ABSTRACT
Despite effective targeted therapy acting on KIT and PDGFRA tyrosine kinases, gastrointestinal stromal tumors (GIST) escape treatment by acquiring mutations conveying resistance to imatinib mesylate (IM). Following the identification of NKp30-based immunosurveillance of GIST and the off-target effects of IM on NK cell functions, we investigated the predictive value of NKp30 isoforms and NKp30 soluble ligands in blood for the clinical response to IM. The relative expression and the proportions of NKp30 isoforms markedly impacted both event-free and overall survival, in two independent cohorts of metastatic GIST. Phenotypes based on disbalanced NKp30B/NKp30C ratio (ΔBClow) and low expression levels of NKp30A were identified in one third of patients with dismal prognosis across molecular subtypes. This ΔBClow blood phenotype was associated with a pro-inflammatory and immunosuppressive tumor microenvironment. In addition, detectable levels of the NKp30 ligand sB7-H6 predicted a worse prognosis in metastatic GIST. Soluble BAG6, an alternate ligand for NKp30 was associated with low NKp30 transcription and had additional predictive value in GIST patients with high NKp30 expression. Such GIST microenvironments could be rescued by therapy based on rIFN-α and anti-TRAIL mAb which reinstated innate immunity.
KEYWORDS: Cancer, immunity, B7-H6, GIST, NK cells, NKp30/NCR3
Abbreviations
- AWD
Alive with disease
- EFS
Event-free survival
- GIST
Gastrointestinal stromal tumor
- IFN-γ
Interferon gamma
- IM
Imatinib mesylate
- NED
Non-evolutive disease
- NK
Natural killer cells
- NKp30/NCR3
Natural cytotoxicity receptor p30
- NKp46/NCR1
Natural cytotoxicity receptor p46
- OS
Overall survival
- PFS
Progression-free survival
- TKI
Tyrosine kinase inhibitor
- TIL
Tumor-infiltrating lymphocytes
- TNF-α
Tumor necrosis factor α
Introduction
Gastrointestinal stromal tumors (GIST) are the most frequent mesenchymal tumor of the gastrointestinal tract, with an annual incidence of around 10–20 cases per million.1 GIST are characterized by activating mutations in KIT or PDGFRA oncogenes, the key molecular drivers of the disease pathogenesis.2 Approximately 75–80% of GIST harbor a KIT mutation, most frequently in the juxtamembrane domain in exon 11, and sometimes in the extracellular domain (exon 9) or in the kinase domain (exons 13 and 17).3 In 2001, the introduction of IM a small molecule inhibitor of KIT and PDGFRA tyrosine kinases, has dramatically improved the prognosis of GIST patients, with the best clinical responses observed in patients bearing the exon 11 KIT mutation.4,5 Hence, IM has become a paradigmatic targeted molecular therapy in cancer and the standard of care for advanced localized and metastatic GIST.6 However, IM is not curative in most GIST patients, requiring continuous treatment in advanced phase, and often associated with the emergence of resistant clones with secondary KIT or PDGFRA mutations.7
Over the last decade, we reported the off-target effects of IM on the immune system. IM targets KIT receptors on dendritic cells (DC), unleashing the DC/NK cell cross-talk, leading to NK cell activation with Th1 cytokine release and enhanced cytotoxicity in mice and humans.8 We previously reported that by 2 months of IM, the increased IFN-γ secretion from circulating NK cells correlated with prolonged survival in metastatic GIST patients treated by IM.9 A comprehensive NK phenotyping in peripheral blood and tumor-infiltrating lymphocytes of GIST revealed that the NK cell cytotoxic receptor NKp30 was specifically downregulated in this disease.9,10 Subsequently, we demonstrated that the alternative splicing of NKp30 exon 4, which affects the signaling and function of NKp30, determined the prognosis of metastatic GIST patients treated with IM.10 There are three major NKp30 isoforms, each one with a specific intracytoplasmic domain, i.e. NKp30A, NKp30B and NKp30C. NKp30A and NKp30B signaling mediates cytotoxicity and IFN-γ/TNF-α production respectively, while the NKp30C isoform induces the production of the immunosuppressive cytokine IL-10. The relative abundance of the mRNA encoding the NKp30C isoform compared to isoform A or B negatively impacts the prognosis of metastatic GIST patients treated with IM.10 Importantly, GIST tumors are infiltrated with NK cells, which mostly reside in peri-tumoral areas and predict event-free-survival in localized stages treated with adjuvant IM.11
Besides NK cells, other immune cells impact the course of GIST. CD4+ and CD8+ T cells, regulatory T cells (Treg) and macrophages have been shown to infiltrate GIST.12,13 In addition, IM induces the apoptosis of Tregs via the inhibition of IDO in KIT exon 11 mutated patients, thus enhancing the CD8+ T cell/Treg ratio and contributing to the intra-tumoral re-location of NK cells.11,14 Taken together, it is clear that the immune system plays a critical role in GIST during oncogene desaddiction by IM.
The aim of the present study was to explore further predictive biomarkers of response to IM pertaining to the NKp30/NKp30 ligand interaction in metastatic GIST. Here, we validated in two independent cohorts that the ratios between activating and inhibiting NKp30 isoforms (ΔBC) predict clinical outcome to IM therapy in metastatic GIST, and we demonstrated that the transcription levels of the NKp30 isoforms impact survival in localized and metastatic GIST patients independently of the KIT mutational status. In addition, ΔBChigh phenotypes contained higher levels of Th1 cytokines in-situ, while ΔBClow tumors released inflammatory and immunosuppressive cytokines and chemokines. Moreover, we show for the first time that the soluble NKp30 ligands sB7-H6 and sBAG6 alone or combined with the transcription levels of NKp30 isoforms, also impact event-free survival in IM-treated metastatic GIST. Altogether, we conclude that the efficacy or resistance of IM can be predicted in distinct subsets of GIST based on four predictors—ΔBC ratio, NKp30 transcription levels, sB7-H6 and sBAG6. Finally, rIFN-α2a and anti-TRAIL mAb could restore NK cell IFN-γ release in tumor beds presenting with NK functional defects. Thus, NKp30 isoforms and NKp30 ligands act as valuable biomarkers predictive of IM efficacy in metastatic GIST. The use of these predictive biomarkers could help stratify patients destined to benefit from combinatorial regimen involving oncogene desaddiction and immunomodulation.
Results
Proportion of NKp30 isoforms predict survival in GIST patients
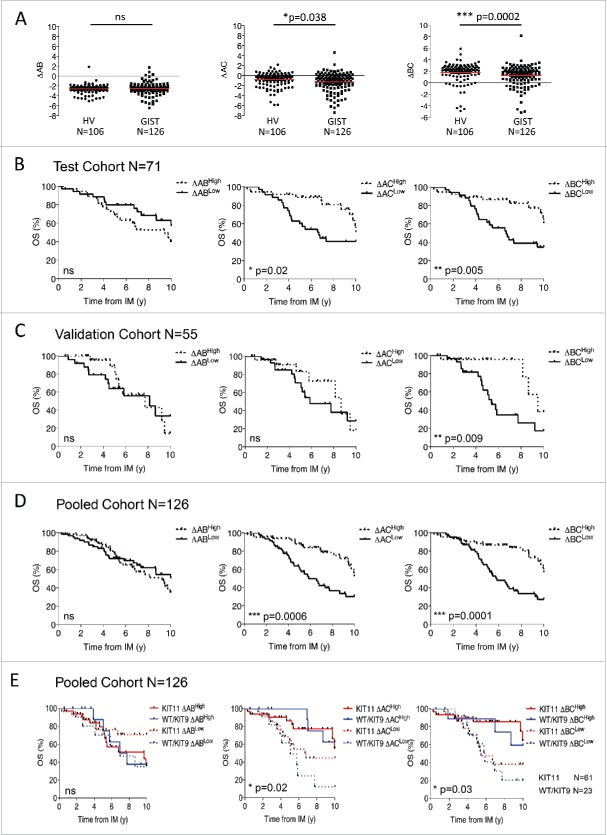
We have previously reported that NKp30 isoform profiles predict the overall survival of metastatic GIST patients.10 These profiles were originally determined by the unsupervised hierarchical clustering of the proportion of the transcriptional level of each NKp30 isoform. However, variable expression of the three NKp30 isoforms (as already known for KIR variegated expression among NK cells) was detected in single NK cells sorted from blood of healthy donors, with the majority of NK cells expressing only one or two NKp30 isoforms (not shown). Therefore, we assessed the relative expression of NKp30 isoforms compared with each other. We calculated the relative expression of the three isoforms (A, B or C) using the “delta” formula ΔNKp30x NKp30y = Ct NKp30y - Ct NKp30x. Importantly, this NKp30 cell profiling determined at time of diagnosis prior to IM therapy is stable over time in a longitudinal follow up for a given patient (Fig. S1A). Interestingly, ΔBC and to a lesser extent ΔAC were significantly lower in metastatic GIST (Table S1) compared with healthy volunteers (HV) while ΔAB was in the same range (Fig. 1A). Moreover, the ΔBC value, defined according to the median level of the cohort (Table S1), best predicted the overall survival of metastatic GIST from the start of IM therapy in both the test cohort (Fig. 1B) and the validation cohort (Fig. 1C). Expectedly, in the whole metastatic GIST cohort of 126 patients, the ΔBC and ΔAC predicted not only the overall survival (Fig. 1D) but also the event-free survival (Fig. S1B) of metastatic GIST at the time of IM therapy, independently of the KIT mutational status (Fig. 1E). Thus, ΔBClow and to some extent ΔAClow NKp30 profiling is associated with poor prognosis despite IM therapy.
Figure 1.
NKp30 isoform Δ ratios predict overall survival in metastatic GIST patients. (A) The NKp30 ΔAB (left panel), ΔAC (middle panel) and ΔBC ratios (right panel) of healthy volunteers (HV, n = 106) and of metastatic GIST patients (n = 126) are shown, non-parametric Mann–Whitney test. (B)–(D). Overall survival from initial imatinib mesylate (IM) treatment of GIST patients according to the median value of ΔAB (left), ΔAC (middle) and ΔBC ratios (right), were assessed by univariate analysis using the Kaplan–Meier method in a test cohort (n = 71, B), validation cohort (n = 55, C) and pooled cohort (n = 126, D). (E) Kaplan–Meier curves of GIST patient overall survival based on the combination of NKp30 Δ ratios and the KIT11 mutational status are shown for the pooled cohort (n = 126), Log-rank (Mantel–cox). *p < 0.05, **p < 0.01, ***p < 0.001. ns = not significant.
The ΔBC phenotype is associated with the tumor immune microenvironment
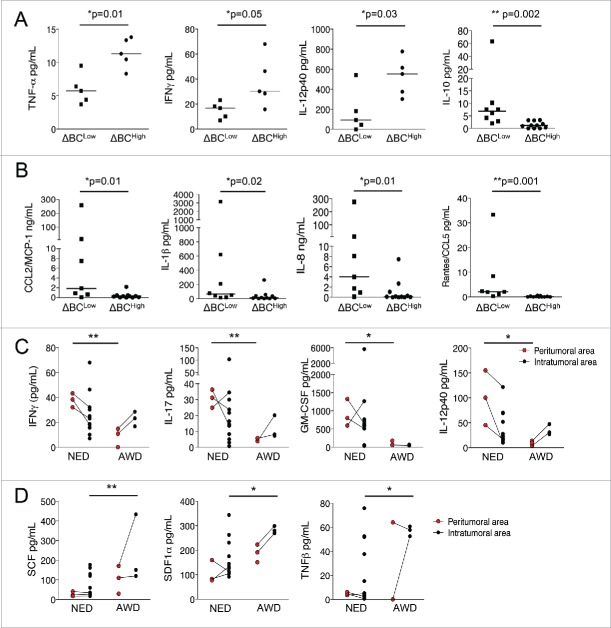
We next investigated the significance of the ΔBC phenotype on the GIST microenvironmental immune tone determined in situ by measuring cytokine and chemokine release from live tumor specimen and by multiplex arrays after laser microdissection of stromal (where NK reside) versus tumor nests. Interestingly, fresh GIST specimens (Table S2) harvested from ΔBChigh patients exhibited significantly higher levels of TNF-α, IFN-γ and IL-12p40 than tumor samples from ΔBClow patients (Fig. 2A), demonstrating the role of NKp30A and NKp30B isoforms in dictating a Th1 profile in situ. In contrast, a pro-inflammatory cytokine/chemokine pattern composed of CCL2/MCP-1, CCL3/MIP-1α (not shown), IL-1β, IL-8 and CCL5 was preferentially released from ΔBClow GIST tumors (Fig. 2B). In addition, higher levels of IL-10 were detected in the tumor fluid of ΔBClow patients (Fig. 2A), confirming the implication of IL-10 with the preferential transcription of the NKp30C isoform in situ.10
Figure 2.
The pro-inflammatory milieu, but not Th1 cytokines are associated with ΔBCLow GIST patients. (A) Cytokine secretion (TNF-α, IFN-γ, IL-12p40) was assessed from frozen tumor samples obtained from ΔBCHigh (n = 5) and ΔBCLow (n = 5) GIST patients, compared using unpaired t-test. (B) Chemokine (CCL2/MCP-1 and CCL5) and cytokine (IL-1β, IL-8 and IL-10 (shown in A)) concentrations were measured from tumor samples fluids of ΔBCHigh (n = 11) and ΔBCLow (n = 7) GIST patients, normalized per mg of total protein/mL (as detailed in M&M) and compared using unpaired t-test. (C)–(D) Cytokine and chemokine secretion, namely IFN-γ, IL-17, GM-CSF, and IL-12p40 (C) as well as SCF, SDF-1α and TNF-β (D), were assessed from micro-dissected stromal and intra-tumoral frozen GIST samples. GIST patients were stratified according to their clinical status, NED—non-evolutive disease (n = 10) and AWD—alive with disease (n = 4), at a median time of 1.2 y post-surgery, Mann–Whitney test. *p < 0.05, **p < 0.01.
Using laser micro-dissection of frozen GIST samples to compare the cytokine pattern of peri-tumoral vs. intra-tumoral areas, we analyzed GIST bearing a bad prognosis (alive with disease (AWD) at a median time of 20 months post-surgery) compared with GIST bearing a favorable prognosis (non-evolutive disease (NED), Table S2). The peri-tumoral area in GIST samples from NED patients contained higher amounts of IFN-γ, IL-17, GM-CSF and IL-12p40 (Fig. 2C) compared with samples from AWD patients (that are mostly ΔBClow). In contrast, inflammatory cytokines and chemokines including SCF, SDF1α and TNF-β (Fig. 2D) (with a trend for G-CSF, M-CSF and CCL27/CTACK (not shown)) were detected in the intra-tumoral area of AWD and ΔBClow GIST patients. These data thereby suggest a contribution of NK cells in dictating the microenvironmental tone of the peri-tumoral area, where they reside at diagnosis.11
Relative expression levels of NKp30 isoforms predict survival in GIST patients
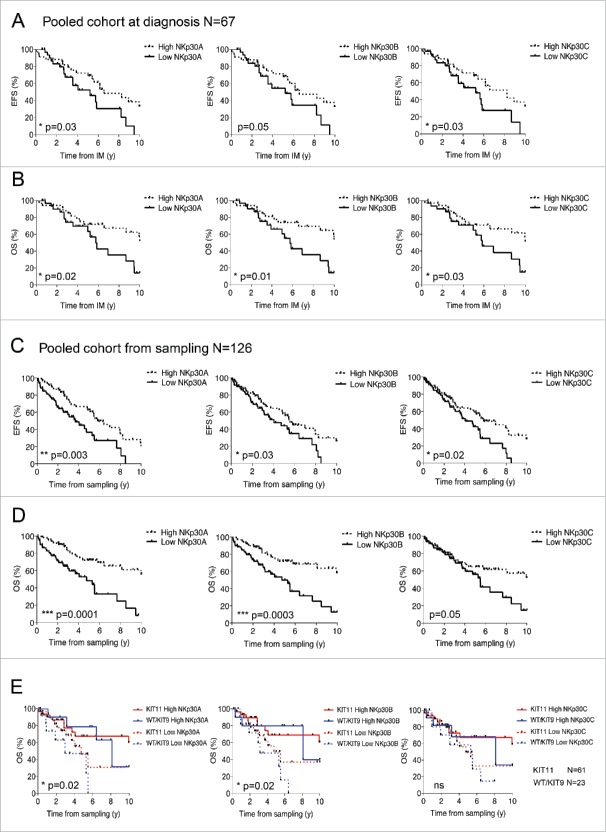
To further analyze the exact contribution of each NKp30 isoform in the dismal prognosis associated with the ΔBClow GIST phenotype, we assessed the transcriptional levels of NKp30A, NKp30B and NKp30C isoforms, normalized to β-2 microglobulin, in peripheral blood mononuclear cells (PBMCs) of 126 metastatic GIST (Table S1), compared with 106 age- and sex-matched HV. The relative expression of NKp30A, B, or C gene products determined at time of diagnosis prior to IM therapy was found to be stable over time, with or without IM, in a longitudinal follow up for a given patient (Fig. S1C). GIST patients presented a deficiency in the expression of all three isoforms (Fig. S2A). The expression levels of NKp30 isoforms correlated with each other but did not reflect the percentages of circulating NK cells ranging from 2–52% of leukocytes (Fig. S3A–B). We then analyzed which of these isoforms are mostly affected in the ΔBClow phenotype of metastatic GIST compared with HV and localized GIST (Table S1). Contrary to the normal pattern where ΔBClow can be attributed to high NKp30C transcription levels (Fig. S2B–C), the ΔBClow phenotype of metastatic GIST is attributable to a drop in the transcription of both NKp30A and NKp30B (Fig. S2D). Next, we analyzed the prognostic value of each individual isoform in the clinical outcome with IM in two independent cohorts of metastatic IM-treated GIST patients (Table S1). The mRNA expression levels of each NKp30 isoform measured at diagnosis (n = 67, Fig. 3A–B) or at time of sampling (n = 126, Fig. 3C–D and Fig. S4) impacted both event free survival (Fig. 3A and C) and overall survival (Fig. 3B, D) if GIST patients were stratified in two groups, based on the criterion of NKp30 isoform expression below or above the median level of both cohorts. Hence, high levels of NKp30A, NKp30B and NKp30C predicted favorable survival of metastatic GIST patients from the time of IM therapy or sampling in both test cohort and validation cohort (Fig. S4B–C). Overall, the prognostic value of NKp30 isoform expression levels on the whole cohort was highly significant and independent of the mutational status of the proto-oncogene KIT (Fig. 3E). To assess the specificity of the prognostic value of NKp30, we analyzed the expression of another NK cell activating receptor NKp46 on a subgroup of 71 GIST patients. Although NKp30 and NKp46 expression levels correlated (Fig. S3B), NKp46 expression failed to influence the overall survival of metastatic GIST patients (Fig. S3C), while that of NKp30 remained predictive in this subgroup (shown for NKp30A, Fig. S3D).
Figure 3.
Relative expression levels of NKp30 isoforms predict EFS and OS in metastatic GIST patients. Event-free survival (A & C) and overall survival (B & D) of metastatic GIST patients according to the median relative expression of NKp30A (left), NKp30B (middle) and NKp30C (right) isoforms, was assessed at the start of imatinib mesylate (IM) therapy at diagnosis (A–B, n = 67) and at the time of sampling (C–D, n = 126) by univariate analysis using the Kaplan–Meier method. (E) Kaplan–Meier curves of overall survival based on combination of the relative expression of NKp30 isoforms with the KIT11 mutation status are shown for the pooled cohort (n = 126). *p < 0.05, **p < 0.01, ***p < 0.001 by Log-rank (Mantel–cox) test.
The prognostic value of NKp30A, NKp30B and NKp30C isoforms was also evaluated in a cohort of locally advanced GIST patients treated by adjuvant or neoadjuvant IM (n = 60, Table S1). Here again, the levels of NKp30A, NKp30B and NKp30C expression were statistically lower in localized GIST than in HV (Fig. S5A) and predicted progression-free survival from the time of IM therapy (Fig. S5B). However, in contrast to metastatic GIST, the NKp30 isoform expression levels were relevant for the prognosis of GIST harboring either a KIT WT or KIT exon 9 mutation, but not those harboring a mutation in KIT exon 11 (Fig. S5C).
Thus, high transcription levels of the NKp30 isoforms had a favorable impact on the survival of localized and metastatic GIST patients from the start, and in the course, of oncogene desaddiction.
Combined relative expression levels and proportions of NKp30 isoforms highlight a subgroup of metastatic GIST patients with poor prognosis
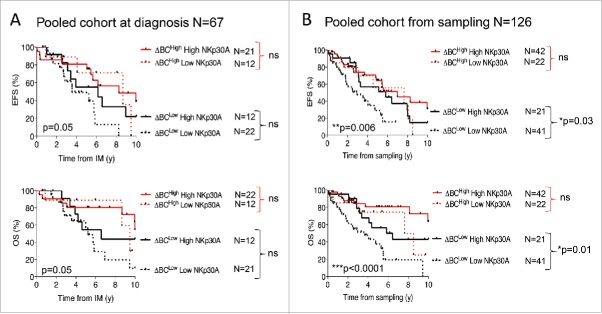
To analyze the prognostic significance of the quantitative and the qualitative distributions of NKp30 isoforms, we tested their coordinated impact on the prognosis in the whole cohort of metastatic GIST patients (Table S1). The ΔBC ratios, combined with low NKp30A (Fig. 4) but not NKp30B or NK30C (Fig. S6), remained prevailing biomarkers dictating GIST event-free survival and overall survival, when evaluated at diagnosis (n = 67, Fig. 4A, upper and lower panels) or at time of sampling (n = 126, Fig. 4B, upper and lower panels). In multivariate analysis using Cox model, only ΔBC ratio, and NKp30A (overall survival), or NKp30C (event-free survival) were retained as independent prognostic factors for survival from the date of sampling, while metastatic sites, nature of mutations, gender were not retained (Table 1).
Figure 4.
NKp30 ΔBC ratio combined with NKp30A isoform relative expression highlight a subgroup of metastatic GIST patients with poor prognosis. Kaplan–Meier curves of event free survival (upper panels) and overall survival (lower panels) obtained by stratifying the pooled metastatic GIST cohort (n = 126) from the beginning of imatinib mesylate (IM) treatment (A) or at the time of sampling (B), into four groups according to the median value of NKp30 ΔBC ratio with the median relative expression of NKp30A isoform. Of note, Fig. S6 shows segregation based on the median value of NKp30 ΔBC ratio with the median relative expression of NKp30B and C isoforms. **p < 0.01, ***p < 0.001 by Log-rank (Mantel–cox) test.
Table 1.
Multivariate analysis of prognosis factors for survival of metastatic GIST patients from the date of sampling (Cox model).
| Parameter | HR (95% CI) | p value | |
|---|---|---|---|
| Event-free survival | |||
| ΔBC | 0.53 (0.32–0.89) | 0.012 | |
| NKp30C | 0.53 (0.31–0.89) | 0.015 | |
| Overall survival | |||
| ΔBC | 0.46 (0.25–0.84) | 0.013 | |
| NKp30A | 0.38 (0.27–0.68) | 0.002 |
Taken together, the ΔBC status is the predominant prognosis biomarker for metastatic GIST, predicting both event-free survival and overall survival in response to IM therapy. For the poor prognostic subgroup harboring a ΔBClow phenotype, consideration of the relative expression of the NKp30A isoform has an added value to identify the most IM-resistant diseases.
Serum levels of soluble NKp30 ligands B7-H6 and BAG6 are biomarkers in metastatic GIST patients
We monitored the serum levels of two NKp30 ligands, sB7-H6 and sBAG6, in metastatic GIST patients at the time of diagnosis and following IM therapy (Table S1). Soluble B7-H6 was detected in the serum of GIST patients particularly prior to surgery (Fig. 5A, left panel), with more abundant levels in metastatic than in localized GIST patients (not shown), while sBAG6 was not associated with the tumor load (Fig. 5A, right panel). In addition, sB7-H6 levels, and not sBAG6, decreased with IM therapy after at least 6 months of administration (Fig. 5B, left and right panel respectively), suggesting that sB7-H6 concentrations reflect tumor burden. Of note, sB7-H6 and sBAG6 did not correlate with the KIT mutational status (not shown). The circulating levels of sB7-H6 at diagnosis were not associated with the NKp30 ΔBC ratio (Fig. 5C, left panel). In contrast, NK cells from patients with detectable Serum levels of sBAG6 expressed significantly lower levels of NKp30A, NKp30B, and to some extent NKp30C isoform mRNA in both localized (Fig. S7A) and metastatic GIST (Fig. S7B) and exhibited a higher probability to fall into the ΔBClow GIST subgroup (Fig. 5C, right panel). In GIST tumor lysates, sBAG6 levels were even higher than the paired plasma concentrations (Fig. S7C), suggesting a gradient from the tumor microenvironment to the lymphoid organs. However, sBAG6 in plasma was not associated with tumor-derived exosomes.15 Indeed the ultracentrifugation of plasma samples from GIST patients allowed the detection of the soluble but not exosomal forms of BAG6 (Fig. S7D).
Figure 5.
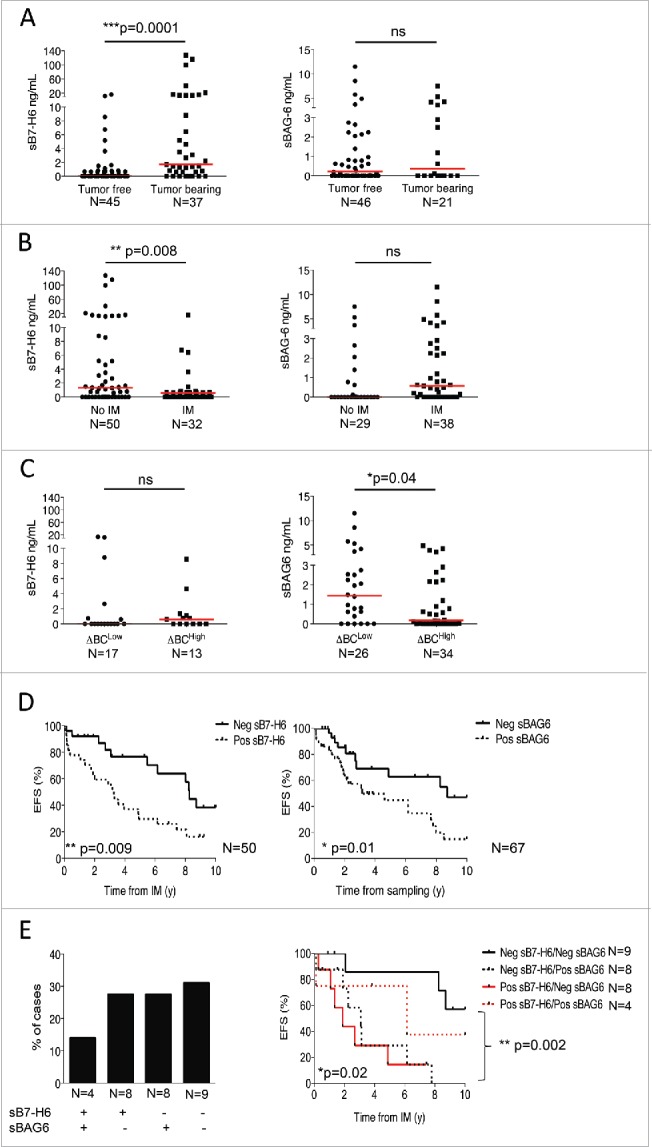
Soluble NKp30 ligands, B7-H6 and BAG6, are biomarkers in metastatic GIST patients. (A)–(C) Levels of sB7-H6 (left panel) and sBAG6 (right panel) measured in metastatic GIST patient serum as related to tumor resection (A), to IM therapy (B) or to the ΔBC ratio (C). (D) Event-free survival of metastatic GIST patients from the time of IM according to the median value of sB7-H6 (n = 50, left panel) and from the time of sampling in the course of IM therapy according to the median value of sBAG6 (n = 67, right panel) was assessed using the Kaplan–Meier method. (E) Representative frequency of metastatic GIST patient presenting no, either one, or both soluble NKp30L at diagnosis, prior to IM therapy (n = 29, left panel). Kaplan–Meier curves of event free survival of 29 metastatic GIST patients from the time of IM according to serum levels of both NKp30 ligands *p < 0.05, **p < 0.01, ***p < 0.001, ns—not significant by non-parametric Mann–Whitney test in (A)–(C); *p < 0.05, **p < 0.01 using the Kaplan–Meier method in (D)–(E).
More importantly, detectable levels of sB7-H6 assessed at diagnosis in 50 cases, before IM therapy, predicted short event-free survival in metastatic GIST patients (Fig. 5D, left panel), independently of the KIT mutational status (not shown). In addition, detectable levels of sBAG6, found in 12 out of 29 of cases at diagnosis failed to predict event-free survival in this small number of metastatic GIST for whom serum was available prior to IM therapy (not shown) but predicted dismal prognosis at any time of sampling during the course of IM administration (Fig. 5D, right panel). In 29 metastatic GIST patients for whom sB7-H6 and sBAG6 levels were evaluable, a minority (4/29) of GIST patients exhibited detectable levels of both sB7-H6 and sBAG6, while most cases presented with the exclusive presence of either one of these NKp30 agonists (Fig. 5E, left panel). Patients presenting no circulating soluble NKp30 ligands (9/29) were of excellent prognosis with IM therapy (Fig. 5E, right panel). The sB7-H6 was the strongest soluble parameter to predict event-free survival, but sBAG6 added prognostic value to this favourable sB7-H6-negative patient subgroup, representing 47% of patients with dismal prognosis (Fig. 5E, right panel).
As controls, the levels of two NKp30-independent markers such as soluble thrombospondin (a marker of Treg induction 16) and osteopontin (a marker of tumor progression 17) had no impact on the event-free survival of metastatic GIST in response to IM (Fig. S8).
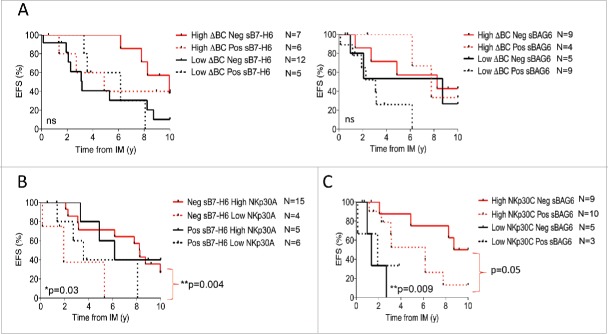
Predictive algorithm of all NKp30-related predictors in metastatic GIST
Importantly, the serum levels of NKp30 ligands did not add any predictive value to the ΔBC ratio (Fig. 6A). In the favorable-prognosis subgroup of sB7-H6 negative individuals, a minority of 13% of patients presenting with low NKp30A (with a trend for NKp30B and NKp30C) transcripts exhibited a shorter event-free survival (Fig. 6B, Fig. S9A, left and right panels). In contrast, sBAG6 added prognostic value to the high NKp30C transcription level subgroup (and to a lesser extent to the high NKp30A and NKp30B transcription subgroups), representing a subset of 26% of patients with dismal prognosis (Fig. 6C, Fig. S9B, left and right panels).
Figure 6.
Event-free survival in metastatic GIST can be best predicted by the coordinated analysis of several NKp30-related parameters. (A) Event-free survival of metastatic GIST patients from the time of IM according to the ΔBClow phenotype and the median values of sB7-H6 (n = 30, left panel) or sBAG6 (n = 27, right panel) was assessed using the Kaplan–Meier method. (B)–(C) Analysis of predictive interactions between the relative expression levels of NKp30 isoforms and serum levels of NKp30 ligands. Event-free survival of metastatic GIST patients from the time of IM according to the sB7-H6 levels and NKp30A relative expression (B) and the median value of the levels of NKp30 isoforms C and sBAG6 levels (C), using the Kaplan–Meier method. Of note, Fig. S10 shows EFS of NKp30L levels with the additional NKp30 isoforms. *p < 0.05, **p < 0.01, ns: not significant by Log-rank (Mantel–Cox) test.
GIST TILs respond to ex vivo stimulation with rIFN-α or anti-TRAIL mAb
We tested the capacity of various immunomodulatory molecules to reinstate local immunity in TILs, including NK cells, with particular attention to ΔBClow phenotypes. Seven primary GIST tumors harvested post-surgery (Table S2) were immuno-phenotyped by flow cytometry to characterize TILs. The frequencies of CD45+ cells (ranging from 10 to 65% (Fig. S10A) comprised various percentages of NK cells (from 1.4% to 11.1% of CD3−CD56+/CD45+, Fig. S10B) and T cells, essentially CD4+ T cells (Fig. S10C), ranging from 14 to 38% of CD45+ cells (not shown)). Most GIST-derived TILs were capable of producing IFN-γ (four out of seven, Fig. S10D) and TNF-α (five out of seven, Fig. S10E) at baseline upon short stimulation with PMA/ionomycin, independently of GIST tumor invasion and T or NK cell frequencies.
TILs were incubated for 24 h with either rIFN-α, the TLR3 ligand Poly I:C, neutralizing anti-FasL mAb, anti-TRAIL mAb or anti-IL-10 mAb (or the corresponding isotype controls), then analyzed for their intracellular concentrations of IFN-γ and TNF-α by flow cytometry. IFN-α2a induced a 2- to 3-fold increase of IFN-γ+ NK cells (in five out of six patients, Fig. S10F). In addition, anti-TRAIL mAb stimulated NK cells (Fig. S10F), CD4+ T cells (Fig. S10G) and, in one patient, CD8+ T cells to produce IFN-γ with up to 6-fold increase in IFN-γ+ NK cells (Fig. S10H). T cell proliferation was monitored after 5 d of stimulation and showed an increase in KI67+CD4+ and KI67+CD8+ T cells following addition of anti-FasL, anti-TRAIL, and anti-IL-10 mAbs (Fig. S10I–J). Thus, TILs derived from ΔBChigh or ΔBClow GIST patients, can be activated ex vivo by a number of immune stimulators.
Discussion
Here, we report that the expression of NKp30 isoforms and soluble NKp30 ligands are strong predictive biomarkers of response to IM therapy in metastatic GIST. The NKp30 isoform ratio (measuring the predominance of the stimulatory NKp30B isoform over the suppressive NKp30C isoform in ΔBChigh patients) and the normal/high level of expression of NKp30 isoforms (NK cell fitness) were found to predict favorable event-free survival and overall survival in metastatic GIST following IM therapy in two distinct series of patients. Consistent with these observations, the soluble NKp30 ligands sB7-H6 and sBAG6, known to dampen NKp30-dependent NK cell functions,18,19 alone or in coordination, also negatively predicted GIST patient event-free survival. The ΔBC ratio and the serum levels of sB7-H6/sBAG6 appear to play somewhat overlapping roles in the predictive model of response to IM. In addition, the relative expression of the rare NKp30A transcripts had an additive value to the ΔBC ratio or to the soluble NKp30 ligand prognostic biomarkers, and helped segregate the cohort into subgroups highlighting those patients most resistant to IM. The correlation of a ΔBClow phenotype with a non-Th1, but pro-inflammatory tumor microenvironment confirms the role of NKp30 in dictating functional cross-talk between cells of the tumor microenvironment as previously shown in GIST 10 and reported by us in high-grade neuroblastoma.18 Encouragingly, the capacity of immunostimulatory molecules, such as IFN-α2a, to reactivate NK cells and T lymphocytes from bad prognosis ΔBClow patients ex vivo, emphasizes the potential benefit of future combination therapies combining immunotherapeutic strategies to oncogene desaddiction-targeted regimen to promote NK and T cell activity in GIST ΔBClow patients, or those patients relapsing after IM monotherapy.
Since tumor cells downregulate MHC class I molecules and express several stress-related ligands, NK cells were thought to be the major players in antitumor immune responses, as shown for virally infected cells.20 However, several murine studies using specific mAbs depleting NK or T cells pointed to a clear role of NK cells against metastases formation rather than in the control of primary tumors.21-23 The prognosis value of NK cell infiltrates in human malignancies has been investigated, using various NK cell-related markers (CD56, CD57, CD16, NKp46) in primary solid tumors after surgical resection. In nearly all the reports, NK cells were underrepresented in the inflammatory infiltrates,24 except in colorectal carcinoma 25 and in GIST 11 where they mostly resided in the peri-tumoral areas.11,26,27 NK cell functions were also seen to be repressed in tumor beds in breast and lung malignancies, due to a downregulation of their natural cytotoxic receptors.26,28,29 Hence, in most malignancies, NK cell abundance in TILs fails to correlate with clinical outcome.24,30-32 This is however a tumor-specific phenomenon, as NK cell receptors (NKp30 or KIR) strongly influenced the prognosis in RCC,33 GIST,11 neuroblastoma,18 and acute myeloid leukemia,34 over NK cells themselves. The biology underlying these different observations across tumor types remains unclear.
In the particular setting of GIST, the natural immune infiltrates, mainly composed of T cells, Tregs, macrophages and NK cells,11-13 are polarized by the standard GIST-targeted therapy, IM. Indeed, IM does activate NK cells to produce IFN-γ via activation of DC/NK cross-talk.8,9 Another BRAF tyrosine kinase inhibitor, vemurafenib, has been recently shown to directly promote NK cell functions that may contribute to the clinical response in melanoma patients.35
We show here that the fitness of NK cells in GIST relies on the ΔBC ratio (measuring the predominance of the stimulatory NKp30B isoform over the suppressive NKp30C isoform) and on the NKp30 mRNA transcriptional levels, independently of the KIT mutational status. The ΔBC ratio represented a strong prognosis biomarker of GIST outcome in metastatic patients. Our group has also demonstrated that the ΔBC ratio dictates the progression-free survival of high-risk neuroblastoma patients after induction chemotherapy.18 In addition, the transcriptional level of NKp30 not only predicted the event-free survival and overall survival of metastatic GIST, but also the progression-free survival of localized WT or KIT exon 9 mutated patients. The combination of both predictors, NKp30 relative isoform ratios and transcription levels, further identifies a subgroup of patients with dismal prognosis (i.e., ΔBClow with low transcription of NKp30 mRNA), who could benefit from a personalized immunotherapeutic strategy aimed at increasing NKp30 isoform transcription. Indeed, IL-2 or IL-15 are two efficient cytokines to upregulate all three NKp30 isoforms in a stoichiometric manner (SR, unpublished data).
In addition, sB7-H6 predicts the event-free survival of metastatic GIST patients independently of the KIT mutational status. We have previously shown that sB7-H6 levels inversely correlate with NKp30 surface expression, and with reduced IFN-γ release by NK cells upon recognition of B7-H6+ tumor target cells.18 Thus, sB7-H6 acts in the periphery, at least in part, by masking the NKp30 receptor and by reducing NKp30-dependent NK cell functions. In contrast to sB7-H6, sBAG6 levels were specifically associated with the downregulation of NKp30 isoform mRNA transcription, without causing it. It has been shown that sBAG6 inhibits NKp30-dependent functions of NK cells.15,36 The presence of sB7-H6 or sBAG6 in the serum detected at diagnosis or at sampling was associated with a reduction in the event-free survival of metastatic GIST patients on IM therapy in univariate analyses.
To combat this, the use of sB7-H6 and/or sBAG6-blocking mAbs, or NKp30-blocking mAbs for ΔBClow patients, could prevent the NKp30-NKp30 ligand-mediated immunosuppression of NK cells.37 B7-H6 bispecific T cell engagers or CAR T cells engineered with antibodies targeting B7-H6 are under investigation.38 A bispecific antibody recognizing NKp46 and a cancer antigen could by-pass the need for the versatile NKp30 receptor.
The correlation of a ΔBClow phenotype with a non-Th1, but pro-inflammatory tumor microenvironment demonstrates the role of NKp30 in dictating functional cross-talk between cells of the tumor microenvironment. More recently, IM was shown to polarize M1 macrophages into unfavorable M2 macrophages,39 a scenario that may worsen the tumor microenvironment and contribute to the poor clinical outcome of ΔBClow GIST patients.
Encouragingly, we also show that GIST-derived TILs, including those from poor-prognosis ΔBClow patients, can be reactivated to produce cytokines and to proliferate upon stimulation with IFN-α2a (as already shown in a pilot study in GIST patients 40), or with blocking mAbs to TRAIL, This emphasizes the potential to develop novel IM-immunotherapeutic combination strategies, which might benefit ΔBClow patients or patients that relapse under IM. In addition, as IM fails to reduce Treg levels in WT GIST, a combined therapy of IM with metronomic cyclophosphamide, anti-CTLA-4, or anti-IDO could have therapeutic benefit in this patient subgroup.14
GIST has come of age as another example of a malignancy entering the arena of immunomodulation, in which personalized immunotherapy can be envisioned based on NKp30 isoform profiling and NKp30 ligand recirculation.
Materials and methods
Patients and specimens
Patient characteristics are depicted in Table S1. The analysis of NKp30 isoform transcripts was performed on 60 localized and a total of 126 metastatic GIST (n = 71 for the test and n = 55 for the validation cohort), while the soluble NKp30 study was performed on 92 metastatic GIST patients with available serum sample. The study on fresh tumors was prospectively performed on 27 GIST patients enrolled for surgery (Table S2). Patient samples were provided by Gustave Roussy Institute (Villejuif, France), Leon Bérard center (Lyon, France), Georges-François Leclerc Center (Dijon, France), University Hospital Jean Minjoz (Besançon, France), Institute Mutualiste Montsouris (Paris, France) and European Hospital Georges Pompidou (Paris, France). The study was approved by the local ethic committee (2007-A00923-50) and informed written consent was obtained from patients. Clinical responses were assessed by computed tomography (CT) scan and the responses were classified according to RECIST.41 One hundred and six healthy volunteers were used as controls (EFS, Créteil and Besançon, France).
Peripheral blood mononuclear cells (PBMC)
PBMC were obtained by Ficoll-Hypaque density gradient (PAA Laboratories), washed twice in Dulbecco's phosphate-buffered saline (PBS, GIBCO Invitrogen), re-suspended in RLT buffer containing β-2 mercaptoethanol (Quiagen) to preserve the RNA quality, and stored at −80°C. Serum or plasma were obtained by centrifugation of whole blood or heparinized-blood respectively (2,000 rpm, 15 min), followed by a second centrifugation (20 min, 5,000 rpm) to remove platelets, and were stored at −80°C.
qRT-PCR
Total cellular RNA was isolated from PBMC and GIST tumor samples with the RNeasy kit (Qiagen) following the manufacturer's recommendations. First strand cDNA was synthesized from 1 µg of total RNA using SuperScript™ III Reverse Transcriptase (Invitrogen) and random primers (Promega) according to the manufacturer's recommendations.
For qRT-PCR, 5 μL of first-strand cDNA was mixed with 12.5 μL of 2X TaqMan Gene Expression Master Mix (Applied Biosystems). For NKp30 isoforms A, B, and C and NKp46: 0.75 μL primers (10 μM) and probe (5 μM); and for the β-2-microglobulin housekeeping gene (β2M): 0.5 μL of primers (10 μM) and probe (5 μM), were used in a final volume of 25 μL. A StepOnePlus System (Applied Biosystems) was used for temperature cycling and real time fluorescence measurement. The PCR conditions were as follows: initial incubation at 50°C for 2 min, denaturation at 95°C for 10 min, followed by 45 cycles at 95°C for 15 s, and 60°C for 1 min. PCR primers and TaqMan probes are listed in Table S3. The relative expression of the gene of interest was calculated with the 2−ΔCt method 42 normalized to the expression level of β2M, with the following formula: 2−ΔCt = 2(Ct[gene of interest] - Ct [housekeeping gene]) × 106. The level of expression of the distinct NKp30 isoforms compared with each other (delta, Δ) was determined using the following formula ΔNKp30x NKp30y = CtNKp30y - CtNKp30x, with Ct = Cycle threshold. The median of each NKp30 isoform pair delta (Δ) was used for the survival curve analysis.
sB7-H6 and sBAG6 serum detection by ELISA
Soluble B7-H6 and BAG6 were assessed from the sera and plasma of GIST patients by an ELISA method. For this, B7-H6 (17B1.3) or BAG6 (3E4) mAb were coated at 5 or 1.5 μg/mL in 0.1 M NaHCO3 solution or PBS respectively overnight at 4°C in 96-well Nunc-Immuno™ plates (Thermo Scientific). Blocking solution (PBS supplemented with 3% or 1% BSA) was then added for 1 h at room temperature or overnight at 4°C. After four washes with PBS plus 0.05% Tween 20, serial dilutions of sB7-H6, sBAG6, or serum samples were incubated for 2 h at room temperature. Then biotinylated B7-H6 (9G9.2, home-made, 1 μg/mL in PBS supplemented with 1% BSA) or polyclonal chicken anti-BAG6 (13pp2, 7.5 µg/mL in PBS, raised against recombinant BAG6 N-terminus) were added to each well for 1 h at room temperature. Streptavidin-conjugated HRP (Sigma Aldrich) or goat anti-chicken-POD was then added for 1 h at room temperature. For sB7-H6 detection, BD Optia TMB substrate (BD Biosciences) was incubated during 15–30 min at room temperature. For sBAG6, tetramethylbenzidine substrate (ThermoScientific; CatNo. #34029) was used and incubated for 20 min at 37°C. The reaction was stopped with 1 M HCl or 0.5 M H2SO4 and the optical density (OD) was measured at 450 nm with an Apollo LB 911 instrument (Berthold) or with a SpectraMax M4 (Molecular Devices).
GIST micro-dissection and chemokine studies
Chemokine and cytokine detection in frozen GIST patient tissue was performed from dissected tissue sections. Tissues were sectioned and dissected using a vacutome (Microm HM550 modified Leica AS LMD system). Hematoxilin staining was used to identify regions of interest. Small pieces of frozen tissue were transferred into 250 µL cold lysis buffer, vortexed, frozen at –80°C (10 min), and thawed on ice. After incubation in a cold ultrasonic bath (10 min), samples were frozen again at −80°C, thawed on ice and centrifuged (13,000 rpm, 20 min, 4°C). The protein concentration of the supernatant was determined and adjusted to 1,000 µg/mL using human serum diluent (BioRad). Cytokine and chemokine concentrations in tissue lysates were quantified by multiplex protein arrays, according to manufacturer's instructions (BioRad).
Statistical analyses
Data were analyzed with Prism 5 software (GraphPad San Diego, CA, USA) and the IBM SPSS 19 software (IBM, Bois-Colombe, France). The unpaired t-test and the non-parametric Mann–Whitney test were used for comparison of the different groups, as indicated in the Figure Legends. Correlations were analyzed by Spearman test, and survival curves were plotted according to the Kaplan–Meier method and compared by log-rank (Mantel–Cox) regression (univariate analysis) or by Firth's penalized-likelihood Cox regression (multivariate analysis). A p value of < 0.05 was considered statistically significant.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Ligue Nationale contre le Cancer (Equipe labellisée); the Foundation ISREC, Lausanne; the Paris Alliance of Cancer Research Institutes (PACRI), the SIRIC-Socrate (grant INCa-DGOS-INSERM 6043), SIRIC-LYRIC (grant INCa-DGOS-INSERM 4664), LABEX Immuno-Oncology, Labex DevweCan (ANR-10-LABX-0061), the NETSARC/RREPS networks, the Swiss Bridge Foundation and the foundation “Ensemble contre le GIST.” EV is supported by European Research Council (THINK advanced grant). KSR and EPvS were supported by the Deutsche Forschungsgemeinschaft (grant KFO286, RP4). SA is the recipient of the 2014 Georges Mathé prize (ESMO grant). We thank Dr Nicolas Delahaye and Dr Cédric Ménard for initiating the study and the tumorothèque of Gustave Roussy and Franche-Comté (BB-0033-00024) for patients sample collection. Thanks to all the participating patients.
References
- 1.Corless CL. Assessing the prognosis of gastrointestinal stromal tumors: a growing role for molecular testing. Am J Clin Pathol 2004; 122:11-3; PMID:15272524; http://dx.doi.org/ 10.1309/BUVLRQBVGU0N0L42 [DOI] [PubMed] [Google Scholar]
- 2.Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer 2011; 11:865-78; PMID:22089421; http://dx.doi.org/ 10.1038/nrc3143 [DOI] [PubMed] [Google Scholar]
- 3.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M et al.. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998; 279:577-80; PMID:9438854; http://dx.doi.org/ 10.1126/science.279.5350.577 [DOI] [PubMed] [Google Scholar]
- 4.Wozniak A, Rutkowski P, Schoffski P, Ray-Coquard I, Hostein I, Schildhaus HU, Le Cesne A, Bylina E, Limon J, Blay JY et al.. Tumor genotype is an independent prognostic factor in primary gastrointestinal stromal tumors of gastric origin: a european multicenter analysis based on ConticaGIST. Clin Cancer Res 2014; 20:6105-16; PMID:25294914; http://dx.doi.org/ 10.1158/1078-0432.CCR-14-1677 [DOI] [PubMed] [Google Scholar]
- 5.Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M et al.. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002; 347:472-80; PMID:12181401; http://dx.doi.org/ 10.1056/NEJMoa020461 [DOI] [PubMed] [Google Scholar]
- 6.ESMO . Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014; 25 Suppl 3:iii21-6; PMID:25210085; http://dx.doi.org/ 10.1093/annonc/mdu255 [DOI] [PubMed] [Google Scholar]
- 7.Pierotti MA, Tamborini E, Negri T, Pricl S, Pilotti S. Targeted therapy in GIST: in silico modeling for prediction of resistance. Nat Rev Clin Oncol 2011; 8:161-70; PMID:21364689; http://dx.doi.org/ 10.1038/nrclinonc.2011.3 [DOI] [PubMed] [Google Scholar]
- 8.Borg C, Terme M, Taieb J, Menard C, Flament C, Robert C, Maruyama K, Wakasugi H, Angevin E, Thielemans K et al.. Novel mode of action of c-kit tyrosine kinase inhibitors leading to NK cell-dependent antitumor effects. J Clin Invest 2004; 114:379-88; PMID:15286804; http://dx.doi.org/ 10.1172/JCI21102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menard C, Blay JY, Borg C, Michiels S, Ghiringhelli F, Robert C, Nonn C, Chaput N, Taïeb J, Delahaye NF et al.. Natural killer cell IFN-gamma levels predict long-term survival with imatinib mesylate therapy in gastrointestinal stromal tumor-bearing patients. Cancer Res 2009; 69:3563-9; PMID:19351841; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-3807 [DOI] [PubMed] [Google Scholar]
- 10.Delahaye NF, Rusakiewicz S, Martins I, Menard C, Roux S, Lyonnet L, Paul P, Sarabi M, Chaput N, Semeraro M et al.. Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumors. Nat Med 2011; 17:700-7; PMID:21552268; http://dx.doi.org/ 10.1038/nm.2366 [DOI] [PubMed] [Google Scholar]
- 11.Rusakiewicz S, Semeraro M, Sarabi M, Desbois M, Locher C, Mendez R, Vimond N, Concha A, Garrido F, Isambert N et al.. Immune infiltrates are prognostic factors in localized gastrointestinal stromal tumors. Cancer Res 2013; 73:3499-510; PMID:23592754; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-0371 [DOI] [PubMed] [Google Scholar]
- 12.Cameron S, Haller F, Dudas J, Moriconi F, Gunawan B, Armbrust T, Langer C, Füzesi L, Ramadori G. Immune cells in primary gastrointestinal stromal tumors. Eur J Gastroenterol Hepatol 2008; 20:327-34; PMID:18334877; http://dx.doi.org/ 10.1097/MEG.0b013e3282f3a403 [DOI] [PubMed] [Google Scholar]
- 13.van Dongen M, Savage ND, Jordanova ES, Briaire-de Bruijn IH, Walburg KV, Ottenhoff TH, Hogendoorn PC, van der Burg SH, Gelderblom H, van Hall T. Anti-inflammatory M2 type macrophages characterize metastasized and tyrosine kinase inhibitor-treated gastrointestinal stromal tumors. Int J Cancer 2010; 127:899-909; PMID:20013807; http://dx.doi.org/ 10.1002/ijc.25113 [DOI] [PubMed] [Google Scholar]
- 14.Balachandran VP, Cavnar MJ, Zeng S, Bamboat ZM, Ocuin LM, Obaid H, Sorenson EC, Popow R, Ariyan C, Rossi F et al.. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med 2011; 17:1094-100; PMID:21873989; http://dx.doi.org/ 10.1038/nm.2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reiners KS, Topolar D, Henke A, Simhadri VR, Kessler J, Sauer M, Bessler M, Hansen HP, Tawadros S, Herling M et al.. Soluble ligands for NK cell receptors promote evasion of chronic lymphocytic leukemia cells from NK cell anti-tumor activity. Blood 2013; 121:3658-65; PMID:23509156; http://dx.doi.org/ 10.1182/blood-2013-01-476606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baumgartner JM, Palmer BE, Banerjee A, McCarter MD. Role of Melanoma Secreted Thrombospondin-1 on Induction of Immunosuppressive Regulatory T Cells through CD47. J Cancer Mol 2008; 4:145-52 [Google Scholar]
- 17.Lee HJ, Yeon JE, Suh SJ, Lee SJ, Yoon EL, Kang K, Yoo YJ, Kim JH, Seo YS, Yim HJ et al.. Clinical utility of plasma glypican-3 and osteopontin as biomarkers of hepatocellular carcinoma. Gut Liver 2014; 8:177-85; PMID:24672660; http://dx.doi.org/ 10.5009/gnl.2014.8.2.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Semeraro M, Rusakiewicz S, Minard-Colin V, Delahaye NF, Enot D, Vely F, Marabelle A, Papoular B, Piperoglou C, Ponzoni M et al.. Clinical impact of the NKp30/B7-H6 axis in high-risk neuroblastoma patients. Sci Transl Med 2015; 7:283ra55; PMID:25877893; http://dx.doi.org/ 10.1126/scitranslmed.aaa2327 [DOI] [PubMed] [Google Scholar]
- 19.Schlecker E, Fiegler N, Arnold A, Altevogt P, Rose-John S, Moldenhauer G, Sucker A, Paschen A, von Strandmann EP, Textor S et al.. Metalloprotease-mediated tumor cell shedding of B7-H6, the ligand of the natural killer cell-activating receptor NKp30. Cancer Res 2014; 74:3429-40; PMID:24780758; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-3017 [DOI] [PubMed] [Google Scholar]
- 20.Seliger B, Ritz U, Ferrone S. Molecular mechanisms of HLA class I antigen abnormalities following viral infection and transformation. Int J Cancer 2006; 118:129-38; PMID:16003759; http://dx.doi.org/ 10.1002/ijc.21312 [DOI] [PubMed] [Google Scholar]
- 21.Aboud M, Kingsmore S, Segal S. Role of natural killer cells in controlling local tumor formation and metastatic manifestation of different 3LL Lewis lung carcinoma cell clones. Nat Immun 1993; 12:17-24; PMID:8431660 [PubMed] [Google Scholar]
- 22.Smyth MJ, Thia KY, Street SE, MacGregor D, Godfrey DI, Trapani JA. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J Exp Med 2000; 192:755-60; PMID:10974040; http://dx.doi.org/ 10.1084/jem.192.5.755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet 2000; 356:1795-9; PMID:11117911; http://dx.doi.org/ 10.1016/S0140-6736(00)03231-1 [DOI] [PubMed] [Google Scholar]
- 24.Desbois M, Rusakiewicz S, Locher C, Zitvogel L, Chaput N. Natural killer cells in non-hematopoietic malignancies. Front Immunol 2012; 3:395; PMID:23269924; http://dx.doi.org/ 10.3389/fimmu.2012.00395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sconocchia G, Zlobec I, Lugli A, Calabrese D, Iezzi G, Karamitopoulou E, Patsouris ES, Peros G, Horcic M, Tornillo L et al.. Tumor infiltration by FcgammaRIII (CD16)+ myeloid cells is associated with improved survival in patients with colorectal carcinoma. Int J Cancer 2011; 128:2663-72; PMID:20715106; http://dx.doi.org/ 10.1002/ijc.25609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Platonova S, Cherfils-Vicini J, Damotte D, Crozet L, Vieillard V, Validire P, Validire P, André P, Dieu-Nosjean MC, Alifano M et al.. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res 2011; 71:5412-22; PMID:21708957; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-4179 [DOI] [PubMed] [Google Scholar]
- 27.Fregni G, Messaoudene M, Fourmentraux-Neves E, Mazouz-Dorval S, Chanal J, Maubec E, Marinho E, Scheer-Senyarich I, Cremer I, Avril MF et al.. Phenotypic and functional characteristics of blood natural killer cells from melanoma patients at different clinical stages. PLoS One 2013; 8:e76928; PMID:24204708; http://dx.doi.org/ 10.1371/journal.pone.0076928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R, Gonçalves A, André P, Romagné F, Thibault G et al.. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest 2011; 121:3609-22; PMID:21841316; http://dx.doi.org/ 10.1172/JCI45816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carrega P, Morandi B, Costa R, Frumento G, Forte G, Altavilla G, Ratto GB, Mingari MC, Moretta L, Ferlazzo G. Natural killer cells infiltrating human nonsmall-cell lung cancer are enriched in CD56 bright CD16(−) cells and display an impaired capability to kill tumor cells. Cancer 2008; 112:863-75; PMID:18203207; http://dx.doi.org/ 10.1002/cncr.23239 [DOI] [PubMed] [Google Scholar]
- 30.Gannon PO, Poisson AO, Delvoye N, Lapointe R, Mes-Masson AM, Saad F. Characterization of the intra-prostatic immune cell infiltration in androgen-deprived prostate cancer patients. J Immunol Methods 2009; 348:9-17; PMID:19552894; http://dx.doi.org/ 10.1016/j.jim.2009.06.004 [DOI] [PubMed] [Google Scholar]
- 31.Marechal R, De Schutter J, Nagy N, Demetter P, Lemmers A, Deviere J, Salmon I, Tejpar S, Van Laethem JL. Putative contribution of CD56 positive cells in cetuximab treatment efficacy in first-line metastatic colorectal cancer patients. BMC Cancer 2010; 10:340; PMID:20591136; http://dx.doi.org/ 10.1186/1471-2407-10-340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin S, Deng Y, Hao JW, Li Y, Liu B, Yu Y, Shi FD, Zhou QH. NK cell phenotypic modulation in lung cancer environment. PLoS One 2014; 9:e109976; PMID:25299645; http://dx.doi.org/ 10.1371/journal.pone.0109976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Remark R, Alifano M, Cremer I, Lupo A, Dieu-Nosjean MC, Riquet M, Crozet L, Ouakrim H, Goc J, Cazes A et al.. Characteristics and clinical impacts of the immune environments in colorectal and renal cell carcinoma lung metastases: influence of tumor origin. Clin Cancer Res 2013; 19:4079-91; PMID:23785047; http://dx.doi.org/ 10.1158/1078-0432.CCR-12-3847 [DOI] [PubMed] [Google Scholar]
- 34.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F et al.. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002; 295:2097-100; PMID:11896281; http://dx.doi.org/ 10.1126/science.1068440 [DOI] [PubMed] [Google Scholar]
- 35.Ferrari de Andrade L, Ngiow SF, Stannard K, Rusakiewicz S, Kalimutho M, Khanna KK, Tey SK, Takeda K, Zitvogel L, Martinet L et al.. Natural killer cells are essential for the ability of BRAF inhibitors to control BRAFV600E-mutant metastatic melanoma. Cancer Res 2014; 74:7298-308; PMID:25351955; http://dx.doi.org/ 10.1158/0008-5472.CAN-14-1339 [DOI] [PubMed] [Google Scholar]
- 36.Pogge von Strandmann E, Simhadri VR, von Tresckow B, Sasse S, Reiners KS, Hansen HP, Rothe A, Böll B, Simhadri VL, Borchmann P et al.. Human leukocyte antigen-B-associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity 2007; 27:965-74; PMID:18055229; http://dx.doi.org/ 10.1016/j.immuni.2007.10.010 [DOI] [PubMed] [Google Scholar]
- 37.Reiners KS, Kessler J, Sauer M, Rothe A, Hansen HP, Reusch U, Hucke C, Köhl U, Dürkop H, Engert A et al.. Rescue of impaired NK cell activity in hodgkin lymphoma with bispecific antibodies in vitro and in patients. Mol Ther 2013; 21:895-903; PMID:23459515; http://dx.doi.org/ 10.1038/mt.2013.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu MR, Zhang T, Gacerez AT, Coupet TA, DeMars LR, Sentman CL. B7H6-Specific Bispecific T Cell Engagers Lead to Tumor Elimination and Host Antitumor Immunity. J Immunol 2015; 194:5305-11; PMID:25911747; http://dx.doi.org/ 10.4049/jimmunol.1402517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cavnar MJ, Zeng S, Kim TS, Sorenson EC, Ocuin LM, Balachandran VP, Seifert AM, Greer JB, Popow R, Crawley MH et al.. KIT oncogene inhibition drives intratumoral macrophage M2 polarization. J Exp Med 2013; 210:2873-86; PMID:24323358; http://dx.doi.org/ 10.1084/jem.20130875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen LL, Chen X, Choi H, Sang H, Chen LC, Zhang H, Gouw L, Andtbacka RH, Chan BK, Rodesch CK et al.. Exploiting antitumor immunity to overcome relapse and improve remission duration. Cancer Immunol Immunother 2012; 61:1113-24; PMID:22198309; http://dx.doi.org/ 10.1007/s00262-011-1185-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC et al.. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92:205-16; PMID:10655437; http://dx.doi.org/ 10.1093/jnci/92.3.205 [DOI] [PubMed] [Google Scholar]
- 42.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 2001; 25:402-8; PMID:11846609; http://dx.doi.org/ 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.