, Vilardi F, Indzhykulian AA, Pangršič T, Santarelli R, Rodriguez‐Ballesteros M, Weber T, Jung S, Cardenas E, Wu X, Wojcik SM, Kwan KY, Del Castillo I, Schwappach B, Strenzke N, Corey DP et al (2016) Tryptophan‐rich basic protein (WRB) mediates insertion of the tail‐anchored protein otoferlin and is required for hair cell exocytosis and hearing. EMBO J 35: 2536–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasunaga S, Grati M, Cohen‐Salmon M, El‐Amraoui A, Mustapha M, Salem N, El‐Zir E, Loiselet J, Petit C (1999) A mutation in OTOF, encoding otoferlin, a FER‐1‐like protein, causes DFNB9, a nonsyndromic form of deafness. Nat Genet 21: 363–369 [DOI] [PubMed] [Google Scholar]
- Yasunaga S, Grati M, Chardenoux S, Smith TN, Friedman TB, Lalwani AK, Wilcox ER, Petit C (2000) OTOF encodes multiple long and short isoforms: genetic evidence that the long ones underlie recessive deafness DFNB9. Am J Hum Genet 67: 591–600 [DOI] [PMC free article] [PubMed] [Google Scholar]


